The Antibacterial Efficacy and In Vivo Toxicity of Sodium Hypochlorite and Electrolyzed Oxidizing (EO) Water-Based Endodontic Irrigating Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Electrolyzed Oxidizing (EO) Water
2.2. Antibacterial Efficacy
2.3. Zebrafish Embryo Toxicity Assays
2.4. Statistical Analysis
3. Results
3.1. Antibacterial Properties for HOCl and NaOCl
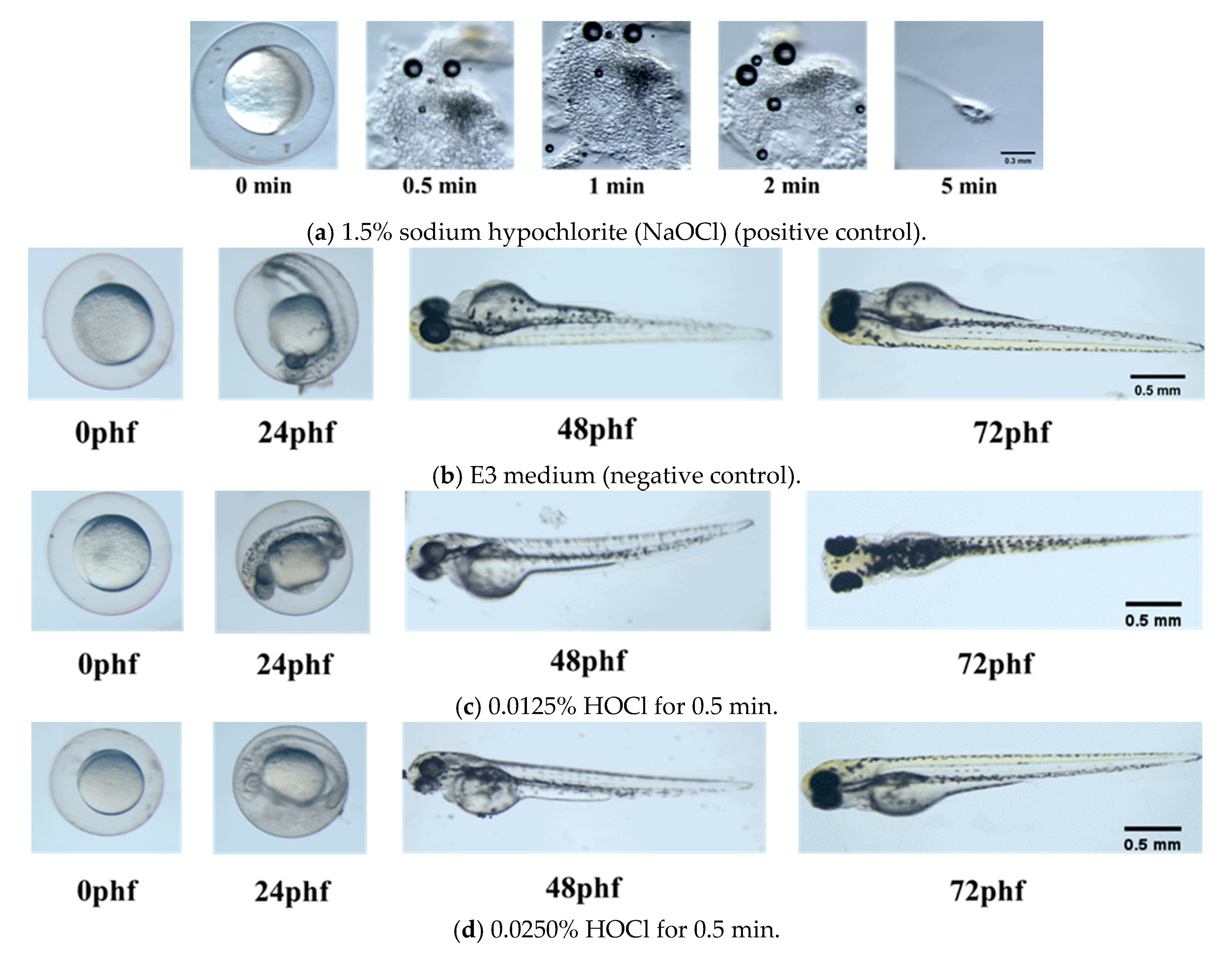
3.2. Zebrafish Embryonic Toxicity Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carrotte, P. Endodontics: Part 7. Preparing the root canal. Br. Dent. J. 2004, 197, 603–613. [Google Scholar] [CrossRef]
- Jurič, I.B.; Anić, I. The Use of Lasers in disinfection and cleanliness of root canals: A review. Acta Stomatol. Croat. 2014, 48, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Guide to Clinical Endodontics, 6th ed.; American Association of Endodontists: Chicago, IL, USA, 2016.
- Rahimi, S.; Janani, M.; Lotfi, M.; Shahi, S.; Aghbali, A.; Vahid Pakdel, M.; Salem Milani, A.; Ghasemi, N. A review of antibacterial agents in endodontic treatment. Iran. Endod. J. 2014, 9, 161–168. [Google Scholar] [PubMed]
- Hidalgo, E.; Bartolome, R.; Dominguez, C. Cytotoxicity mechanisms of sodium hypochlorite in cultured human dermal fibroblasts and its bactericidal effectiveness. Chem. Biol. Interact. 2002, 139, 265–282. [Google Scholar] [CrossRef]
- Crincoli, V.; Scivetti, M.; Di Bisceglie, M.B.; Pilolli, G.P.; Favia, G. Unusual case of adverse reaction in the use of sodium hypochlorite during endodontic treatment: A case report. Quintessence Int. 2008, 39, e70–e73. [Google Scholar]
- AAE Clinical Considerations for a Regenerative Procedure; American Association of Endodontists: Chicago, IL, USA, 2018; p. 1.
- Farren, S.T.; Sadoff, R.S.; Penna, K.J. Sodium hypochlorite chemical burn. Case report. N. Y. State Dent. J. 2008, 74, 61–62. [Google Scholar] [PubMed]
- Grawehr, M.; Sener, B.; Waltimo, T.; Zehnder, M. Interactions of ethylenediamine tetraacetic acid with sodium hypochlorite in aqueous solutions. Int. Endod. J. 2003, 36, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Giardino, L.; Palazzi, F.; Shalavi, S.; Alikhani, M.Y.; Giudice, G.L.; Davoodpour, N. Effect of sodium hypochlorite on the substantivity of chlorhexidine. Int. J. Clin. Dent. 2013, 6, 173–178. [Google Scholar]
- Rossi-Fedele, G.; Doğramaci, E.J.; Guastalli, A.R.; Steier, L.; de Figueiredo, J.A. Antagonistic interactions between sodium hypochlorite, chlorhexidine, EDTA, and citric acid. J. Endod. 2012, 38, 426–431. [Google Scholar] [CrossRef]
- Rahman, S.; Khan, I.; Oh, D.H. Electrolyzed Water as a Novel Sanitizer in the Food Industry: Current Trends and Future Perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef]
- Emswiler-Rose, B.; Kotula, A.W. Inhibition of bacterial growth by two chlorine sources in a model system. J. Food Sci. 1984, 49, 931–933. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Massarsky, A.; Kozal, J.S.; Di Giulio, R.T. Glutathione and zebrafish: Old assays to address a current issue. Chemosphere 2017, 168, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Legislation for the protection of animals used for scientific purposes. In Directive 2010/63/EU; European Commission: Brussels, Belgium, 2010.
- Rotstein, I.; Ingle, J.I. Ingle’s Endodontics, 7th ed.; PMPH USA: Raleigh, NC, USA, 2019; ISBN 978-1-60795-192-6. [Google Scholar]
- Asgary, S.; Fazlyab, M.; Sabbagh, S.; Eghbal, M.J. Outcomes of different vital pulp therapy techniques on symptomatic permanent teeth: A case series. Iran. Endod. J. 2014, 9, 295–300. [Google Scholar]
- Ricucci, D.; Siqueira, J.F., Jr.; Li, Y.; Tay, F.R. Vital Pulp: Therapy:hitsopathology and histobacteriology-based guidelines to treat teeth with deep caries and pulp exposure. J. Dent. 2019, 86, 41–52. [Google Scholar] [CrossRef]
- Guida, A. Mechanism of action of sodium hypochlorite and its effects on dentin. Minerva Stomatol. 2006, 55, 471–482. [Google Scholar]
- Al-Kilani, M.G.; Whitworth, J.M.; Dummer, P.M. Preliminary in vitro evaluation of Carisolv as a root canal irrigant. Int. Endod. J. 2003, 36, 433–440. [Google Scholar] [CrossRef]
- Cvek, M. A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J. Endod. 1978, 4, 232–237. [Google Scholar] [CrossRef]
- Munson, M.A.; Banerjee, A.; Watson, T.F.; Wade, W.G. Molecular analysis of the microflora associated with dental caries. J. Clin. Microbiol. 2004, 42, 3023–3029. [Google Scholar] [CrossRef]
- Garcia, S.S.; Blackledge, M.S.; Michalek, S.; Su, L.; Ptacek, T.; Eipers, P.; Morrow, C.; Lefkowitz, E.J.; Melander, C.; Wu, H. Targeting of streptococcus mutans biofilm by a novel small molecule prevents dental caries and preserves the oral microbiome. J. Dent. Res. 2017, 96, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Ng, Y.L.; Gulabivala, K.; Moles, D.R.; Spratt, D.A. Susceptibilties of two enterococcus faecalis phenotypes to root canal medications. J. Endod. 2005, 31, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tulsani, S.G.; Chikkanarasaiah, N.; Bethur, S. An in vivo comparison of antimicrobial efficacy of sodium hypochlorite and biopure MTAD against enterococcus faecalis in primary teeth: A qPCR study. J. Clin. Pediatr. Dent. 2014, 39, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Tian, Y.; Zhao, C.; Qu, T.; Ma, C.; Liu, X.; Yu, Q. Bactericidal effect of strong acid electrolyzedwater against flow enterococcus faecalis biofilms. J. Endod. 2016, 42, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Vissers, M.C.; Winterbourn, C.C. Living with a killer: The effects of hypochlorous acid on mammalian cells. IUBMB Life 2000, 50, 259–266. [Google Scholar] [CrossRef]
- Lapenna, D.; Cuccurullo, F. Hypochlorous acid and its pharmacological antagonism: An update picture. Gen. Pharmacol. 1996, 27, 1145–1147. [Google Scholar] [CrossRef]
- Barrette, W.C.; Hannum, D.M.; Wheeler, W.D.; Hurst, J.K. General mechanism for the bacterial toxicity of hypochlorous acid: Abolition of ATP production. Biochemistry 1989, 28, 9172–9178. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.; Murray, P.E.; Garcia-Godoy, F.; Namerow, K.N. Effect of aquatine endodontic cleanser on smear layer removal in the root canals of ex vivo human teeth. J. Appl. Oral Sci. 2010, 18, 403–408. [Google Scholar] [CrossRef]
- Gomi, K.; Makino, T.; Suzuki, S.; Hasegawa, M.; Maeda, N.; Arai, T. Microbicidal and cytotoxic effects of functional water in vitro. Quintessence Int. 2010, 41, e166–e172. [Google Scholar]
- Nocca, G.; Ahmed, H.M.A.; Martorana, G.E.; Callà, C.; Gambarini, G.; Rengo, S.; Spagnuolo, G. Chromographic Analysis and Cytotoxic Effects of Chlorhexidine and Sodium Hypochlorite Reaction Mixtures. J. Endod. 2017, 43, 1545–1552. [Google Scholar] [CrossRef]
- Hargreaves, K.M.; Geisler, T.; Henry, M.; Wang, Y. Regeneration potential of the young permanent tooth: What does the future hold? J. Endod. 2008, 34, S51–S56. [Google Scholar] [CrossRef]
- Matalová, E.; Lungová, V.; Sharpe, P. References in The Chapter 26—Development of Tooth and Associated Structures. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Academic Press: London, UK, 2015; pp. 335–346. ISBN 978-0-12-397157-9. [Google Scholar]
- Wang, X.; Thibodeau, B.; Trope, M.; Lin, L.M.; Huang, G.T.J. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. J. Endod. 2010, 36, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.M.; Goodis, H.E.; Tay, F.R. Seltzer and Bender’s Dental Pulp, 2nd ed.; Quintessence Publishing USA: Batavia, IL, USA, 2012; ISBN 978-0-86715-480-1. [Google Scholar]
- Torabinejad, M.; Alexander, A.; Vahdati, S.A.; Grandhi, A.; Baylink, D.; Shabahang, S. Effect of residual dental pulp tissue on regeneration of dentin-pulp complex: An in vivo investigation. J. Endod. 2018, 44, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Grossman, L.I.; Meiman, B.W. Solution of pulp tissue by chemical agents. J. Endod. 1982, 8, S10–S12. [Google Scholar] [CrossRef]
- Kaufman, A.Y.; Keila, S. Hypersensitivity to sodium hypochlorite. J. Endod. 1989, 15, 224–226. [Google Scholar] [CrossRef]
- Estrela, C.; Estrela, C.R.; Barbin, E.L.; Spano, J.C.; Marchesan, M.A.; Pecora, J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Poletto, D.; Poletto, A.C.; Cavalaro, A.; Machado, R.; Cosme-Silva, L.; Garbelini, C.C.D.; Hoeppner, M.G. Smear layer removal by different chemical solutions used with or without ultrasonic activation after post preparation. Restor. Dent. Endod. 2017, 42, 324–331. [Google Scholar] [CrossRef]
- Spencer, H.; Ike, V.; Brennan, P. Review: The use of sodium hypochlorite in endodontics—Potential complications and their management. Br. Dent. J. 2007, 202, 555–559. [Google Scholar] [CrossRef]
- Becking, A.G. Complications in the use of sodium hypochlorite during endodontic treatment. Oral Surg. Oral Med. Oral Path. 1991, 71, 346–348. [Google Scholar] [CrossRef]
- Mozo, S.; Llena, C.; Forner, L. Review of ultrasonic irrigation in endodontics: Increasing action of irrigating solutions. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e512–e516. [Google Scholar] [CrossRef]
- Alovisi, M.; Pasqualini, D.; Musso, E.; Bobbio, E.; Giuliano, C.; Mancino, D.; Scotti, N.; Berutti, E. Influence of contracted endodontic access on root canal geometry: An in vitro study. J. Endod. 2018, 44, 614–620. [Google Scholar] [CrossRef]
- Metzger, Z. The self-adjusting file (SAF) system: An evidence-based update. J. Conserv. Dent. 2014, 17, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Gluskin, A.H.; Peters, C.I.; Peters, O.A. Minimally invasive endodontics: Challenging prevailing paradigms. Br. Dent. J. 2014, 216, 347–353. [Google Scholar] [CrossRef] [PubMed]
| (a) Enterococcus Faecalis (ATCC® 29212) | ||||
| Samples | Treatment Time (min) | Before (cfu/mL) | After (cfu/mL) | Bacterial Reduction (%) |
| 1.50% NaOCl | 5.0 | 3.8 × 105 | <1 | >99.9 |
| 5.25% NaOCl | 0.5 | 3.8 × 105 | <1 | >99.9 |
| 5.25% NaOCl | 1.0 | 3.8 × 105 | <1 | >99.9 |
| 0.0250% HOCl | 0.5 | 3.8 × 105 | <1 | >99.9 |
| 0.0250% HOCl | 1.0 | 3.8 × 105 | <1 | >99.9 |
| 0.0125% HOCl | 0.5 | 3.8 × 105 | <1 | >99.9 |
| 0.0125% HOCl | 1.0 | 3.8 × 105 | <1 | >99.9 |
| (b) Streptococcus Mutans (ATCC® 25175) | ||||
| Samples | Treatment Time (min) | Before (cfu/mL) | After (cfu/mL) | Bacterial Reduction (%) |
| 0.0250% HOCl | 0.5 | 4.3 × 105 | <1 | >99.9 |
| 0.0250% HOCl | 1.0 | 4.3 × 105 | <1 | >99.9 |
| 0.0125% HOCl | 0.5 | 4.3 × 105 | <1 | >99.9 |
| 0.0125% HOCl | 1.0 | 4.3 × 105 | <1 | >99.9 |
| Samples | 0.5 min Soaking Time | 1 min Soaking Time | ||
|---|---|---|---|---|
| Survival Rate (%) at 72 h | Body Length (mm) at 72 h | Survival Rate (%) at 72 h | Body Length (mm) at 72 h | |
| E3 Medium | 83.3 ± 5.8 a | 3.55 ± 0.19 b | 83.3 ± 5.8 a | 3.57 ± 0.22 b |
| 0.0125% HOCl | 73.3 ± 5.8 a | 3.45 ± 0.18 b | 76.7 ± 15.3 a,c | 3.22 ± 0.18 b |
| 0.0250% HOCl | 66.7 ± 25.2 a | 3.41 ± 0.19 b | 50.0 ± 17.3 c | 3.23 ± 0.36 b |
| 1.5% NaOCl | 0 | N.A. | 0 | N.A. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, S.-C.; Teng, N.-C.; Chu, C.C.; Chu, Y.-T.; Chen, C.-H.; Chang, L.-Y.; Hsu, C.-Y.; Huang, C.-S.; Hsiao, G.Y.-W.; Yang, J.-C. The Antibacterial Efficacy and In Vivo Toxicity of Sodium Hypochlorite and Electrolyzed Oxidizing (EO) Water-Based Endodontic Irrigating Solutions. Materials 2020, 13, 260. https://doi.org/10.3390/ma13020260
Hsieh S-C, Teng N-C, Chu CC, Chu Y-T, Chen C-H, Chang L-Y, Hsu C-Y, Huang C-S, Hsiao GY-W, Yang J-C. The Antibacterial Efficacy and In Vivo Toxicity of Sodium Hypochlorite and Electrolyzed Oxidizing (EO) Water-Based Endodontic Irrigating Solutions. Materials. 2020; 13(2):260. https://doi.org/10.3390/ma13020260
Chicago/Turabian StyleHsieh, Sung-Chih, Nai-Chia Teng, Chia Chun Chu, You-Tai Chu, Chung-He Chen, Liang-Yu Chang, Chieh-Yun Hsu, Ching-Shuan Huang, Grace Ying-Wen Hsiao, and Jen-Chang Yang. 2020. "The Antibacterial Efficacy and In Vivo Toxicity of Sodium Hypochlorite and Electrolyzed Oxidizing (EO) Water-Based Endodontic Irrigating Solutions" Materials 13, no. 2: 260. https://doi.org/10.3390/ma13020260
APA StyleHsieh, S.-C., Teng, N.-C., Chu, C. C., Chu, Y.-T., Chen, C.-H., Chang, L.-Y., Hsu, C.-Y., Huang, C.-S., Hsiao, G. Y.-W., & Yang, J.-C. (2020). The Antibacterial Efficacy and In Vivo Toxicity of Sodium Hypochlorite and Electrolyzed Oxidizing (EO) Water-Based Endodontic Irrigating Solutions. Materials, 13(2), 260. https://doi.org/10.3390/ma13020260





