In Situ Growth of Ca2+-Based Metal–Organic Framework on CaSiO3/ABS/TPU 3D Skeleton for Methylene Blue Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
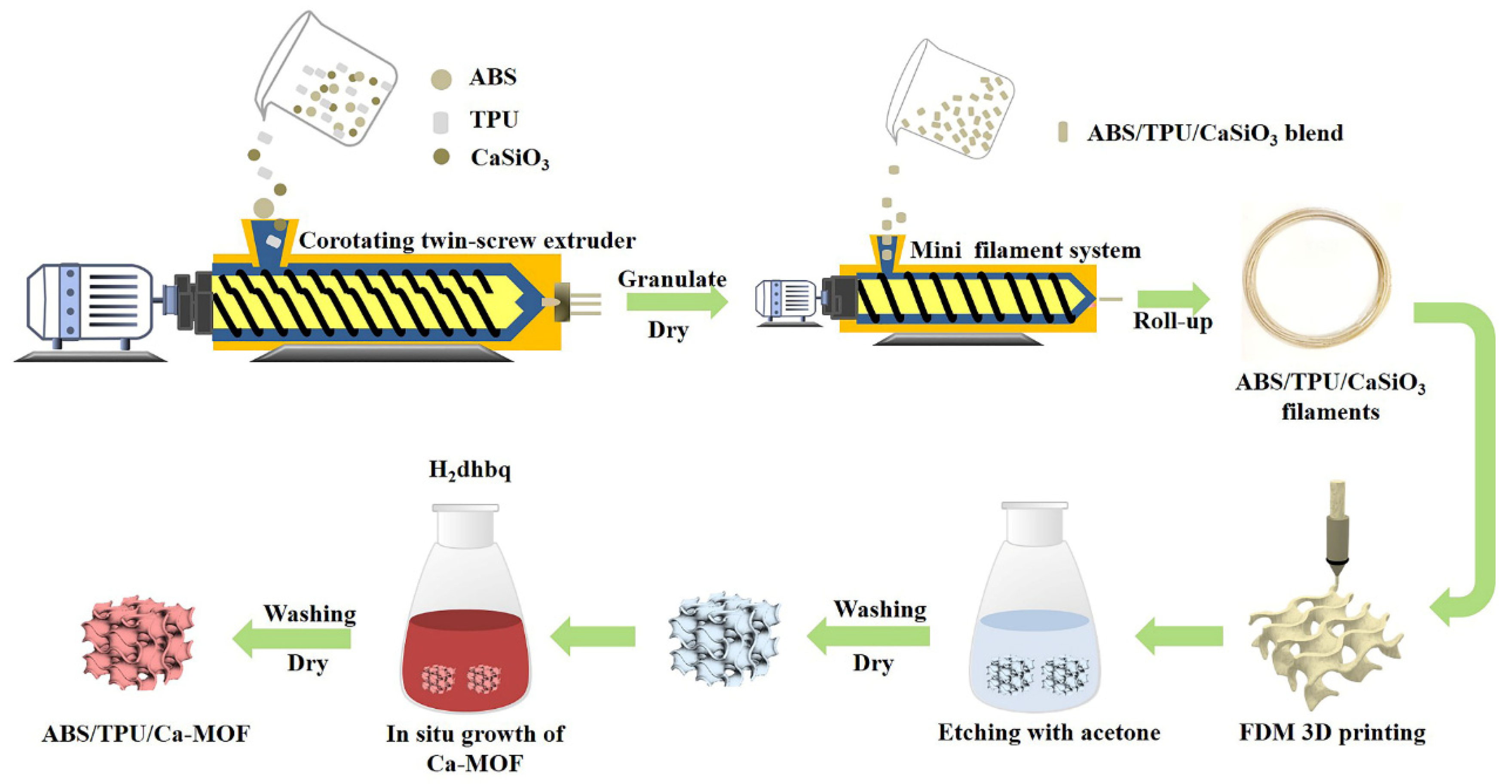
2.2. Immobilization of CaSiO3 on the ABS/TPU 3D Filaments
2.3. Preparation of CaSiO3/ABS/TPU 3D Skeleton by FDM Printing Process
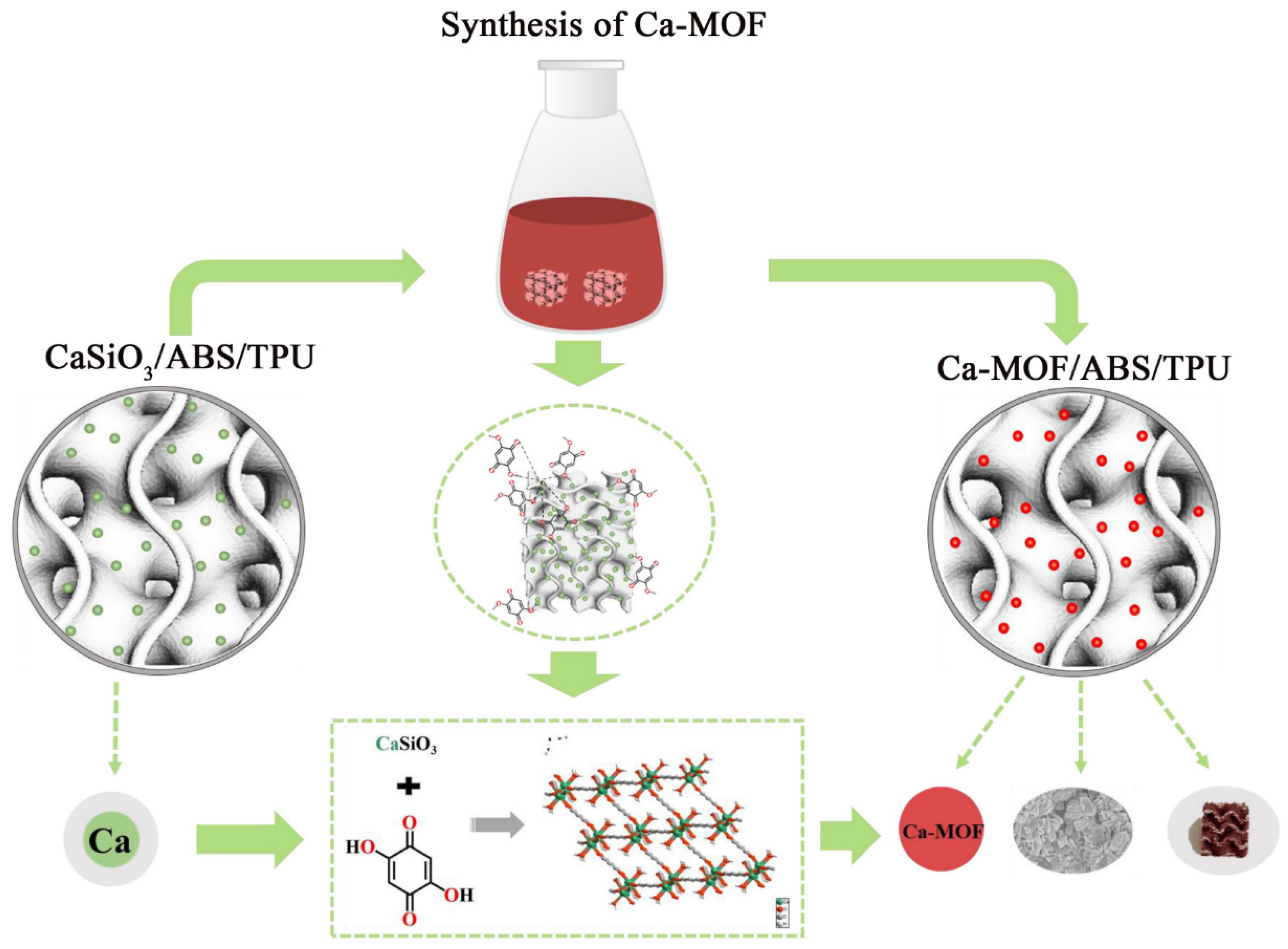
2.4. Preparation of Ca-MOF/ABS/TPU 3D Skeleton
2.5. Adsorption of Methylene Blue
2.6. Reusability
2.7. Characterization
3. Results and Discussion
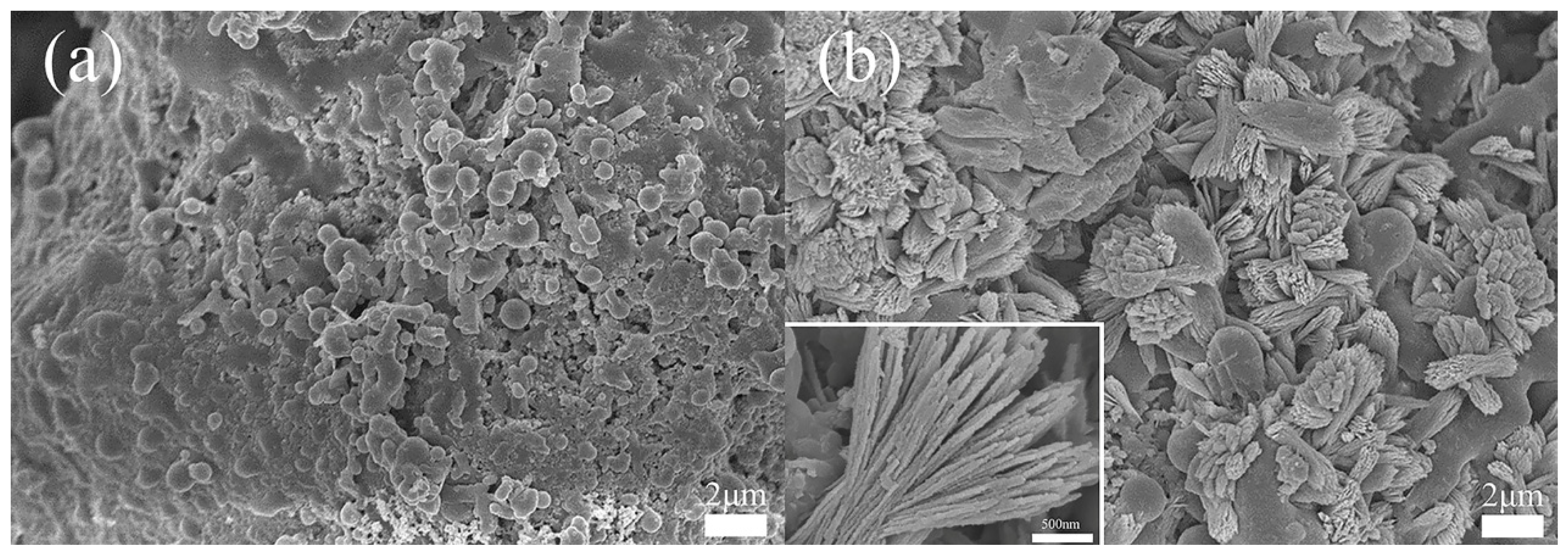
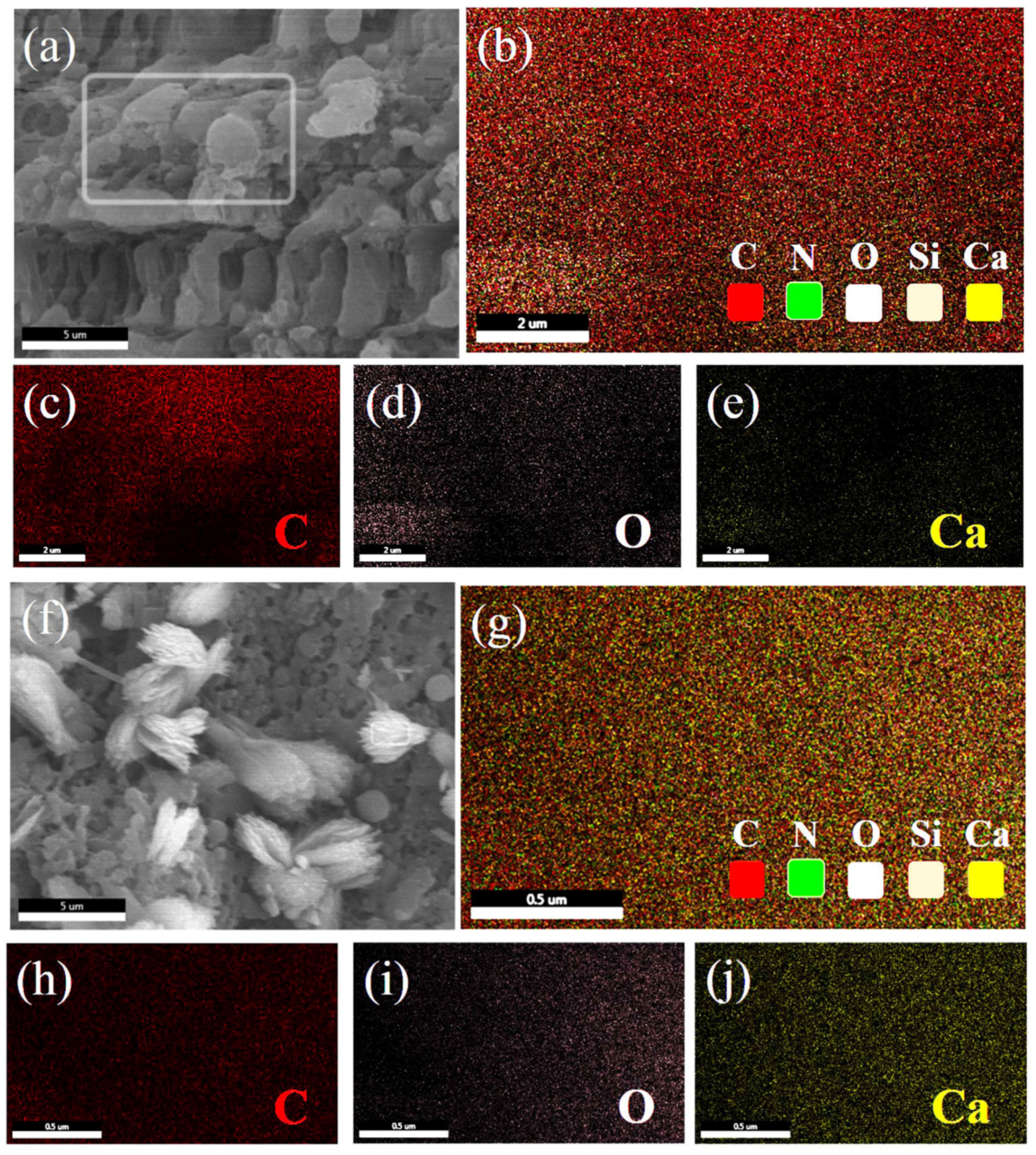
3.1. Characterization of Ca-MOF/ABS/TPU 3D Skeleton
3.2. Effects of Adsorption Conditions
3.3. Adsorption Thermodynamics
3.4. Adsorption Kinetics
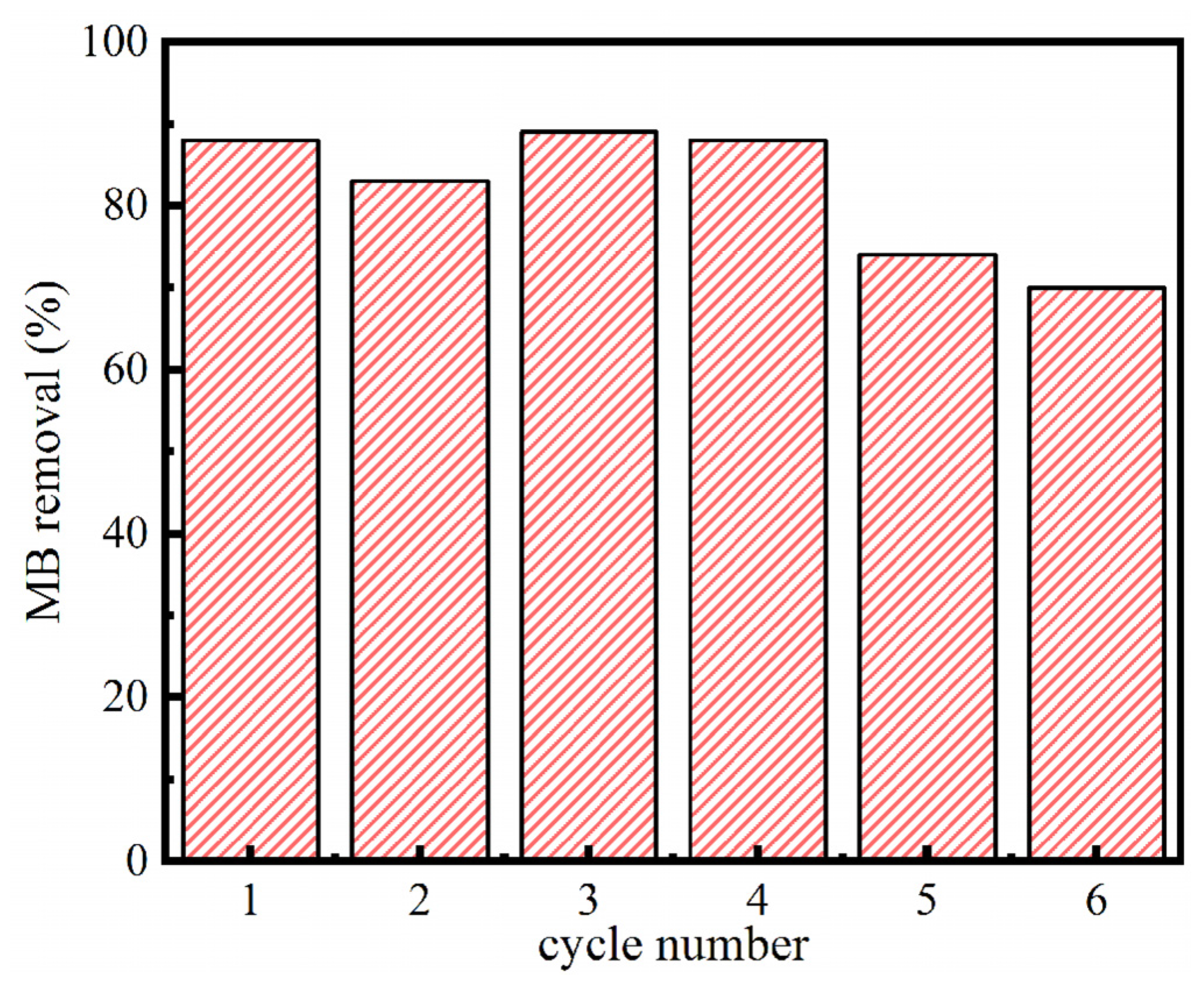
3.5. Reusability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.; Jiang, X.; Yin, C.; Zhang, B.; Zhang, Q. Facile fabrication of hierarchical porous ZIF-8 for enhanced adsorption of antibiotics. J. Hazard. Mater. 2019, 367, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Xu, J.; Bu, X.H. Recent advances about metal-organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31. [Google Scholar] [CrossRef]
- Ahmed, I.; Jhung, S.H. Applications of metal-organic frameworks in adsorption/separation processes via hydrogen bonding interactions. Chem. Eng. J. 2017, 310, 197–215. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal-organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Qu, T.G.; Hao, X.-M.; Wang, H.; Cui, X.-G.; Chen, F.; Wu, Y.-B.; Yang, D.; Zhang, M.; Guo, W.-L. A luminescent 2D zinc(II) metal-organic framework for selective sensing of Fe(III) ions and adsorption of organic dyes. Polyhedron 2018, 156, 208–217. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, V.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination polymers based on highly emissive ligands: Synthesis and functional properties. Materials 2020, 13, 67. [Google Scholar] [CrossRef]
- Lv, C.; Li, W.; Zhou, Y.; Li, J.; Lin, Z. A new porous Ca (II)-organic framework with acylamide decorated pores for highly efficient CO2 capture. Inorg. Chem. Commun. 2018, 99, 40–43. [Google Scholar]
- Pei, P.; Tian, Z.; Zhu, Y. 3D printed mesoporous bioactive glass/metal-organic framework scaffolds with antitubercular drug delivery. Micropor. Mesopor. Mat. 2018, 272, 24–30. [Google Scholar] [CrossRef]
- Zheng, J.P.; Ou, S.; Zhao, M.; Wu, C.D. A highly sensitive luminescent Dye@MOF composite for probing different volatile organic compounds. Chem Plus Chem 2016, 81, 758–763. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244–245, 444–456. [Google Scholar] [CrossRef]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, selective heavy metal removal from water by a metal-organic framework/polydopamine composite. ACS Cent. Sci 2018, 4, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Fan, L.; Zhao, F.; Xiao, J.; Yang, Y.; Lai, A.; Zhou, S.F.; Zhan, G. 3D-Printed metal-organic frameworks within biocompatible polymers as excellent adsorbents for organic dyes removal. J. Hazard Mater. 2020, 384, 121418. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, D.F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Lawson, S.; Hajari, A.; Rownaghi, A.A.; Rezaei, F. MOF immobilization on the surface of polymer-cordierite composite monoliths through in-situ crystal growth. Sep. Purif. Technol. 2017, 183, 173–180. [Google Scholar] [CrossRef]
- Xia, X.; Xu, X.; Lin, C.; Yang, Y.; Zeng, L.; Zheng, Y.; Wu, X.; Li, W.; Xiao, L.; Qian, Q.; et al. Microalgal-immobilized biocomposite scaffold fabricated by fused deposition modeling 3D printing technology for dyes removal. ES Mater. Manuf. 2020, 7, 40–50. [Google Scholar] [CrossRef]
- Shapira, A.; Noor, N.; Oved, H.; Dvir, T. Transparent support media for high resolution 3D printing of volumetric cell-containing ECM structures. Biomed. Mater. 2020, 15, 10. [Google Scholar] [CrossRef]
- Li, K.; de Mimerand, Y.D.; Jin, X.Y.; Yi, J.G.; Guo, J. Metal oxide (ZnO and TiO2) and Fe-based metal-organic-framework nanoparticles on 3D-printed fractal polymer surfaces for photocatalytic degradation of organic pollutants. ACS Appl. Nano Mater. 2020, 3, 2830–2845. [Google Scholar] [CrossRef]
- Lyu, Z.; Lim, G.J.H.; Guo, R.; Kou, Z.; Wang, T.; Guan, C.; Ding, J.; Chen, W.; Wang, J. 3D-Printed MOF-derived hierarchically porous frameworks for practical high-energy density Li-O2 batteries. Adv. Funct. Mater. 2019, 29, 1806658. [Google Scholar] [CrossRef]
- Bible, M.; Sefa, M.; Fedchak, J.A.; Scherschligt, J.; Natarajan, B.; Ahmed, Z.; Hartings, M.R. 3D-printed acrylonitrile butadiene styrene-metal organic framework composite materials and their gas storage properties. 3D. Print. Addit. Manuf. 2018, 5, 63–72. [Google Scholar] [CrossRef]
- Thakkar, H.; Eastman, S.; Al-Naddaf, Q.; Rownaghi, A.A.; Rezaei, F. 3D-printed metal-organic framework monoliths for gas adsorption processes. ACS Appl. Mater. Inter. 2017, 9, 35908–35916. [Google Scholar] [CrossRef]
- Lawson, S.; Griffin, C.; Rapp, K.; Rownaghi, A.A.; Rezaei, F. Amine-functionalized MIL-101 monoliths for CO2 removal from enclosed environments. Energy Fuels 2019, 33, 2399–2407. [Google Scholar] [CrossRef]
- Evans, K.A.; Kennedy, Z.C.; Arey, B.W.; Christ, J.F.; Schaef, H.T.; Nune, S.K.; Erikson, R.L. Chemically active, porous 3D-printed thermoplastic composites. ACS Appl. Mater. Inter. 2018, 10, 15112–15121. [Google Scholar] [CrossRef] [PubMed]
- Lawson, S.; Al-Naddaf, Q.; Krishnamurthy, A.; Amour, M.S.; Griffin, C.; Rownaghi, A.A.; Knox, J.C.; Rezaei, F. UTSA-16 growth within 3D-printed Co-Kaolin monoliths with high selectivity for CO2/CH4, CO2/N2, and CO2/H2 separation. ACS Appl. Mater. Inter. 2018, 10, 19076–19086. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Xu, C.; Chen, F.; Wang, Y.; Li, L.; Meng, Q.; Zhang, R. Renewable metal–organic-frameworks-coated 3D printing film for removal of malachite green. RSC Adv. 2017, 7, 49947–49952. [Google Scholar] [CrossRef]
- Li, R.; Yuan, S.; Zhang, W.; Zheng, H.; Zhu, W.; Li, B.; Zhou, M.; Wing-Keung Law, A.; Zhou, K. 3D printing of mixed matrix films based on metal-organic frameworks and thermoplastic polyamide 12 by selective laser sintering for water applications. ACS Appl. Mater. Inter. 2019, 11, 40564–40574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Li, M.; Sun, K.; Liu, C.J. Three-dimensional printed acrylonitrile butadiene styrene framework coated with Cu-BTC metal-organic frameworks for the removal of methylene blue. Sci Rep. 2014, 4, 5939. [Google Scholar] [CrossRef]
- Halevi, O.; Tan, J.M.R.; Lee, P.S.; Magdassi, S. Hydrolytically stable MOF in 3D-printed structures. Adv. Sustain. Syst. 2018, 2, 5. [Google Scholar] [CrossRef]
- Margariti, A.; Rapti, S.; Katsenis, A.D.; Friščić, T.; Georgiou, Y.; Manos, M.J.; Papaefstathiou, G.S. Cu2+ sorption from aqueous media by a recyclable Ca2+ framework. Inorg. Chem. Front. 2017, 4, 773–781. [Google Scholar] [CrossRef]
- Pournara, A.D.; Margariti, A.; Tarlas, G.D.; Kourtelaris, A.; Petkov, V.; Kokkinos, C.; Economou, A.; Papaefstathiou, G.S.; Manos, M.J. A Ca2+ MOF combining highly efficient sorption and capability for voltammetric determination of heavy metal ions in aqueous media. J. Mater. Chem. A 2019, 7, 15432–15443. [Google Scholar] [CrossRef]
- Sumida, K.; Hu, M.; Furukawa, S.; Kitagawa, S. Structuralization of Ca2+-based metal-organic frameworks prepared via coordination replication of calcium carbonate. Inorg. Chem. 2016, 55, 3700–3705. [Google Scholar] [CrossRef]
- George, P.; Das, R.K.; Chowdhury, P. Facile microwave synthesis of Ca-BDC metal organic framework for adsorption and controlled release of Curcumin. Micropor. Mesopor. Mat. 2019, 281, 161–171. [Google Scholar] [CrossRef]
- Lothenbach, B.; Nied, D.; L’Hopital, E.; Achiedo, G.; Dauzeres, A. Magnesium and calcium silicate hydrates. Cement Concr. Res. 2015, 77, 60–68. [Google Scholar] [CrossRef]
- Yang, S.Y.; Castilleja, J.R.; Barrera, E.V.; Lozano, K. Thermal analysis of an acrylonitrile-butadiene-styrene/SWNT composite. Polym. Degrad. Stab. 2004, 83, 383–388. [Google Scholar] [CrossRef]
- Lian, J.; Zhou, F.; Chen, B.; Yang, M.; Wang, S.; Liu, Z.; Niu, S. Enhanced adsorption of molybdenum (VI) onto drinking water treatment residues modified by thermal treatment and acid. J. Clean. Prod. 2020, 244, 118719. [Google Scholar] [CrossRef]
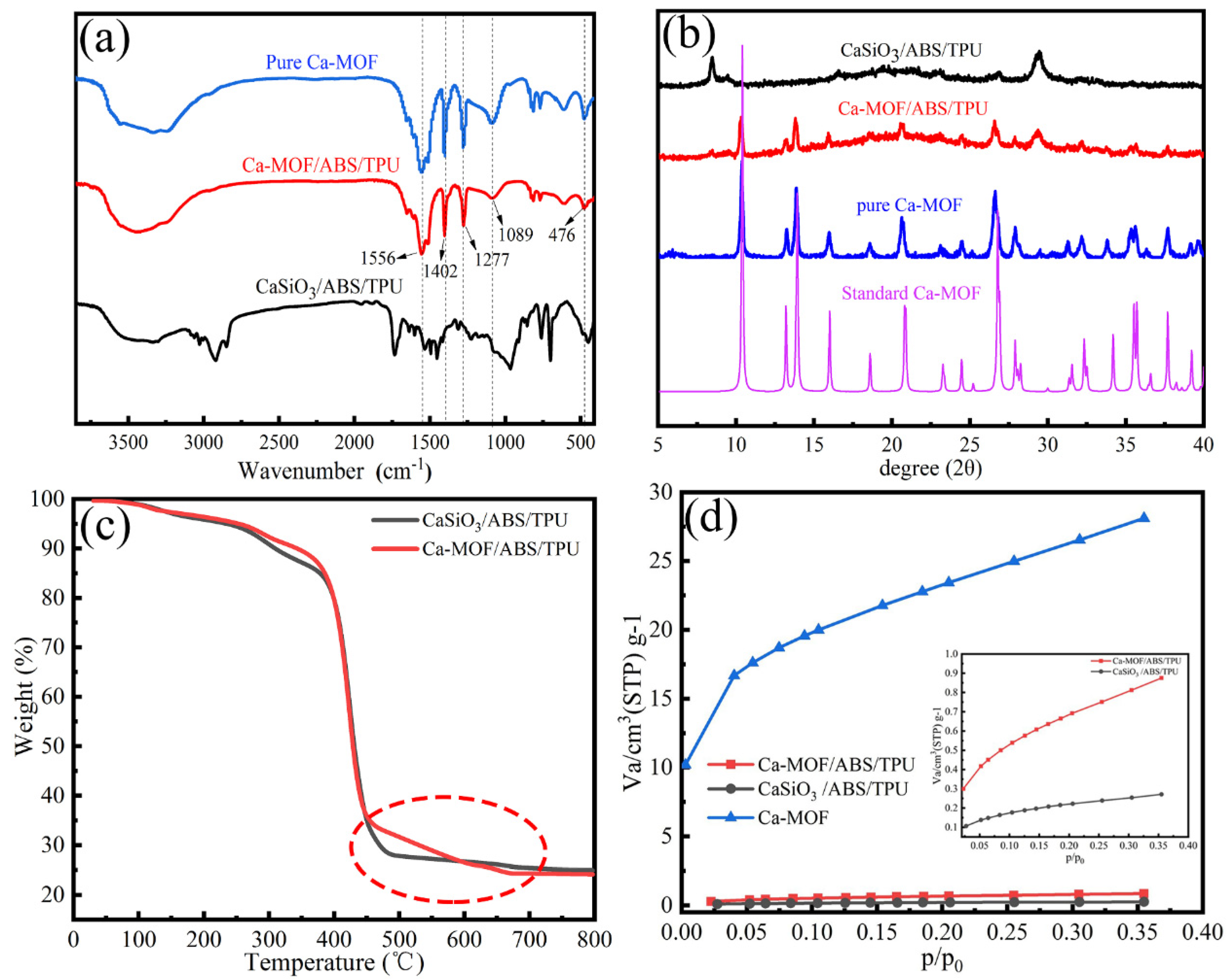

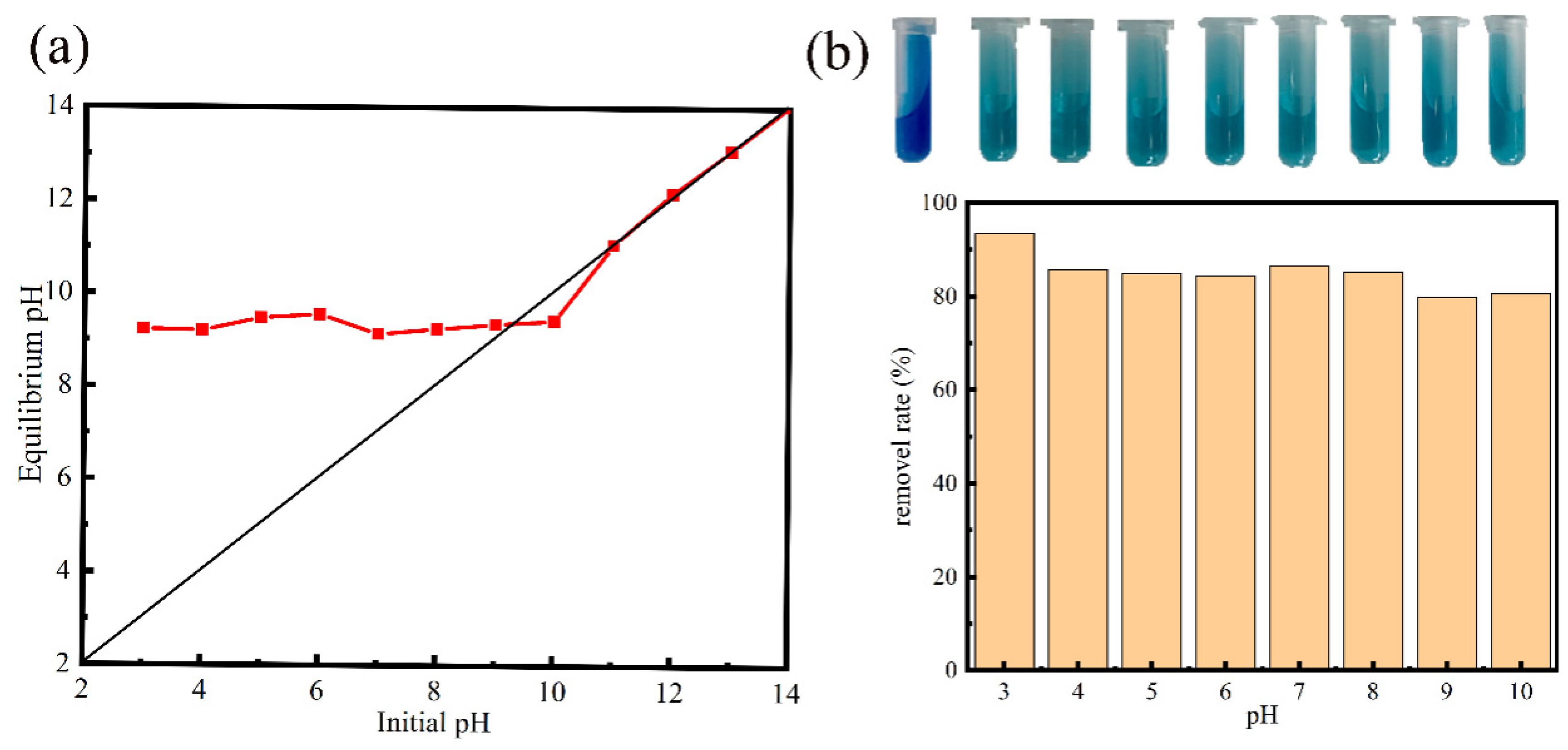
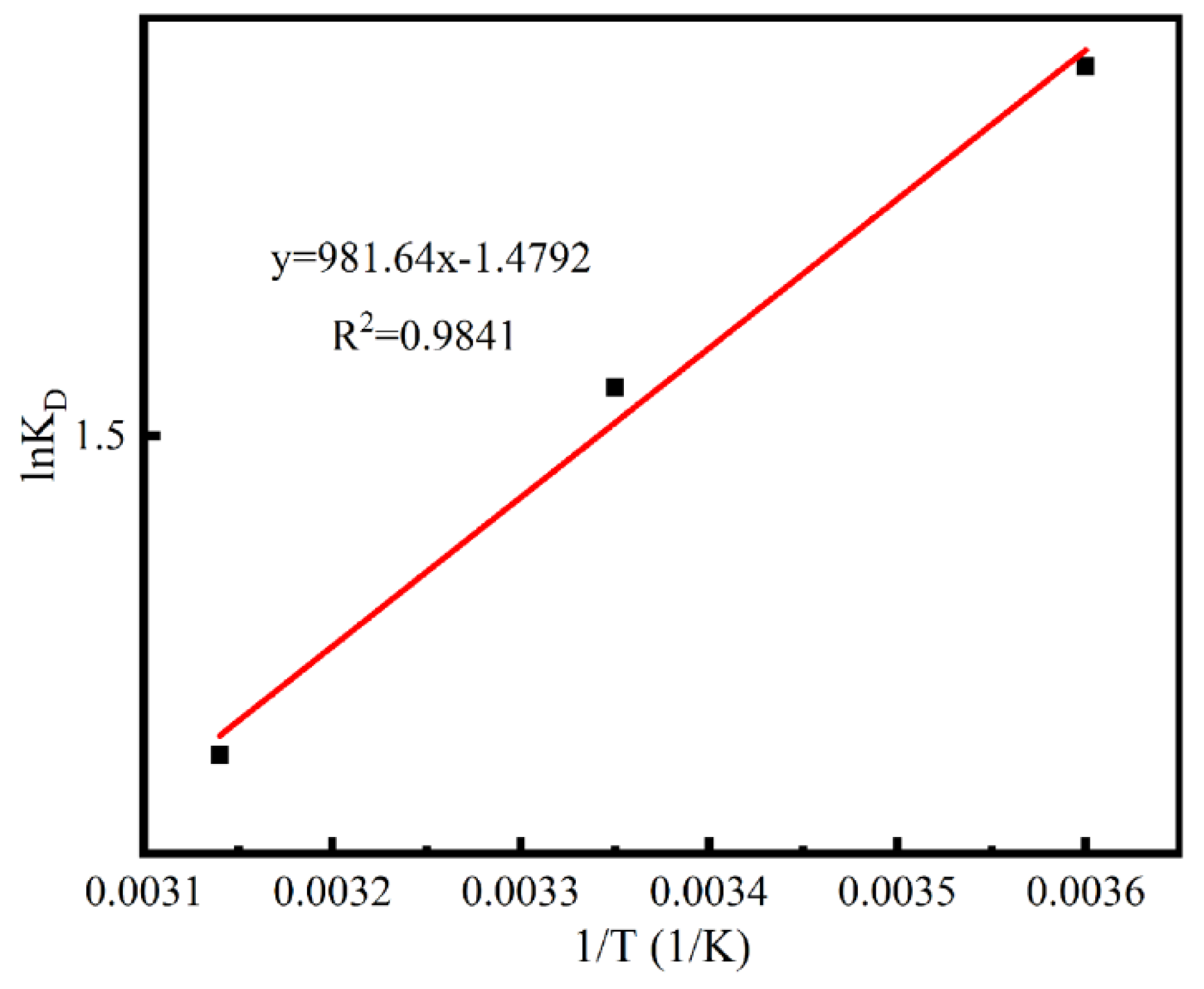
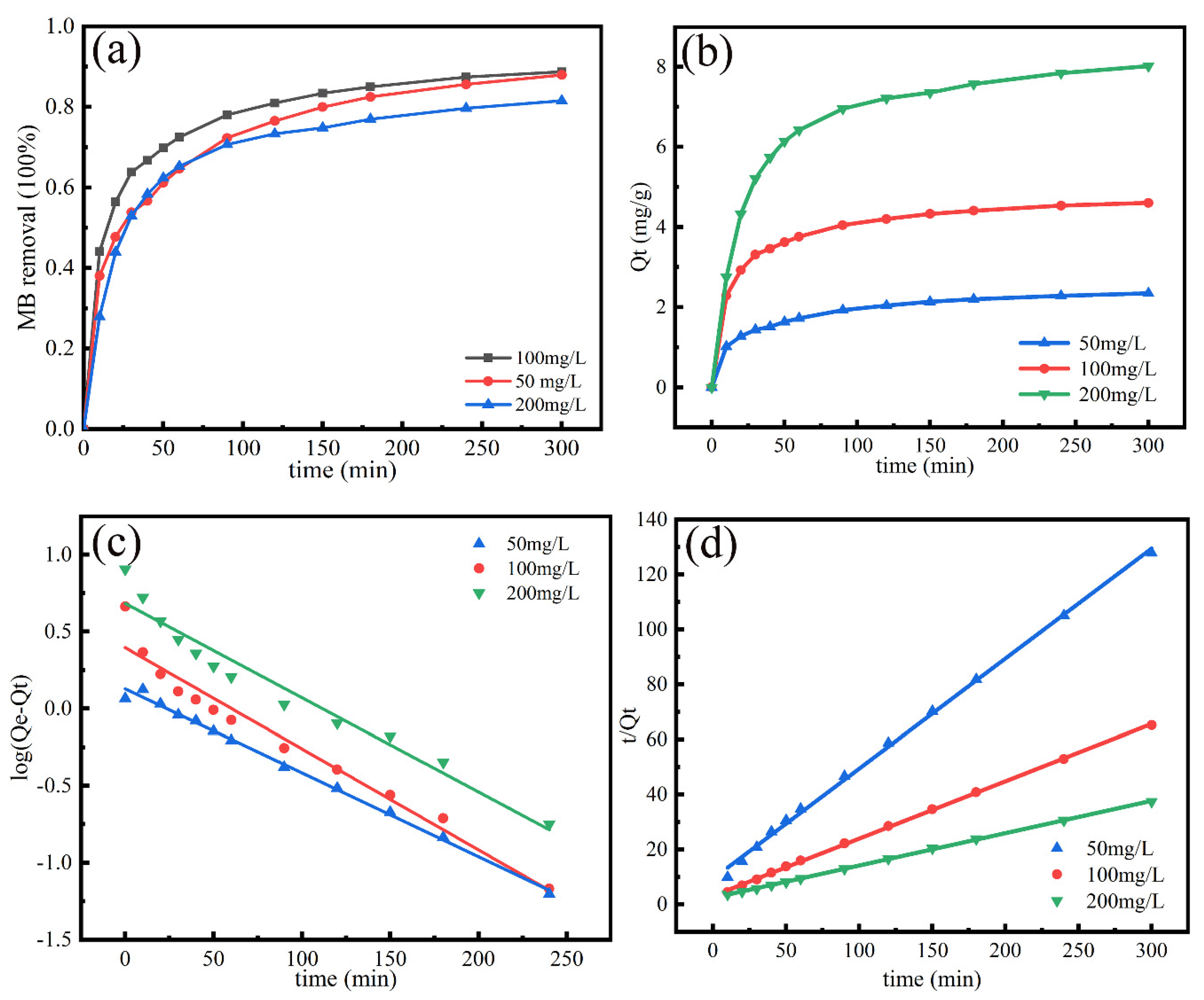
| Temperature (K) | ∆G (kJ/mol) | ∆H (kJ/mol) | ∆S (J/mol K) |
|---|---|---|---|
| 278 | −4.740 | −8.161 | −12.30 |
| 298 | −4.494 | ||
| 308 | −4.248 |
| Model | Parameter Description | Experimental Data | |||
|---|---|---|---|---|---|
| Parameters | C0 (mg/L) | ||||
| 50 | 100 | 200 | |||
| Pseudo-first-order model: | qe: maximum adsorption capacity, mg/g | qe,exp | 2.35 | 4.6 | 8.18 |
| qe,cal | 1.34 | 2.48 | 4.82 | ||
| k1: the rate constant of pseudo first-order adsorption, min−1 | k1 | 0.01255 | 0.0151 | 0.01409 | |
| R2 | 0.995 | 0.957 | 0.9492 | ||
| Pseudo-second-order model: | k2: the rate constant of pseudo-second-order adsorption, (g/(mg·min)) | qe,exp | 2.35 | 4.6 | 8.18 |
| qe,cal | 2.49 | 4.79 | 8.49 | ||
| k2 | 0.45231 | 0.22649 | 0.12686 | ||
| R2 | 0.9981 | 0.9995 | 0.9997 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Xia, X.; Li, W.; Xiao, L.; Sun, X.; Luo, F.; Chen, Q.; Qian, Q. In Situ Growth of Ca2+-Based Metal–Organic Framework on CaSiO3/ABS/TPU 3D Skeleton for Methylene Blue Removal. Materials 2020, 13, 4403. https://doi.org/10.3390/ma13194403
Liu Z, Xia X, Li W, Xiao L, Sun X, Luo F, Chen Q, Qian Q. In Situ Growth of Ca2+-Based Metal–Organic Framework on CaSiO3/ABS/TPU 3D Skeleton for Methylene Blue Removal. Materials. 2020; 13(19):4403. https://doi.org/10.3390/ma13194403
Chicago/Turabian StyleLiu, Zhen, Xinshu Xia, Wei Li, Liren Xiao, Xiaoli Sun, Fubin Luo, Qinghua Chen, and Qingrong Qian. 2020. "In Situ Growth of Ca2+-Based Metal–Organic Framework on CaSiO3/ABS/TPU 3D Skeleton for Methylene Blue Removal" Materials 13, no. 19: 4403. https://doi.org/10.3390/ma13194403
APA StyleLiu, Z., Xia, X., Li, W., Xiao, L., Sun, X., Luo, F., Chen, Q., & Qian, Q. (2020). In Situ Growth of Ca2+-Based Metal–Organic Framework on CaSiO3/ABS/TPU 3D Skeleton for Methylene Blue Removal. Materials, 13(19), 4403. https://doi.org/10.3390/ma13194403






