In Vitro Biological Characterization of Silver-Doped Anodic Oxide Coating on Titanium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AgNP Synthesis
2.3. AgNP Characterization
2.4. Plasma Electrolytic Oxidation
2.5. Surface Characterization
2.6. Bacterial Adhesion test
2.7. Cell Culture Biocompatibility Test
2.8. Collagen Deposition Assay
2.9. Statistics
3. Results
3.1. Structural and Chemical Composition of AgNPs and PEO Coating
3.2. In Vitro Investigations
3.2.1. Cell Proliferation and Collagen Production Assays
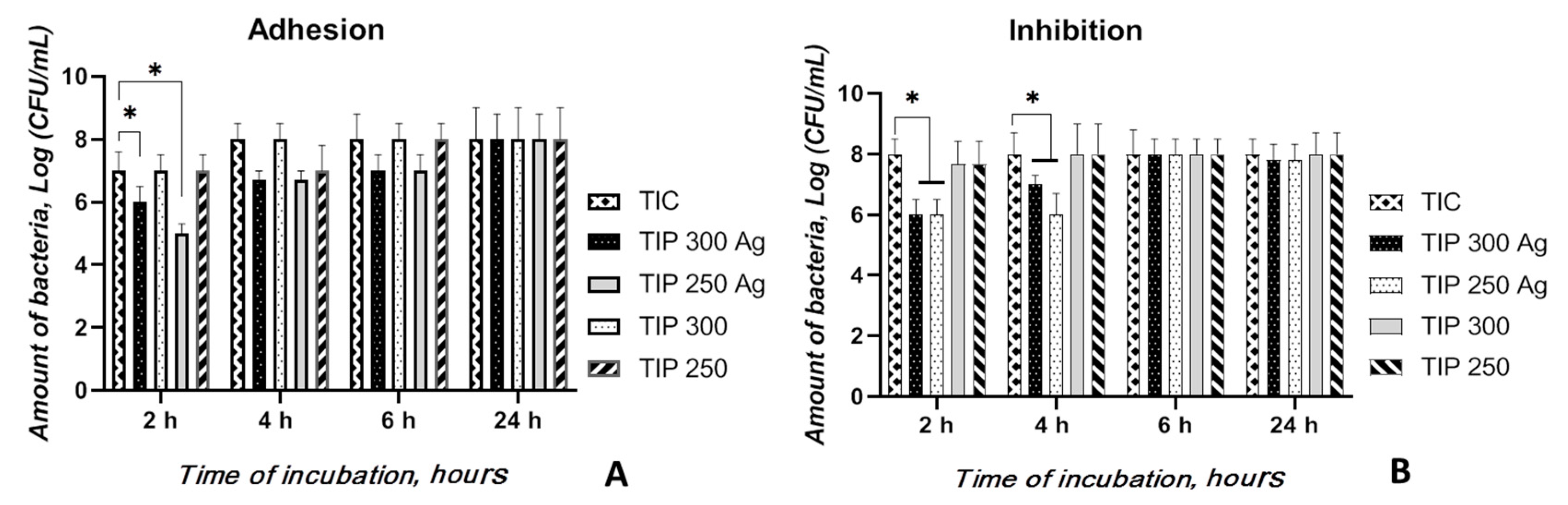
3.2.2. Bacterial Adhesion and Inhibition Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parnia, F.; Yazdani, J.; Javaherzadeh, V.; Maleki Dizaj, S. Overview of Nanoparticle Coating of Dental Implants for Enhanced Osseointegration and Antimicrobial Purposes. J. Pharm. Pharm. Sci. 2017, 20, 148–160. [Google Scholar] [CrossRef]
- Sendyk, D.I.; Rovai, E.S.; Pannuti, C.M.; Deboni, M.C.Z.; Sendyk, W.R.; Wennerberg, A. Dental implant loss in older versus younger patients: A systematic review and meta-analysis of prospective studies. J. Oral Rehabil. 2017, 44, 229–236. [Google Scholar] [CrossRef]
- Mombelli, A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol. 2000 2002, 28, 177–189. [Google Scholar] [CrossRef]
- Broggini, N.; McManus, L.M.; Hermann, J.S.; Medina, R.; Schenk, R.K.; Buser, D.; Cochran, D.L. Peri-implant inflammation defined by the implant-abutment interface. J. Dent. Res. 2006, 85, 473–478. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Current trends in metallic orthopedic biomaterials: From additive manufacturing to bio-functionalization, infection prevention, and beyond. Int. J. Mol. Sci. 2018, 19, 2684. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Ralf, S.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed Res. Int. 2016. [Google Scholar] [CrossRef]
- Grischke, J.; Eberhard, J.; Stiesch, M. Antimicrobial dental implant functionalization strategies—A systematic review. Dent. Mater. J. 2016, 35, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Oshida, Y.; Tuna, E.B.; Aktören, O.; Gençay, K. Dental implant systems. Int. J. Mol. Sci. 2010, 11, 1580–1678. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Donghyun, L.; Choi, Y.S.; Jeon, H.B.; Lee, C.-H.; Moon, J.-H.; Kwona, I.K. Mesoporous TiO2 implants for loading high dosage of antibacterial agent. Appl. Surf. Sci. 2014, 303, 140–146. [Google Scholar] [CrossRef]
- Sukhorukova, I.V.; Sheveyko, A.N.; Kiryukhantsev-Korneev, P.V.; Levashov, E.A.; Shtansky, D.V. In vitro bioactivity study of TiCaPCO(N) and Ag-doped TiCaPCO(N) films in simulated body fluid. J. Biomed. Mater. Res. Part B 2017, 105, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Janković, A.; Eraković, S.; Ristoscu, S.C.; Mihailescu Serban, N.; Duta, L.; Visan, A.; Stan, G.E.; Popa, A.C.; Husanu, M.A.; Luculescu, C.R. Structural and biological evaluation of lignin addition to simple and silver-doped hydroxyapatite thin films synthesized by matrix-assisted pulsed laser evaporation. J. Mater. Sci. Mater. Med. 2015, 26, 5333. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.J.; Tsai, M.-T.; Shieh, T.-M.; Huang, H.-L.; Hsu, J.-T.; Ko, Y.-C.; Fuh, L.J. In vitro antibacterial activity and cytocompatibility of bismuth doped micro-arc oxidized titanium. J. Biomater. 2013, 27, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Cao, H.; Qiao, Y.; Meng, F.; Zhu, H.; Liu, X. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surf. B Biointerfaces 2014, 117, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Moreno, A.M.; Pacha-Olivenza, M.A.; Saldaña, L.; Pérez-Giraldo, C.; Bruque, J.M.; Vilaboa, N.; González-Martín, M.L. In vitro biocompatibility and bacterial adhesion of physico-chemically modified Ti6Al4V surface by means of UV irradiation. Acta Biomater. 2009, 5, 181–192. [Google Scholar] [CrossRef]
- Westas, E.; Gillstedt, M.; Lönn-Stensrud, J.; Bruzell, E.; Andersson, M. Biofilm formation on nanostructured hydroxyapatite-coated titanium. J. Biomed. Mater. Res. Part A 2014, 102, 1063–1070. [Google Scholar] [CrossRef]
- Xie, K.; Zhou, Z.; Guo, Y.; Wang, L.; Li, G.; Zhao, S.; Liu, X.; Li, J.; Jiang, W.; Wu, S. Long-Term Prevention of Bacterial Infection and Enhanced Osteoinductivity of a Hybrid Coating with Selective Silver Toxicity. Adv. Healthc. Mater. 2019, 8, 1465. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, D. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef]
- Van Hengel, I.A.J.; Riool, M.; Fratila-Apachitei, L.E.; Witte-Bouma, J.; Farrell, E.; Zadpoor, A.A.; Zaat, S.A.J.; Apachitei, I. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials 2017, 140, 1–15. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2015, 61, 965–978. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Necula, B.S.; Apachitei, I.; Tichelaar, F.D.; Fratila-Apachitei, L.E.; Duszczyk, J. An electron microscopical study on the growth of TiO2-Ag antibacterial coatings on Ti6Al7Nb biomedical alloy. Acta Biomater. 2011, 7, 2751–2757. [Google Scholar] [CrossRef]
- Necula, B.S.; Van Leeuwen, J.P.T.M.; Fratila-Apachitei, L.E.; Zaat, S.A.J.; Apachitei, I.; Duszczyk, J. In vitro cytotoxicity evaluation of porous TiO2-Ag antibacterial coatings for human fetal osteoblasts. Acta Biomater. 2012, 8, 4191–4197. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Hou, P.; Ni, J.; Han, P.; Chai, Y.; Zhang, X. Ag-Incorporated FHA Coating on Pure Mg: Degradation and in Vitro Antibacterial Properties. ACS Appl. Mater. Interfaces 2016, 8, 5093–5103. [Google Scholar] [CrossRef] [PubMed]
- Kazek-Kȩsik, A.; Kalemba-Rec, I.; Simka, W. Anodization of a medical-grade Ti-6Al-7Nb alloy in a Ca(H2PO2)2-hydroxyapatite suspension. Materials 2019, 12, 3002. [Google Scholar] [CrossRef]
- Zhu, X.; Kim, K.H.; Jeong, Y. Anodic oxide films containing Ca and P of titanium biomaterial. Biomaterials 2001, 22, 2199–2206. [Google Scholar] [CrossRef]
- Oleshko, O.; Deineka, V.; Husak, Y.; Korniienko, V.; Mishchenko, O.; Holubnycha, V.; Pisarek, M.; Michalska, J.; Kazek-Kęsik, A.; Jakóbik-Kolon, A.; et al. Ag Nanoparticle-Decorated Oxide Coatings Formed via Plasma Electrolytic Oxidation on ZrNb Alloy. Materials 2019, 12, 3742. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Pardo, A.; Matykina, E. Bioactive multi-elemental PEO-coatings on titanium for dental implant applications. Mater. Sci. Eng. C 2019, 97, 738–752. [Google Scholar] [CrossRef]
- Gordillo-Delgado, F.; Moya-Betancourt, S.; Parra-López, A.; Garcia-Giraldo, J.A.; Torres-Cerón, D. S-incorporated TiO2 coatings grown by plasma electrolytic oxidation for reduction of Cr(VI)-EDTA with sunlight. Environ. Sci. Pollut. Res. 2019, 26, 4253–4259. [Google Scholar] [CrossRef]
- Tsuji, G.; Hattori, T.; Kato, M.; Hakamata, W.; Inoue, H.; Naito, M.; Kurihara, M.; Demizu, Y.; Shoda, T. Design and synthesis of cell-permeable fluorescent nitrilotriacetic acid derivatives. Bioorganic Med. Chem. 2018, 26, 5494–5498. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, F.; Liu, Y.; Lv, J.; Wu, G.; Liu, Y.; Ma, R.; An, Y.; Shi, L. Nitrilotriacetic Acid-Functionalized Glucose-Responsive Complex Micelles for the Efficient Encapsulation and Self-Regulated Release of Insulin. Langmuir 2018, 34, 12116–12125. [Google Scholar] [CrossRef] [PubMed]
- Simka, W.; Mishchenko, O.; Pogorielov, M. Polish Patent no. P.430333, 2019.
- Korniienko, V.; Oleshko, O.; Husak, Y.; Deineka, V.; Holubnycha, V.; Mishchenko, O.; Kazek-Kęsik, A.; Jakóbik-Kolon, A.; Pshenychnyi, R.; Leśniak-Ziółkowska, K. Formation of a Bacteriostatic Surface on ZrNb Alloy via Anodization in a Solution Containing Cu Nanoparticles. Materials 2020, 13, 3913. [Google Scholar] [CrossRef] [PubMed]
- Trackman, P.C.; Saxena, D.; Bais, M.V. TGF-β1- and CCN2-stimulated sirius red assay for collagen accumulation in cultured cells. Methods Mol. Biol. 2017, 1489, 481–485. [Google Scholar] [PubMed]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Particle Fibre Toxicol. 2014, 11, 11–28. [Google Scholar] [CrossRef]
- Han, X.; Gelein, R.; Corson, N.; Wade-Mercer, P.; Jiang, J.; Biswas, P.; Finkelstein, J.N.; Elder, A.; Oberdorster, G. Validation of an LDH assay for assessing nanoparticle toxicity. Toxicology 2011, 287, 99–104. [Google Scholar] [CrossRef]
- Holder, A.L.; Marr, L.C. Toxicity of silver nanoparticles at the air-liquid interface. Biomed Res Int. 2013, 2013, 328934. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Wang, C.; Li, H.C.; Wang, T.; Liao, C.Y.; Cui, L.; Zhou, Q.F.; Yan, B.; Jiang, G.B. Impact of silver nanoparticles on human cells: Effect of particle size. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Simka, W.; Krząkała, A.; Masełbas, M.; Dercz, G.; Szade, J.; Winiarski, A.; Michalska, J. Formation of bioactive coatings on Ti–13Nb–13Zr alloy for hard tissue implants. RSC Adv. 2013, 3, 11195–11204. [Google Scholar] [CrossRef]
- Gambardella, A.; Berni, M.; Graziani, G.; Kovtun, A.; Liscio, A.; Russo, A.; Visani, A.; Bianchi, M. Nanostructured Ag thin films deposited by pulsed electron ablation. Appl. Surf. Sci. 2019, 475, 917–925. [Google Scholar] [CrossRef]
- Bernia, M.; Carrano, I.; Kovtun, A.; Russo, A.; Visani, A.; Dionigi, C.; Liscio, A.; Valle, F.; Gambardella, A. Monitoring morphological and chemical properties during silver solid-state dewetting. Appl. Surf. Sci. 2019, 498, 143890. [Google Scholar] [CrossRef]
- Lin, W.-T.; Zhang, Y.-Y.; Tan, H.-L.; Ao, H.-Y.; Duan, Z.-L.; He, G.; Tang, T.-T. Inhibited bacterial adhesion and biofilm formation on quaternized chitosan-loaded titania nanotubes with various diameters. Materials 2016, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Eggert, F.M.; Levin, L.L. Biology of teeth and implants: The external environment, biology of structures, and clinical aspects. Quintessence Int. 2018, 49, 301–312. [Google Scholar]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.H. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef]
- Babuska, V. Evaluating the osseointegration of nanostructured titanium implants in animal models: Current experimental methods and perspectives (Review). Biointerphases 2016, 11, 030801. [Google Scholar] [CrossRef]
- Stangl, R.; Pries, A.; Loos, B.; Muller, M.; Erben, R.G. Influence of pores created by laser superfinishing on osseointegration of titanium alloy implants. J. Biomed. Mater. Res. 2004, 69A, 444–453. [Google Scholar] [CrossRef]
- Li, C.W.; Fu, R.; Yu, C.; Li, Z.; Guan, H.; Hu, D.; Zhao, D.; Lu, L. Silver nanoparticle/chitosan oligosaccharide/poly(vinyl alcohol) nanofibers as wound dressings: A preclinical study. Int. J. Nanomed. 2013, 8, 4131–4145. [Google Scholar]
- Sussman, E.M.; Jayanti, P.; Dair, B.J.; Casey, B.J. Assessment of total silver and silver nanoparticle extraction from medical devices. Food Chem. Toxicol. 2015, 85, 10–19. [Google Scholar] [CrossRef]
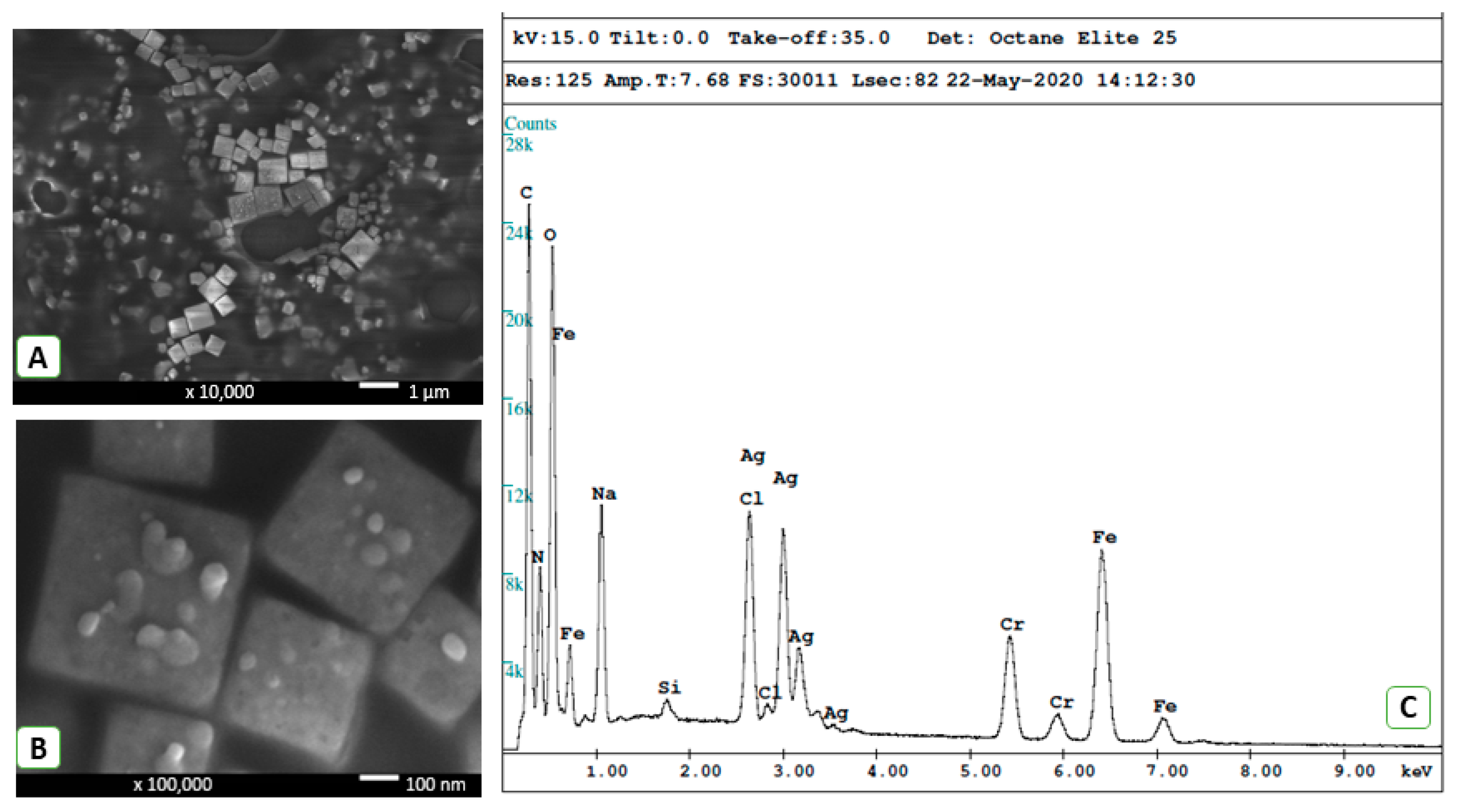
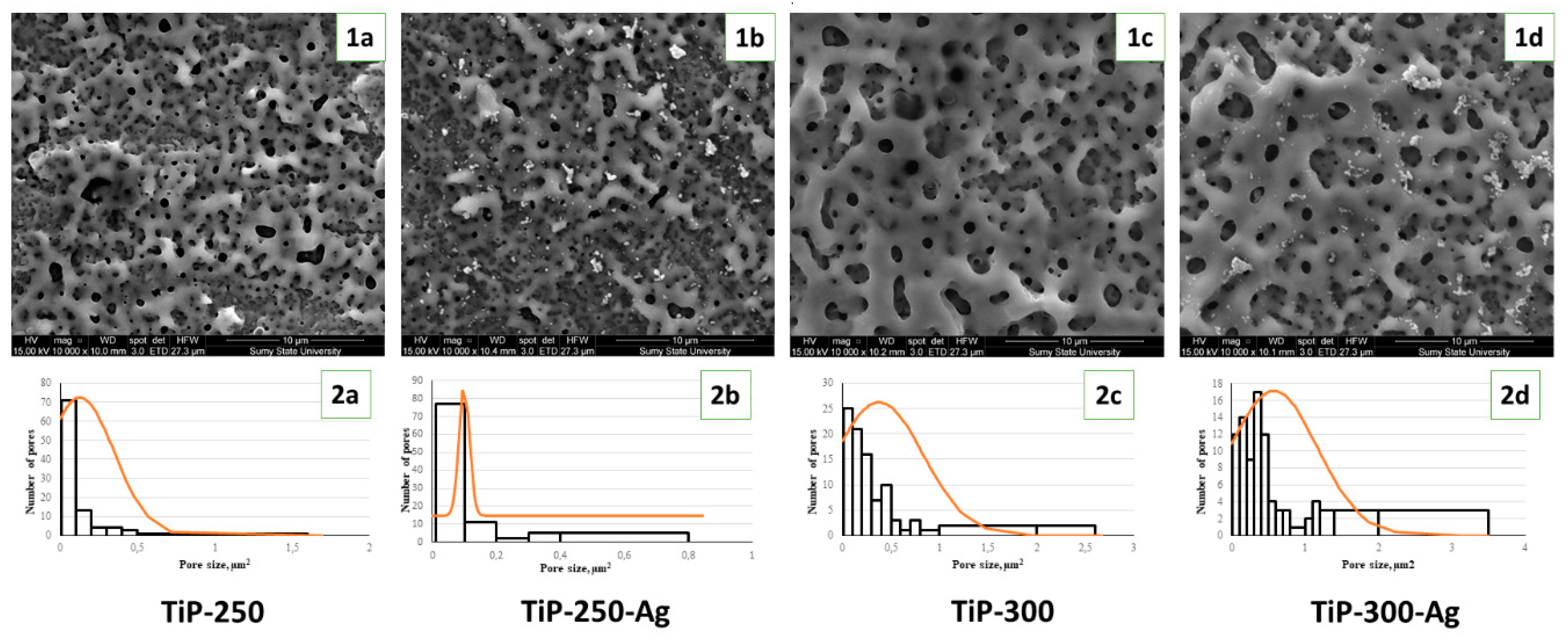
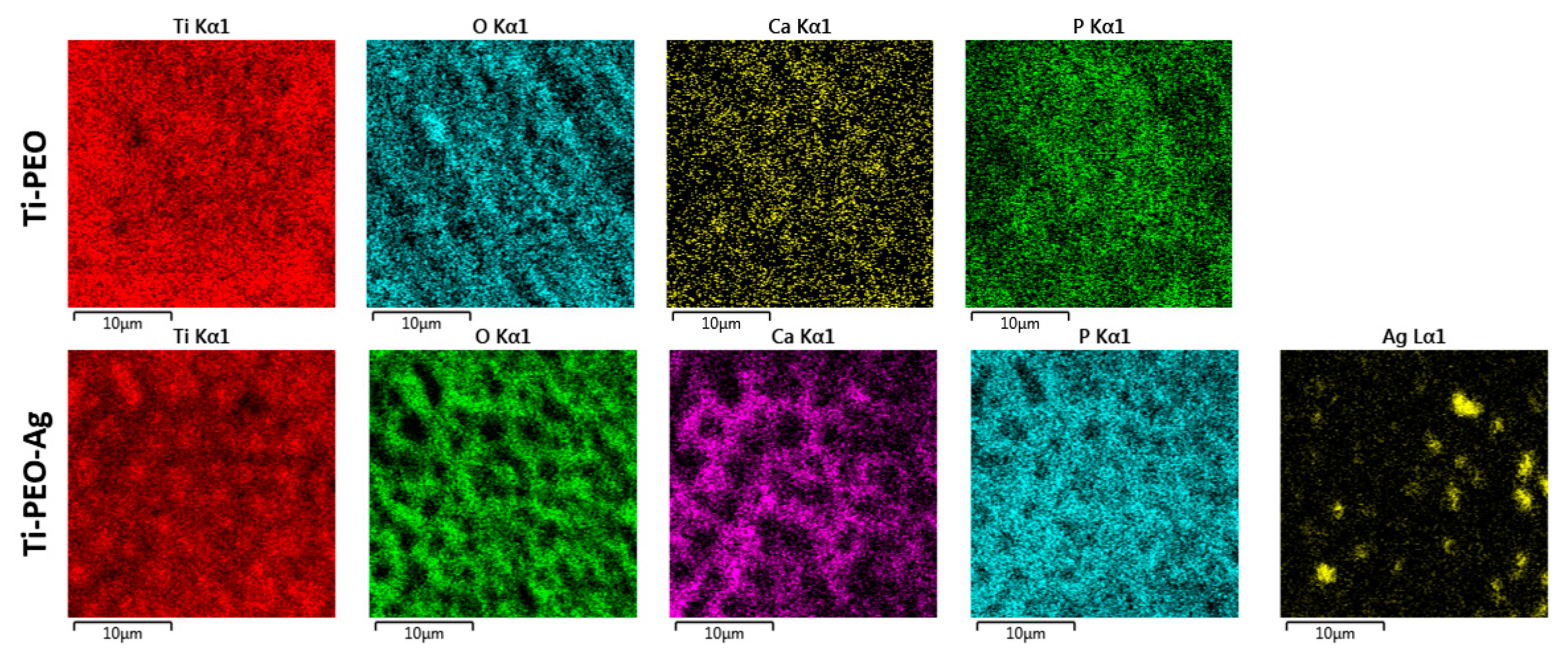
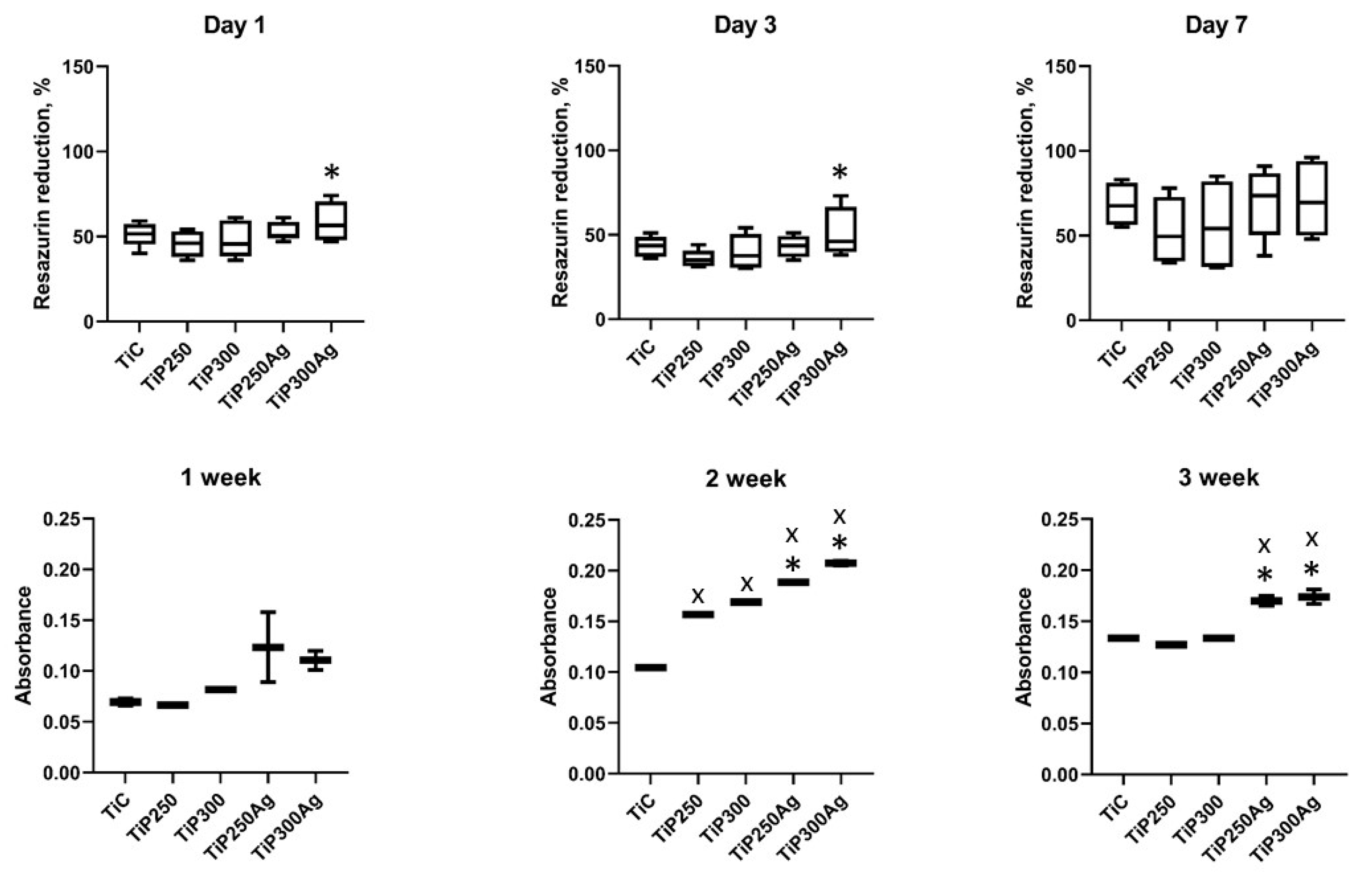
| Sample | Ti | O | C | Ca | P | Ag |
|---|---|---|---|---|---|---|
| TiP-250 | 52.6 | 12.3 | 5.2 | 16.8 | 13.1 | - |
| TiP-250-Ag | 49.2 | 9.8 | 7.2 | 17.6 | 15.9 | 0.3 |
| TiP-300 | 57.3 | 7.5 | 4.0 | 19.7 | 11.5 | - |
| TiP-300-Ag | 54.8 | 7.9 | 6.1 | 18.3 | 12.2 | 0.7 |
| Parameter | TiC | TiP-250 | TiP-250-Ag | TiP-300 | TiP-300-Ag |
|---|---|---|---|---|---|
| Contact angle (°) | 97.3 ± 5.8 | 54.9 ± 6.3 * | 58.5 ± 3.6 * | 34.2 ± 4.0 *£ | 31.9 ± 5.1 *£ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleshko, O.; Liubchak, I.; Husak, Y.; Korniienko, V.; Yusupova, A.; Oleshko, T.; Banasiuk, R.; Szkodo, M.; Matros-Taranets, I.; Kazek-Kęsik, A.; et al. In Vitro Biological Characterization of Silver-Doped Anodic Oxide Coating on Titanium. Materials 2020, 13, 4359. https://doi.org/10.3390/ma13194359
Oleshko O, Liubchak I, Husak Y, Korniienko V, Yusupova A, Oleshko T, Banasiuk R, Szkodo M, Matros-Taranets I, Kazek-Kęsik A, et al. In Vitro Biological Characterization of Silver-Doped Anodic Oxide Coating on Titanium. Materials. 2020; 13(19):4359. https://doi.org/10.3390/ma13194359
Chicago/Turabian StyleOleshko, Oleksandr, Iryna Liubchak, Yevheniia Husak, Viktoriia Korniienko, Aziza Yusupova, Tetiana Oleshko, Rafal Banasiuk, Marek Szkodo, Igor Matros-Taranets, Alicja Kazek-Kęsik, and et al. 2020. "In Vitro Biological Characterization of Silver-Doped Anodic Oxide Coating on Titanium" Materials 13, no. 19: 4359. https://doi.org/10.3390/ma13194359
APA StyleOleshko, O., Liubchak, I., Husak, Y., Korniienko, V., Yusupova, A., Oleshko, T., Banasiuk, R., Szkodo, M., Matros-Taranets, I., Kazek-Kęsik, A., Simka, W., & Pogorielov, M. (2020). In Vitro Biological Characterization of Silver-Doped Anodic Oxide Coating on Titanium. Materials, 13(19), 4359. https://doi.org/10.3390/ma13194359







