Cobalt Chromium Molybdenum Surface Modifications Alter the Osteogenic Differentiation Potential of Human Mesenchymal Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. CoCrMo Alloy Surface Modifications
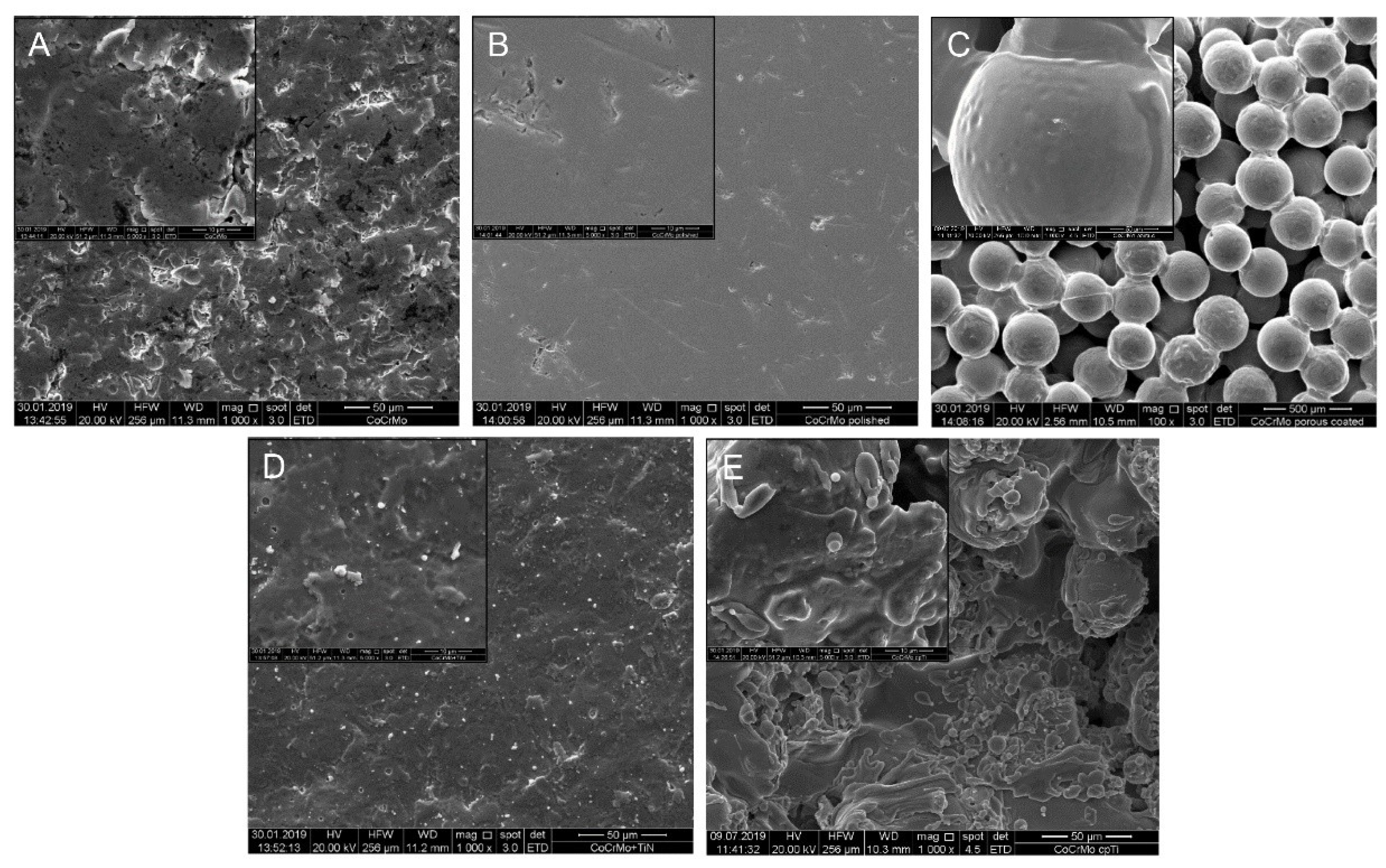
2.2. Scanning Electron Microscopy (SEM)
2.3. Intraoral Tissue Harvest and Cell Culture
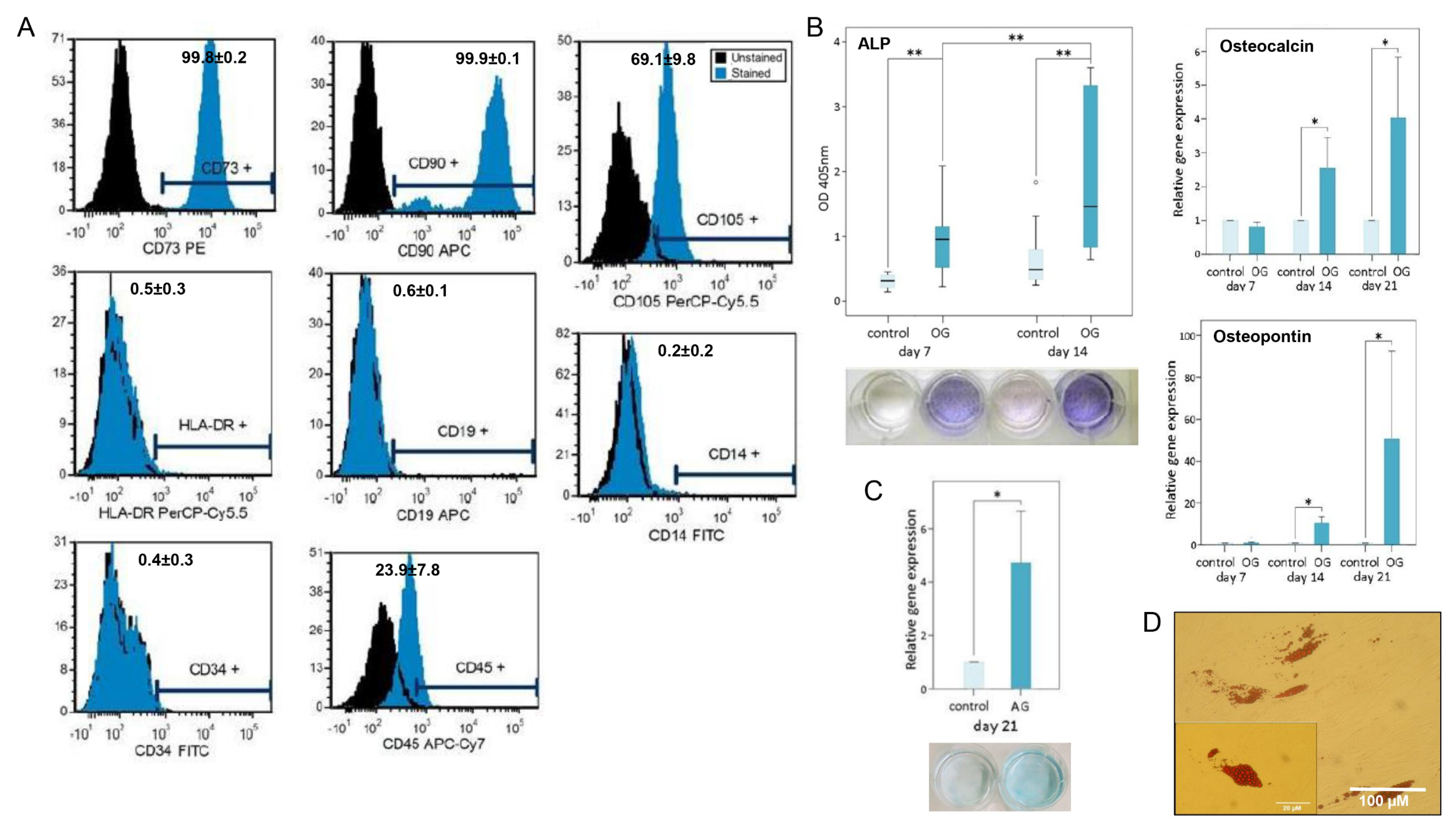
2.4. Flow Cytometry
2.5. Multilineage Differentiation Analysis
2.6. Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
2.7. xMAP Human Bone Metabolism Magnetic Bead Panel
2.8. Statistical Analysis
3. Results
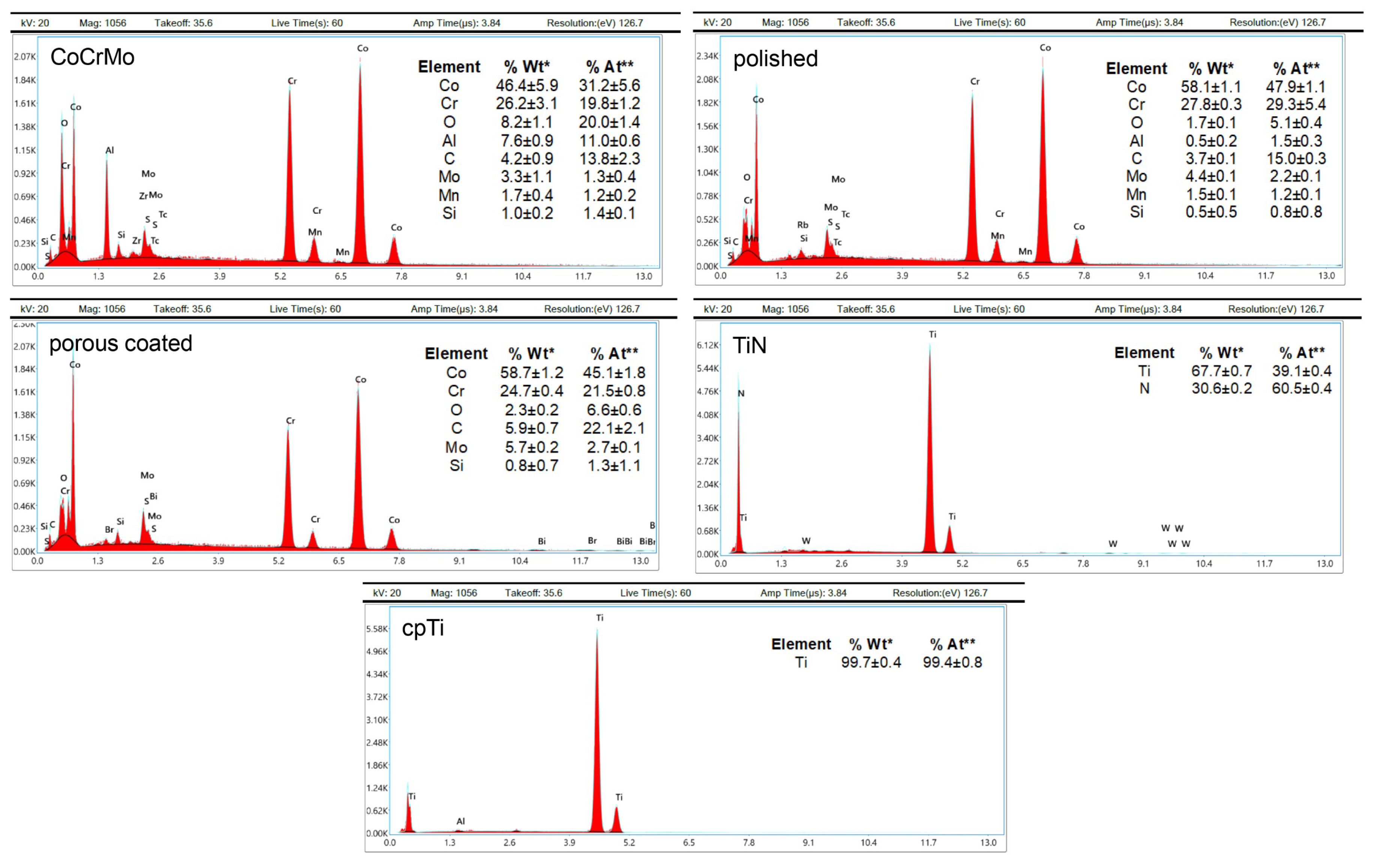
3.1. Surface Characteristics
3.2. MSPC Characterization and Multilineage Differentiation Analysis
3.3. Expression of Osteogenic Markers on Different CoCrMo Surface Modifications
3.4. The Role of Integrin α5β1 Subunits on Different CoCrMo Surface Modifications
3.5. Expression of Bone Biology Markers on Different CoCrMo Surface Modifications
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Q.Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, V.E. Uncemented porous-coated anatomical total hip replacement. J. Bone Jt. Surg. 1990, 72, 45. [Google Scholar]
- Habibovic, P.P.; de Groot, K. Osteoinductive biomaterials properties and relevance in bone repair. J. Tissue Eng. Regen. Med. 2007, 1, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Linez, P.; Bigerelle, M.; Le Maguer, D.; Le Maguer, A.; Hardouin, P.; Hildebrand, H.; Iost, A.; Leroy, J. The relative influence of the topography and chemistry of TiAl6V4 surfaces on osteoblastic cell behaviour. Biomaterials 2000, 21, 1567–1577. [Google Scholar] [CrossRef]
- Ozdemir, Z.; Ozdemir, A.; Basim, G.B. Application of chemical mechanical polishing process on titanium based implants. Mater. Sci. Eng. C 2016, 68, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Li, Y.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qi, W.; Xie, L.; Yang, X.; Li, J.; Zhang, Y. Microstructure and Corrosion Behavior of (CoCrFeNi)95Nb₅ High-Entropy Alloy Coating Fabricated by Plasma Spraying. Materials 2019, 12, 694. [Google Scholar] [CrossRef]
- Liang, R.; Xu, Y.; Zhao, M.; Han, G.; Li, J.; Wu, W.; Dong, M.; Yang, J.; Liu, Y. Properties of silver contained coatings on CoCr alloys prepared by vacuum plasma spraying. Mater. Sci. Eng. C 2020, 106, 110156. [Google Scholar] [CrossRef]
- Meyer, U.; Büchter, A.; Wiesmann, H.P.; Joos, U.; Jones, D.B. Basic reactions of osteoblasts on structured material surfaces. Eur. Cell Mater. 2005, 9, 39–49. [Google Scholar] [CrossRef]
- Davies, J.E. In vitro modeling of the bondimplant interface. Anat. Rec. 1996, 245245, 426–445. [Google Scholar] [CrossRef]
- Frosch, K.H.; Barvencik, F.; Lohmann, C.H.; Viereck, V.; Siggelkow, H.; Breme, J.; Dresing, K.; Stürmer, K. Migration, matrix production and lamellar bone formation of human osteoblast-like cells in porous titanium implants. Cells Tissues Organs 2002, 170, 214–227. [Google Scholar] [CrossRef]
- Luthringer, B.J.; Katha, U.M.; Willumeit, R. Phosphatidylethanolamine biomimetic coating increases mesenchymal stem cell osteoblastogenesis. J. Mater. Sci. Mater. Med. 2014, 11, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Abagnale, G.; Steger, M.; Nguyen, V.H.; Hersch, N.; Sechi, A.S.; Joussen, S.; Denecke, B.; Merkel, R.; Hoffmann, B.; Dreser, A.; et al. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials 2015, 61, 316–326. [Google Scholar] [CrossRef]
- Ducy, P. CBFA1: A molecular switch in osteoblast biology. Dev. Dyn. 2000, 219, 461–471. [Google Scholar] [CrossRef]
- Atmani, H.; Audrain, C.; Mercier, L.; Chappard, D.; Baslé, M.F. Phenotypic effects of continuous or discontinuous treatment with dexamethasone and/or calcitriol on osteoblasts differentiated from rat bone marrow stromal cells. J. Cell. Biochem. 2002, 85, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Bellows, C.; Heersche, J.; Aubin, J.E. Determination of the capacity for proliferation and differentiation of osteoprogenitor cells in the presence and absence of dexamethasone. Dev. Boil. 1990, 140, 132–138. [Google Scholar] [CrossRef]
- Muschler, G.F.; Boehm, C.; Easley, K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: The influence of aspiration volume. J. Bone Jt. Surg. Am. 1997, 79, 1699–1709. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef]
- Hamidouche, Z.; Fromigué, O.; Ringe, J.; Häupl, T.; Vaudin, P.; Pagès, J.-C.; Srouji, S.; Livne, E.; Marie, P.J. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 18587–18591. [Google Scholar] [CrossRef]
- Marie, P.J. Targeting integrins to promote bone formation and repair. Nat. Rev. Endocrinol. 2013, 9, 288–295. [Google Scholar] [CrossRef]
- Lohberger, B.; Stuendl, N.; Glaenzer, D.; Rinner, B.; Donohue, N.; Lichtenegger, H.C.; Ploszczanski, L.; Leithner, A. CoCrMo surface modifications affect biocompatibility, adhesion, and inflammation in human osteoblasts. Sci. Rep. 2020, 10, 1682. [Google Scholar] [CrossRef]
- Klontzas, M.E.; Reakasame, S.; Silva, R.; Morais, J.C.; Vernardis, S.; Macfarlane, R.J.; Heliotis, M.; Tsiridis, E.; Panoskaltsis, N.; Boccaccini, A.R.; et al. Oxidized alginate hydrogels with the GHK peptide enhance cord blood mesenchymal stem cell osteogenesis: A paradigm for metabolomics-based evaluation of biomaterial design. Acta Biomater. 2019, 88, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef]
- Otto, F.; Lübbert, M.; Stock, M. Upstream and downstream targets of RUNX proteins. J. Cell. Biochem. 2003, 89, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Wang, D.; Benson, M.D.; Karsenty, G.; Franceschi, R.T. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J. Biol. Chem. 1998, 273, 32988–32994. [Google Scholar] [CrossRef]
- Jagodzinski, M.; Drescher, M.; Zeichen, J.; Hankemeier, S.; Krettek, C.; Bosch, U.; Van Griensven, M. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur. Cell Mater. 2004, 7, 35–41. [Google Scholar] [CrossRef]
- Qi, H.; Aguiar, D.J.; Williams, S.M.; La Pean, A.; Pan, W.; Verfaillie, C.M. Identification of genes responsible for osteoblast differentiation from human mesodermal progenitor cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3305–3310. [Google Scholar] [CrossRef]
- Standal, T.; Borset, M.; Sundan, A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp. Oncol. 2004, 26, 179–184. [Google Scholar]
- Ryoo, H.M.; Hoffmann, H.M.; Beumer, T.; Frenkel, B.; Towler, D.A.; Stein, G.S.; Stein, J.L.; van Wijnen, A.J.; Lian, J.B. Stage-specific expression of Dlx-5 during osteoblast differentiation: Involvement in regulation of osteocalcin gene expression. Mol. Endocrinol. 1997, 11, 1681–1694. [Google Scholar] [CrossRef]
- Wada, S.; Fukawa, T.; Kamiya, S. Osteocalcin and bone. Clin. Calcium 2007, 17, 1673–1677. [Google Scholar] [PubMed]
- Logan, N.; Sherif, A.; Cross, A.J.; Collins, S.N.; Traynor, A.; Bozec, L.; Parkin, I.P.; Brett, P. TiO2-coated CoCrMo: Improving the osteogenic differentiation and adhesion of mesenchymal stem cells in vitro. J. Biomed. Mater. Res. Part A 2015, 103, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhuang, X.; Tang, K.; Wu, P.; Guo, X.; Yin, L.; Cao, H.; Zou, D. Intrinsic Surface Effects of Tantalum and Titanium on Integrin α5β1/ERK1/2 Pathway-Mediated Osteogenic Differentiation in Rat Bone Mesenchymal Stromal Cells. Cell Physiol. Biochem. 2018, 51, 589–609. [Google Scholar] [CrossRef] [PubMed]
- Logan, N.; Bozec, L.; Traynor, A.; Brett, P. Mesenchymal stem cell response to topographically modified CoCrMo. J. Biomed. Mater. Res. A 2015, 103, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.L.; Hankenson, K.D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 2011, 112, 3491–3501. [Google Scholar] [CrossRef]
- Jonitz-Heincke, A.; Sellin, M.-L.; Seyfarth, A.; Peters, K.; Mueller-Hilke, B.; Fiedler, T.; Bader, R.; Klinder, A.; Heincke, J.-; Hilke, M. Analysis of Cellular Activity Short-Term Exposure to Cobalt and Chromium Ions in Mature Human Osteoblasts. Materials 2019, 12, 2771. [Google Scholar] [CrossRef]
- Ormsby, R.T.; Solomon, L.B.; Yang, D.; Crotti, T.N.; Haynes, D.R.; Findlay, D.M.; Atkins, G. Osteocytes respond to particles of clinically-relevant conventional and cross-linked polyethylene and metal alloys by up-regulation of resorptive and inflammatory pathways. Acta Biomater. 2019, 87, 296–306. [Google Scholar] [CrossRef]
- Upadhyay, J.; Farr, O.M.; Mantzoros, C.S. The role of leptin in regulating bone metabolism. Metabolism 2015, 64, 105–113. [Google Scholar] [CrossRef]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef]
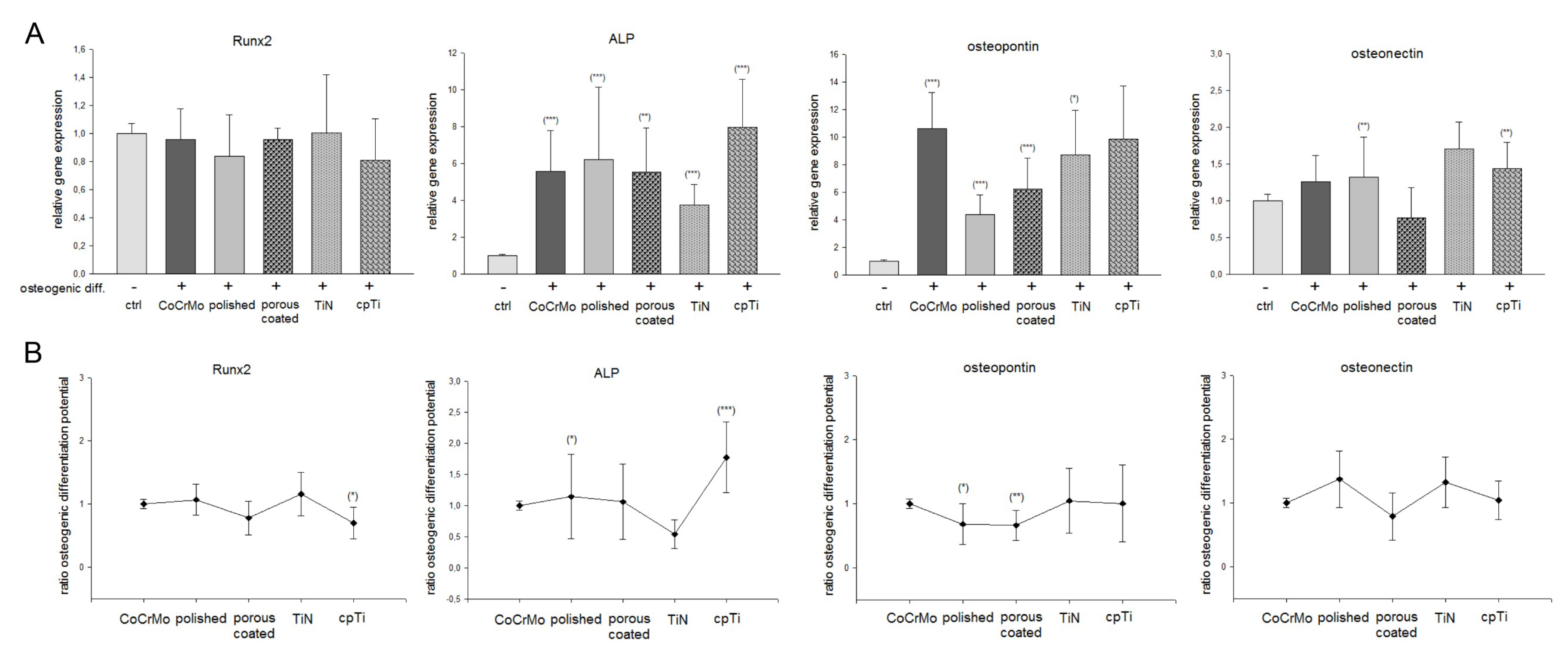
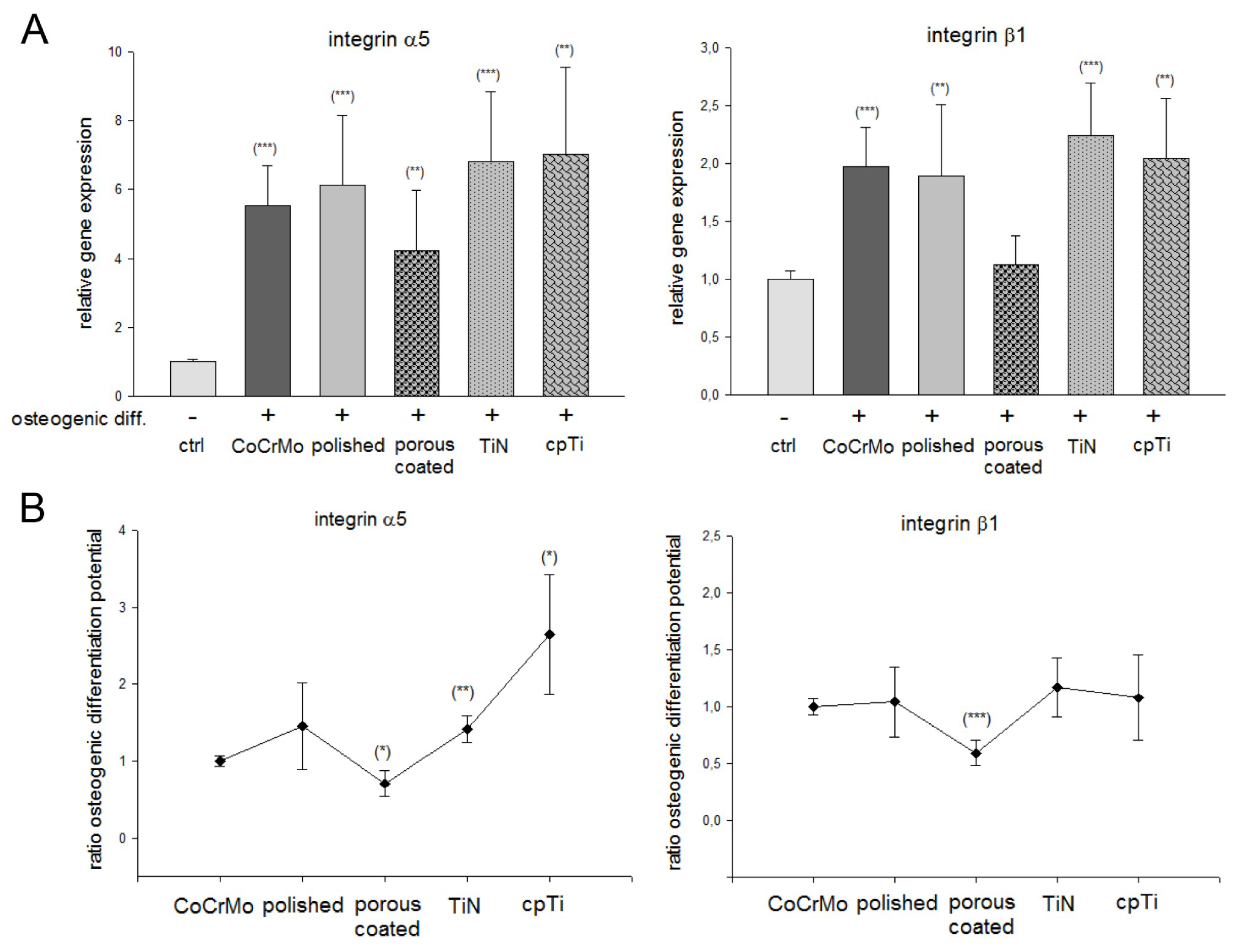
| Gene. | Ctrl | Osteogenic Differentiated | ||||
| ad Figure 3 | CoCrMo | Polished | Porous | TiN | cpTi | |
| Runx2 (Figure 3A) | 1 ± 0.1 | 0.95 ± 0.2 | 0.84 ± 0.3 | 0.96 ± 0.1 | 1.00 ± 0.4 | 0.81 ± 0.3 |
| Runx2 (Figure 3B) | 1 ± 0.1 | 1.07 ± 0.2 | 0.77 ± 0.3 | 1.16 ± 0.3 | 0.69 ± 0.2 * | |
| ALP (Figure 3A) | 1 ± 0.1 | 5.17 ± 2.4 *** | 5.92 ± 4.1 ** | 5.66 ± 3.3 *** | 3.93 ± 1.8 *** | 7.74 ± 2.8 *** |
| ALP (Figure 3B) | 1 ± 0.1 | 1.62 ± 0.4* | 1.26 ± 0.7 | 1.24 ± 0.3 | 1.93 ± 0.5 *** | |
| Osteopontin (Figure 3A) | 1 ± 0.1 | 10.63 ± 4.0 *** | 4.38 ± 1.4 *** | 5.73 ± 4.2 * | 8.74 ± 3.2 *** | 9.38 ± 5.6 |
| Osteopontin (Figure 3B) | 1 ± 0.1 | 0.68 ± 0.3 * | 0.66 ± 0.2 ** | 1.05 ± 0.5 | 0.84 ± 0.7 | |
| Osteonectin (Figure 3A) | 1 ± 0.1 | 1.26 ± 0.3 | 1.33 ± 0.5 | 0.77 ± 0.4 | 1.74 ± 0.4 ** | 1.44 ± 0.3 ** |
| Osteonectin (Figure 3B) | 1 ± 0.1 | 1.37 ± 0.5 | 0.79 ± 0.4 | 1.32 ± 0.4 | 1.04 ± 0.3 | |
| Ctrl | Osteogenic Differentiated | |||||
| ad Figure 4 | CoCrMo | Polished | Porous | TiN | cpTi | |
| Integrin α5 (Figure 4A) | 1 ± 0.1 | 5.53 ± 1.2 *** | 6.13 ± 2.0 *** | 4.22 ± 1.8 ** | 6.83 ± 2.0 *** | 7.03 ± 2.5 ** |
| Integrin α5 (Figure 4B) | 1 ± 0.1 | 1.41 ± 0.6 | 0.69 ± 0.2 * | 1.35 ± 0.3 * | 2,65 ± 0.8 | |
| Integrin β1 (Figure 4A) | 1 ± 0.1 | 1.97 ± 0.3 *** | 1.89 ± 0.6 ** | 1.17 ± 0.2 | 2.25 ± 0.4 *** | 2.16 ± 0.5 ** |
| Integrin β1 (Figure 3B) | 1 ± 0.1 | 1.04 ± 0.3 | 0.59 ± 0.11 *** | 1.17 ± 0.3 | 1.07 ± 0.3 | |
| pg/mL | |||||
|---|---|---|---|---|---|
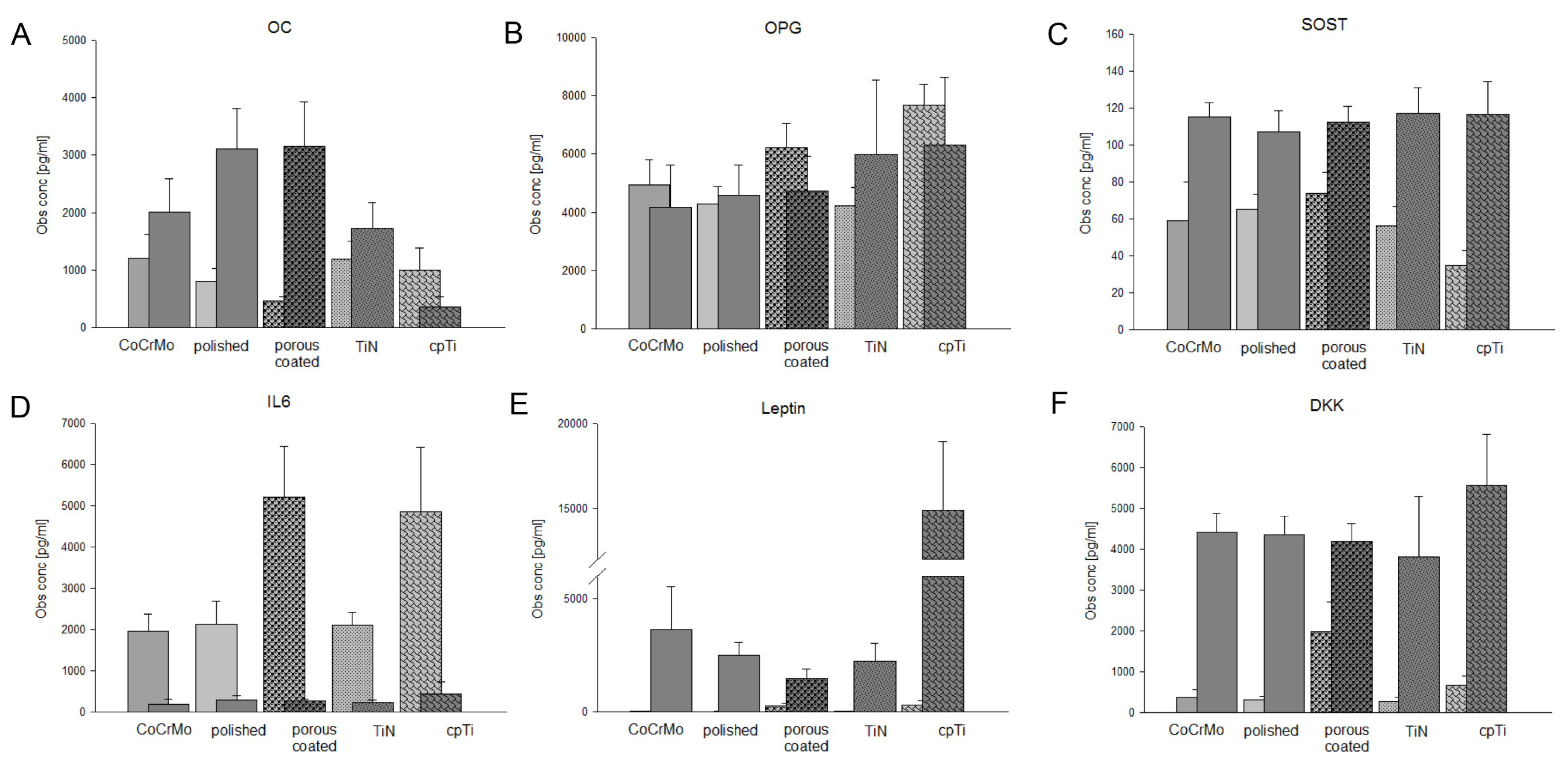
| Gene | CoCrMo | Polished | Porous | TiN | cpTi |
| OC undiff | 1200 ± 419 | 805 ± 218 | 467 ± 69 | 1195 ± 306 | 999 ± 379 |
| OC OG | 2003 ± 591 | 3158 ± 775 * | 1727 ± 447 * | 3117 ± 699 * | 354 ± 184 * |
| SM undiff. | n.s. | # | n.s. | n.s. | |
| SM OG | n.s. | n.s. | n.s. | ## | |
| OPG undiff | 4934 ± 879 | 4279 ± 593 | 6221 ± 840 | 4224 ± 639 | 7674 ± 710 |
| OPG OG | 4176 ± 1462 | 4740 ± 1178 | 5985 ± 2565 | 4583 ± 1043 | 6315 ± 2307 |
| SM undiff. | n.s. | n.s | n.s. | ## | |
| SM OG | n.s. | n.s | n.s. | n.s. | |
| SOST undiff | 58.8 ± 21 | 65.1 ± 8 | 73.6 ± 11.8 | 56.2 ± 10.5 | 35.0 ± 7.9 |
| SOST OG | 115 ± 7.9 * | 112.5 ± 8.6 *** | 117.1 ± 13.8 * | 107.3 ± 11.3 ** | 116.7 ± 17.6 ** |
| SM undiff. | n.s. | # | n.s. | n.s. | |
| SM OG | n.s. | n.s. | n.s. | n.s. | |
| IL6 undiff | 1957 ± 426 | 2114 ± 575 | 5208 ± 1230 | 2099 ± 324 | 4857 ± 1556 |
| IL6 OG | 187 ± 128 *** | 262 ± 57 * | 218 ± 76 * | 295 ± 91 ** | 435 ± 296 * |
| SM undiff. | n.s. | # | n.s. | # | |
| SM OG | n.s. | n.s. | n.s. | n.s. | |
| Leptin undiff | 27.3 ± 4.5 | 17.7 ± 4.6 | 267 ± 107 | 33 ± 12.3 | 313 ± 175 |
| Leptin OG | 3642 ± 1877 *** | 1478 ± 405 ** | 2230 ± 811 ** | 2498 ± 549 *** | 14911 ± 4053 *** |
| SM undiff. | # | # | n.s. | ## | |
| SM OG | n.s. | n.s. | n.s. | ## | |
| DKK undiff | 371 ± 196 | 311 ± 79 | 1978 ± 737 | 266 ± 106 | 659 ± 232 |
| DKK OG | 4410 ± 468 *** | 4179 ± 449 *** | 3816 ± 1482 | 4350 ± 641 *** | 5557 ± 1266 ** |
| SM undiff. | n.s. | # | n.s. | n.s. | |
| SM OG | n.s. | n.s. | n.s. | n.s. | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohberger, B.; Eck, N.; Glaenzer, D.; Lichtenegger, H.; Ploszczanski, L.; Leithner, A. Cobalt Chromium Molybdenum Surface Modifications Alter the Osteogenic Differentiation Potential of Human Mesenchymal Stem Cells. Materials 2020, 13, 4292. https://doi.org/10.3390/ma13194292
Lohberger B, Eck N, Glaenzer D, Lichtenegger H, Ploszczanski L, Leithner A. Cobalt Chromium Molybdenum Surface Modifications Alter the Osteogenic Differentiation Potential of Human Mesenchymal Stem Cells. Materials. 2020; 13(19):4292. https://doi.org/10.3390/ma13194292
Chicago/Turabian StyleLohberger, Birgit, Nicole Eck, Dietmar Glaenzer, Helga Lichtenegger, Leon Ploszczanski, and Andreas Leithner. 2020. "Cobalt Chromium Molybdenum Surface Modifications Alter the Osteogenic Differentiation Potential of Human Mesenchymal Stem Cells" Materials 13, no. 19: 4292. https://doi.org/10.3390/ma13194292
APA StyleLohberger, B., Eck, N., Glaenzer, D., Lichtenegger, H., Ploszczanski, L., & Leithner, A. (2020). Cobalt Chromium Molybdenum Surface Modifications Alter the Osteogenic Differentiation Potential of Human Mesenchymal Stem Cells. Materials, 13(19), 4292. https://doi.org/10.3390/ma13194292






