Physical–Mechanical Characteristics and Microstructure of Ti6Al7Nb Lattice Structures Manufactured by Selective Laser Melting
Abstract
1. Introduction
2. Experimental Section
2.1. Powder
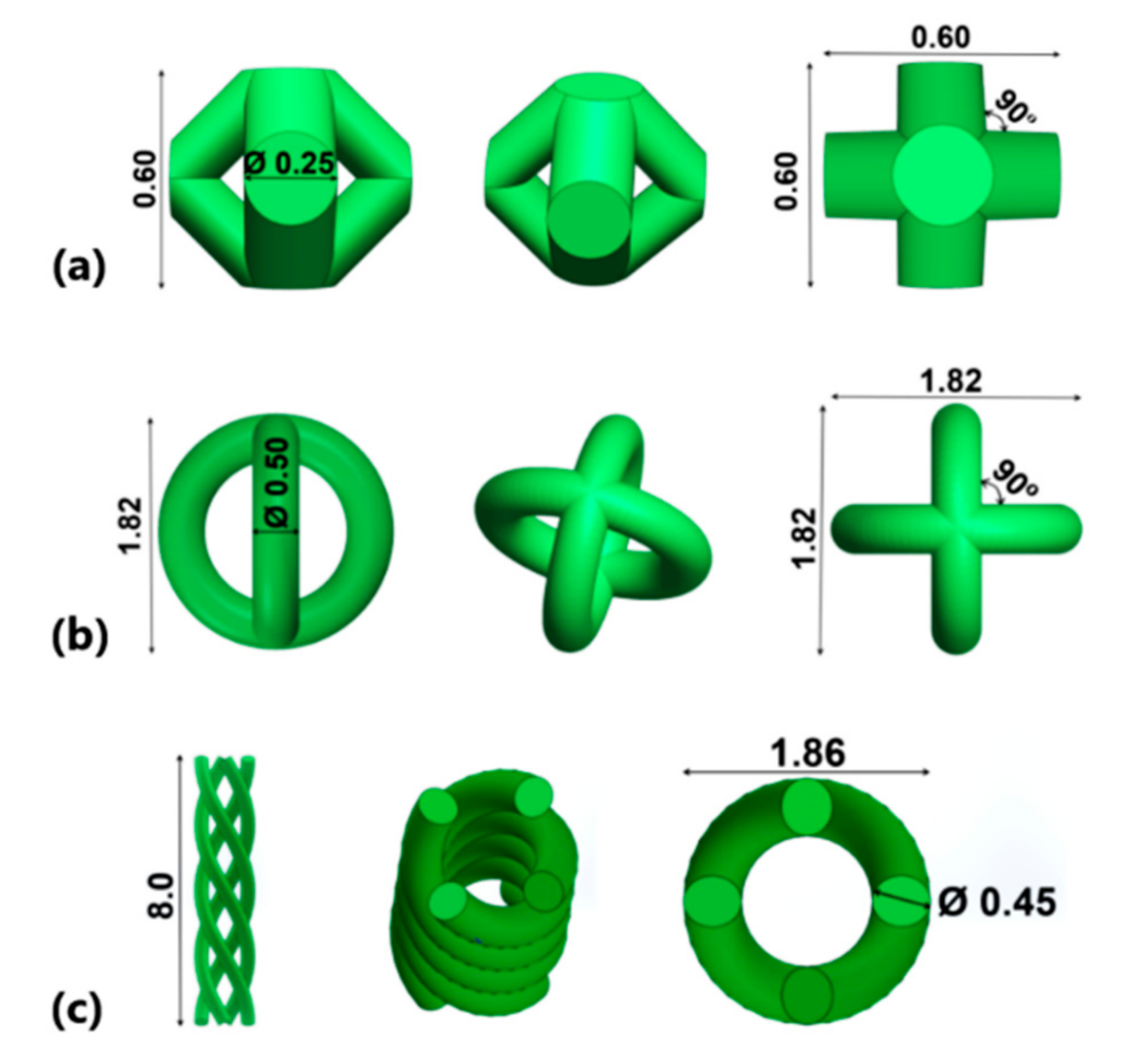
2.2. Cell Design
2.3. SLM Manufacturing
2.4. Mechanical Testing and Estimation Equations
2.5. Microstructure and Metallographic Analyses
3. Results and Discussion
3.1. Strut Diameter and Porosity Level
3.2. Compression Properties
3.3. Correlation between Gibson–Ashby Mathematical Model and Compressive Properties
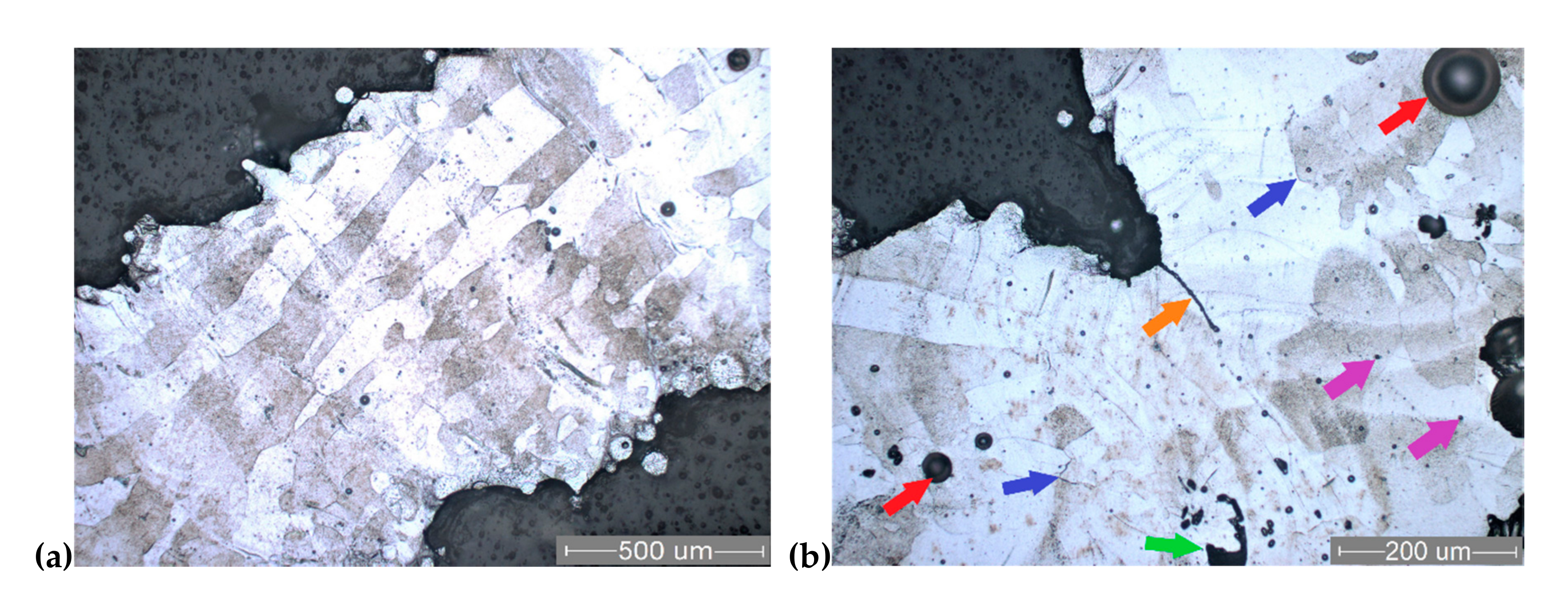
3.4. SEM Analysis of Struts
3.5. Microstructure
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lietaert, K.; Cutolo, A.; Boey, D.; Van Hooreweder, B. Fatigue life of additively manufactured Ti6Al4V scaffolds under tension-tension, tension-compression and compression-compression fatigue load. Nat. Sci. Rep. 2018, 8, 4957. [Google Scholar] [CrossRef] [PubMed]
- Popan, I.A.; Carean, A.; Luca, A.; Ceclan, V.; Balc, N. Research on 3D metal sculpturing by water jet cutting versus CNC machining. Acad. J. Manuf. Eng. 2013, 11, 74–79. [Google Scholar]
- Luca, A.; Balc, N.; Popan, A.; Ceclan, V.; Panc, N. Improving the quality of the parts made by rapid metal casting process. Acad. J. Manuf. Eng. 2014, 12, 82–86. [Google Scholar]
- Monková, K.; Monka, P.; Vegnerová, P.; Čep, R.; Müllerová, J.; Bražina, D.; Duspara, M. Factor analysis of the abrasive waterjet factors affecting the surface roughness of titanium. Teh. Vjesn. 2011, 18, 73–77. [Google Scholar]
- Varga, G.; Torok, T.; Felho, C.; Orosz-Szirmai, G.; Rez, I. Surface features of chromium alloyed carbon steel specimens after salt-spray tests in NaCl solution. Adv. Prod. Eng. Manag. 2019, 14, 449–460. [Google Scholar] [CrossRef]
- Grobelny, P.; Legutko, S.; Habrat, W.; Furmanski, L. Investigations of surface topography of titanium alloy manufactured with the use of 3D print. IOP Conf. Ser. Mater. Sci. Eng. 2018, 393, 012108. [Google Scholar] [CrossRef]
- Saboori, A.; Gallo, D.; Biamino, S.; Fino, P.; Lombardi, M. An Overview of Additive Manufacturing of Titanium Components by Directed Energy Deposition: Microstructure and Mechanical Properties. Appl. Sci. 2017, 7, 883. [Google Scholar] [CrossRef]
- Wally, Z.J.; Haque, A.M.; Feteira, A.; Claeyssens, F.; Goodall, R.; Reilly, G.C. Selective laser melting processed Ti6Al4V lattices with graded porosities for dental applications. J. Mech. Behav. Biomed. Mater. 2019, 90, 20–29. [Google Scholar] [CrossRef]
- Weißmann, V.; Wieding, J.; Hansmann, H.; Laufer, N.; Wolf, A.; Bader, R. Specific Yielding of Selective Laser-Melted Ti6Al4V Open-Porous Scaffolds as a Function of Unit Cell Design and Dimensions. Metals 2016, 6, 166. [Google Scholar] [CrossRef]
- Yan, C.; Hao, L.; Hussein, A.; Young, P.; Raymont, D. Advanced lightweight 316L stainless steel cellular lattice structures fabricated via selective laser melting. Mater. Des. 2014, 55, 533–541. [Google Scholar] [CrossRef]
- Sallica-Leva, E.; Jardini, A.L.; Fogagnolo, J.B. Microstructure and mechanical behavior of porous Ti6Al4V parts obtained by selective laser melting. J. Mech. Behav. Biomed. Mater. 2013, 26, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Burton, H.E.; Eisenstein, N.M.; Lawless, B.M.; Jamshidi, P.; Segarra, M.A.; Addison, O.; Shepherd, D.E.; Attallah, M.M.; Grover, L.M.; Cox, S.C. The design of additively manufactured lattices to increase the functionality of medical implants. Mater. Sci. Eng. C 2019, 94, 901–908. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Campoli, G.; Yavari, S.A.; Sajadi, B.; Wauthlé, R.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Mechanical behavior of regular open-cell porous biomaterials made of diamond lattice unit cells. J. Mech. Behav. Biomed. Mater. 2014, 34, 106–115. [Google Scholar] [CrossRef]
- Brandt, M. Laser Additive Manufacturing-Materials, Design, Technologies, and Applications, 1st ed.; Woodhead Publishing: Cambridge, UK, 2017. [Google Scholar] [CrossRef]
- Yavari, S.A.; Wauthlé, R.; van der Stok, J.; Riemslag, A.C.; Janssen, M.; Mulier, M.; Kruth, J.P.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Fatigue behavior of porous biomaterials manufactured using selective laser melting. Mater. Sci. Eng. C 2013, 33, 4849–4858. [Google Scholar] [CrossRef]
- Nakano, T.; Ishimoto, T. Powder-based Additive Manufacturing for development of Tailor-made implants for orthopedic applications. KONA Powder Part. J. 2015, 32, 75–84. [Google Scholar] [CrossRef]
- Wang, H.; Su, K.; Su, L.; Liang, P.; Ji, P.; Wang, C. The effect of 3D-printed Ti6Al4V scaffolds with various macropore structures on osteointegration and osteogenesis: A biomechanical evaluation. J. Mech. Behav. Biomed. Mater. 2018, 88, 488–496. [Google Scholar] [CrossRef]
- El-Sayed, M.A.; Essa, K.; Ghazy, M.; Hassanin, H. Design optimization of additively manufactured titanium lattice structures for biomedical implants. Int. J. Adv. Manuf. Technol. 2020. [Google Scholar] [CrossRef]
- Han, C.; Li, Y.; Wang, Q.; Wen, S.; Wei, Q.; Yan, C.; Hao, L.; Liu, J.; Shi, Y. Continuous functionally graded porous titanium scaffolds manufactured by selective laser melting for bone implants. J. Mech. Behav. Biomed. Mater. 2018, 80, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wei, Q.; Cheng, L.; Li, S.; Shi, Y. Effects of scan line spacing on pore characteristics and mechanical properties of porous Ti6Al4V implants fabricated by selective laser melting. Mater. Des. 2014, 63, 185–193. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Martinez, E.; Medina, F.; Wicker, R.B. Next Generation Orthopaedic Implants by Additive Manufacturing Using Electron Beam Melting. Int. J. Biomater. 2012, 2012, 245727. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, J.; Choong, P.F. Three-dimensional printed calcaneal prosthesis following total calcanectomy. Int. J. Surg. Case. Rep. 2015, 10, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann. Maxillofac. Surg. 2014, 4, 9–18. [Google Scholar] [CrossRef]
- Armencea, G.; Cosma, C.; Dinu, C.; Onisor, F.; Lazar, M.; Berce, P.; Balc, N.; Baciut, M.; Bran, S. Technical queries of a 3D design custom-made implant made from titanium particles for maxillofacial bone reconstruction. Part. Sci. Technol. 2019, 38, 676–684. [Google Scholar] [CrossRef]
- Yavari, S.A.; Ahmadi, S.M.; van der Stok, J.; Wauthlé, R.; Riemslag, A.C.; Janssen, M.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Effects of bio-functionalizing surface treatments on the mechanical behavior of open porous titanium biomaterials. J. Mech. Behav. Biomed. Mater. 2014, 36, 109–119. [Google Scholar] [CrossRef]
- Du Plessis, A.; Kouprianoff, D.P.; Yadroitsava, I.; Yadroitsev, I. Mechanical properties and in-situ deformation imaging of micro-lattices manufactured by laser based powder bed fusion. Materials 2018, 11, 1663. [Google Scholar] [CrossRef]
- Feng, Q.; Tang, Q.; Liu, Z.; Liu, Y.; Setchi, R. An investigation of the mechanical properties of metallic lattice structures fabricated using selective laser melting. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2016, 232, 1719–1730. [Google Scholar] [CrossRef]
- Liu, F.; Ran, Q.; Zhao, M.; Zhang, T.; Zhang, D.Z.; Su, Z. Additively Manufactured Continuous Cell-Size Gradient Porous Scaffolds: Pore Characteristics, Mechanical Properties and Biological Responses in Vitro. Materials 2020, 13, 2589. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, Y.; Ao, Y.; Han, C.; Wang, Q.; Li, Y.; Liu, J.; Wei, Q.; Zhang, Z. Effect of pore geometry on the fatigue properties and cell affinity of porous titanium scaffolds fabricated by selective laser melting. J. Mech. Behav. Biomed. Mater. 2018, 88, 478–487. [Google Scholar] [CrossRef]
- Ganbold, B.; Heo, S.-J.; Koak, J.-Y.; Kim, S.-K.; Cho, J. Human Stem Cell Responses and Surface Characteristics of 3D Printing Co-Cr Dental Material. Materials 2019, 12, 3419. [Google Scholar] [CrossRef]
- Hedayati, R.; Ahmadi, S.M.; Lietaert, K.; Pouran, B.; Li, Y.; Weinans, H.; Rans, C.D.; Zadpoor, A.A. Isolated and modulated effects of topology and material type on the mechanical properties of additively manufactured porous biomaterials. J. Mech. Behav. Biomed. Mater. 2018, 79, 254–263. [Google Scholar] [CrossRef]
- Čapek, J.; Machová, M.; Fousová, M.; Kubásek, J.; Vojtěch, D.; Fojt, J.; Jablonska, E.; Lipov, J.; Ruml, T. Highly porous, low elastic modulus 316L stainless steel scaffold prepared by selective laser melting. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Balc, N.; Gebhardt, A.; Abbas, K. Basic research on lattice structures focused on the reliance of the cross sectional area and additional coatings. MATEC Web Conf. 2017, 94, 03008. [Google Scholar] [CrossRef][Green Version]
- Monkova, K.; Monka, P. Some Aspects Influencing Production of Porous Structures with Complex Shapes of Cells. Int. Conf. Adv. Manuf. Eng. Technol. 2017, 267–276. [Google Scholar] [CrossRef]
- Chlebus, E.; Kuźnicka, B.; Kurzynowski, T.; Dybala, B. Microstructure and mechanical behaviour of Ti-6Al-7Nb alloy produced by selective laser melting. Mater. Character. 2011, 62, 488–495. [Google Scholar] [CrossRef]
- Kumar, U.; Gope, D.K.; Srivastava, J.P.; Chattopadhyaya, S.; Das, A.K.; Krolczyk, G. Experimental and Numerical Assessment of Temperature Field and Analysis of Microstructure and Mechanical Properties of Low Power Laser Annealed Welded Joints. Materials 2018, 11, 1514. [Google Scholar]
- Pawlak, A.; Szymczyk, P.; Ziolkowski, G.; Chlebus, E.; Dybala, B. Fabrication of microscaffolds from Ti-6Al-7Nb alloy by SLM. Rapid Prototyp. J. 2015, 21, 393–401. [Google Scholar] [CrossRef]
- ASTM B213-17, Standard Test Methods for Flow Rate of Metal Powders Using the Hall Flowmeter Funnel; ASTM International: Montgomery County, PA, USA, 2018.
- Marcu, T.; Todea, M.; Gligor, I.; Berce, P.; Popa, C. Effect of surface conditioning on the flowability of Ti6Al7Nb powder for selective laser melting applications. Appl. Surf. Sci. 2012, 258, 3276–3282. [Google Scholar] [CrossRef]
- Yang, C.; Xu, K.; Xie, S. Comparative Study on the Uniaxial Behaviour of Topology-Optimised and Crystal-Inspired Lattice Materials. Metals 2020, 10, 491. [Google Scholar] [CrossRef]
- Saboori, A.; Aversa, A.; Marchese, G.; Biamino, S.; Lombardi, M.; Fino, P. Application of Directed Energy Deposition-Based Additive Manufacturing in Repair. Appl. Sci. 2019, 9, 3316. [Google Scholar] [CrossRef]
- Marcu, T.; Menapace, C.; Girardini, L.; Leordean, D.; Popa, C. Selective laser melting of Ti6Al7Nb with hydroxyapatite addition. Rapid Prototyp. J. 2014, 20, 301–310. [Google Scholar] [CrossRef]
- Zhang, L.C.; Attar, H. Selective Laser Melting of Titanium Alloys and Titanium Matrix Composites for Biomedical Applications: A Review. Adv. Eng. Mater. 2016, 18, 463–475. [Google Scholar] [CrossRef]
- Todea, M.; Vulpoi, A.; Popa, C.; Berce, P.; Simon, S. Effect of different surface treatments on bioactivity of porous titanium implants. J. Mater. Sci. Technol. 2019, 35, 418–426. [Google Scholar] [CrossRef]
- Pal, S.; Gubeljak, N.; Hudak, R.; Lojen, G.; Rajtukova, V.; Predan, J.; Kokol, V.; Drstvensek, I. Tensile properties of selective laser melting products affected by building orientation and energy density. Mater. Sci. Eng. A 2019, 743, 637–647. [Google Scholar] [CrossRef]
- Cosma, C.; Kessler, J.; Gebhardt, A.; Campbell, I.; Balc, N. Improving the Mechanical Strength of Dental Applications and Lattice Structures SLM Processed. Materials 2020, 13, 905. [Google Scholar] [CrossRef]
- DIN 50106:2016, Testing of Metallic Materials—Compression Test at Room Temperature; DIN: Berlin, Germany, 2016.
- Nouri, A.; Hodgson, P.D.; Wen, C. Biomimetic Porous Titanium Scaffolds for Orthopedic and Dental Applications. Biomim. Learn. Nat. 2010, 415–450. [Google Scholar] [CrossRef]
- Li, J.P.; Li, S.H.; Van Blitterswijk, C.A.; De Groot, K. A novel porous Ti6Al4V: Characterization and cell attachment. J. Biomed. Mater. Res. 2005, 73, 223–233. [Google Scholar] [CrossRef]
- Aly, M.S.; Bleck, W.; Scholz, P.F. How metal foams behave if the temperature rises. Met. Powder Rep. 2005, 60, 38–45. [Google Scholar] [CrossRef]
- Dey, A.; Mukhopadhyay, A.K. Nanoindentation of Natural Materials: Hierarchical and Functionally Graded Microstructures; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Fan, Z.; Rho, J.Y. Effects of viscoelasticity and time-dependent plasticity on nanoindentation measurements of human cortical bone. J. Biomed. Mater. Res. 2003, 67, 208–214. [Google Scholar] [CrossRef]
- Keaveny, T.; Morgan, F.; Yeh, C.O. Bone Mechanics. In Biomedical Engineering and Design; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Morgan, E.F.; Keaveny, T.M. Dependence of yield strain of human trabecular bone on anatomic site. J. Biomech. 2001, 34, 569–577. [Google Scholar] [CrossRef]
- Simmons, C.A.; Matlis, S.; Thornton, A.J.; Chen, S.; Wang, C.Y.; Mooney, D.J. Cyclic strain enhances matrix mineralization by adult human mesenchymal stem cells via the extracellular signal-regulated kinase (ERK1/2) signaling pathway. J. Biomech. 2003, 36, 1087–1096. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, N. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Low Modulus Titanium Alloys for Inhibiting Bone Atrophy. Biomater. Sci. Eng. 2011, 249–268. [Google Scholar] [CrossRef]
- Herle, S.; Marcu, C.; Benea, H.; Miclea, M.; Robotin, R. Simulation-Based Stress Analysis for a 3D Modeled Humerus-Prosthesis Assembly. Innov. Comput. Sci. Softw. Eng. 2010, 343–348. [Google Scholar] [CrossRef]
- Peters, A.E.; Akhtar, R.; Comerford, E.J.; Bates, K.T. The effect of ageing and osteoarthritis on the mechanical properties of cartilage and bone in the human knee joint. Nat. Sci. Rep. 2018, 8, 5931. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Huang, T.; Kou, H.; Zhou, L. Compression fatigue behavior and failure mechanism of porous titanium for biomedical applications. J. Mech. Behav. Biomed. Mater. 2017, 65, 814–823. [Google Scholar] [CrossRef]
- Maconachie, T.; Leary, M.; Lozanovski, B.; Zhang, X.; Qian, M.; Faruque, O.; Brandt, M. SLM lattice structures: Properties, performance, applications and challenges. Mater. Des. 2019, 183, 108137. [Google Scholar] [CrossRef]
- Van Hooreweder, B.; Apers, Y.; Lietaert, K.; Kruth, J.P. Improving the fatigue performance of porous metallic biomaterials produced by Selective Laser Melting. Acta Biomater. 2017, 47, 193–202. [Google Scholar] [CrossRef]
- Liu, P.S.; Ma, X.M. Property relations based on the octahedral structure model with body-centered cubic mode for porous metal foams. Mater. Des. 2020, 188. [Google Scholar] [CrossRef]
- Tkac, J.; Samborski, S.; Monkova, K.; Debski, H. Analysis of mechanical properties of a lattice structure produced with the additive technology. Compos. Struct. 2020, 242. [Google Scholar] [CrossRef]
- Attar, H.; Calin, M.; Zhang, C.; Scudino, S.; Eckert, J. Manufacture by selective laser melting and mechanical behavior of commercially pure titanium. Mater. Sci. Eng. A 2014, 593, 170–177. [Google Scholar] [CrossRef]
- Mierzejewska, Ż.A.; Hudák, R.; Sidun, J. Mechanical Properties and Microstructure of DMLS Ti6Al4V Alloy Dedicated to Biomedical Applications. Materials 2019, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Liverani, E.; Toschi, S.; Ceschini, L.; Fortunato, A. Effect of Selective Laser Melting (SLM) process parameters on microstructure and mechanical properties of 316L austenitic stainless steel. J. Mater. Process. Technol. 2017, 249, 255–263. [Google Scholar] [CrossRef]
- Miron, A.E.; Moldovan, M.; Prejmerean, C.A.; Prodan, D.; Vlassa, M.; Filip, M.; Badea, M.E.; Moldovan, M.A. New Antimicrobial Biomaterials for the Reconstruction of Craniofacial Bone Defects. Coatings 2020, 10, 678. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Mester, A.; Benea, H.; Oltean-Dan, D.; Onisor, F.; Baciut, M.; Bran, S. Cannabinoids and bone regeneration. Drug Metab. Rev. 2019, 51, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Nat. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef]
- Dascalu, L.M.; Moldovan, M.; Prodan, D.; Ciotlaus, I.; Popescu, V.; Baldea, I.; Carpa, R.; Sava, S.; Chifor, R.; Badea, M.E. Assessment and Characterization of Some New Photosensitizers for Antimicrobial Photodynamic Therapy (aPDT). Materials 2020, 13, 3012. [Google Scholar] [CrossRef]
- Tlotleng, M.; Akinlabi, E.; Shukla, M.; Pityana, S. Microstructures, hardness and bioactivity of hydroxyapatite coatings deposited by direct laser melting process. Mater. Sci. Eng. C 2014, 43, 189–198. [Google Scholar] [CrossRef]







| Laser Power (W) | Scanning Speed (mm/s) | Hatch Distance (mm) | Spot Size (µm) | Layer Thickness (µm) | Energy Density (J/mm3) |
|---|---|---|---|---|---|
| 180 | 300 | 0.11 | 45 | 50 | 109.1 |
| Lattice Topology | Nominal Strut Diameter (mm) | Actual Strut Diameter (mm) |
|---|---|---|
| BCC | 0.25 | 0.29 (±0.04) |
| PCI | 0.50 | 0.55 (±0.05) |
| PHS | 0.45 | 0.49 (±0.04) |
| Physical–Mechanical Characteristic | Fully Dense | BCC | PCI | PHS | Cortical Bone * | Trabecular Bone * |
|---|---|---|---|---|---|---|
| Young Modulus (GPa) | 104 (±3.8) | 8.2 (±1.7) | 28.6 (±3.6) | 6.1 (±1.2) | 10–29 | 0.8–5 |
| Compressive Strength (MPa) | 790 (±26) | 59.4 (±6.4) | 279.0 (±27.5) | 38.8 (±4.7) | 135–205 | 2–7 |
| Compressive Strain (%) | 1.5 (±0.2) | 8.9 (±1.6) | 10.2 (±2.6) | 5.9 (±1.1) | 1–5 | up to 1 |
| Material | Lattice Topology | Porosity (%) | Strut Diameter (mm) | Compressive Strength (MPa) | Strain (%) | Young Modulus (GPa) | Source |
|---|---|---|---|---|---|---|---|
| Ti6Al7Nb | BCC, PCI, PHS | 56 | 0.3–0.6 | 38–279 | 6–10 | 6–28 | This study |
| Ti6Al4V | Cubic, Twisted | 49–66 | 0.9–1.8 | 215–409 | 2.8–4.2 | 10–26 | [9] |
| Simple cubic | 63.8 | 0.5–0.6 | 219 | N/A | 8.7 | [11] | |
| Schwartz primitive, Cylinder grid | 70 | 0.3–0.6 | 120–140 | 10–12 | 1.9–2.4 | [12] | |
| Diamond | 65 | 0.3–0.4 | 99–150 | 10 | 3–4 | [13] | |
| Reinforced BCC and FCC | 65 | 0.3 | 35–140 | 8–13 | 2–3 | [14] | |
| Dodecahedron | 65–69 | 0.20–0.25 | 78–117 | 4–5 | 2.6–3.5 | [15] | |
| Diamond | 55–65 | 0.6–1 | 68–228 | N/A | 4.2–8.3 | [18] |
| Physical–Mechanical Characteristics | BCC | PCI | PHS | |
|---|---|---|---|---|
| Actual Porosity (%) | ~56 | |||
| Pore Size (mm) | 0.40–0.51 | 0.85–0.91 | 0.50–0.62 | |
| Value of m exponent | 0.41 | 1.72 | 0.28 | |
| Young modulus (GPa) | Theo. (Equation (2)) | 8.1 | 33.9 | 5.5 |
| Exp. | 8.2 | 28.6 | 6.1 | |
| Compressive strength (MPa) | Theo. (Equation (3)) | 61.5 | 258.2 | 42.0 |
| Exp. | 59.4 | 279.0 | 38.8 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosma, C.; Drstvensek, I.; Berce, P.; Prunean, S.; Legutko, S.; Popa, C.; Balc, N. Physical–Mechanical Characteristics and Microstructure of Ti6Al7Nb Lattice Structures Manufactured by Selective Laser Melting. Materials 2020, 13, 4123. https://doi.org/10.3390/ma13184123
Cosma C, Drstvensek I, Berce P, Prunean S, Legutko S, Popa C, Balc N. Physical–Mechanical Characteristics and Microstructure of Ti6Al7Nb Lattice Structures Manufactured by Selective Laser Melting. Materials. 2020; 13(18):4123. https://doi.org/10.3390/ma13184123
Chicago/Turabian StyleCosma, Cosmin, Igor Drstvensek, Petru Berce, Simon Prunean, Stanisław Legutko, Catalin Popa, and Nicolae Balc. 2020. "Physical–Mechanical Characteristics and Microstructure of Ti6Al7Nb Lattice Structures Manufactured by Selective Laser Melting" Materials 13, no. 18: 4123. https://doi.org/10.3390/ma13184123
APA StyleCosma, C., Drstvensek, I., Berce, P., Prunean, S., Legutko, S., Popa, C., & Balc, N. (2020). Physical–Mechanical Characteristics and Microstructure of Ti6Al7Nb Lattice Structures Manufactured by Selective Laser Melting. Materials, 13(18), 4123. https://doi.org/10.3390/ma13184123










