Recent Progress in Processing Functionally Graded Polymer Foams
Abstract
1. Introduction
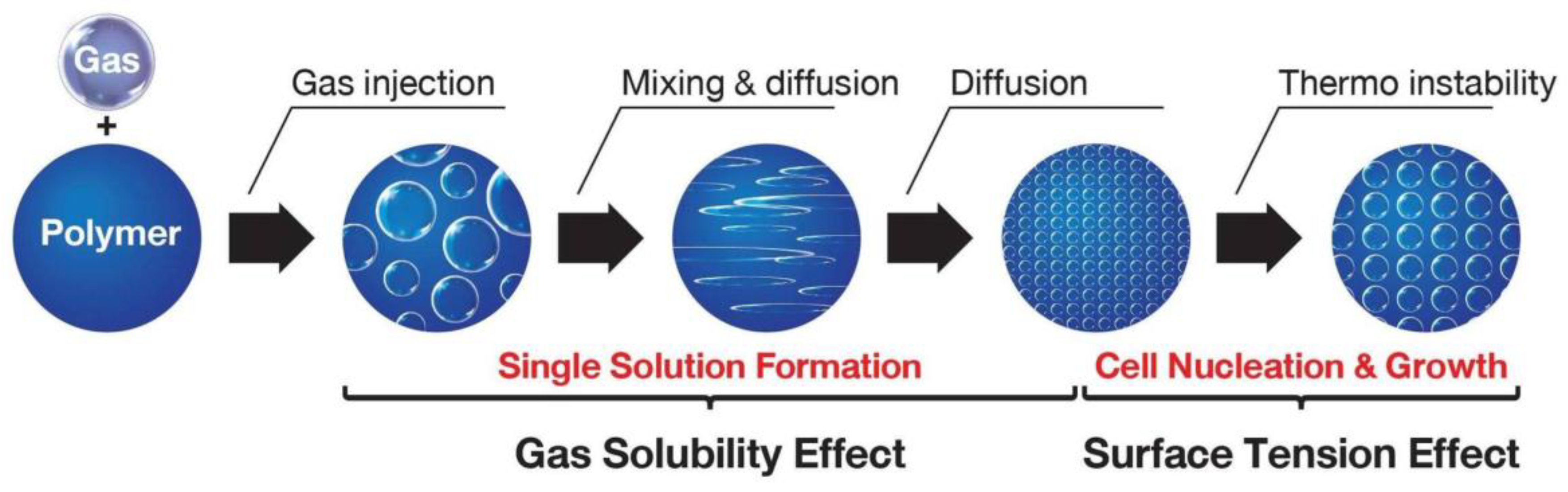
2. Polymer Foaming Process
- (i)
- the degree of crystallinity of the polymer matrix,
- (ii)
- the amount of the dissolved gas (fluid),
- (iii)
- the degree of saturation of gas in the polymer,
- (iv)
- the interfacial energy of polymer/gas (fluid), and
- (v)
- the plasticization profile of the polymer/gas system (i.e., the melting point and the glass transition temperature, Tg, of polymer matrix).
3. Thermodynamic Aspects and Computer Modeling of Polymer Foam Processing
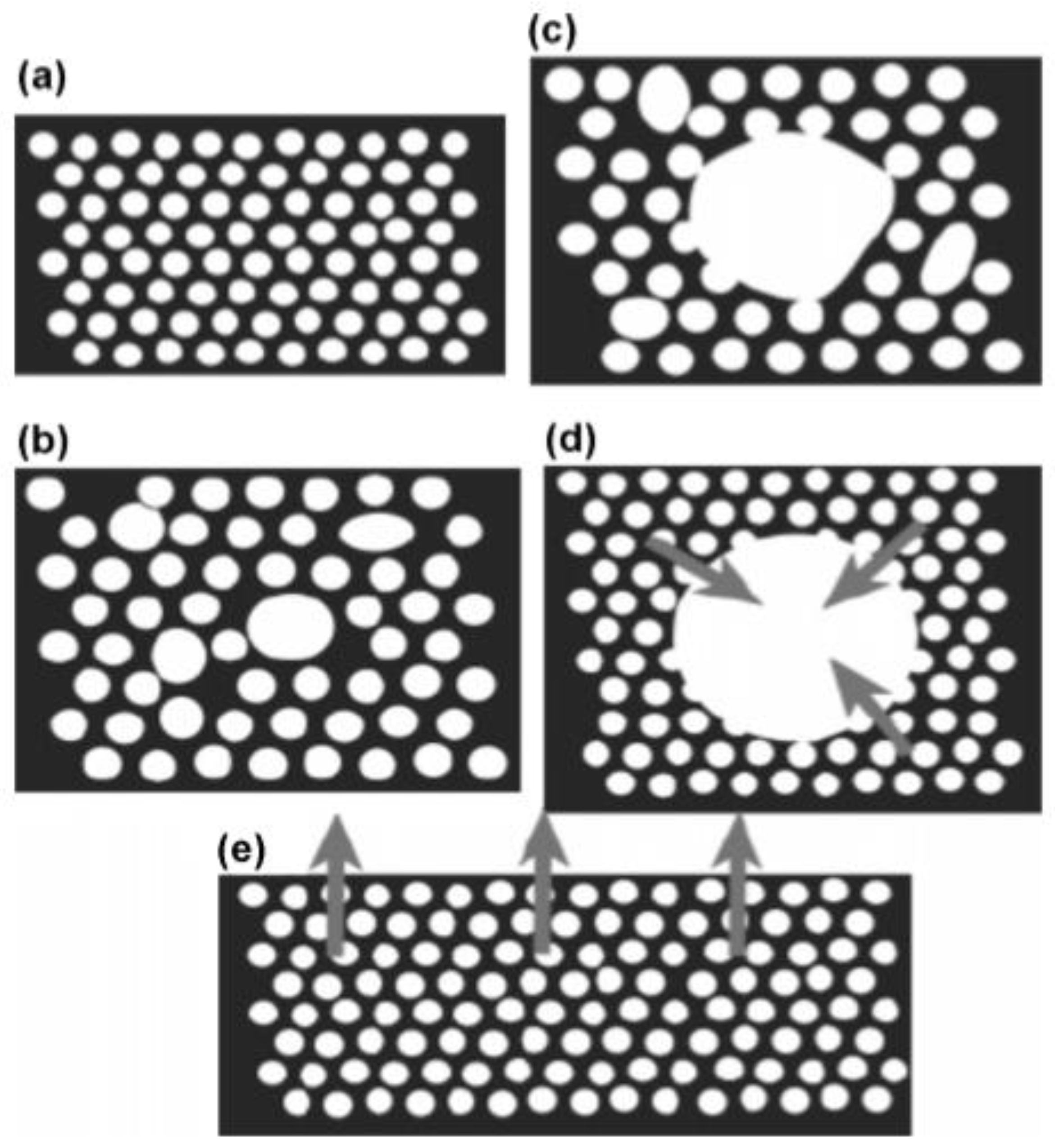
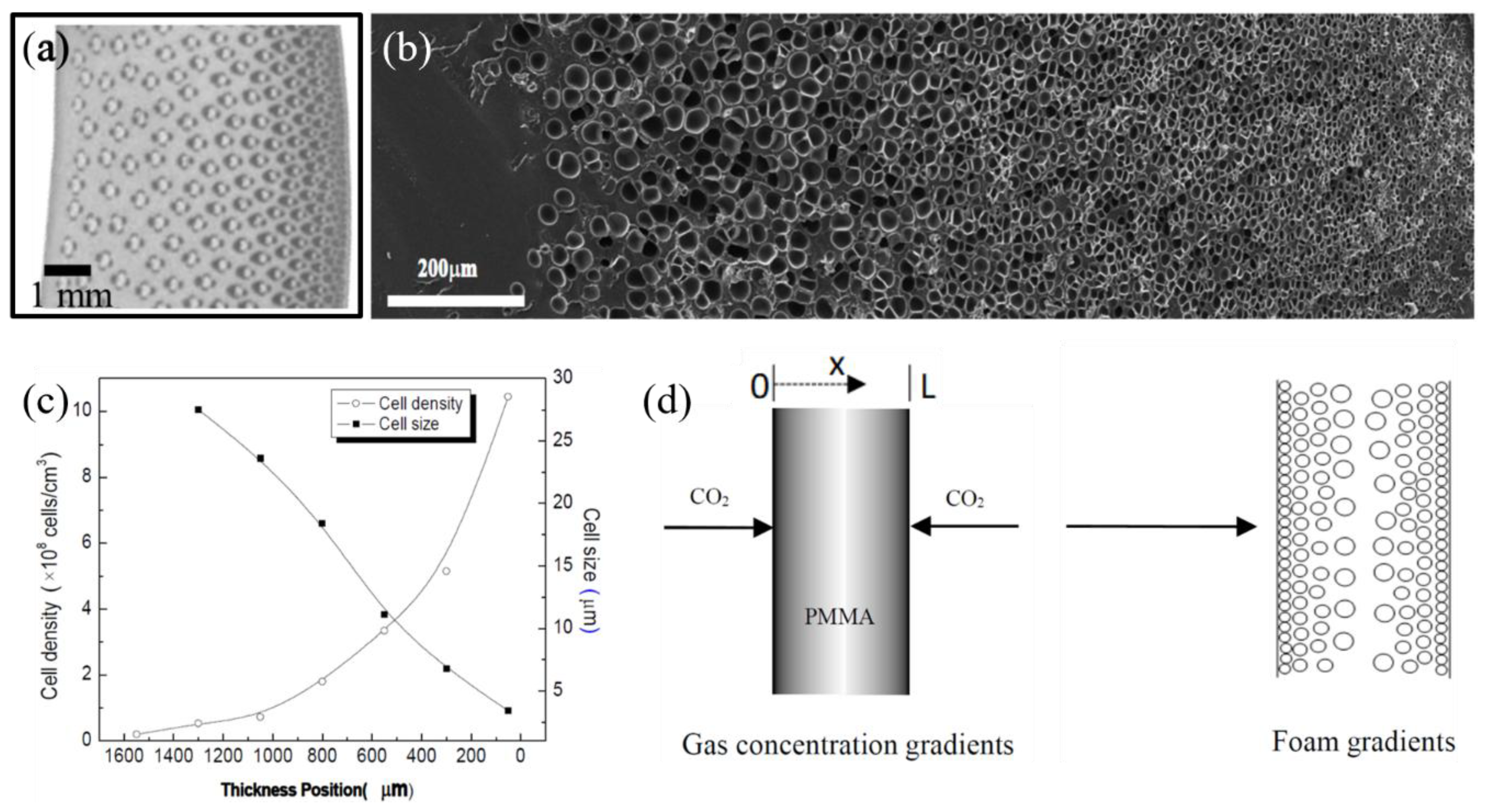
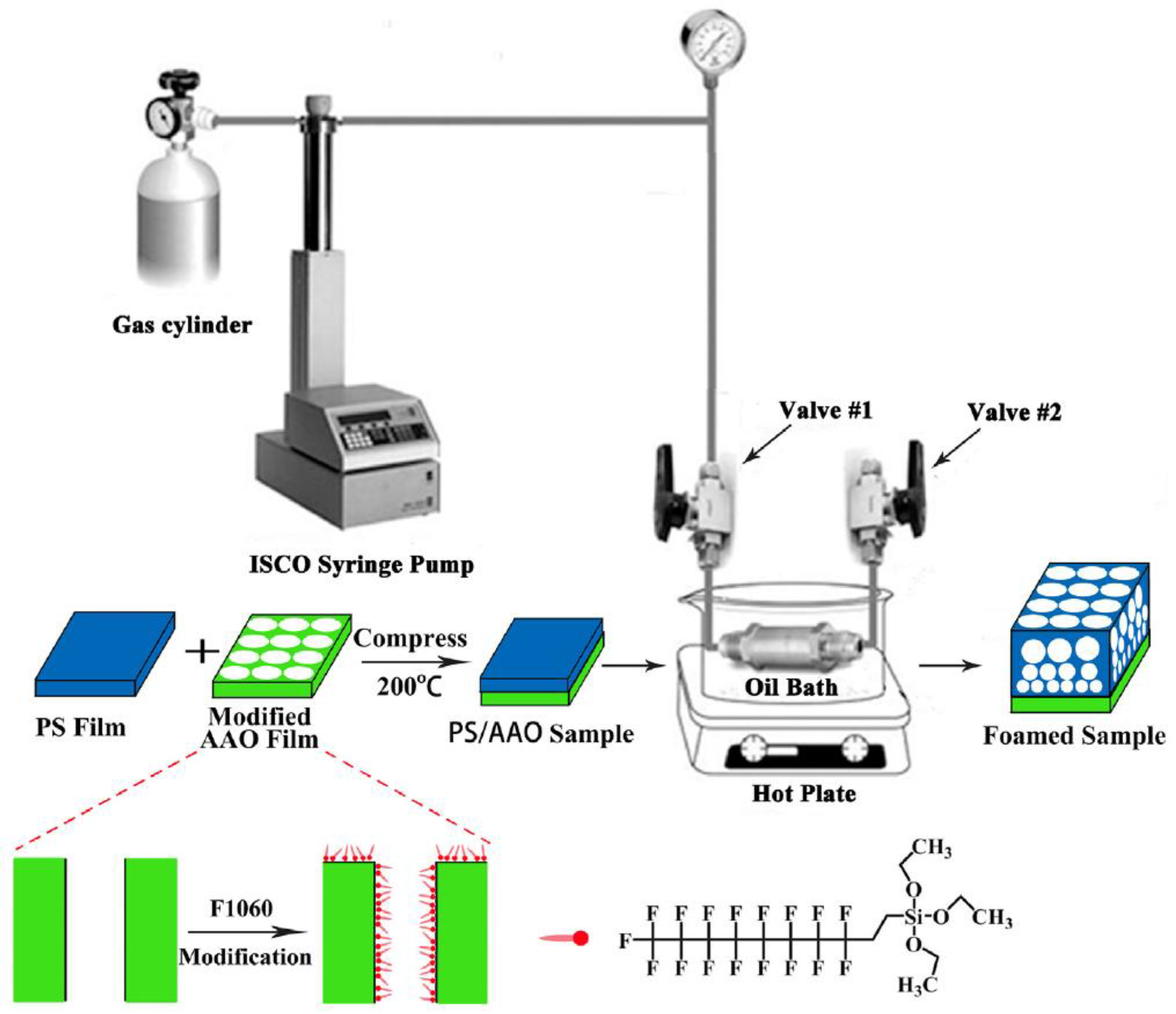
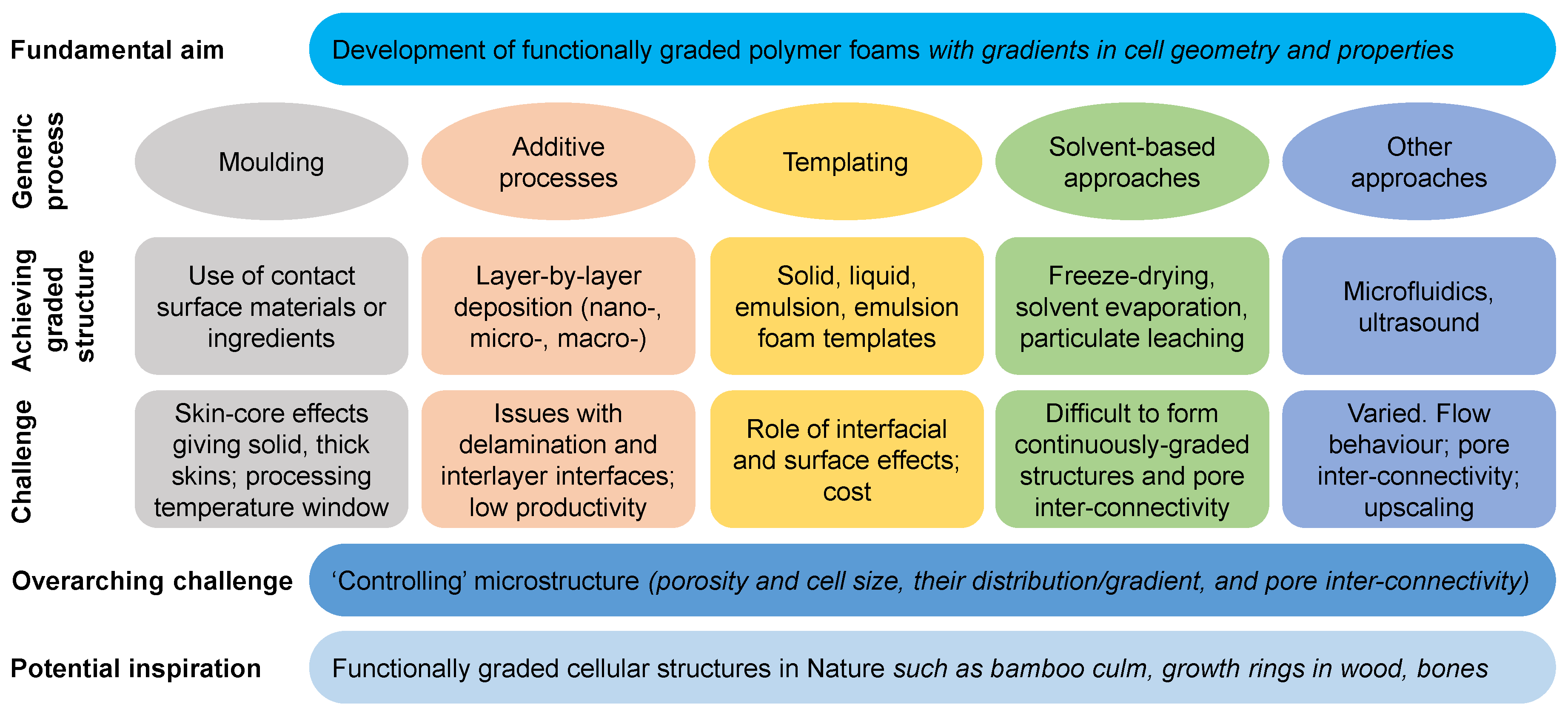
4. Recent Processes to Produce Functionally Graded Foams
5. Conclusions and Future Research Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- CES EduPack 2019; Ansys (Granta Design Limited): Cambridge, UK, 2020.
- Fan, D.; Li, M.; Qiu, J.; Xing, H.; Jiang, Z.; Tang, T. Novel method for preparing auxetic foam from closed–cell polymer foam based on the steam penetration and condensation process. ACS Appl. Mater. Interfaces 2018, 10, 22669–22677. [Google Scholar] [CrossRef]
- De Luca Bossa, F.; Verdolotti, L.; Russo, V.; Campaner, P.; Minigher, A.; Lama, G.C.; Boggioni, L.; Tesser, R.; Lavorgna, M. Upgrading sustainable polyurethane foam based on greener polyols: Succinic–based polyol and mannich–based polyol. Materials 2020, 13, 3170. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, Y.; Guo, X.; Chen, L.; Jia, D. The synergistic effect of ionic liquid–modified expandable graphite and intumescent flame–retardant on flame–retardant rigid polyurethane foams. Materials 2020, 13, 3095. [Google Scholar] [CrossRef]
- Suresh, K.I. Rigid polyurethane foams from cardanol: Synthesis, structural characterization, and evaluation of polyol and foam properties. ACS Sustain. Chem. Eng. 2013, 1, 232–242. [Google Scholar] [CrossRef]
- Abbes, B.; Lacoste, C.; Bliard, C.; Maalouf, C.; Simescu-Lazar, F.; Bogard, F.; Polidori, G. Novel extruded starch–beet pulp composites for packaging foams. Materials 2020, 13, 1571. [Google Scholar] [CrossRef]
- Dugad, R.; Radhakrishna, G.; Gandhi, A. Recent advancements in manufacturing technologies of microcellular polymers: A review. J. Polym. Res. 2020, 27, 182. [Google Scholar] [CrossRef]
- Zhang, N.; Cao, H. Enhancement of the antibacterial activity of natural rubber latex foam by blending It with chitin. Materials 2020, 13, 1039. [Google Scholar] [CrossRef]
- Walter, M.; Friess, F.; Krus, M.; Zolanvari, S.M.H.; Grun, G.; Krober, H.; Pretsch, T. Shape memory polymer foam with programmable apertures. Polymers 2020, 12, 1914. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.L.; Zhao, M.; Park, M.; Park, S.J. Recent trends of foaming in polymer processing: A review. Polymers 2019, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef]
- Caballe-Serrano, J.; Zhang, S.; Sculean, A.; Staehli, A.; Bosshardt, D.D. Tissue integration and degradation of a porous collagen–based scaffold used for soft tissue augmentation. Materials 2020, 13, 2420. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and synthetic polymers for bone scaffolds optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Trade Map. List of Exporters for the Selected Product: Product: 4008 Plates, Sheets, Strip, Rods and Profile Shapes, of Vulcanised Rubber (Excluding Hard Rubber); International Trade Centre: Geneva, Switzerland, 2019. [Google Scholar]
- Mohebbi, A.; Mighri, F.; Ajji, A.; Rodrigue, D. Current issues and challenges in polypropylene foaming: A review. Cell. Polym. 2015, 34, 299–338. [Google Scholar] [CrossRef]
- Liao, X.; Nawaby, A.V. The sorption behaviors in PLLA–CO2 system and its effect on foam morphology. J. Polym. Res. 2012, 19, 9827. [Google Scholar] [CrossRef]
- Galakhova, A.; Santiago-Calvo, M.; Tirado-Mediavilla, J.; Villafane, F.; Rodriguez-Perez, M.A.; Riess, G. Identification and quantification of cell gas evolution in rigid polyurethane foams by novel GCMS methodology. Polymers 2019, 11, 1192. [Google Scholar] [CrossRef]
- Zhao, S.; Pan, C.; Xin, Z.; Li, Y.; Qin, W.; Zhou, S. 13X zeolite as difunctional nucleating agent regulating the crystal form and improving the Foamability of blocked copolymerized polypropylene in supercritical CO2 foaming process. J. Polym. Res. 2019, 26, 58. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Polyetherimide foams filled with low content of graphene nanoplatelets prepared by scCO2 dissolution. Polymers 2019, 11, 328. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, J.; Jiang, P.; Yu, J. Synthesis, characteristic of a novel flame retardant containing phosphorus, silicon and its application in ethylene vinyl–acetate copolymer (EVM) rubber. J. Polym. Res. 2010, 17, 891–902. [Google Scholar] [CrossRef]
- Suksup, R.; Sun, Y.; Sukatta, U.; Smitthipong, W. Foam rubber from centrifuged and creamed latex. J. Polym. Eng. 2019, 39, 336–342. [Google Scholar] [CrossRef]
- Phomrak, S.; Nimpaiboon, A.; Newby, B.Z.; Phisalaphong, M. Natural rubber latex foam reinforced with micro and nanofibrillated cellulose via Dunlop method. Polymers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Lang, X.H.; Wang, D.; Prakashan, K.; Zhang, X.; Zhang, Z.X. Microcellular chlorinated polyethylene (CM) rubber foam by using N2 as blowing agent. J. Polym. Res. 2017, 24, 175. [Google Scholar] [CrossRef]
- Karim, A.F.A.; Ismail, H.; Ari, Z.M. Properties and characterization of Kenaf–Filled natural rubber latex foam. Bioresources 2016, 11, 1080–1091. [Google Scholar]
- Rathnayake, W.G.I.U.; Ismail, H.; Baharin, A.; Bandara, C.D.; Rajapakse, S. Enhancement of the antibacterial activity of natural rubber latex foam by the incorporation of zinc oxide nanoparticles. J. Appl. Polym. Sci. 2013, 131, 131. [Google Scholar] [CrossRef]
- Pinto, J.; Escudero, J.; Solόrzano, E.; Rodriguez-Perez, M.A. A novel route to produce structural polymer foams with a controlled solid skin–porous core structure based on gas diffusion mechanisms. J. Sandw. Struct. Mater. 2020, 22, 822–832. [Google Scholar] [CrossRef]
- Kumar, V.; Suh, N.P. A process for making microcellular thermoplastic parts. Polym. Eng. Sci. 1990, 30, 1323–1329. [Google Scholar] [CrossRef]
- Goel, S.K.; Beckman, E.J. Generation of microcellular polymeric foams using supercritical carbon dioxide. I: Effect of pressure and temperature on nucleation. Polym. Eng. Sci. 1994, 34, 1137–1147. [Google Scholar] [CrossRef]
- Ratcha, A.; Samart, C.; Yoosuk, B.; Sawada, H.; Reubroycharoen, P.; Kongparakul, S. Polyisoprene modified poly(alkyl acrylate) foam as oil sorbent material. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Ratcha, A.; Yoosuk, B.; Kongparakul, S. Grafted methyl methacrylate and butyl methacrylate onto natural rubber foam for oil sorbent. Adv. Mater. Res. 2014, 844, 385–390. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Sanxaridou, G.; Pavlidou, E.; Panayiotou, C. Foaming of polymers with supercritical fluids: A thermodynamic investigation. J. Supercrit. Fluids 2016, 110, 240–250. [Google Scholar] [CrossRef]
- Reglero Ruiz, J.A.; Vincent, M.; Agassant, J.-F.; Sadik, T.; Pillon, C.; Carrot, C. Polymer foaming with chemical blowing agents: Experiment and modeling. Polym. Eng. Sci. 2015, 55, 2018–2029. [Google Scholar] [CrossRef]
- Thompson, R.B.; Park, C.B.; Chen, P. Reduction of polymer surface tension by crystallized polymer nanoparticles. J. Chem. Phys. 2010, 133, 144913. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhang, M.; Guo, C.; Shi, L.; Lin, P. Experimental investigations on the effects of bottom ventilation on the fire behavior of natural rubber latex foam. Appl. Therm. Eng. 2018, 133, 201–210. [Google Scholar] [CrossRef]
- Klempner, D.; Frisch, K.C. Handbook of Polymeric Foams and Foam Technology; Hanser Munich etc.: Birmingham, UK, 1991; p. 404. [Google Scholar]
- Kudori, S.N.I.; Ismail, H.; Shuib, R.K. Kenaf core and bast loading vs. properties of natural rubber latex foam (NRLF). BioResources 2019, 14, 1765–1780. [Google Scholar]
- Surya, I.; Kudori, S.N.I.; Ismail, H. Effect of partial replacement of kenaf by empty fruit bunch (EFB) on the properties of natural rubber latex foam (NRLF). BioResources 2019, 14, 9375–9391. [Google Scholar]
- Panploo, K.; Chalermsinsuwan, B.; Poompradub, S. Natural rubber latex foam with particulate fillers for carbon dioxide adsorption and regeneration. RSC Adv. 2019, 9, 28916–28923. [Google Scholar] [CrossRef]
- Rathnayake, I.U.; Ismail, H.; De Silva, C.R.; Darsanasiri, N.D.; Bose, I. Antibacterial effect of Ag–doped TiO2 nanoparticles incorporated natural rubber latex foam under visible light conditions. Iran. Polym. J. 2015, 24, 1057–1068. [Google Scholar] [CrossRef]
- Rathnayake, W.G.I.U.; Ismail, H.; Baharin, A.; Darsanasiri, A.G.N.D.; Rajapakse, S. Synthesis and characterization of nano silver based natural rubber latex foam for imparting antibacterial and anti–fungal properties. Polym. Test. 2012, 31, 586–592. [Google Scholar] [CrossRef]
- Stalder, A.F.; Melchior, T.; Müller, M.; Sage, D.; Blu, T.; Unser, M. Low–bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Colloids Surf. A 2010, 364, 72–81. [Google Scholar] [CrossRef]
- Wulf, M.; Michel, S.; Grungke, K.; Del Rio, O.I.; Kwok, D.Y.; Neumann, A.W. Simultaneous determination of surface tension and density of polymer melts using axisymmetric drop shape analysis. J. Colloid Interface Sci. 1999, 210, 172–181. [Google Scholar] [CrossRef]
- Ramasamy, S.; Ismail, H.; Munusamy, Y. Tensile and morphological properties of rice husk powder filled natural rubber latex foam. Polym. Technol. Eng. 2012, 51, 1524–1529. [Google Scholar] [CrossRef]
- Oliveira-Salmazo, L.; Lopez-Gil, A.; Silva-Bellucci, F.; Job, A.E.; Rodriguez-Perez, M. A Natural rubber foams with anisotropic cellular structures: Mechanical properties and modeling. Ind. Ind. Crop. Prod. 2016, 80, 26–35. [Google Scholar] [CrossRef]
- Sandhu, I.; Kala, M.; Thangadurai, M.; Singh, M.; Alegaonkar, P.; Saroha, D.R. Experimental study of blast wave mitigation in open cell foams. Mater. Today Proc. 2018, 5, 28170–28179. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Burley, A.; Feng, L.; Yeh, S.-K.; Miyazono, K.; Nirmal-Kumar, S.; Kusaka, I.; Koelling, K. Development of CO2 for polymer foam applications. J. Supercrit. Fluids 2009, 47, 493–499. [Google Scholar] [CrossRef]
- Frerich, S.C. Biopolymer foaming with supercritical CO2—Thermodynamics, foaming behaviour and mechanical characteristics. J. Supercrit. Fluids 2015, 96, 349–358. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Panayiotou, C. Foaming of chitin hydrogels processed by supercritical carbon dioxide. J. Supercrit. Fluids 2008, 47, 302–308. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Panayiotou, C. Designing Issues in Polymer Foaming with Supercritical Fluids. Macromol. Symp. 2013, 331–332, 109–114. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, G.; Yang, Q.; Shi, X.; Shi, A. Fabrication of microcellular polycarbonate foams with unimodal or bimodal cell–size distributions using supercritical carbon dioxide as a blowing agent. J. Cell. Plast. 2014, 50, 55–79. [Google Scholar] [CrossRef]
- Yeh, S.-K.; Liu, W.-H.; Huang, Y.-M. Carbon dioxide–blown expanded polyamide bead foams with bimodal cell structure. Ind. Eng. Chem. Res. 2019, 58, 2958–2969. [Google Scholar] [CrossRef]
- Trofa, M.; Di Maio, E.; Maffettone, P.L. Multi–graded foams upon time–dependent exposition to blowing agent. Chem. Eng. J. 2019, 362, 812–817. [Google Scholar] [CrossRef]
- Sumey, J.L.; Sarver, J.A.; Kiran, E. Foaming of polystyrene and poly(methyl methacrylate) multilayered thin films with supercritical carbon dioxide. J. Supercrit. Fluids 2019, 145, 243–252. [Google Scholar] [CrossRef]
- Cusson, E.; Akbarzadeh, A.H.; Therriault, D.; Rodrigue, D. Density graded polyethylene foams: Effect of processing conditions on mechanical properties. Cell. Polym. 2019, 38, 3–14. [Google Scholar] [CrossRef]
- Bates, S.R.G.; Farrow, I.R.; Trask, R.S. Compressive behaviour of 3D printed thermoplastic polyurethane honeycombs with graded densities. Mater. Des. 2019, 162, 130–142. [Google Scholar] [CrossRef]
- Esmailzadeh, M.; Manesh, H.D.; Zebarjad, S.M. Fabrication and characterization of functional graded polyurethane foam (FGPUF). Polym. Adv. Technol. 2018, 29, 182–189. [Google Scholar] [CrossRef]
- Jahwari, F.A.l.; Huang, Y.; Naguib, H.E.; Lo, J. Relation of impact strength to the microstructure of functionally graded porous structures of acrylonitrile butadiene styrene (ABS) foamed by thermally activated microspheres. Polymer 2016, 98, 270–281. [Google Scholar] [CrossRef]
- Heim, H.-P.; Tromm, M. Injection molded components with functionally graded foam structures–Procedure and essential results. J. Cell. Plast. 2016, 52, 299–319. [Google Scholar] [CrossRef]
- Ghaffari, S.; Naguib, H.E.; Park, C.B.; Atalla, N. Design and development of novel bio–based functionally graded foams for enhanced acoustic capabilities. J. Mater. Sci. 2015, 50. [Google Scholar]
- Zhou, C.; Wang, P.; Li, W. Fabrication of functionally graded porous polymer via supercritical CO2 foaming. Compos. B Eng. 2011, 42, 318–325. [Google Scholar] [CrossRef]
- Yao, J.; Rodrigue, D. Density graded polyethylene foams produced by compression moulding using a chemical blowing agent. Cell. Polym. 2012, 31, 189–206. [Google Scholar] [CrossRef]
- Stubenrauch, C.; Menner, A.; Bismarck, A.; Drenckhan, W. Emulsion and foam templating—Promising routes to tailor–made porous polymers. Angew. Chem. Int. Ed. 2018, 57, 10024–10032. [Google Scholar] [CrossRef]
- Andrieux, S.; Quell, A.; Stubenrauch, C.; Drenckhan, W. Liquid foam templating—A route to tailor–made polymer foams. Adv. Colloid Interface 2018, 256, 276–290. [Google Scholar] [CrossRef]
- Lee, J.J.; Cho, M.Y.; Kim, B.H.; Lee, S. Development of eco–friendly polymer foam using overcoat technology of deodorant. Materials 2018, 11, 1898. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, J.; Voutilainen, M.; Virtanen, P.; Lassila, L.; Fardim, P. Cellulose fibre–reinforced biofoam for structural applications. Materials 2017, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Forest, C.; Chaumont, P.; Cassagnau, P.; Swoboda, B.; Sonntag, P. Polymer nano–foams for insulating applications prepared from CO2 foaming. Prog. Polym. Sci. 2015, 41, 122–145. [Google Scholar] [CrossRef]
- Chollakup, R.; Smitthipong, W.; Chworos, A. Specific interaction of DNA–functionalized polymer colloid. Polym. Chem. 2010, 1, 658–662. [Google Scholar] [CrossRef]
- Chollakup, R.; Smitthipong, W.; Chworos, A. DNA–functionalized polystyrene particles and their controlled self–assembly. RSC Adv. 2014, 4, 30648–30653. [Google Scholar] [CrossRef]
- Strachota, B.; Morand, A.; Dybal, J.; Matejka, L. Control of gelation and properties of reversible Diels–Alder networks: Design of a self–healing network. Polymers 2019, 11, 930. [Google Scholar] [CrossRef]
- Stephanou, P.S.; Tsimouri, I.C.; Mavrantzas, V.G. Simple, Accurate and user–friendly differential constitutive model for the rheology of entangled polymer melts and solutions from nonequilibrium thermodynamics. Materials 2020, 13, 2867. [Google Scholar] [CrossRef]
- Sauceau, M.; Fages, J.; Common, A.; Nikitine, C.; Rodier, E. New challenges in polymer foaming: A review of extrusion processes assisted by supercritical carbon dioxide. Prog. Polym. Sci. 2011, 36, 749–766. [Google Scholar] [CrossRef]
- Pakornpadungsit, P.; Smitthipong, W.; Chworos, A. Self–assembly nucleic acid–based biopolymers: Learn from the nature. J. Polym. Res. 2018, 25, 45. [Google Scholar] [CrossRef]
- Yokoyama, H.; Sugiyama, K. Nanocellular structures in block copolymers with CO2–philic blocks using CO2 as a blowing agent: Crossover from micro- to nanocellular structures with depressurization temperature. Macromolecules 2005, 38, 10516–10522. [Google Scholar] [CrossRef]
- Jiang, Y.; Greco, C.; Daoulas, K.h.; Chen, J.Z.Y. Thermodynamics of a compressible Maier–Saupe model based on the self–consistent field theory of wormlike polymer. Polymers 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Mondy, L.; Rao, R.; Grillet, A.; Adolf, D.; Brotherton, C.; Russick, E.; Cote, R.; Castaňeda, J.; Thompson, K.; Bourdon, C.; et al. Experiments for Foam Model Development and Validation; Sandia National Laboratories: Albuquerque, NM, USA, 2008.
- Seo, D.; Youn, J.R.; Tucker III, C.L. Numerical simulation of mold filling in foam reaction injection molding. Int. J. Numer. Methods Fluids 2003, 42, 1105–1134. [Google Scholar] [CrossRef]
- Prud’homme, R.; Khan, S.A. Foams: Theory, Measurements, and Applications; Marcel Dekker, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Gibson, L.J.; Ashby, M.F. Cellular solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- May, C. Epoxy Resins: Chemistry and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Lin, H.; Lv, L.; Zhang, J.; Wang, Z. Energy–absorbing performance of graded Voronoi foams. J. Cell. Plast. 2019, 55, 589–613. [Google Scholar] [CrossRef]
- Liu, H.; Ding, S.; Ng, B.F. Impact response and energy absorption of functionally graded foam under temperature gradient environment. Compos. B Eng. 2019, 172, 516–532. [Google Scholar] [CrossRef]
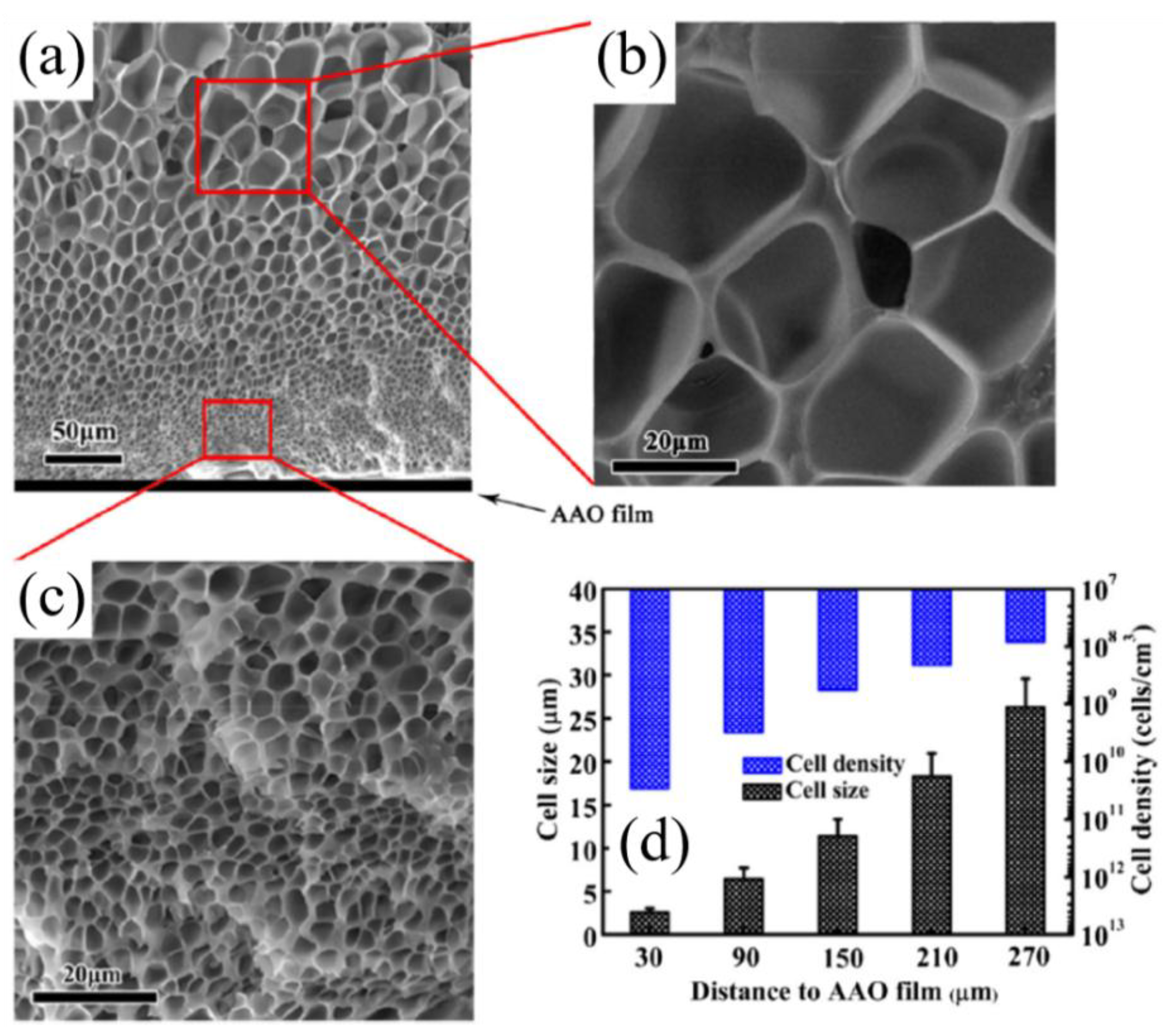
- Yu, J.; Song, L.; Chen, F.; Fan, P.; Sun, L.; Zhong, M.; Yang, J. Preparation of polymer foams with a gradient of cell size: Further exploring the nucleation effect of porous inorganic materials in polymer foaming. Mater. Today Commun. 2016, 9, 1–6. [Google Scholar] [CrossRef]
- Timothy, J.J.; Meschke, G. A cascade continuum micromechanics model for the effective elastic properties of porous materials. Int. J. Solids Struct. 2016, 83, 1–12. [Google Scholar] [CrossRef]
- Bashir, A.S.; Munusamy, Y.; Chew, T.L.; Ismail, H.; Ramasamy, S. Mechanical, thermal, and morphological properties of (eggshell powder)–filled natural rubber latex foam. J. Vinyl Addit. Technol. 2015, 23, 3–12. [Google Scholar] [CrossRef]
- Sadik, T.; Pillon, C.; Carrot, C.; Ruiz, J.A.R.; Vincent, M.; Billon, M. Propylene structural foam: Measurements of the core, skin, and overall mechanical properties with evaluation of predictive models. J. Cell. Plast. 2017, 53, 25–44. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.; Xiong, Y.; Luo, G.; Shen, Q.; Zhang, L. Preparation and characterization of PMMA graded microporous foams via one–step supercritical carbon dioxide foaming. J. Phys. Conf. Ser. 2013, 419. [Google Scholar] [CrossRef]
- Elsing, J.; Quell, A.; Stubenrauch, C. Toward functionally graded polymer foams using microfluidics. Adv. Eng. Mater. 2017, 19. [Google Scholar] [CrossRef]
- Yang, C.; Wang, M.; Zhao, Z.; Wang, M.; Wu, G. A new promising nucleating agent for polymer foaming: Effects of hollow molecular–sieve particles on polypropylene supercritical CO2 microcellular foaming. RSC Adv. 2018, 8, 20061–20067. [Google Scholar] [CrossRef]
- Llewelyn, G.; Rees, A.; Griffiths, C.A.; Jacobi, M. A novel hybrid foaming method for low–pressure microcellular foam production of unfilled and talc–filled copolymer polypropylenes. Polymers 2019, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Onder, O.C.; Yilgor, E.; Yilgor, I. Preparation of monolithic polycaprolactone foams with controlled morphology. Polymer 2018, 136, 166–178. [Google Scholar] [CrossRef]
- Song, W.; Barber, K.; Lee, K.Y. Heat–induced bubble expansion as a route to increase the porosity of foam–templated bio–based macroporous polymers. Polymer 2017, 118, 97–106. [Google Scholar] [CrossRef]
- Vahidifar, A.; Khorasani, S.N.; Park, C.B.; Naguib, H.E.; Khonakdar, H.A. Fabrication and characterization of closed–cell rubber foams based on natural rubber/carbon black by one–step foam processing. Ind. Eng. Chem. Res. 2016, 55, 2407–2416. [Google Scholar] [CrossRef]
- Ramage, M.H.; Burridge, H.; Busse-Wicher, M.; Fereday, G.; Reynolds, T.; Shah, D.U.; Wu, G.; Yu, L.; Fleming, P.; Densley-Tingley, D.; et al. The wood from the trees: The use of timber in construction. Renew. Sustain. Energy Rev. 2017, 68, 333–359. [Google Scholar] [CrossRef]
- Shah, D.U.; Reynolds, T.P.S.; Ramage, M.H. The strength of plants: Theory and experimental methods to measure the mechanical properties of stems. J. Exp. Bot. 2017, 68, 4497–4516. [Google Scholar] [CrossRef]
- Ciechanska, D. Multifunctional bacterial cellulose/chitosan composite materials for medical applications. Fibres Text. East. Eur. 2004, 12, 69–72. [Google Scholar]
- Dominic CD, M.; Joseph, R.; Begum, P.; Joseph, M.; Padmanabhan, D.; Morris, L.A.; Kumar, A.S.; Formela, K. Cellulose nanofibers isolated from the cuscuta reflexa plant as a green reinforcement of natural rubber. Polymers 2020, 12, 814. [Google Scholar] [CrossRef]
- Abdul Azam, F.A.; Rajendran Royan, N.R.; Yuhana, N.Y.; Mohd Radzuan, N.A.; Ahmad, S.; Sulong, A.B. Fabrication of porous recycled HDPE biocomposites foam: Effect of rice husk filler contents and surface treatments on the mechanical properties. Polymers 2020, 12, 475. [Google Scholar] [CrossRef]
- Phomrak, S.; Phisalaphong, M. Lactic acid modified natural rubber–bacterial cellulose composites. Appl. Sci. 2020, 10, 3583. [Google Scholar] [CrossRef]
- Ismail, H.; Edyham, M.; Wirjosentono, B. Bamboo fibre filled natural rubber composites: The effects of filler loading and bonding agent. Polym. Test. 2002, 21, 139–144. [Google Scholar] [CrossRef]
- Karmarkar, A.; Chauhan, S.; Modak, J.M.; Chanda, M. Mechanical properties of wood–fiber reinforced polypropylene composites: Effect of a novel compatibilizer with isocyanate functional group. Compos. Part A Appl. Sci. Manuf. 2007, 38, 227–233. [Google Scholar] [CrossRef]
- Gao, C.; Xiao, W.; Ji, G.; Zhang, Y.; Cao, Y.; Han, L. Regularity and mechanism of wheat straw properties change in ball milling process at cellular scale. Bioresour. Technol. 2017, 241, 214–219. [Google Scholar] [CrossRef]
- Zhou, L.; He, H.; Li, M.-C.; Song, K.; Cheng, H.; Wu, Q. Morphological influence of cellulose nanoparticles (CNs) from cottonseed hulls on rheological properties of polyvinyl alcohol/CN suspensions. Carbohydr. Polym. 2016, 153, 445–454. [Google Scholar] [CrossRef]
- Ling, Z.; Edwards, J.V.; Guo, Z.; Prevost, N.T.; Nam, S.; Wu, Q.; French, A.D.; Xu, F. Structural variations of cotton cellulose nanocrystals from deep eutectic solvent treatment: Micro and nano scale. Cellulose 2019, 26, 861–876. [Google Scholar] [CrossRef]
- Phisalaphong, M.; Suwanmajo, T.; Sangtherapitikul, P. Novel nanoporous membranes from regenerated bacterial cellulose. J. Appl. Polym. Sci. 2008, 107, 292–299. [Google Scholar] [CrossRef]
- Liu, M.; Wang, H.; Han, J.; Niu, Y. Enhanced hydrogenolysis conversion of cellulose to C2–C3 polyols via alkaline pretreatment. Carbohydr. Polym. 2012, 89, 607–612. [Google Scholar] [CrossRef]
- Nomura, S.; Kugo, Y.; Erata, T. 13C NMR and XRD studies on the enhancement of cellulose II crystallinity with low concentration NaOH post–treatments. Cellulose 2020, 27, 3553–3563. [Google Scholar] [CrossRef]
- Williams, T.; Hosur, M.; Theodore, M.; Netravali, A.; Rangari, V.; Jeelani, S. Time effects on morphology and bonding ability in mercerized natural fibers for composite reinforcement. Int. J. Polym. Sci. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.C.; Tzounis, L.; Pongwisuthiruchte, A.; Potiyaraj, P. Effect of various surface treatments on the performance of jute fibers filled natural rubber (NR) composites. Polymers 2020, 12, 369. [Google Scholar] [CrossRef] [PubMed]
- Pakornpadungsit, P.; Prasopdee, T.; Swainson, N.M.; Chworos, A.; Smitthipong, W. DNA:chitosan complex, known as a drug delivery system, can create a porous scaffold. Polym. Test. 2020, 83, 106333. [Google Scholar] [CrossRef]
- Chollakup, R.; Uttayarat, P.; Chworos, A.; Smitthipong, W. Noncovalent sericin–chitosan scaffold: Physical properties and low cytotoxicity effect. Int. J. Mol. Sci. 2020, 21, 775. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-M.; He, L.-Z.; Zhao, M.; Yang, D.; Liu, Y. Biological properties of the chitosan–gelatin sponge wound dressing. Carbohydr. Polym. 2007, 69, 583–589. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M., Jr. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Bodhibukkana, C.; Srichana, T.; Kaewnopparat, S.; Tangthong, N.; Bouking, P.; Martin, G.P.; Suedee, R. Composite membrane of bacterially–derived cellulose and molecularly imprinted polymer for use as a transdermal enantioselective controlled–release system of racemic propranolol. J. Control. Release 2006, 113, 43–56. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose–Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Ari, Z.; Zakaria, Z.; Tay, L.; Lee, S. Effect of foaming temperature and rubber grades on properties of natural rubber foams. J. Appl. Polym. Sci. 2008, 107, 2531–2538. [Google Scholar]

| Types of Polymer Foam | Exported Value in Million USD (% of Total) | |||
|---|---|---|---|---|
| 2017 | 2018 | |||
| Polystyrene foam | 1.276 | (10.3%) | 1.339 | (9.9%) |
| Polyvinyl chloride foam | 1.799 | (14.5%) | 2.001 | (14.9%) |
| Polyurethanes foam | 3.860 | (31.1%) | 4.167 | (30.9%) |
| Other plastic foams | 4.426 | (35.7%) | 4.852 | (36.0%) |
| Rubber foams | 1.053 | (8.5%) | 1.110 | (8.2%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suethao, S.; Shah, D.U.; Smitthipong, W. Recent Progress in Processing Functionally Graded Polymer Foams. Materials 2020, 13, 4060. https://doi.org/10.3390/ma13184060
Suethao S, Shah DU, Smitthipong W. Recent Progress in Processing Functionally Graded Polymer Foams. Materials. 2020; 13(18):4060. https://doi.org/10.3390/ma13184060
Chicago/Turabian StyleSuethao, Supitta, Darshil U. Shah, and Wirasak Smitthipong. 2020. "Recent Progress in Processing Functionally Graded Polymer Foams" Materials 13, no. 18: 4060. https://doi.org/10.3390/ma13184060
APA StyleSuethao, S., Shah, D. U., & Smitthipong, W. (2020). Recent Progress in Processing Functionally Graded Polymer Foams. Materials, 13(18), 4060. https://doi.org/10.3390/ma13184060






