Solid-State Li-Ion Batteries Operating at Room Temperature Using New Borohydride Argyrodite Electrolytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid-State Electrolytes
2.2. Cathode Materials
2.3. Cell Assembly
2.4. Electrochemical Measurements
3. Results
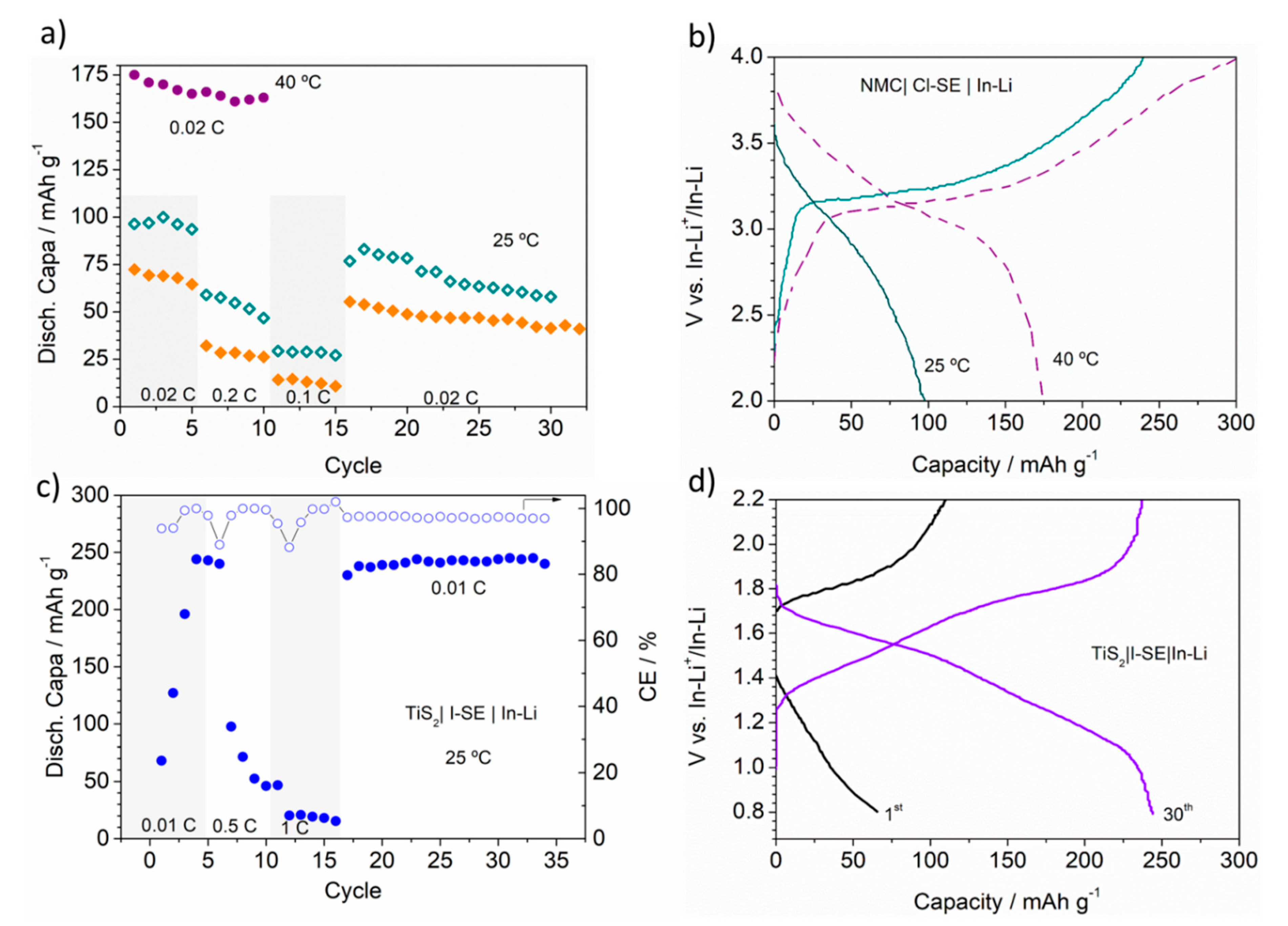
3.1. High-Voltage NMC│SE│In-Li Cell
3.2. TiS2│I-SE│In-Li Cell
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dudney, N.J.; West, W.C.; Nanda, J. Handbook of Solid State Batteries, 2nd ed.; World Scientific: Singapore, 2015; ISBN 978-981-4651-89-9. [Google Scholar]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Takada, K. Progress and prospective of solid-state lithium batteries. Acta Mater. 2013, 61, 759–770. [Google Scholar] [CrossRef]
- Lau, J.; DeBlock, R.H.; Butts, D.M.; Ashby, D.S.; Choi, C.S.; Dunn, B.S. Sulfide solid electrolytes for lithium battery applications. Adv. Energy Mater. 2018, 8, 1800933. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Sulfide Solid Electrolyte with Favorable Mechanical Property for All-Solid-State Lithium Battery. Available online: https://www.nature.com/articles/srep02261 (accessed on 23 April 2020).
- Randau, S.; Weber, D.A.; Kötz, O.; Koerver, R.; Braun, P.; Weber, A.; Ivers-Tiffée, E.; Adermann, T.; Kulisch, J.; Zeier, W.G.; et al. Benchmarking the performance of all-solid-state lithium batteries. Nat. Energy 2020, 5, 259–270. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Kitaura, H.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. Electrochemical performance of all-solid-state lithium secondary batteries with Li-Ni-Co-Mn oxide positive electrodes. Electrochim. Acta 2010, 55, 8821–8828. [Google Scholar] [CrossRef]
- Machida, N.; Kashiwagi, J.; Naito, M.; Shigematsu, T. Electrochemical properties of all-solid-state batteries with ZrO2-coated LiNi1/3Mn1/3Co1/3O2 as cathode materials. Solid State Ion. 2012, 225, 354–358. [Google Scholar] [CrossRef]
- Okada, K.; Machida, N.; Naito, M.; Shigematsu, T.; Ito, S.; Fujiki, S.; Nakano, M.; Aihara, Y. Preparation and electrochemical properties of LiAlO2-coated Li(Ni1/3Mn1/3Co1/3)O2 for all-solid-state batteries. Solid State Ion. 2014, 255, 120–127. [Google Scholar] [CrossRef]
- Meng, H.; Huang, B.; Yin, J.; Yao, X.; Xu, X. Synthesis and electrochemical properties of LiNi1/3Co1/3Mn1/3O2 cathodes in lithium-ion and all-solid-state lithium batteries. Ionics 2015, 21, 43–49. [Google Scholar] [CrossRef]
- Deiseroth, H.-J.; Kong, S.-T.; Eckert, H.; Vannahme, J.; Reiner, C.; Zaiß, T.; Schlosser, M. Li6PS5X: A Class of Crystalline Li-Rich Solids With an Unusually High Li+ Mobility. Angew. Chem. Int. Ed. 2008, 47, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.D.; Kim, J.-S.; Choi, S.; Kim, S.; Jeon, M.; Jung, H.-G.; Chung, K.Y.; Lee, J.-H.; Kim, B.-K.; Lee, J.-H.; et al. Superionic halogen-rich Li-argyrodites using in situ nanocrystal nucleation and rapid crystal growth. Nano Lett. 2020, 20, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Koerver, R.; Aygün, I.; Leichtweiß, T.; Dietrich, C.; Zhang, W.; Binder, J.O.; Hartmann, P.; Zeier, W.G.; Janek, J. Capacity fade in solid-state batteries: Interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes. Chem. Mater. 2017, 29, 5574–5582. [Google Scholar] [CrossRef]
- Li, W.J.; Hirayama, M.; Suzuki, K.; Kanno, R. Fabrication and all solid-state battery performance of TiS2/Li10GeP2S12 composite electrodes. Mater. Trans. 2016, 57, 549–552. [Google Scholar] [CrossRef]
- Shin, B.R.; Nam, Y.J.; Oh, D.Y.; Kim, D.H.; Kim, J.W.; Jung, Y.S. Comparative study of TiS2/Li-In all-solid-state lithium batteries using glass-ceramic Li3PS4 and Li10GeP2S12 solid electrolytes. Electrochim. Acta 2014, 146, 395–402. [Google Scholar] [CrossRef]
- Auvergniot, J.; Cassel, A.; Ledeuil, J.-B.; Viallet, V.; Seznec, V.; Dedryvère, R. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries. Chem. Mater. 2017, 29, 3883–3890. [Google Scholar] [CrossRef]
- de Jongh, P.E.; Blanchard, D.; Matsuo, M.; Udovic, T.J.; Orimo, S. Complex hydrides as room-temperature solid electrolytes for rechargeable batteries. Appl. Phys. A 2016, 122, 251. [Google Scholar] [CrossRef]
- Matsuo, M.; Orimo, S. Lithium fast-ionic conduction in complex hydrides: Review and prospects. Adv. Energy Mater. 2011, 1, 161–172. [Google Scholar] [CrossRef]
- Latroche, M.; Blanchard, D.; Cuevas, F.; El Kharbachi, A.; Hauback, B.C.; Jensen, T.R.; de Jongh, P.E.; Kim, S.; Nazer, N.S.; Ngene, P.; et al. Full-cell hydride-based solid-state Li batteries for energy storage. Int. J. Hydrogen Energy 2019, 44, 7875–7887. [Google Scholar] [CrossRef]
- Ha Dao, A.; López-Aranguren, P.; Černý, R.; Guiader, O.; Zhang, J.; Cuevas, F.; Latroche, M.; Jordy, C. Improvement of the ionic conductivity on new substituted borohydride argyrodites. Solid State Ion. 2019, 339, 114987. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Chiang, Y.-M. Characterization of electronic and ionic transport in Li1-xNi0.33Mn0.33Co0.33O2 (NMC333) and Li1-xNi0.50Mn0.20Co0.30O2 (NMC523) as a function of Li content. J. Electrochem. Soc. 2016, 163, A1512–A1517. [Google Scholar] [CrossRef]
- Logothetis, E.M.; Kaiser, W.J.; Kukkonen, C.A.; Faile, S.P.; Colella, R.; Gambold, J. Hall coefficient and reflectivity evidence that TiS2 is a semiconductor. J. Phys. C Solid State Phys. 1979, 12, L521–L526. [Google Scholar] [CrossRef]
- Julien, C.; Nazri, G.-A. Solid state batteries: Materials design and optimization. In The Springer International Series in Engineering and Computer Science; Springer: New York, NY, USA, 1994; Volume 271, ISBN 978-0-7923-9460-0. [Google Scholar]
- Kaiser, N.; Spannenberger, S.; Schmitt, M.; Cronau, M.; Kato, Y.; Roling, B. Ion transport limitations in all-solid-state lithium battery electrodes containing a sulfide-based electrolyte. J. Power Sources 2018, 396, 175–181. [Google Scholar] [CrossRef]
- Santhosha, A.L.; Medenbach, L.; Buchheim, J.R.; Adelhelm, P. The indium−lithium electrode in solid-state lithium-ion batteries: Phase formation, redox potentials, and interface stability. Batter. Supercaps 2019, 2, 524–529. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Sakaguchi, I.; Zhang, L.; Ma, R.; Fukuda, K.; Osada, M.; Sasaki, T. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem. Commun. 2007, 9, 1486–1490. [Google Scholar] [CrossRef]
- Ohta, N.; Takada, K.; Zhang, L.; Ma, R.; Osada, M.; Sasaki, T. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification. Adv. Mater. 2006, 18, 2226–2229. [Google Scholar] [CrossRef]
- Haruyama, J.; Sodeyama, K.; Tateyama, Y. Cation mixing properties toward Co diffusion at the LiCoO2 cathode/sulfide electrolyte interface in a solid-state battery. ACS Appl. Mater. Interfaces 2017, 9, 286–292. [Google Scholar] [CrossRef]
- Ohtomo, T.; Hayashi, A.; Tatsumisago, M.; Tsuchida, Y.; Hama, S.; Kawamoto, K. All-solid-state lithium secondary batteries using the 75Li2S·25P2S5 glass and the 70Li2S·30P2S5 glass–ceramic as solid electrolytes. J. Power Sources 2013, 233, 231–235. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Interfacial observation between LiCoO2 electrode and Li2S−P2S5 solid electrolytes of all-solid-state lithium secondary batteries using transmission electron microscopy. Chem. Mater. 2010, 22, 949–956. [Google Scholar] [CrossRef]
- Woo, J.H.; Trevey, J.E.; Cavanagh, A.S.; Choi, Y.S.; Kim, S.C.; George, S.M.; Oh, K.H.; Lee, S.-H. Nanoscale interface modification of LiCoO2 by Al2O3 atomic layer deposition for solid-state Li batteries. J. Electrochem. Soc. 2012, 159, A1120–A1124. [Google Scholar] [CrossRef]
- Sakuda, A.; Yamauchi, A.; Yubuchi, S.; Kitamura, N.; Idemoto, Y.; Hayashi, A.; Tatsumisago, M. Mechanochemically prepared Li2S-P2S5-LiBH4 solid electrolytes with an argyrodite structure. ACS Omega 2018, 3, 5453–5458. [Google Scholar] [CrossRef]
- Unemoto, A.; Wu, H.; Udovic, T.J.; Matsuo, M.; Ikeshoji, T.; Orimo, S. Fast lithium-ionic conduction in a new complex hydride-sulphide crystalline phase. Chem. Commun. 2016, 52, 564–566. [Google Scholar] [CrossRef]
- López-Aranguren, P.; Berti, N.; Dao, H.A.; Zhang, J.; Cuevas, F.; Latroche, M.; Jordy, C. An all-solid-state metal hydride—Sulfur lithium-ion battery. J. Power Sources 2017, 357, 56–60. [Google Scholar] [CrossRef]
- Takahashi, K.; Hattori, K.; Yamazaki, T.; Takada, K.; Matsuo, M.; Orimo, S.; Maekawa, H.; Takamura, H. All-solid-state lithium battery with LiBH4 solid electrolyte. J. Power Sources 2013, 226, 61–64. [Google Scholar] [CrossRef]
- Sveinbjörnsson, D.; Christiansen, A.S.; Viskinde, R.; Norby, P.; Vegge, T. The LiBH4-LiI solid solution as an electrolyte in an all-solid-state battery. J. Electrochem. Soc. 2014, 161, A1432–A1439. [Google Scholar] [CrossRef]
- Unemoto, A.; Nogami, G.; Tazawa, M.; Taniguchi, M.; Orimo, S. Development of 4V-Class bulk-type all-solid-state lithium rechargeable batteries by a combined use of complex hydride and sulfide electrolytes for room temperature operation. Mater. Trans. 2017, 58, 1063–1068. [Google Scholar] [CrossRef]
- Unemoto, A.; Ikeshoji, T.; Yasaku, S.; Matsuo, M.; Stavila, V.; Udovic, T.J.; Orimo, S. Stable interface formation between TiS2 and LiBH4 in bulk-type all-solid-state lithium batteries. Chem. Mater. 2015, 27, 5407–5416. [Google Scholar] [CrossRef]
- Kim, S.; Toyama, N.; Oguchi, H.; Sato, T.; Takagi, S.; Ikeshoji, T.; Orimo, S. Fast lithium-ion conduction in atom-deficient closo-type complex hydride solid electrolytes. Chem. Mater. 2018, 30, 386–391. [Google Scholar] [CrossRef]
- Tang, W.S.; Matsuo, M.; Wu, H.; Stavila, V.; Zhou, W.; Talin, A.A.; Soloninin, A.V.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; et al. Liquid-like ionic conduction in solid lithium and sodium monocarba-closo-decaborates near or at room temperature. Adv. Energy Mater. 2016, 6, 1502237. [Google Scholar] [CrossRef]
- El Kharbachi, A.; Hu, Y.; Sørby, M.H.; Mæhlen, J.P.; Vullum, P.E.; Fjellvåg, H.; Hauback, B.C. Reversibility of metal-hydride anodes in all-solid-state lithium secondary battery operating at room temperature. Solid State Ion. 2018, 317, 263–267. [Google Scholar] [CrossRef]
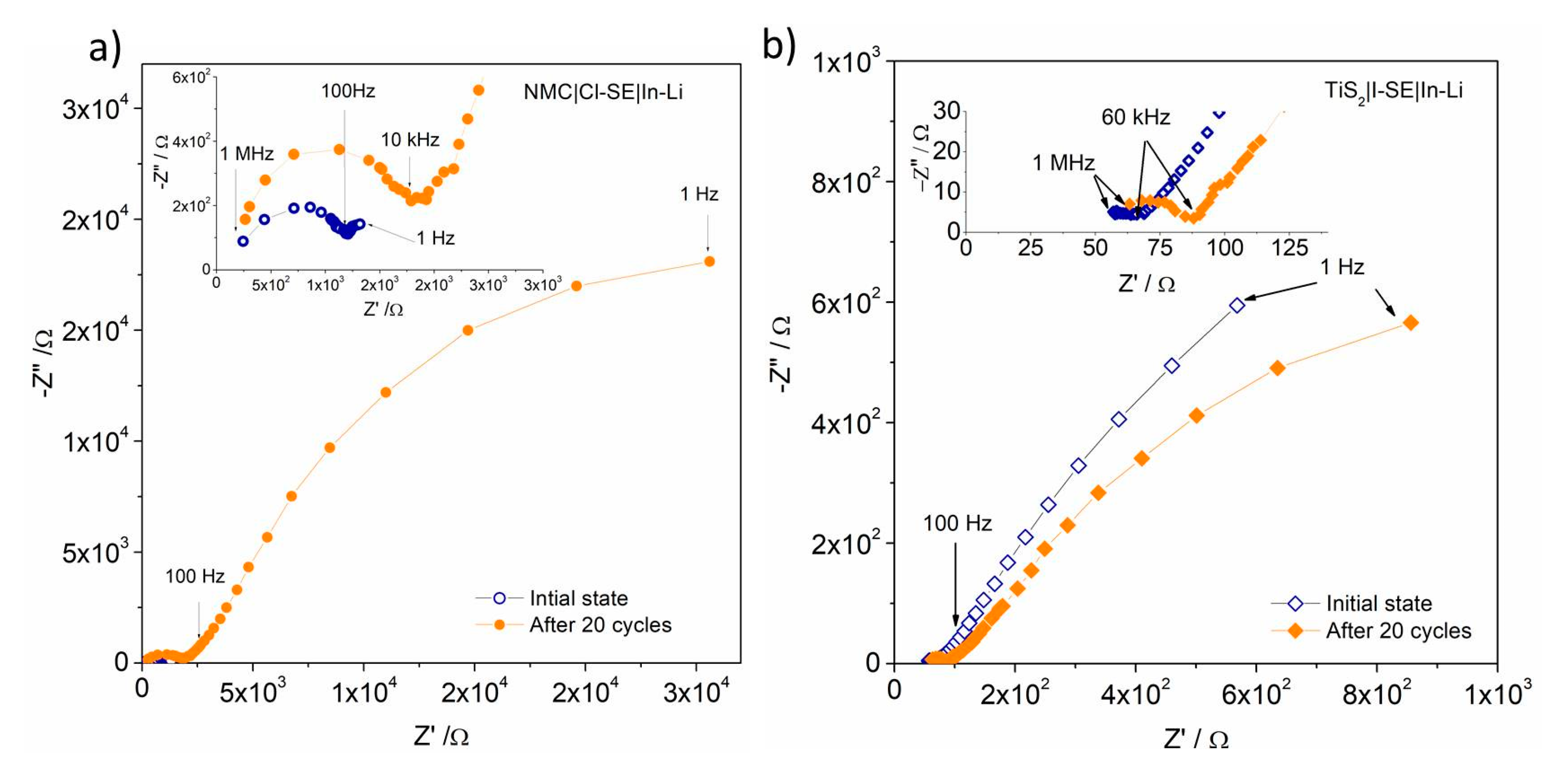

| Cell Type | Voltage | Capacity | Capacity | σ | CE | R1 (ini./20 c.) | Zw1 (ini./20 c.) | Zw2 (ini./20 c.) |
|---|---|---|---|---|---|---|---|---|
| V | mAhg−1 | mAhg−1 | ×104 Scm−1 | % | Ω | Ω | kΩ | |
| NMC│Cl-SE│In-Li | 3.2–3.8 | 100 @ C/50 | 20 @ C/10 | 4.1 | ~90 | 170/150 | 1040/1858 | 2/47 |
| NMC│I-SE│In-Li | 3.2–3.8 | 75 @ C/50 | 10 @ C/10 | 7.6 | ~90 | 120/340 | 1270/1780 | 56/63 |
| TiS2│I-SE│In-Li | 1.3–1.9 | 240 @ C/100 | 20 @ C | 7.6 | 97 | 50/50 | 170/118 | 3.35/1.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dao, A.H.; López-Aranguren, P.; Zhang, J.; Cuevas, F.; Latroche, M. Solid-State Li-Ion Batteries Operating at Room Temperature Using New Borohydride Argyrodite Electrolytes. Materials 2020, 13, 4028. https://doi.org/10.3390/ma13184028
Dao AH, López-Aranguren P, Zhang J, Cuevas F, Latroche M. Solid-State Li-Ion Batteries Operating at Room Temperature Using New Borohydride Argyrodite Electrolytes. Materials. 2020; 13(18):4028. https://doi.org/10.3390/ma13184028
Chicago/Turabian StyleDao, Anh Ha, Pedro López-Aranguren, Junxian Zhang, Fermín Cuevas, and Michel Latroche. 2020. "Solid-State Li-Ion Batteries Operating at Room Temperature Using New Borohydride Argyrodite Electrolytes" Materials 13, no. 18: 4028. https://doi.org/10.3390/ma13184028
APA StyleDao, A. H., López-Aranguren, P., Zhang, J., Cuevas, F., & Latroche, M. (2020). Solid-State Li-Ion Batteries Operating at Room Temperature Using New Borohydride Argyrodite Electrolytes. Materials, 13(18), 4028. https://doi.org/10.3390/ma13184028






