A Facile Method to Prepare a Superhydrophobic Magnesium Alloy Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Sample Characterization
3. Results and Discussion
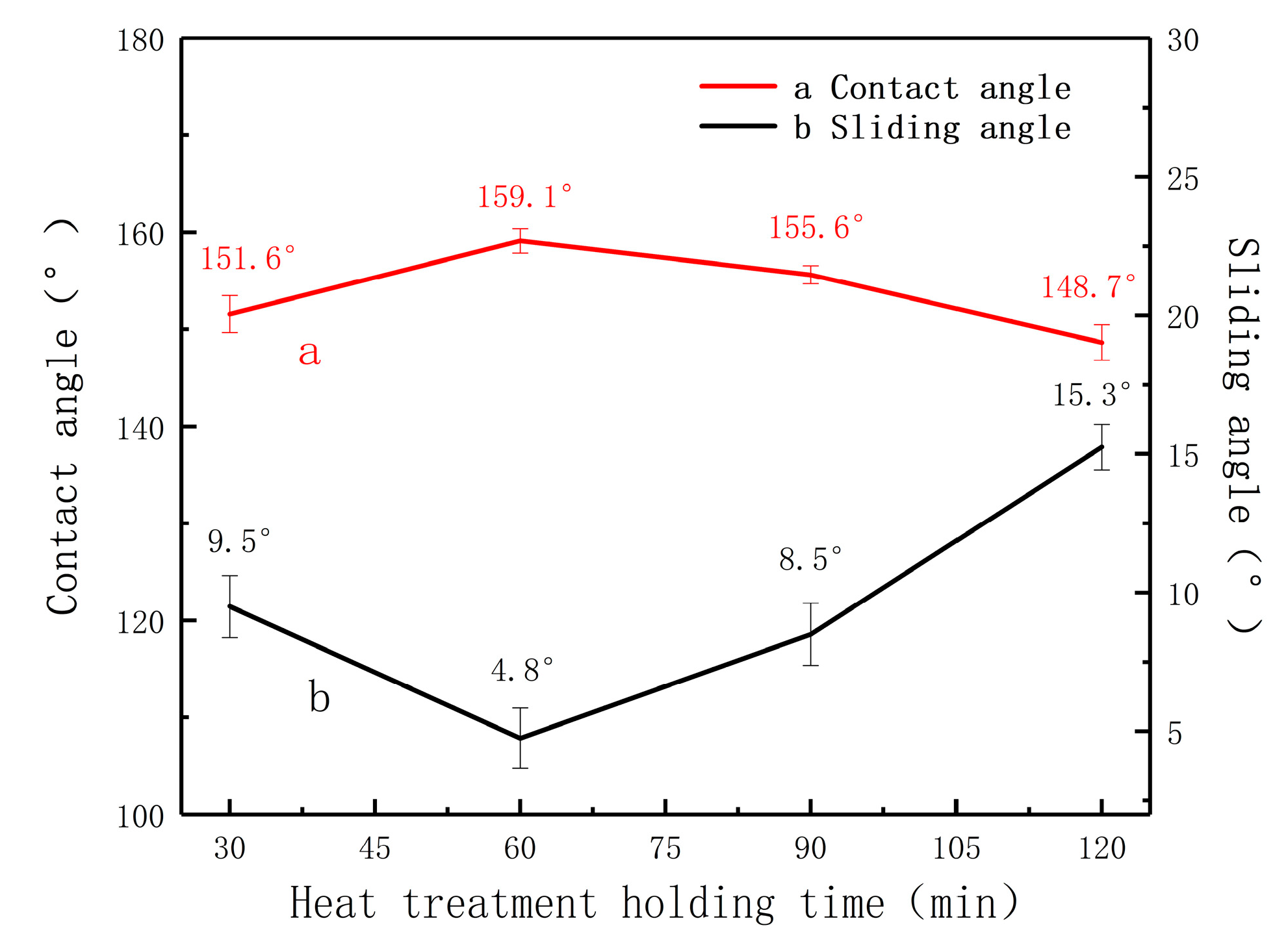
3.1. Wettability
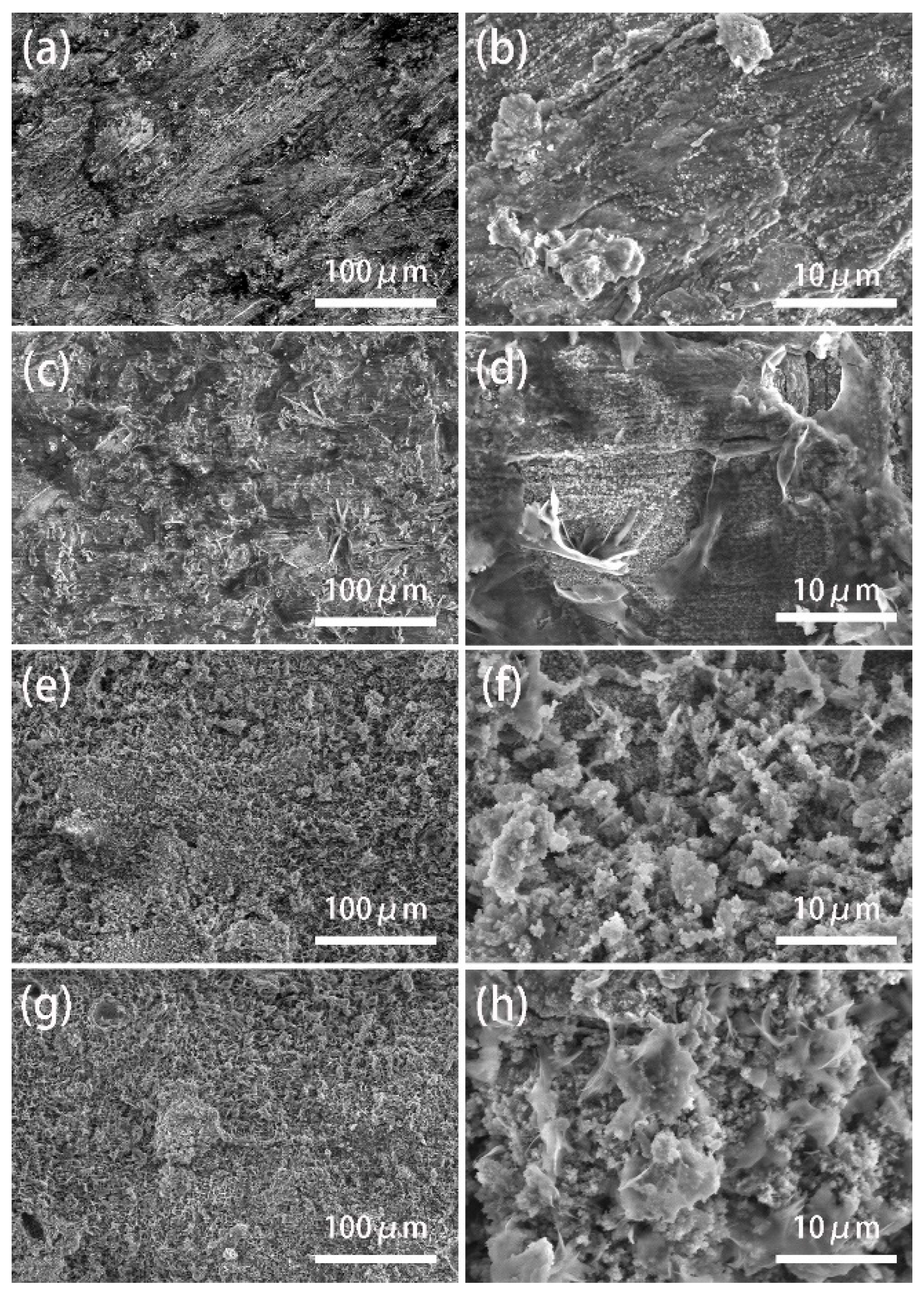
3.2. Surface Morphology
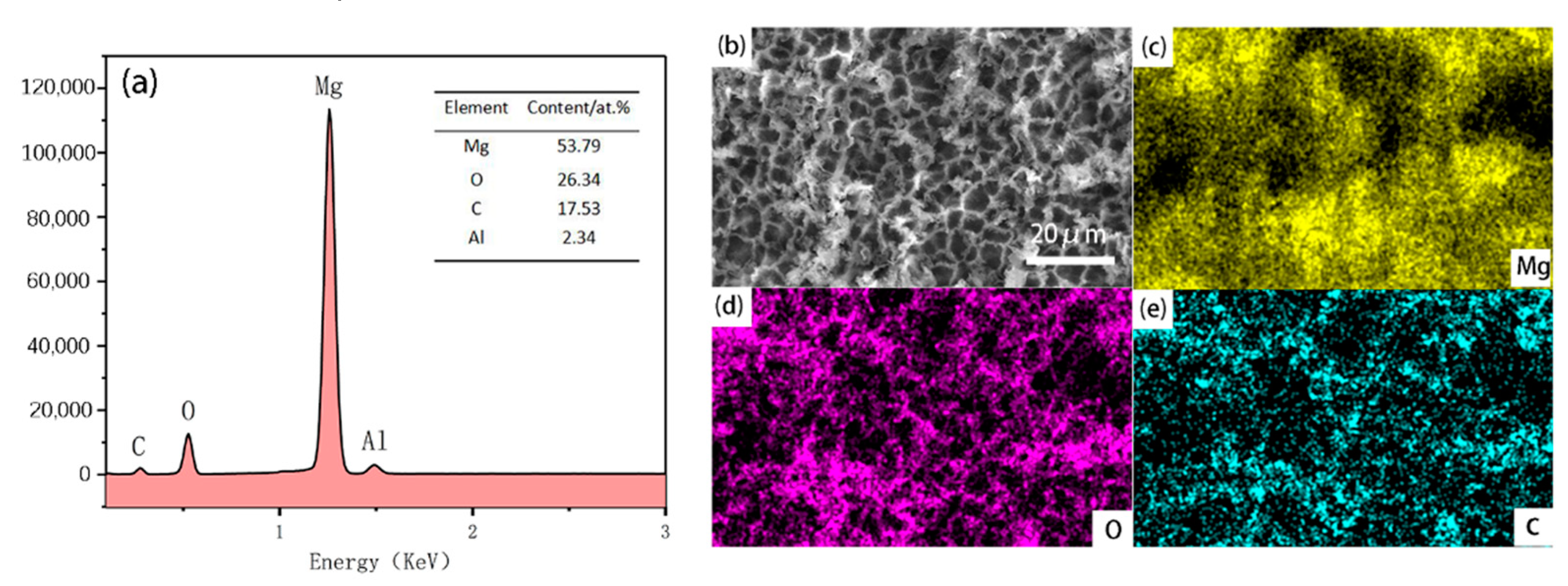
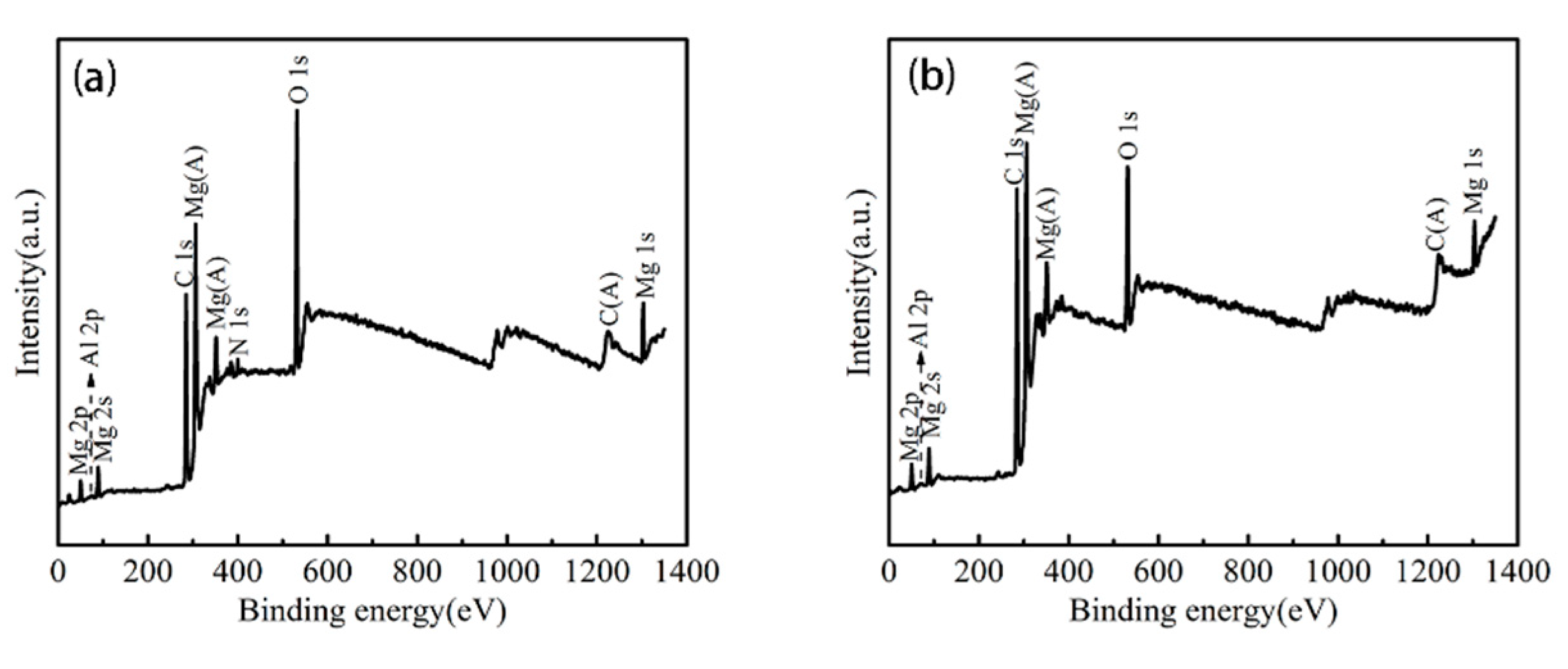
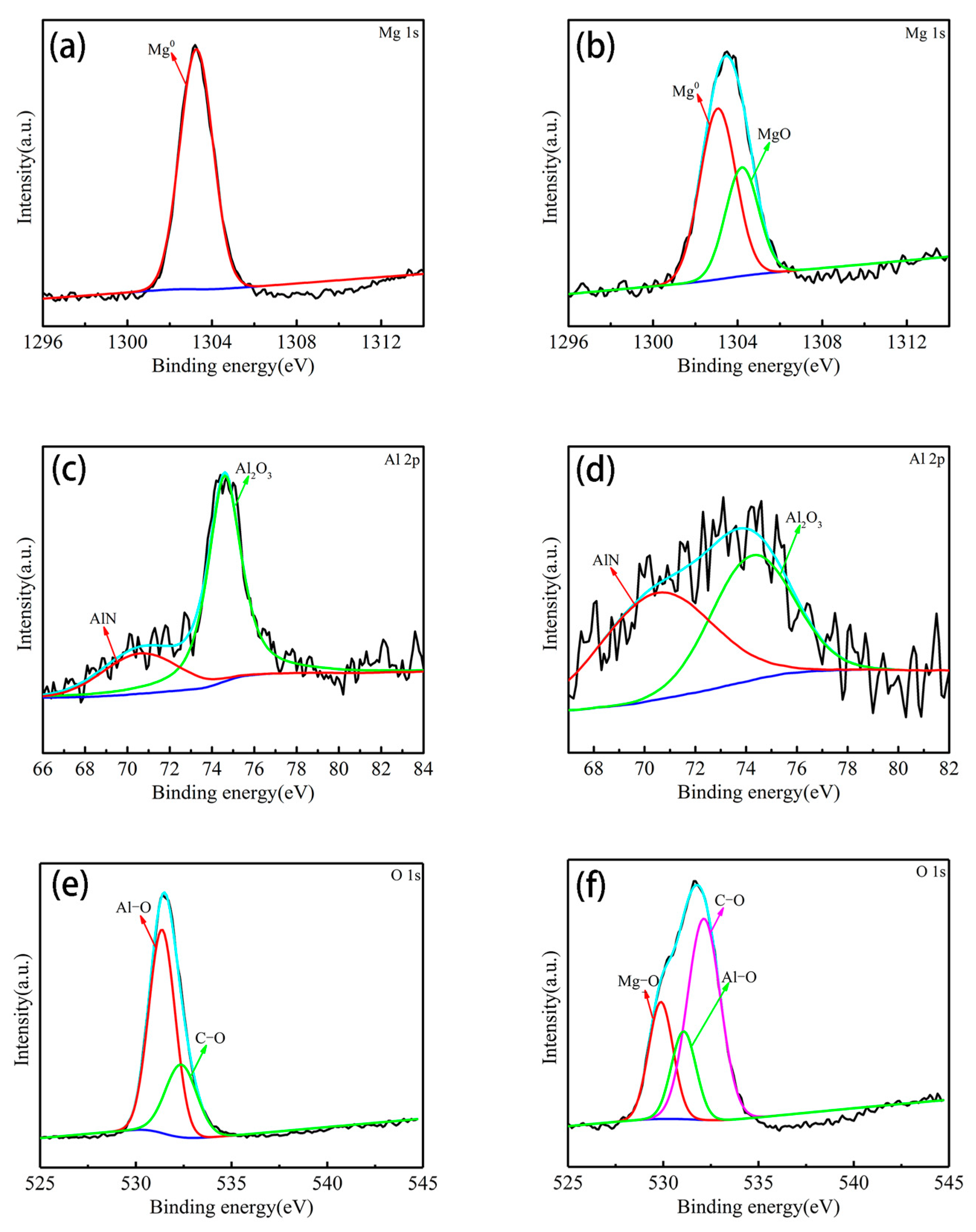
3.3. Surface Composition Analysis
3.4. Corrosion Behavior
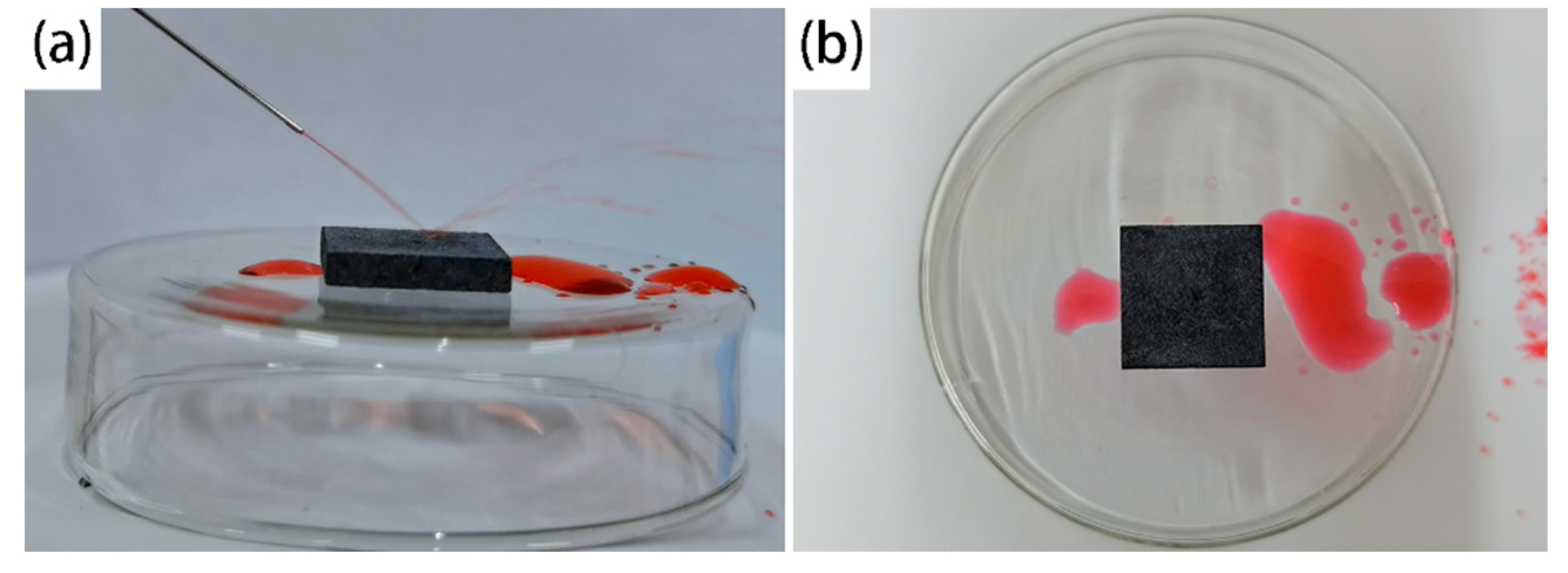
3.5. Self-Cleaning Effect
3.6. Bouncing Performance Test
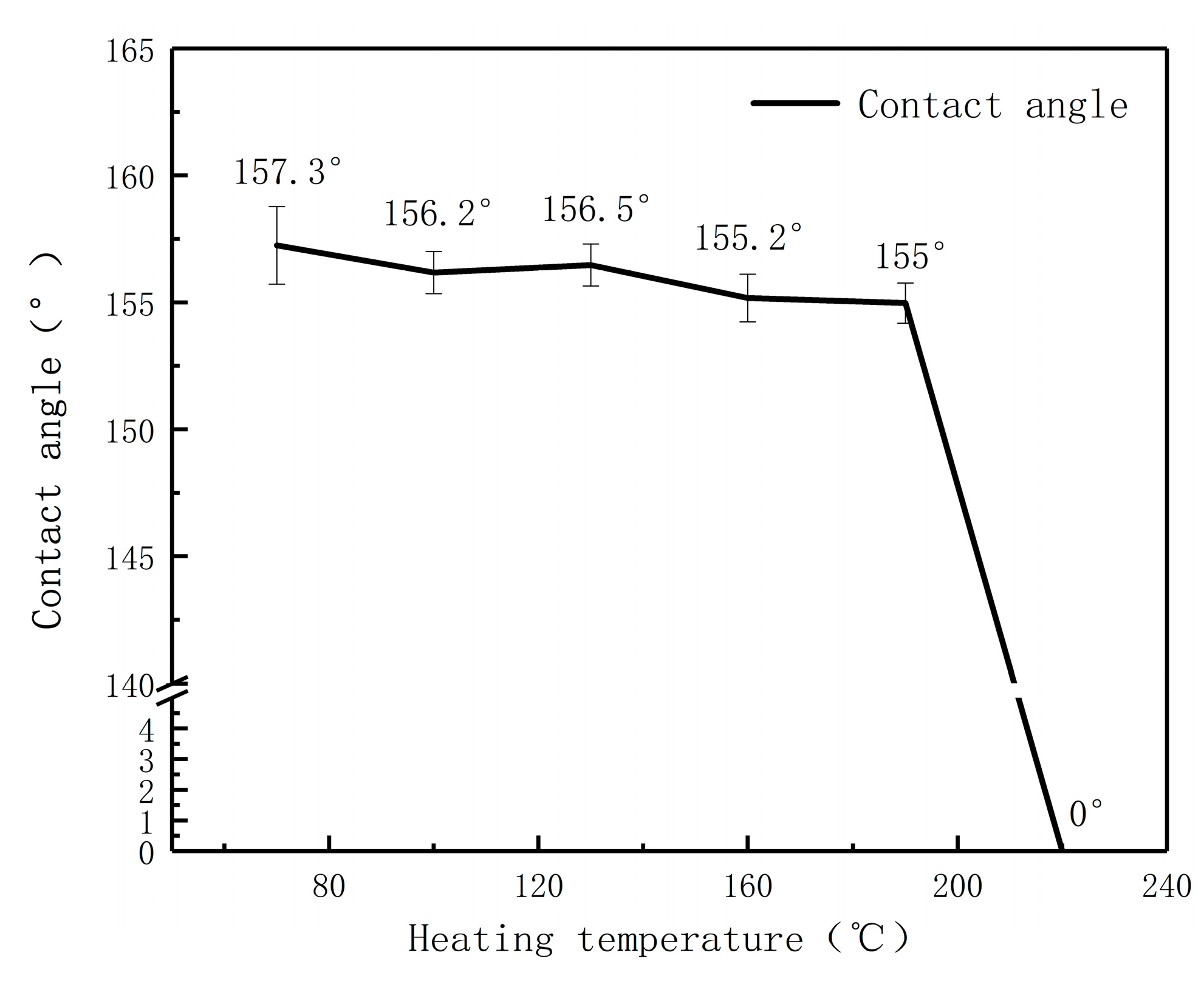
3.7. Thermal Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, L.; Yao, X.; Zheng, Y. Direction-dependent adhesion of water strider’s legs for water-walking. Solid State Sci. 2012, 14, 1146–1151. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Gao, X.; Yan, X.; Yao, X.; Xu, L.; Zhang, K.; Zhang, J.; Yang, B.; Jiang, L. The Dry-Style Antifogging Properties of Mosquito Compound Eyes and Artificial Analogues Prepared by Soft Lithography. Adv. Mater. 2007, 19, 2213–2217. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coat. 2020, 142, 105557. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Bai, Y.; Zhang, J.; Han, Z.; Ren, L. Fabrication of biomimetic hydrophobic patterned graphene surface with ecofriendly anti-corrosion properties for Al alloy. Colloids Surf. A Physicochem. Eng. Asp. 2016, 500, 64–71. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.; Han, X.; Zhao, Y. A stable hierarchical superhydrophobic coating on pipeline steel surface with self-cleaning, anticorrosion, and anti-scaling properties. Colloids Surf. A: Physicochem. Eng. Asp. 2016, 503, 43–52. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Li, J.; Liu, T.; Pu, J.; Zhao, H.; Wang, L. One-step synthesis of superhydrophobic polyhedral oligomericsilsesquioxane-graphene oxide and its application in anti-corrosion and anti-wear fields. Corros. Sci. 2019, 147, 9–21. [Google Scholar] [CrossRef]
- Pakzad, H.; Liravi, M.; Moosavi, A.; Nouri-Borujerdi, A.; Najafkhani, H. Fabrication of durable superhydrophobic surfaces using PDMS and beeswax for drag reduction of internal turbulent flow. Appl. Surf. Sci. 2020, 513, 145754. [Google Scholar] [CrossRef]
- Alinovi, E.; Bottaro, A. Apparent slip and drag reduction for the flow over superhydrophobic and lubricant-impregnated surfaces. Phys. Rev. Fluids 2018, 3, 124002. [Google Scholar] [CrossRef]
- Rastegari, A.; Akhavan, R. The common mechanism of turbulent skin-friction drag reduction with superhydrophobic longitudinal microgrooves and riblets. J. Fluid Mech. 2018, 838, 68–104. [Google Scholar] [CrossRef]
- Qu, M.; Liu, S.; He, J.; Feng, J.; Yao, Y.; Hou, L.; Ma, X.; Liu, X. Fabrication of recyclable superhydrophobic materials with self-cleaning and mechanically durable properties on various substrates by quartz sand and polyvinylchloride. RSC Adv. 2016, 6, 79238–79244. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, B.; Zhang, M.; Li, X.; Yang, J. A novel colorful sepiolite-based superhydrophobic coating with excellent mechanical and chemical stability and self-cleaning property. Mater. Lett. 2019, 254, 340–343. [Google Scholar] [CrossRef]
- Ruan, M.; Li, W.; Wang, B.; Deng, B.; Ma, F.; Yu, Z. Preparation and Anti-icing Behavior of Superhydrophobic Surfaces on Aluminum Alloy Substrates. Langmuir 2013, 29, 8482–8491. [Google Scholar] [CrossRef] [PubMed]
- Ganne, A.; Lebed, V.O.; Gavrilov, A.I. Combined wet chemical etching and anodic oxidation for obtaining the superhydrophobic meshes with anti-icing performance. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 150–155. [Google Scholar] [CrossRef]
- Shen, L.; Xu, M.; Jiang, W.; Qiu, M.; Fan, M.; Ji, G.; Tian, Z.-J. A novel superhydrophobic Ni/Nip coating fabricated by magnetic field induced selective scanning electrodeposition. Appl. Surf. Sci. 2019, 489, 25–33. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Wang, W.; Xu, C.; Ren, L. Superhydrophobic Copper Surface Textured by Laser for Delayed Icing Phenomenon. Langmuir 2020, 36, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Yu, S.; Zhu, G.; Zhou, X. Fabrication of superhydrophobic surface on aluminum alloy 6061 by a facile and effective anodic oxidation method. Surf. Coat. Technol. 2019, 380, 125078. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, S.; Guan, S.; Lv, Z.; Liu, E.; Zhao, Y. Fabrication and characterization of superhydrophobic TiO2 nanotube coating by a facile anodic oxidation approach. Surf. Coat. Technol. 2018, 354, 83–91. [Google Scholar] [CrossRef]
- Su, J.; Tian, H.; Jiang, N. TRPIV experimental investigational of the effect of retrograde vortex on drag-reduction mechanism over superhydrophobic surfaces. Chin. J. Theor. Appl. Mech. 2016, 48, 1033–1039. [Google Scholar]
- Ou, J.; Zhu, W.; Xie, C.; Xue, M. Mechanically Robust and Repairable Superhydrophobic Zinc Coating via a Fast and Facile Method for Corrosion Resisting. Materials 2019, 12, 1779. [Google Scholar] [CrossRef]
- Ben, S.; Zhou, T.; Ma, H.; Yao, J.; Ning, Y.; Tian, D.; Liu, K.; Jiang, L. Multifunctional Magnetocontrollable Superwettable-Microcilia Surface for Directional Droplet Manipulation. Adv. Sci. 2019, 6, 1900834. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Rafsanjani-Abbasi, A.; Rahimi, E.; Shalchian, H.; Vahdati-Khaki, J.; Babakhani, A.; Hosseinpour, S.; Davoodi, A. Recycled Cobalt from Spent Li-ion Batteries as a Superhydrophobic Coating for Corrosion Protection of Plain Carbon Steel. Materials 2018, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, D.; Zhang, G.; Zhang, Z.; Zhang, S.; Tang, A.; Pan, F. Fabrication and characterization of Mg-M layered double hydroxide films on anodized magnesium alloy AZ31. Appl. Surf. Sci. 2018, 431, 177–186. [Google Scholar] [CrossRef]
- Xie, J.; Hu, J.; Fang, L.; Liao, X.; Du, R.; Wu, F.; Wu, L. Facile fabrication and biological properties of super-hydrophobic coating on magnesium alloy used as potential implant materials. Surf. Coat. Technol. 2020, 384, 125223. [Google Scholar] [CrossRef]
- Joo, J.; Kim, D.; Moon, H.-S.; Kim, K.; Lee, J. Durable anti-corrosive oil-impregnated porous surface of magnesium alloy by plasma electrolytic oxidation with hydrothermal treatment. Appl. Surf. Sci. 2020, 509, 145361. [Google Scholar] [CrossRef]
- Zeng, Z.; Stanford, N.; Davies, C.; Nie, J.F.; Birbilis, N. Magnesium extrusion alloys: A review of developments and prospects. Int. Mater. Rev. 2018, 64, 27–62. [Google Scholar] [CrossRef]
- Udhayan, R.; Bhatt, D.P. On the corrosion behaviour of magnesium and its alloys using electrochemical techniques. J. Power Sour. 1996, 63, 103–107. [Google Scholar] [CrossRef]
- Li, H.; Feng, X.; Peng, Y.; Zeng, R.-C. Durable lubricant-infused coating on a magnesium alloy substrate with anti-biofouling and anti-corrosion properties and excellent thermally assisted healing ability. Nanoscale 2020, 12, 7700–7711. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W.; Su, B.-L. Superhydrophobic surfaces: From natural to biomimetic to functional. J. Colloid Interface Sci. 2011, 353, 335–355. [Google Scholar] [CrossRef]
- Chobaomsup, V.; Metzner, M.; Boonyongmaneerat, Y. Superhydrophobic surface modification for corrosion protection of metals and alloys. J. Coat. Technol. Res. 2020, 17, 583–595. [Google Scholar] [CrossRef]
- Yin, B.; Fang, L.; Hu, J.; Tang, A.-Q.; Wei, W.-H.; He, J. Preparation and properties of super-hydrophobic coating on magnesium alloy. Appl. Surf. Sci. 2010, 257, 1666–1671. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Yu, S.; Han, Z.; Ren, L. A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar] [CrossRef]
- Liu, A.-H.; Xu, J.L. Preparation and corrosion resistance of superhydrophobic coatings on AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 2287–2293. [Google Scholar] [CrossRef]
- Kang, Z.; Li, W. Facile and fast fabrication of superhydrophobic surface on magnesium alloy by one-step electrodeposition method. J. Ind. Eng. Chem. 2017, 50, 50–56. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Q.; Chen, R.; Liu, J.; Zhang, H.; Li, R.; Takahashi, K.; Liu, P.; Wang, J. Fabrication of ZIF-8@SiO2 Micro/Nano Hierarchical Superhydrophobic Surface on AZ31 Magnesium Alloy with Impressive Corrosion Resistance and Abrasion Resistance. ACS Appl. Mater. Interfaces 2017, 9, 11106–11115. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.F.; Lei, J.L. Self-cleaning and Self-healing Protective Coating on Magnesium Alloy. Surf. Tech. 2019, 48, 27–33. [Google Scholar]
- Ou, J.; Hu, W.; Li, C.; Wang, Y.; Xue, M.; Wang, F.; Li, W. Tunable Water Adhesion on Titanium Oxide Surfaces with Different Surface Structures. ACS Appl. Mater. Interfaces 2012, 4, 5737–5741. [Google Scholar] [CrossRef]
- Jena, G.; Thinaharan, C.; George, R.; Philip, J. Robust nickel-reduced graphene oxide-myristic acid superhydrophobic coating on carbon steel using electrochemical codeposition and its corrosion resistance. Surf. Coat. Technol. 2020, 397, 125942. [Google Scholar] [CrossRef]
- Jbeily, M.; Schwieger, C.; Kressler, J. Mixed Langmuir monolayers of perfluorostearic acid and stearic acid studied by epifluorescence microscopy using fluorinated rhodamines and infrared reflection absorption spectroscopy (IRRAS). Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 274–285. [Google Scholar] [CrossRef]
- Griffth, E.C.; Adams, E.; Allen, H.C.; Vaida, V. Hydrophobic collapse of a steric acid film by adsorbed L-phenylalanine at the air-water interface. J. Phys. Chem. B 2012, 116, 7849–7857. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Natarajan, R.; Kala, A. FTIR spectra and mechanical strength analysis of some selected rubber derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, D.; Yang, G.; Zhang, Q.; Shen, J.; Lu, J.; Zhang, K. Highly Exothermic and Superhydrophobic Mg/Fluorocarbon Core/Shell Nanoenergetic Arrays. ACS Appl. Mater. Interfaces 2014, 6, 10497–10505. [Google Scholar] [CrossRef] [PubMed]
- Yathisha, R.; Nayaka, Y.A.; Purushothama, H.; Manjunatha, P.; Basavarajappa, K.; Vinay, M. Investigation the influence of Zn2+ doping on the photovoltaic properties (DSSCs) of MgO nanoparticles. J. Mol. Struct. 2020, 1217. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Jaw, K.-S.; Hsu, C.-K.; Lee, J.-S. The thermal decomposition behaviors of stearic acid, paraffin wax and polyvinyl butyral. Thermochim. Acta 2001, 367, 165–168. [Google Scholar] [CrossRef]


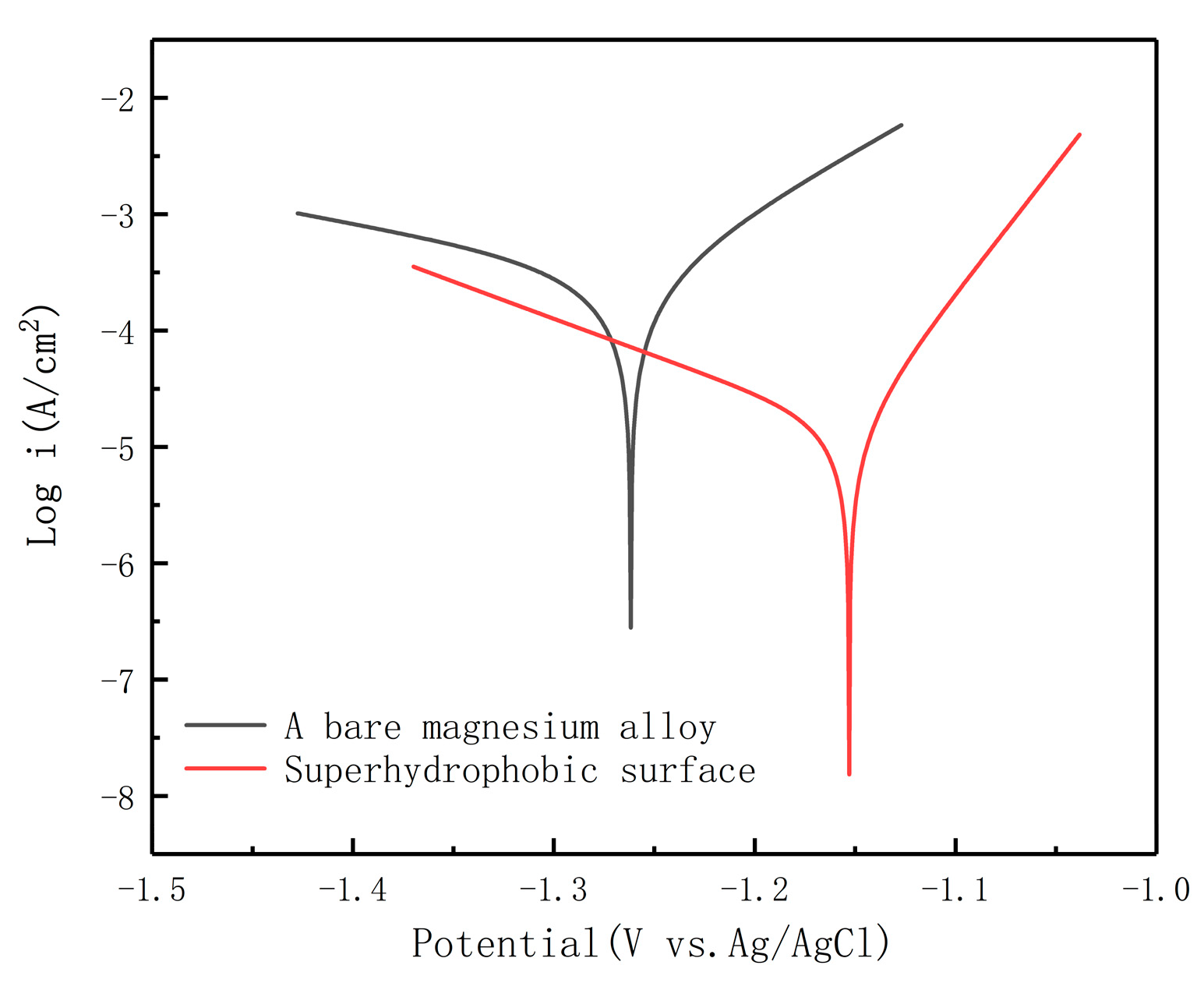

| Sample | Ecorr (V vs. Ag/AgCl) | Icorr (A/cm2) | η (%) |
|---|---|---|---|
| AZ91D | −1.2525 | 3.1713 × 10−4 | —— |
| SMA | −1.1495 | 9.7053 × 10−6 | 96.94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Jia, H. A Facile Method to Prepare a Superhydrophobic Magnesium Alloy Surface. Materials 2020, 13, 4007. https://doi.org/10.3390/ma13184007
Zhu J, Jia H. A Facile Method to Prepare a Superhydrophobic Magnesium Alloy Surface. Materials. 2020; 13(18):4007. https://doi.org/10.3390/ma13184007
Chicago/Turabian StyleZhu, Jiyuan, and Haojie Jia. 2020. "A Facile Method to Prepare a Superhydrophobic Magnesium Alloy Surface" Materials 13, no. 18: 4007. https://doi.org/10.3390/ma13184007
APA StyleZhu, J., & Jia, H. (2020). A Facile Method to Prepare a Superhydrophobic Magnesium Alloy Surface. Materials, 13(18), 4007. https://doi.org/10.3390/ma13184007





