The Phase Relations of the Co-Ni-In Ternary System at 673 K and 873 K and Magnetic Properties of Their Compounds
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
3.1. Phase Analysis
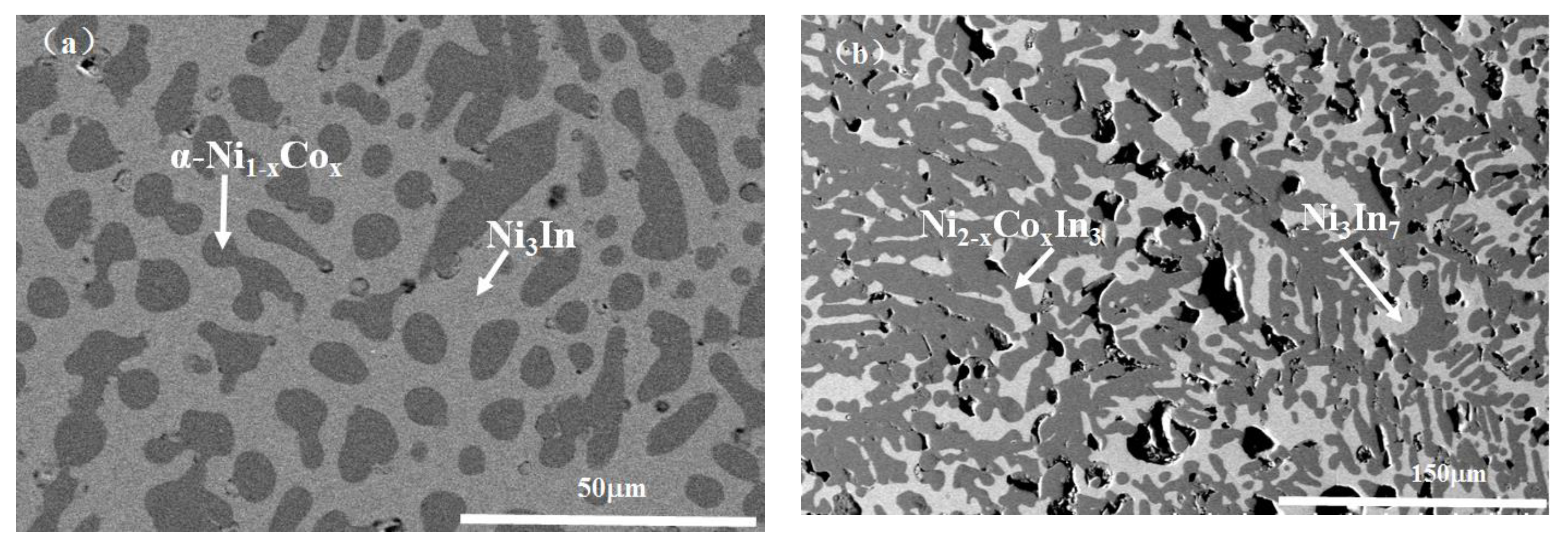
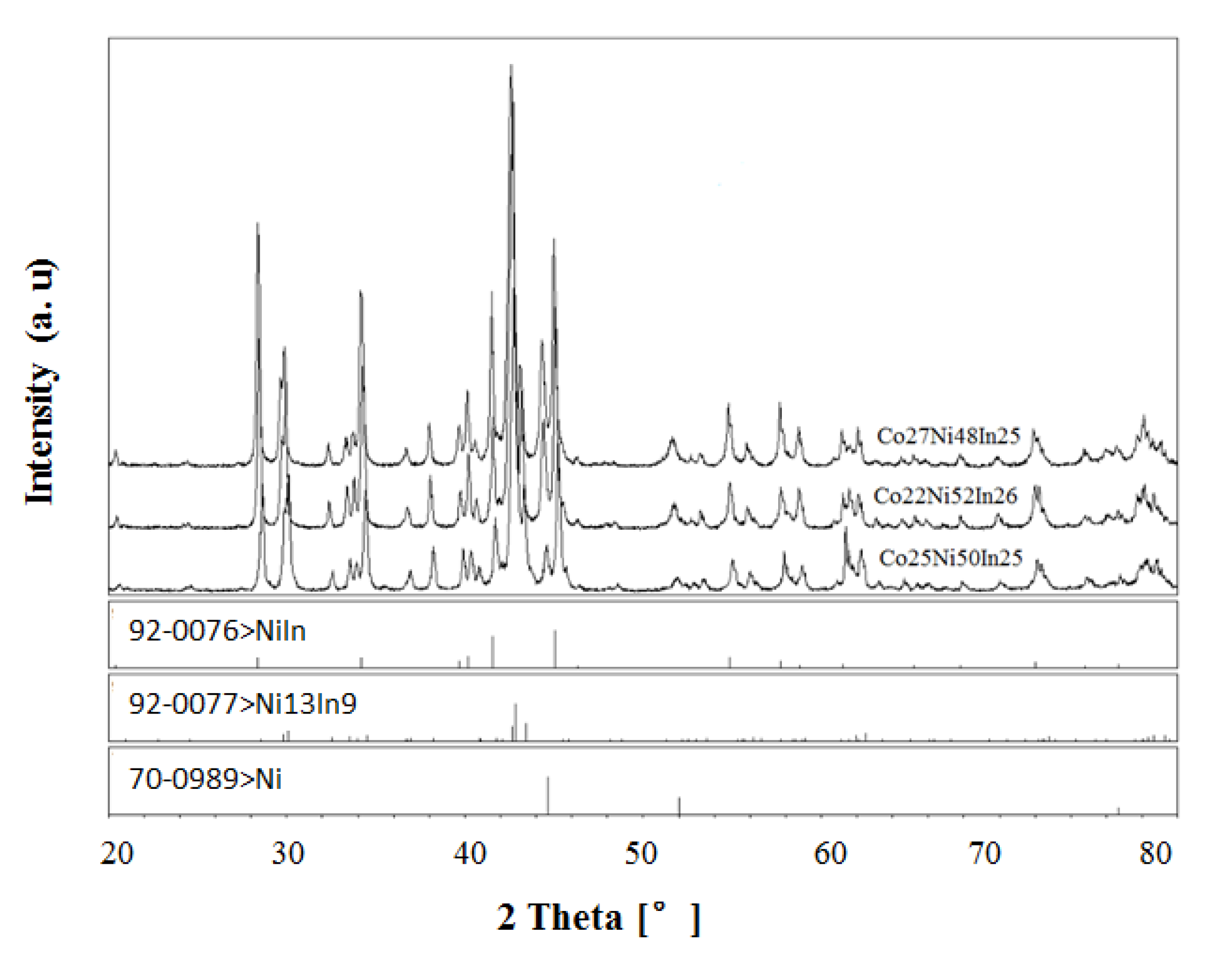
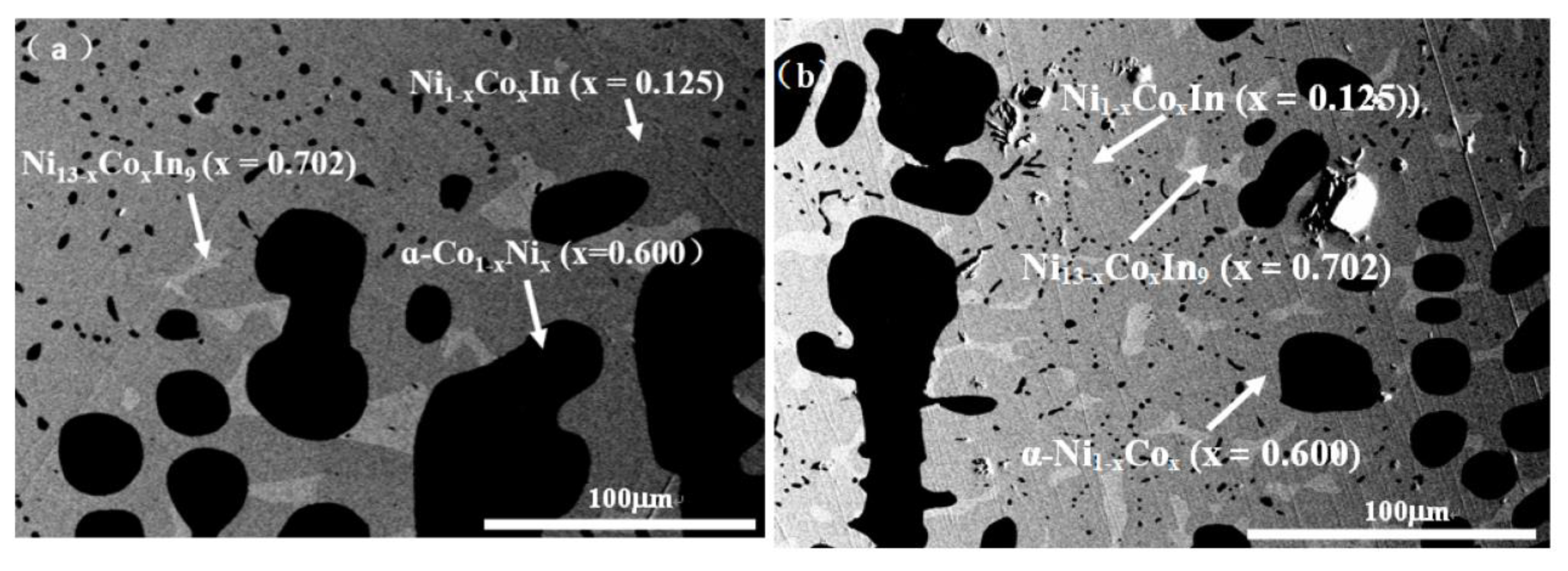
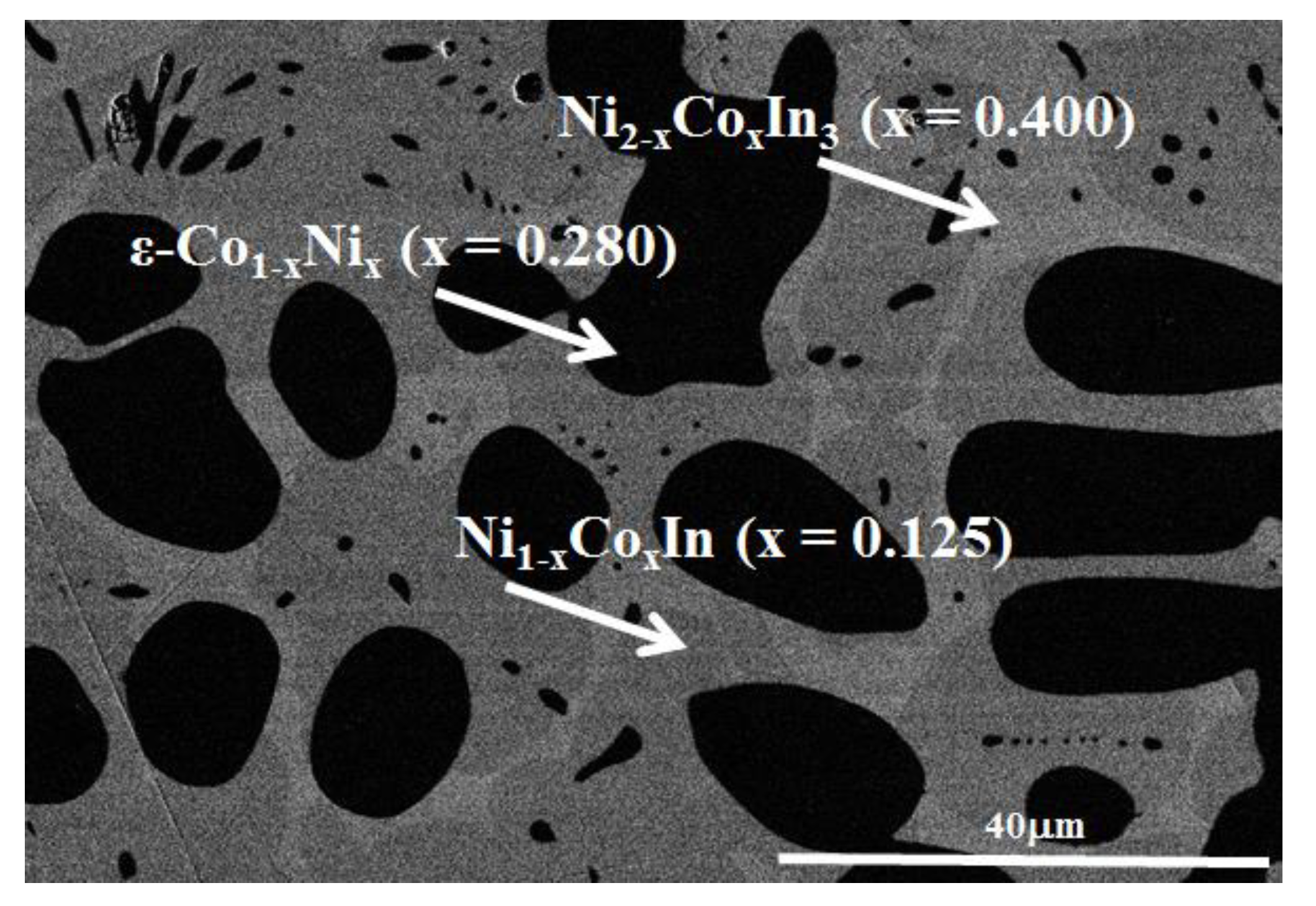
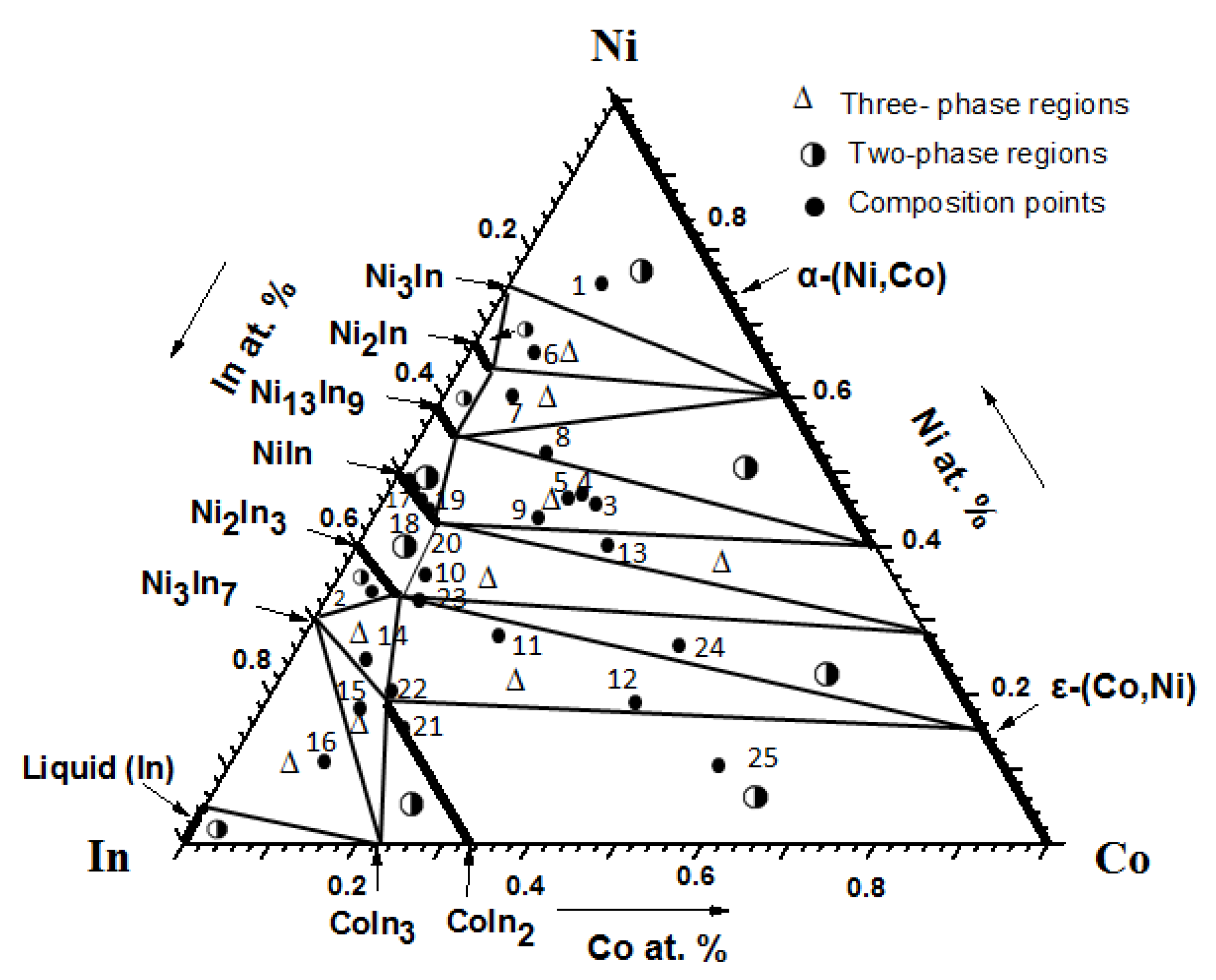
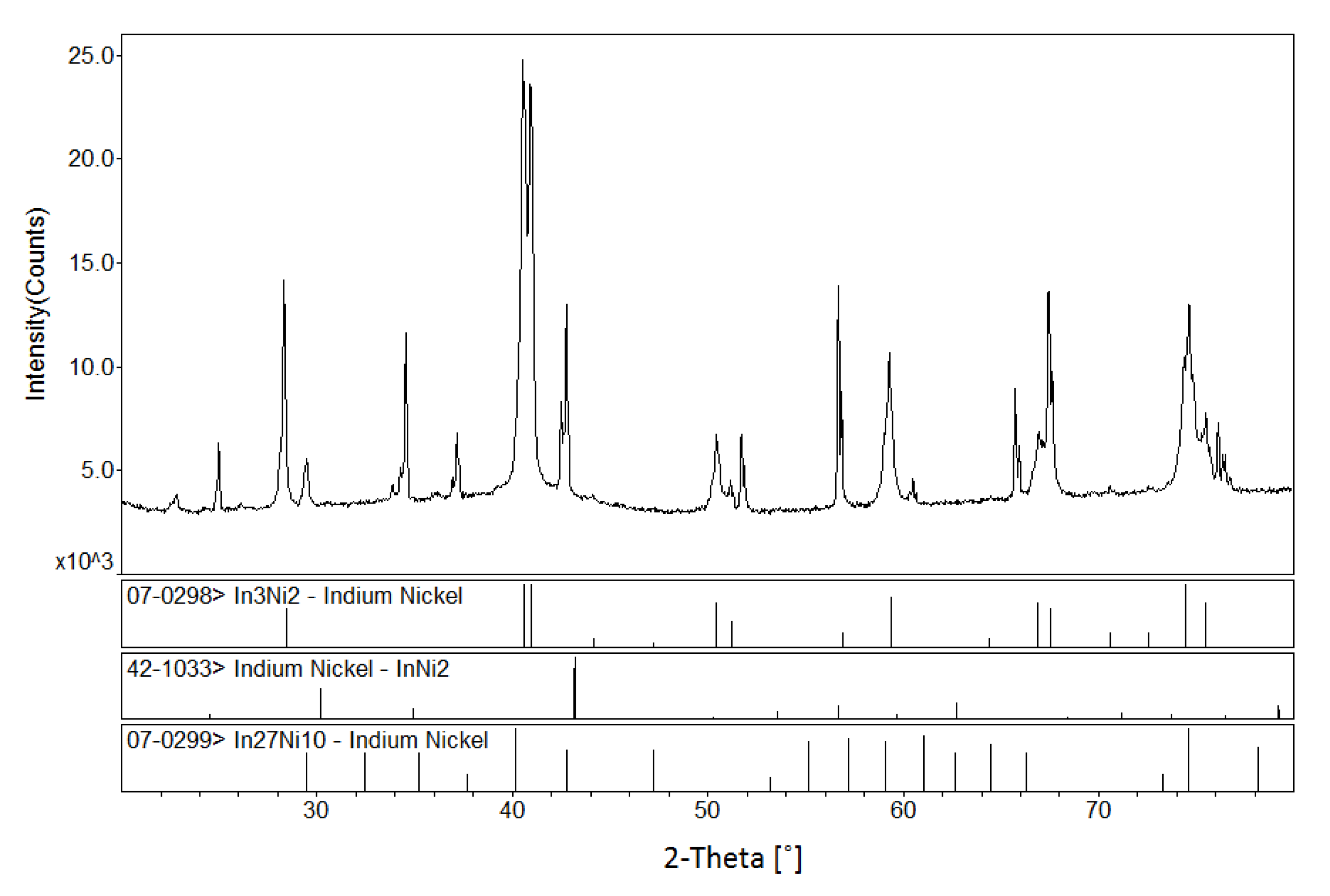
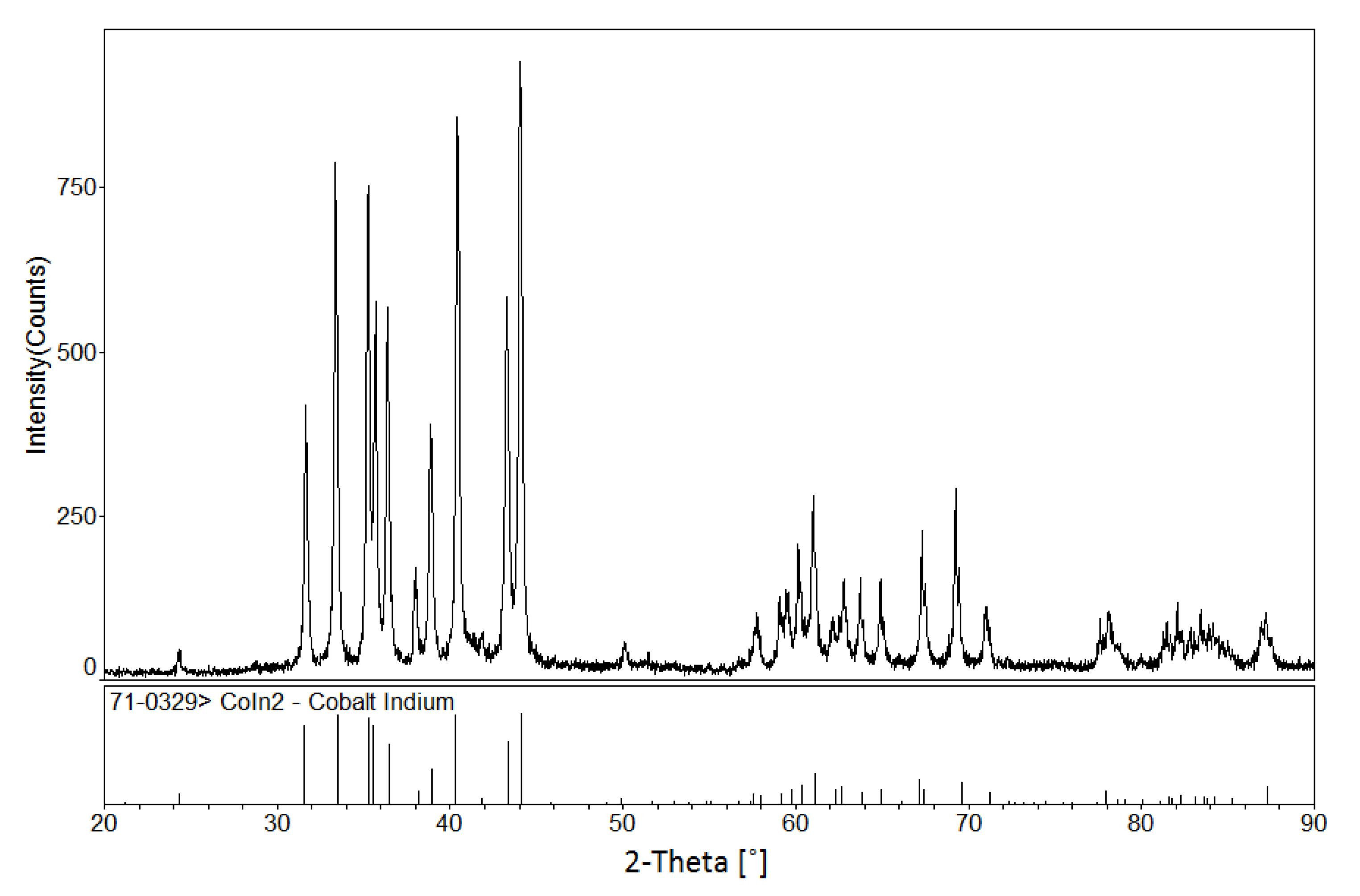
3.1.1. Phase Analysis at 673 K
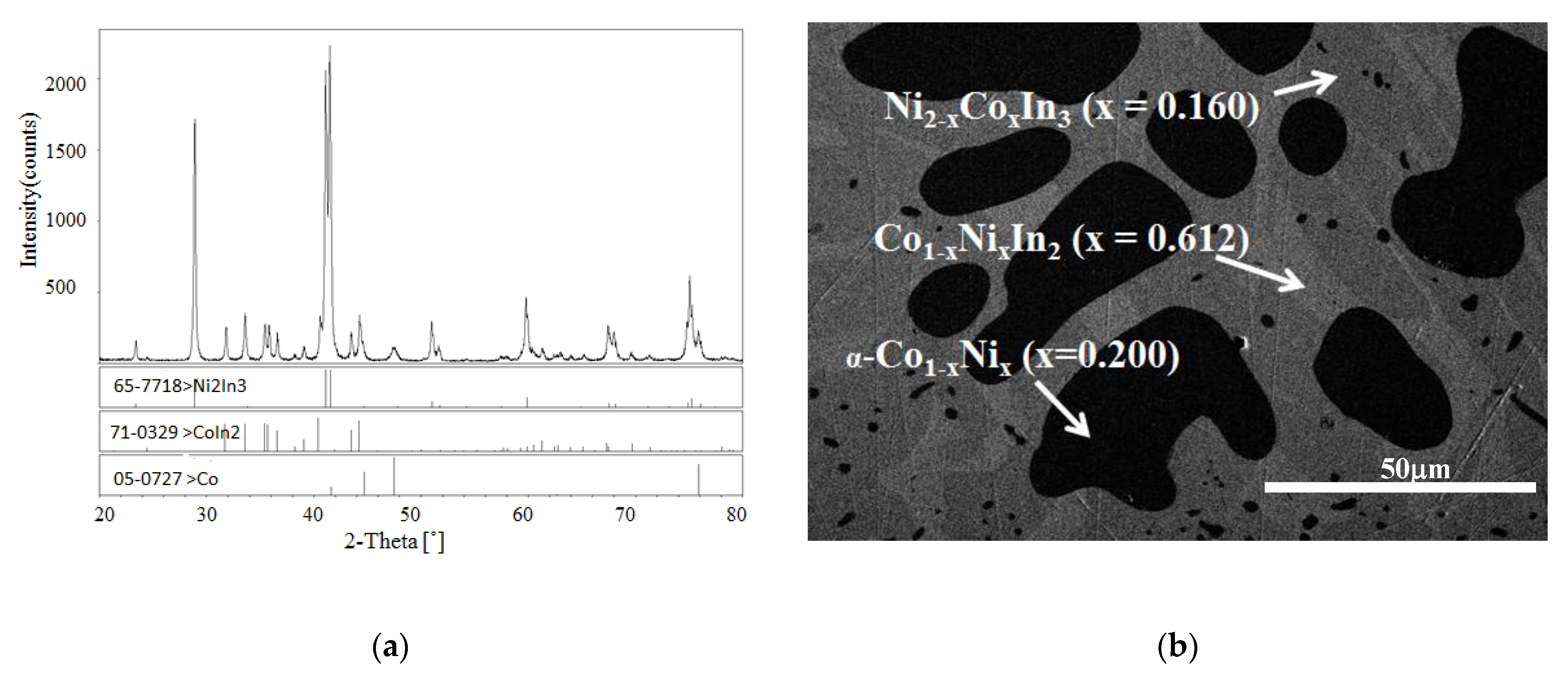
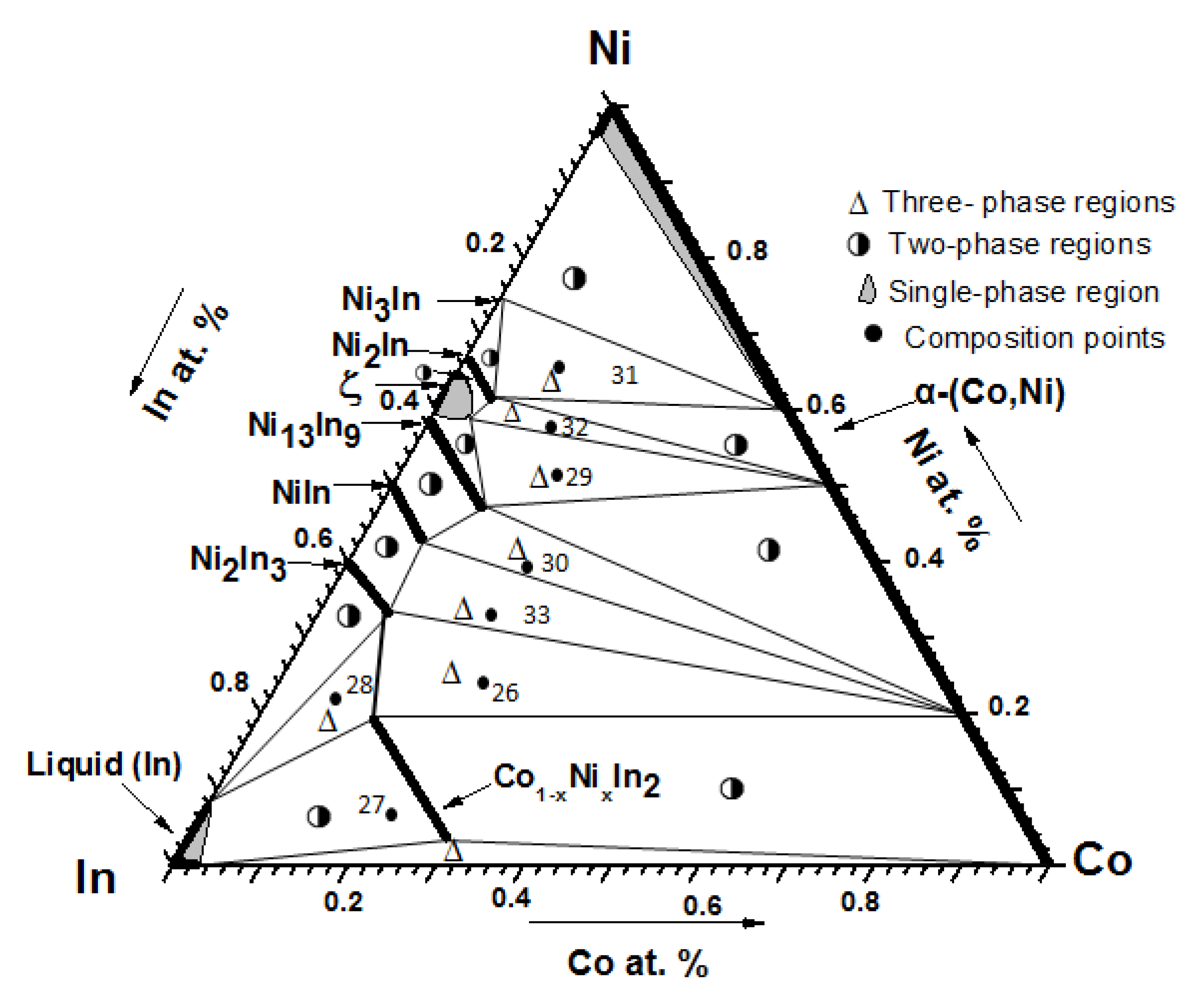
3.1.2. Phase Analysis at 873 K
3.2. Solid Solubility
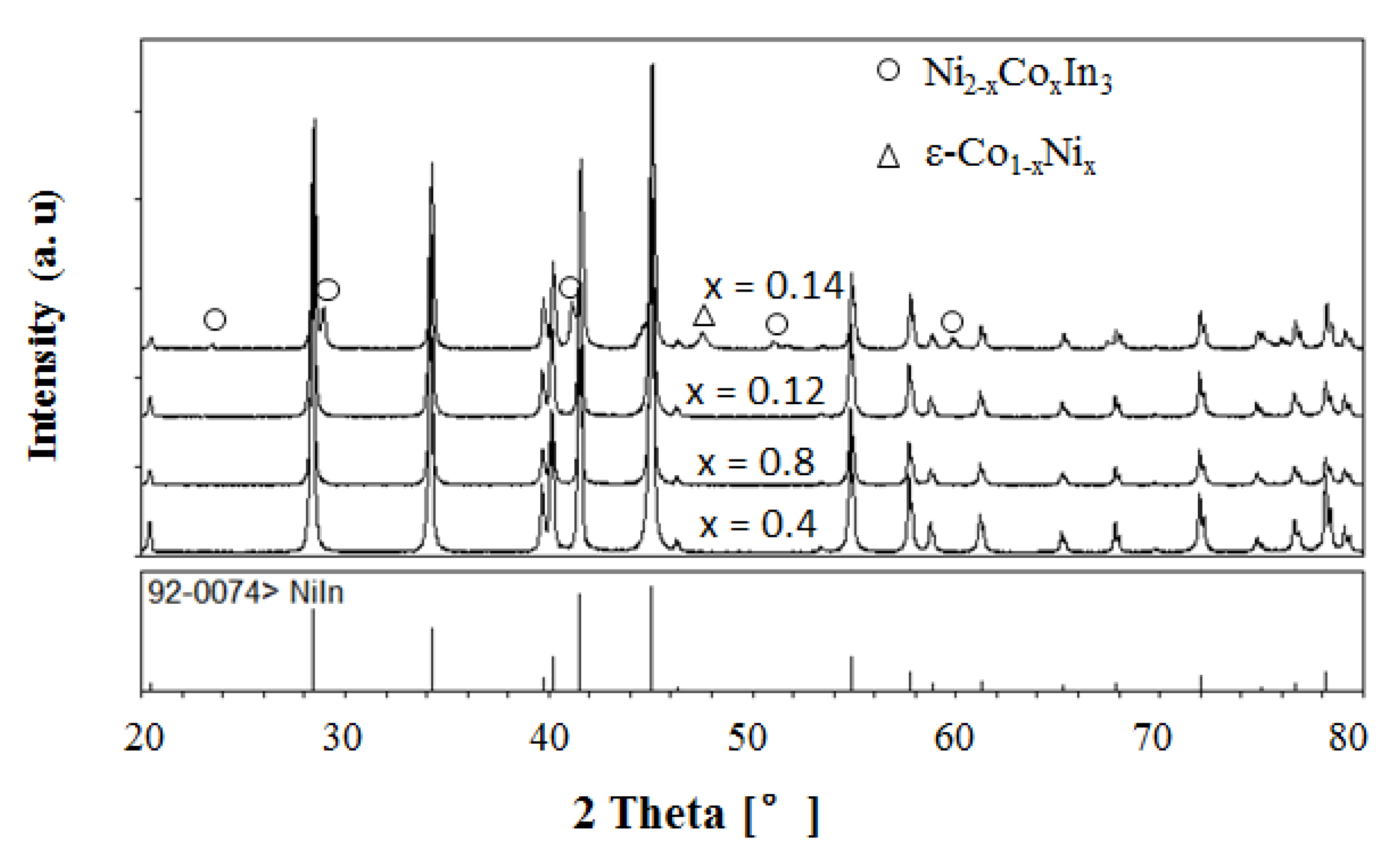
3.2.1. Solid Solubility at 673 K
3.2.2. Solid Solubility at 873 K
3.3. Isothermal Sections of the Co-Ni-In Ternary System at 673 K and 873 K
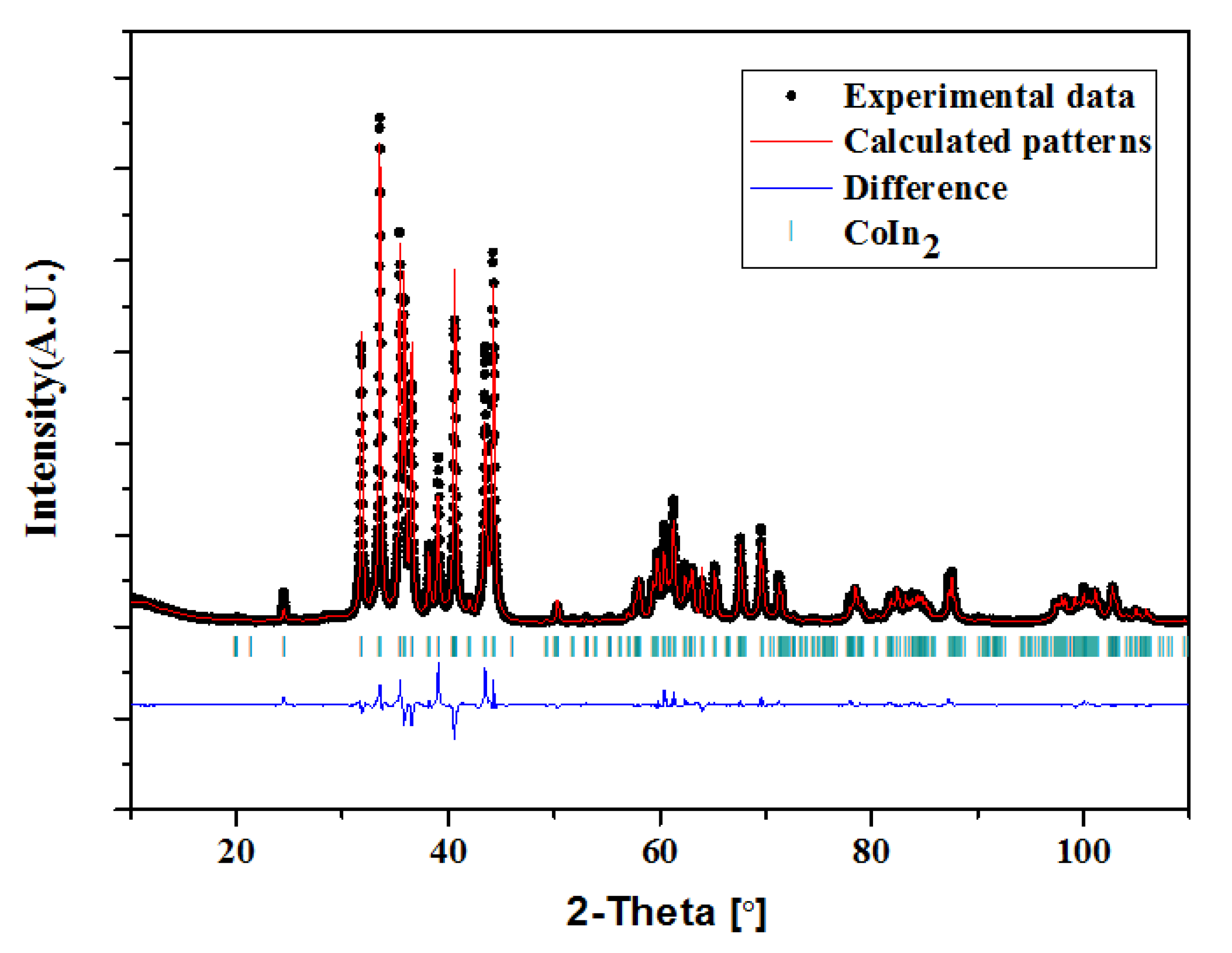
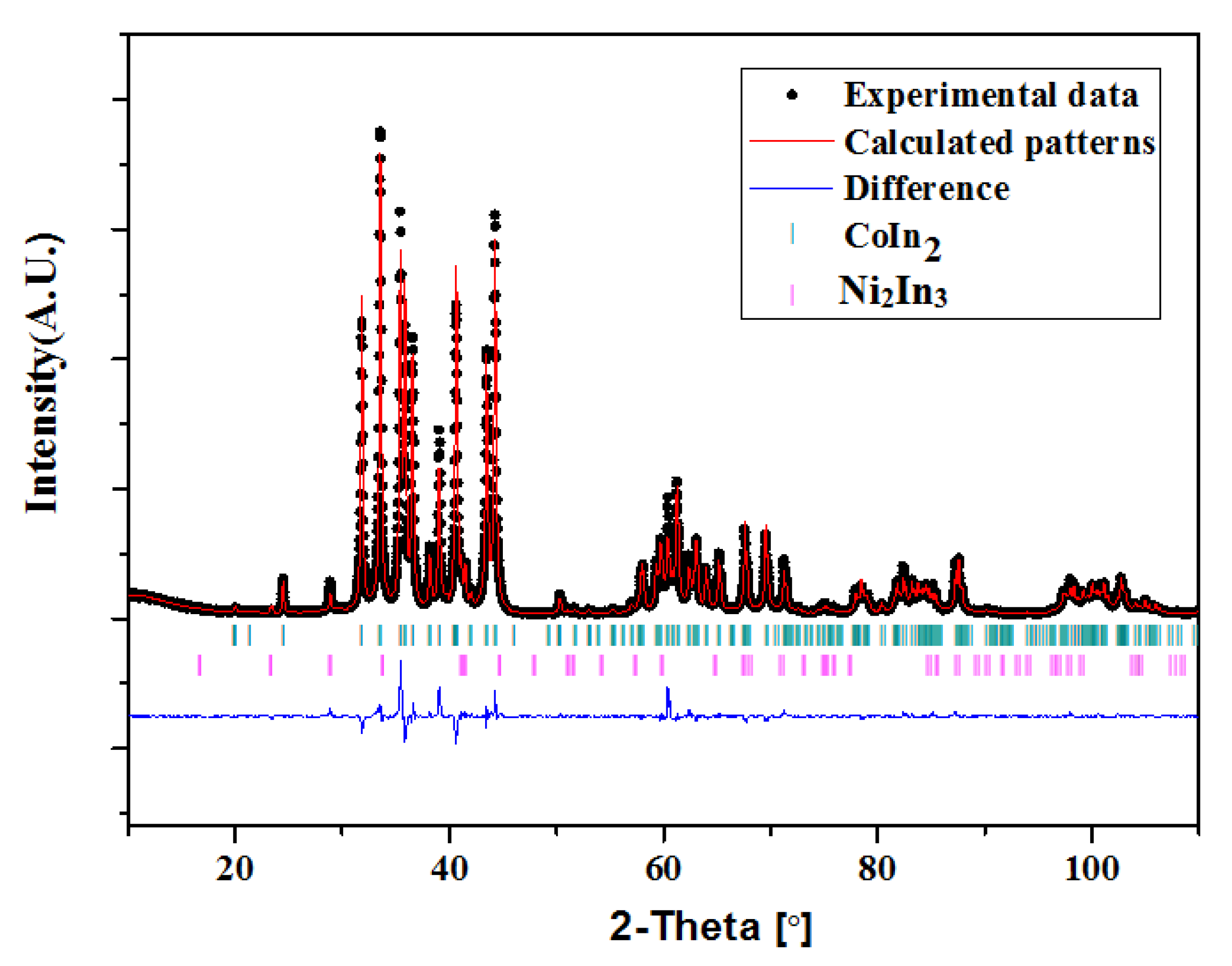
3.4. Crystal Structure
3.4.1. Crystal Structure of Ni2−xCoxIn3 (x = 0.200)
3.4.2. Crystal Structure of Co1−xNixIn2 (x = 0.540, 0.580)
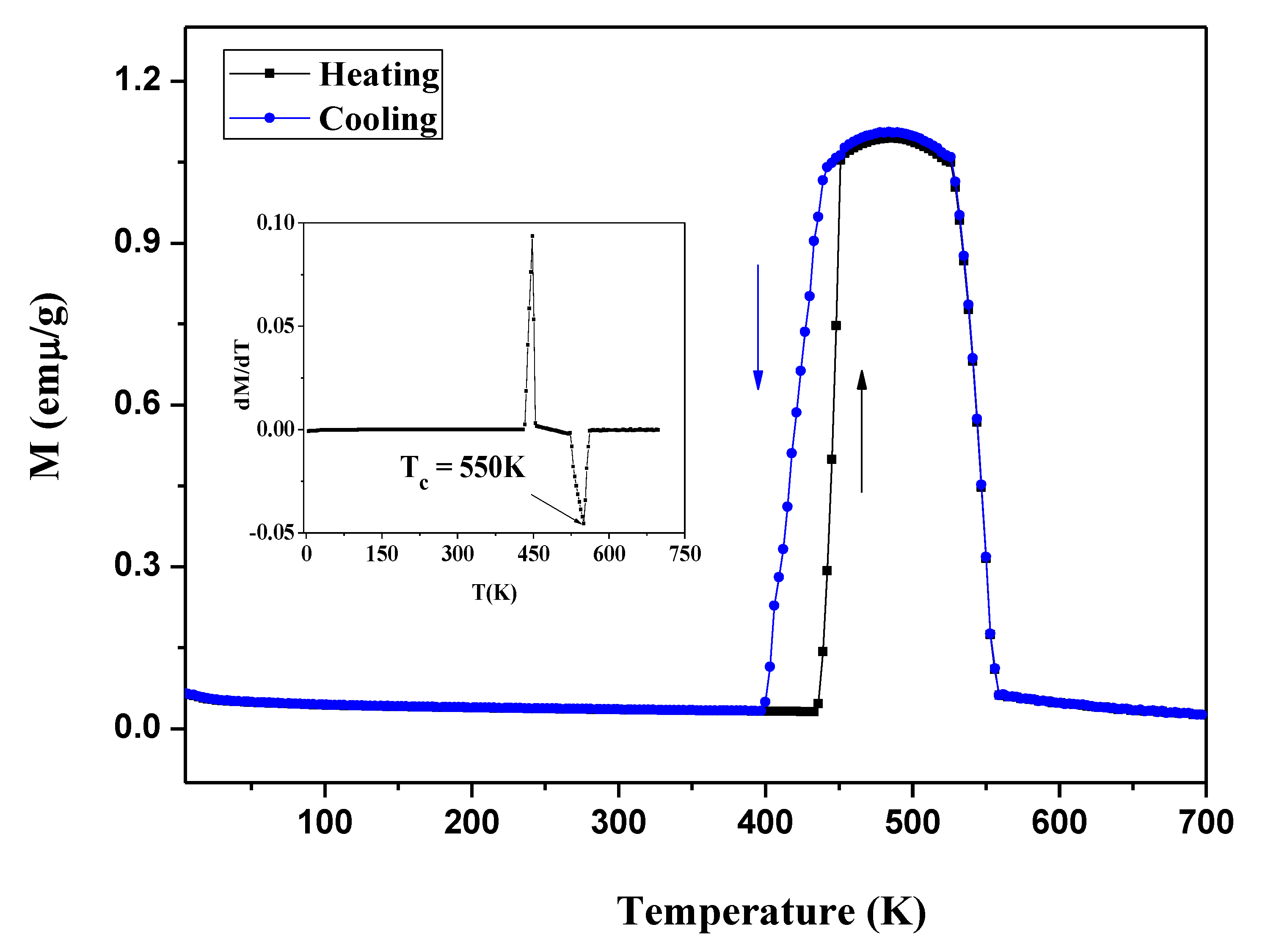
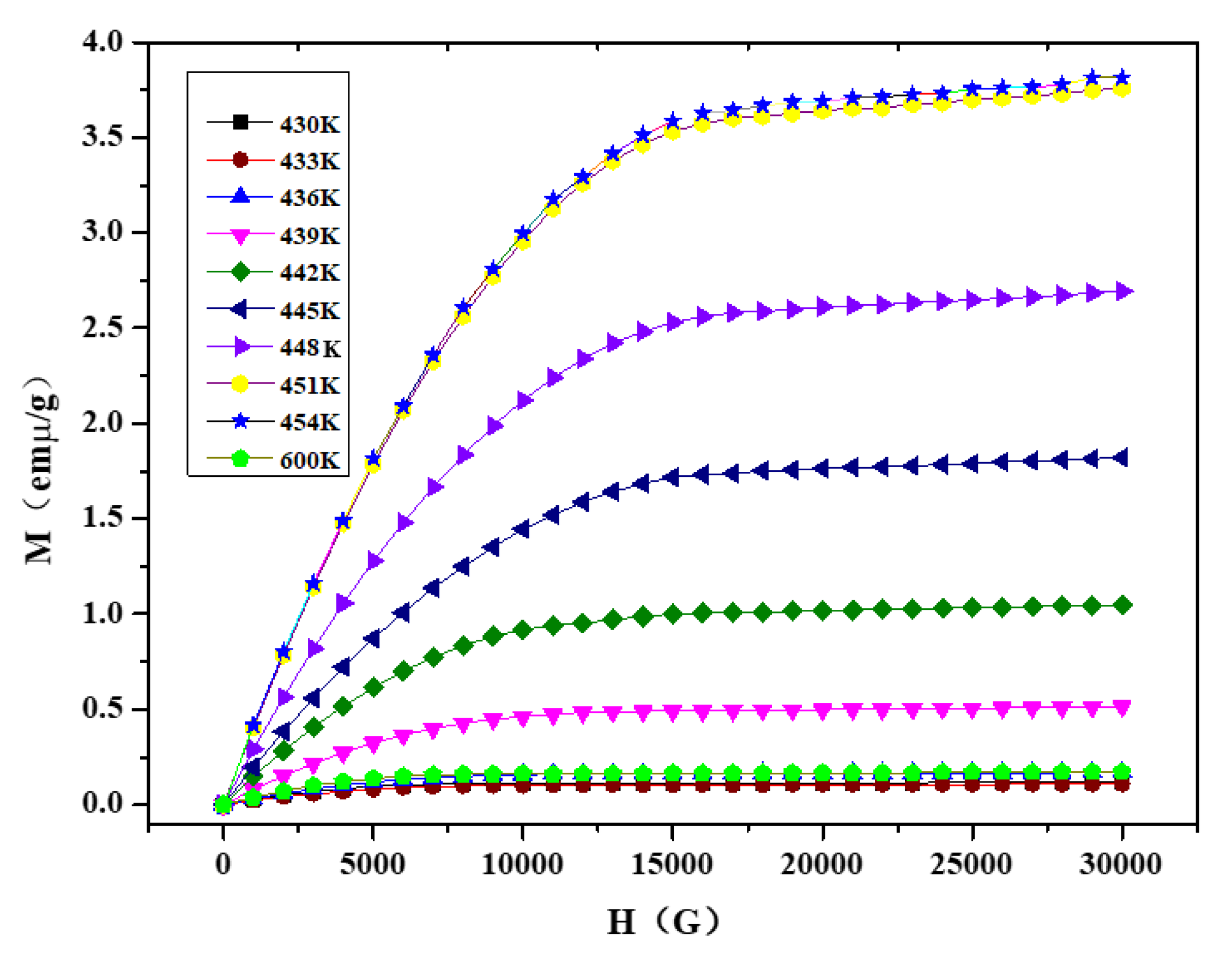
3.5. Magnetic Properties
3.5.1. Magnetic Properties of Ni2−xCoxIn3 (x = 0.200)
3.5.2. Magnetic Properties of Co1−xNixIn2 (x = 0.540)
4. Conclusions
- 1.
- There are eight Co-Ni-In binary compounds existing at 673 K and six binary compounds existing at 873 K. Compared to the phases at 673 K, the binary compounds Ni3In7, CoIn3, and CoIn2 disappeared, and ξ exists at 873 K.
- 2.
- The solid solubility range of Co1−xNixIn2 was found to be 0–18.64% at. % Ni at 673 K, while it shifted to the range of 3–20.1% at. % Ni at 873 K due to the absence of CoIn2 at 873 K.
- 3.
- The isothermal section of the phase diagram of the Co-Ni-In system at 673 K consists of 11 single-phase regions, 21 two-phase regions, and 9 three-phase regions, and the isothermal section at 873 K contains 8 single-phase regions, 16 two-phase regions, and 8 three-phase regions. Three-phase regions disappear due to the absence of the binary compounds Ni3In7 and CoIn3 at 873 K. The three-phase region Ni13 − xCoxIn9 (x = 0.702) + Ni1−xCoxIn2 (x = 0.091) + α-Ni1−xCox (x = 0.400) breaks into two three-phase regions due to the ξ phase existing at 873 K.
- 4.
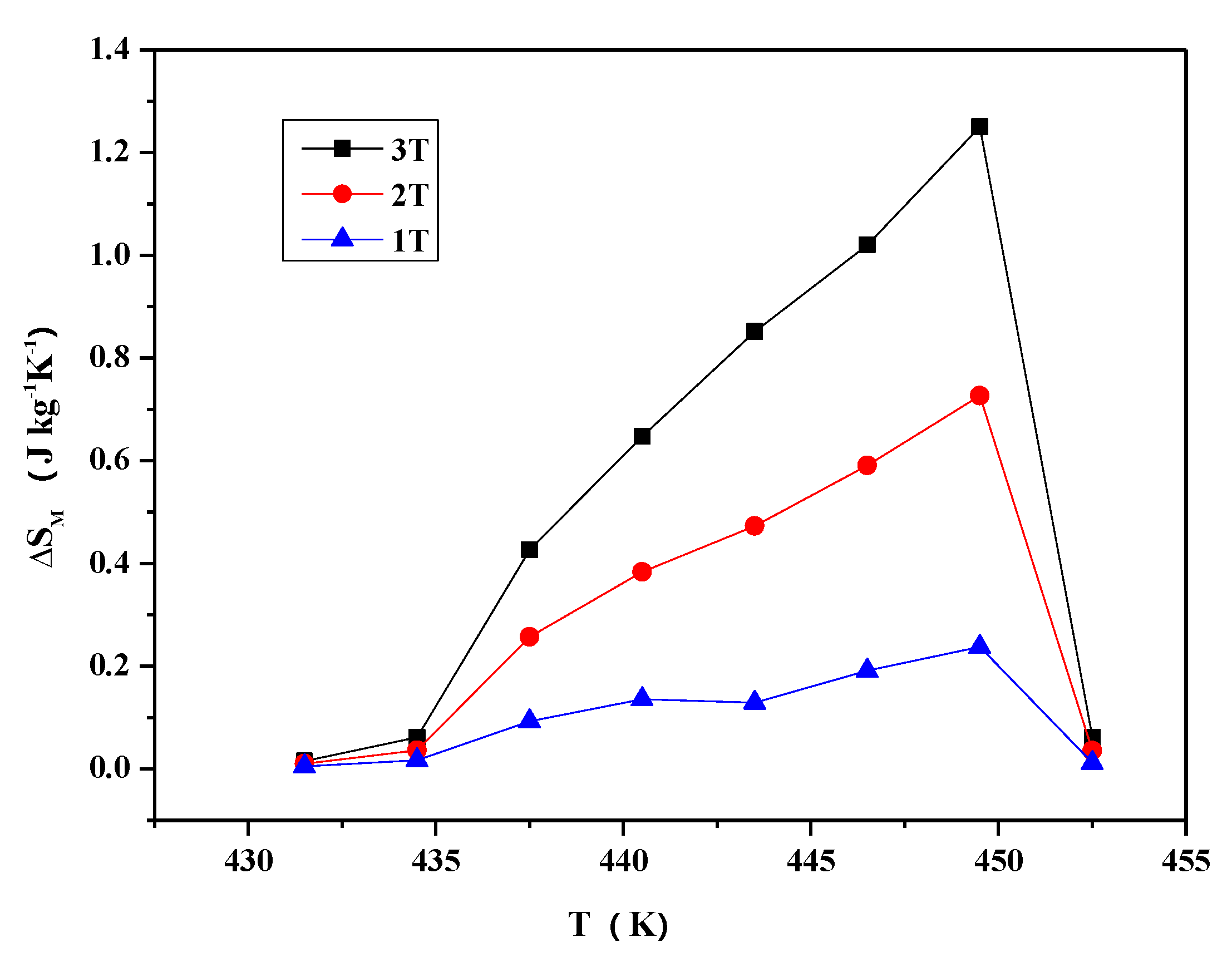
- The MT curves of Ni2−xCoxIn3 (x = 0.200) and Co1−xNixIn2 (x = 0.540), which are similar to the Ni-Mn-A (A = Ga, Sn, In), do not undergo martensitic transformation at temperatures, which results in a sharp change in magnetization. The maximum magnetic entropy changes of Ni2−xCoxIn3 (x = 0.200) and Co1−xNixIn2 (x = 0.540) under 3T in 430 K–454 K and 454 K–472 K are 1.25 J kg−1K−1, 1.475 J kg−1K−1, respectively.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Imano, Y.; Ito, W.; Sutou, Y.; Morito, H.; Okamoto, S.; Kitakami, O.; Oikawa, K.; Fujita, A.; Kanomata, T.; Ishida, K. Magnetic-field-induced shape recovery by reverse phase transformation. Nature 2006, 439, 957–960. [Google Scholar]
- Liu, J.; Gottschall, T.; Skokov, K.P.; James, D.M.; Oliver, G. Giant magnetocaloric effect driven by structural transitions. Nat. Mater. 2012, 11, 620–626. [Google Scholar] [CrossRef]
- Bonnot, E.; Romero, R.; Manosa, L.; Vives, E.; Planes, A. Elastocaloric effect associated with the martensitic transition in shape-memory alloys. Phys. Rev. Lett. 2008, 100, 125901. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.X.; Yu, S.Y.; Chen, J.L.; Cao, Z.X.; Wu, G.H. Giant magnetothermal conductivity in the Ni-Mn-In ferromagnetic shape memory alloys. Appl. Phys. Lett. 2007, 91, 012510. [Google Scholar] [CrossRef]
- Krenke, T.; Duman, E.; Acet, M.; Wassermann, E.F. Magnetic superelasticity and inverse magnetocaloric effect in Ni-Mn-In. Phys. Rev. B 2007, 75, 104414. [Google Scholar] [CrossRef]
- Mañosa, L.; González-Alonso, D.; Planes, A.; Bonnot, E.; Barrio, M.; Tamarit, J.; Aksoy, S.; Acet, M. Giant solid-state barocaloric effect in the Ni-Mn-In magnetic shape-memory alloy. Nat. Mater. 2010, 9, 478–481. [Google Scholar] [CrossRef]
- Wang, B.M.; Liu, Y.; Ren, P.; Xia, B.; Ruan, K.B.; Yi, J.B.; Ding, J.; Li, X.G.; Wang, L. Large Exchange Bias after Zero-Field Cooling from an Unmagnetized State. Phys. Rev. Lett. 2011, 106, 077203. [Google Scholar] [CrossRef]
- Ito, W.; Imano, Y.; Kainuma, R.; Sutou, Y.; Oikawa, K.; Ishida, K. Martensitic and Magnetic Transformation Behaviors in Heusler-Type NiMnIn and NiCoMnIn Metamagnetic Shape Memory Alloys. Metall. Mater. Trans. 2007, 38, 759–766. [Google Scholar] [CrossRef]
- Wu, Z.G.; Liu, Z.H.; Yang, H.; Liu, Y.N.; Wu, G.H. Metamagnetic phase transformation in Mn50Ni37In10Co3 polycrystalline alloy. Appl. Phys. Lett. 2011, 98, 061904. [Google Scholar] [CrossRef]
- Wu, Z.G.; Liu, Z.H.; Yang, H.; Liu, Y.N.; Wu, G.H. Effect of Co addition on martensitic phase transformation and magnetic properties of Mn50Ni40-xIn10Cox polycrystalline alloys. Intermetallics 2011, 19, 1839–1848. [Google Scholar] [CrossRef]
- He, X.J.; Wei, S.X.; Kang, Y.R.; Zhang, Y.L.; Cao, Y.M.; Xu, K.; Li, Z.; Jing, C. Enhanced barocaloric effect produced by hydrostatic pressure-induced martensitic transformation for Ni44.6Co5.5Mn35.5In14.4 Heusler alloy. Scr. Mater. 2018, 145, 58–61. [Google Scholar] [CrossRef]
- Zhang, X.X.; Qian, M.F.; Miao, S.P.; Su, R.Z.; Liu, Y.F.; Geng, L.; Sun, J.F. Enhanced magnetic entropy change and working temperature interval in Ni-Mn-In-Co alloys. J. Alloys Compd. 2016, 656, 154–158. [Google Scholar] [CrossRef]
- Monroe, J.A.; Karaman, I.; Basaran, B.; Ito, W.; Umetsu, R.Y.; Kainuma, R.; Koyama, K.; Chumlyakov, Y.I. Direct measurement of large reversible magnetic-field-induced strain in Ni-Co-Mn-In metamagnetic shape memory alloys. Acta Mater. 2012, 60, 6883–6891. [Google Scholar] [CrossRef]
- Liu, J.; Aksoy, S.; Scheerbaum, N.; Acet, M.; Gutfleisch, O. Large magnetostrain in polycrystalline Ni-Mn-In-Co. Appl. Phys. Lett. 2009, 95, 232515. [Google Scholar]
- Madiligama, A.S.B.; Ari-Gur, P.; Shavrov, V.G.; Koledov, V.V.; Calder, S.; Mashirov, A.V.; Kamantsev, A.P.; Dilmieva, E.T.; Gonzalez-Legarreta, L.; Grande, B.H.; et al. Crystalline structure and magnetic behavior of the Ni41Mn39In12Co8 alloy demonstrating giant magnetocaloric effect. Smart Mater. Struct. 2016, 25, 085013. [Google Scholar] [CrossRef]
- Gottschall, T.; Skokov, K.P.; Frincu, B.; Gutfleisch, O. Large reversible magnetocaloric effect in Ni-Mn-In-Co. Appl. Phys. Lett. 2015, 106, 021901. [Google Scholar] [CrossRef]
- Huang, L.; Cong, D.Y.; Ma, L.; Nie, Z.H.; Wang, Z.L.; Suo, H.L.; Ren, Y.; Wang, Y.D. Large reversible magnetocaloric effect in a Ni-Co-Mn-In magnetic shape memory alloy. Appl. Phys. Lett. 2016, 108, 032405. [Google Scholar] [CrossRef]
- Cheng, F.; Gao, L.M.; Wang, Y.; Wang, J.T.; Liao, X.Q.; Yang, S. Large refrigeration capacity in a Ni42Co8Mn37.7In12.3 magnetocaloric alloy. J. Magn. Magn. Mater. 2019, 478, 234–238. [Google Scholar] [CrossRef]
- Karaca, H.E.; Karaman, I.; Basaran, B.; Ren, Y.; Chumlyakov, Y.I.; Maier, H.J. Magnetic Field-Induced Phase Transformation in NiMnCoIn Magnetic Shape-Memory Alloys—A New Actuation Mechanism with Large Work Output. Adv. Funct. Mater. 2009, 19, 983–998. [Google Scholar] [CrossRef]
- Wang, Y.D.; Huang, E.W.; Ren, Y.; Nie, Z.H.; Wang, G.; Liu, Y.D.; Deng, J.N.; Choo, H.; Liaw, P.K.; Brown, D.E.; et al. In situ high-energy X-ray studies of magnetic-field-induced phase transition in a ferromagnetic shape memory Ni-Co-Mn-In alloy. Acta Mater. 2008, 56, 913–923. [Google Scholar] [CrossRef]
- Pérez-Landazábal, J.I.; Recarte, V.; Sánchez-Alarcos, V.; Gómez-Polo, C.; Kustov, S.; Cesari, E. Magnetic field induced martensitic transformation linked to the arrested austenite in a Ni-Mn-In-Co shape memory alloy. Appl. Phys. Lett. 2011, 109, 093515. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, H.; Xiao, F.; Bian, X.H.; Wang, P. Improvement of mechanical property and large shape recovery of sintered Ni45Mn36.6In13.4Co5 alloy. J. Alloys Compd. 2018, 765, 264–270. [Google Scholar] [CrossRef]
- Madiligama, A.; Ari-Gur, P.; Ren, Y.; Koledov, V.V.; Dilmieva, E.T.; Kamantsev, A.P.; Mashirov, A.V.; Shavrov, V.G.; Gonzalez-Legarreta, L.; Grande, B.H. Thermal and magnetic hysteresis associated with martensitic and magnetic phase transformations in Ni52Mn25In16Co7 Heusler alloy. J. Magn. Magn. Mater. 2017, 442, 25–35. [Google Scholar] [CrossRef]
- Turabi, A.S.; Karaca, H.E.; Tobe, H.; Basaran, B.; Aydogdu, Y.; Chumlyakov, Y.I. Shape memory effect and superelasticity of NiMnCoIn metamagnetic shape memory alloys under high magnetic field. Scr. Mater. 2016, 111, 110–113. [Google Scholar] [CrossRef]
- Ao, W.Q.; Liu, F.S.; Li, J.Q.; Du, Y.; Liu, F.L. Isothermal section of the Ni-Mn-In ternary system at 773 K. J. Alloys Compd. 2015, 622, 149–154. [Google Scholar] [CrossRef]
- Miyamoto, T.; Nagasako, M.; Kainuma, R. Phase equilibria in the Ni-Mn-In alloy system. J. Alloys Compd. 2013, 549, 57–63. [Google Scholar] [CrossRef]
- Gupta, K.P. The Co-Mn-Ni (Cobalt-Manganese-Nickel) system. J. Phase Equilib. 1999, 20, 527–532. [Google Scholar] [CrossRef]
- Zhou, Y.; Nash, P.; Dai, X.; Li, D.; Silva, A.; Cardoso, G. Phase equilibria in the Co-Mn-Ni system. J. Alloys Compd. 2018, 777, 1274–1285. [Google Scholar] [CrossRef]
- Nash, P.; Nash, A. Phase Diagrams of Binary Nickel Alloy; ASM International: Materials Park, OH, USA, 1991; p. 229. [Google Scholar]
- Okamoto, H. Co-In. J. Phase Equilib. Diffus. 1997, 18, 3. [Google Scholar]
- Durussel, P.; Burri, G.; Feschotte, E. The binary system Ni-In. J. Alloys Compd. 1997, 257, 253–258. [Google Scholar] [CrossRef]
- Bai, J.; Xu, N.; Raulot, J.M.; Zhang, Y.D.; Esling, C. First-principles investigations of crystallographic, magnetic, and electronic structures in Ni2XIn (X = Mn, Fe, and Co). Appl. Phys. 2012, 112, 114901. [Google Scholar] [CrossRef]
- Villars, P. Pearson’s Handbook, Desk Edition: Crystallographic Data for Intermetallic Phases; ASM International: Materials Park, OH, USA, 1997; p. 1. [Google Scholar]
- Roisnel, T.; Rodriguez-Carvajal, J. WinPLOTR: A windows tool for powder diffraction patterns analysis. Mater. Sci. 2001, 378–381, 118–123. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Schmetterer, C.; Zemanova, A.; Flandorfer, H. Phase equilibria in the ternary In-Ni-Sn system at 700 °C. Intermetallics 2013, 35, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Howe, R.A.; Enderby, J.E. The thermoelectric power of cerium, lanthanum, nickel and cobalt in the vicinity of the melting point. J. Phys. F Met. Phys. 1973, 3, 12–14. [Google Scholar] [CrossRef]
- Maira, G.K.; Orton, B.R.; Riviere, J.C. An XPS study of indium through the melting point. J. Phys. F Met. Phys. 1987, 17, 10. [Google Scholar] [CrossRef]
- Seguí, C.; Cesari, E.; Pons, J. Intermartensitic Transformations in Ni-Mn-Ga Alloys: A General View. Adv. Mater. Res. 2008, 52, 47–55. [Google Scholar] [CrossRef]
- Krenke, T.; Acet, M.; Wassermann, E.F. Ferromagnetism in the austenitic and martensitic states of Ni-Mn-In alloys. Phys. Rev. B 2006, 7, 174413. [Google Scholar] [CrossRef]
- Krenke, T.; Acet, M.; Wassermann, E.F. Martensitic transitions and the nature of ferromagnetism in the austenitic and martensitic states of Ni-Mn-Sn alloys. Phys. Rev. B 2005, 72, 014412. [Google Scholar] [CrossRef]







| Compounds | Structure Type | Space Group | Lattice Parameters (nm) | References | |||
|---|---|---|---|---|---|---|---|
| a | b | c | β(°) | ||||
| CoIn2 | CuMg2 | Fddd | 0.9402 | 0.5282 | 1.7846 | 90 | [30] |
| CoIn3 | Mg | P63/mmc | 0.6829 | 0.6829 | 0.7094 | 90 | [30] |
| Ni4In | W | Imm | 0.2929 | 0.2929 | 0.2929 | 90 | [33] |
| Ni3In | Ni3Sn | P63/mmc | 0.5320 | 0.5320 | 0.4242 | 90 | [31] |
| Ni3In | AuCu3 | Pmm | 0.3750 | 0.3750 | 0.3750 | 90 | [31] |
| ζ | NiAs | P63/mmc | 0.4189 | 0.4189 | 0.5123 | 90 | [31] |
| Ni2In | Ni2In | P63/mmc | 0.4179 | 0.4179 | 0.5131 | 90 | [31] |
| Ni13In9 | Ni13In9 | C2/m | 1.4646 | 0.8329 | 0.8977 | 35.35 | [31] |
| Ni13In7 | AsNi | P63/mmc | 0.4178 | 0.4178 | 0.5137 | 90 | [33] |
| NiIn | CoSn | P6/mmm | 0.5243 | 0.5243 | 0.4342 | 90 | [31] |
| NiIn | CsCl | Pmm | 0.3092 | 0.3092 | 0.3092 | 90 | [31] |
| Ni2In3 | Al3Ni2 | Pm1 | 0.4390 | 0.4390 | 0.5201 | 90 | [31] |
| Ni3In7 | Cu5Zn8 | I4m | 0.9180 | 0.9180 | 0.9180 | 90 | [33] |
| Ni2CoIn | BiF3 | Fmm | 0. 5944 | 0. 5944 | 0. 5944 | 90 | [32] |
| Phase Regions | Samples | Alloy Compositions | Phase Components |
|---|---|---|---|
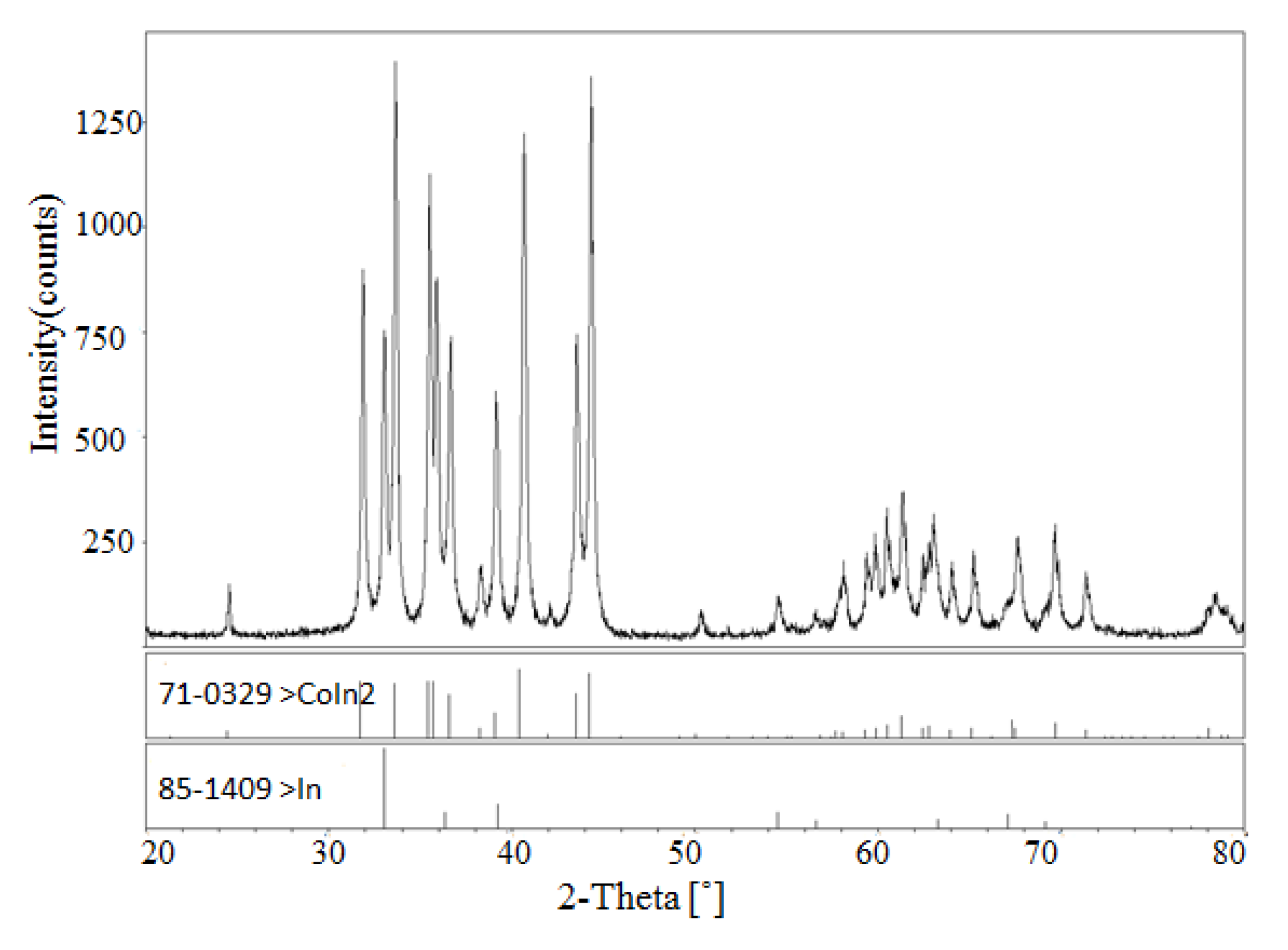
| 1 | No. 6 | Co10Ni68In22 | Ni2−xCoxIn(x = 0.091) + Ni3In+ α-Ni1−xCox (x = 0.400) |
| 2 | No. 7 | Co10Ni62In28 | Ni13 − xCoxIn9 (x = 0.702) + Ni2−xCoxIn (x = 0.091) + α-Ni1−xCox (x = 0.400) |
| 3 | No. 9 | Co20Ni45In35 | Ni13 − xCoxIn9 (x = 0.702) + Ni1−xCoxIn (x = 0.125) + α-Co1−xNix(x = 0.600) |
| 4 | No. 10 | Co10Ni40In50 | Ni1−xCoxIn (x = 0.125) + Ni2−xCoxIn3 (x = 0.400) + ε-Co1−xNix (x = 0.280) |
| 5 | No. 11 | Co40Ni20In40 | Co1−xNixIn2 (x = 0.565) + Ni2−xCoxIn3 (x = 0.400) + ε-Co1−xNix (x = 0.280) |
| 6 | No. 13 | Co30Ni40In30 | Ni1−xCoxIn (x = 0.125) + α-Co1−xNix (x = 0.600) +ε-Co1−xNix (x = 0.280) |
| 7 | No. 14 | Co10Ni24In66 | Co1−xNixIn2 (x = 0.565) + Ni2−xCoxIn3 (x = 0.400) + Ni3In7 |
| 8 | No. 15 | Co16Ni12In72 | Co1−xNixIn2 (x = 0.565) + CoIn3 + Ni3In7 |
| 9 | No. 16 | Co10Ni12In78 | CoIn3 + Ni3In7 + In (Liquid) |
| Phase Regions | Samples | Alloy Compositions | Phase Components |
|---|---|---|---|
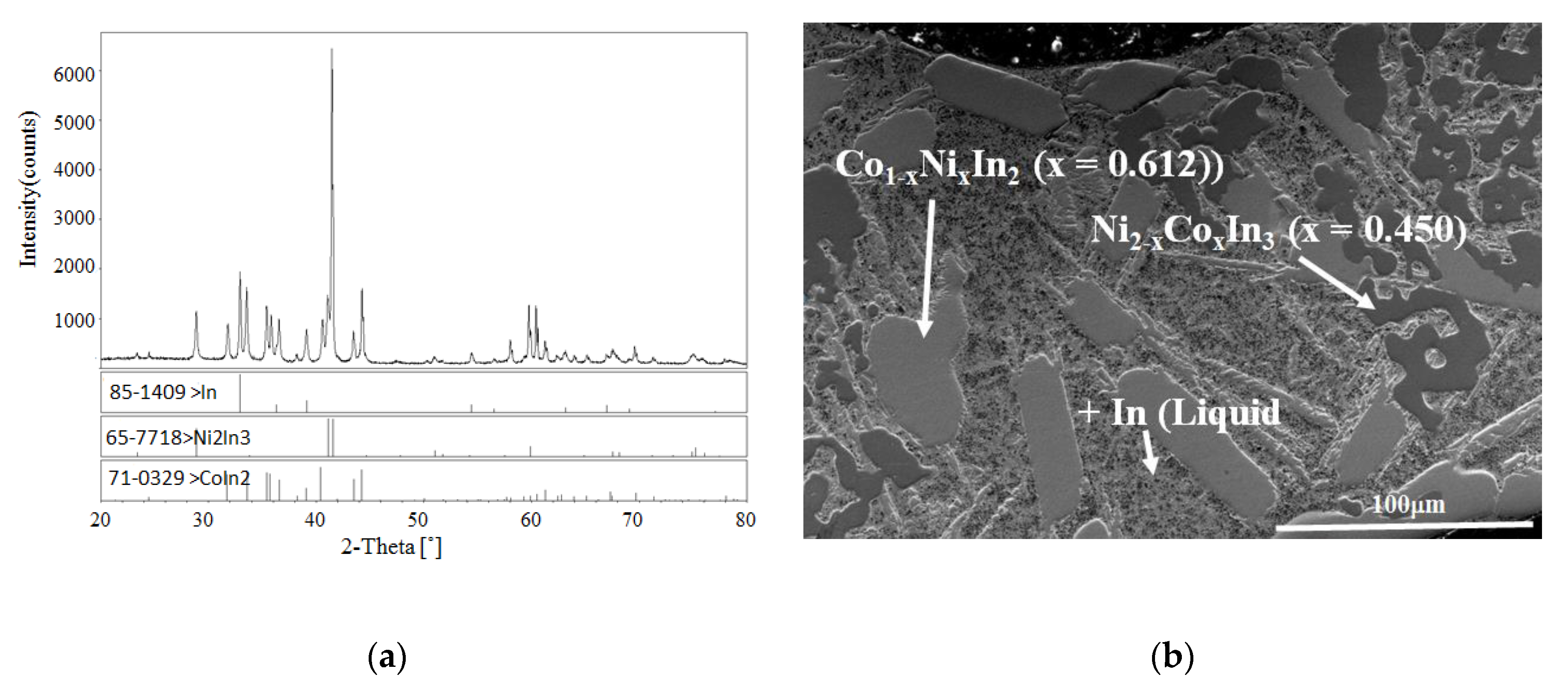
| I | No. 26 | Co28Ni24In48 | Co1−xNixIn2 (x = 0.612) + Ni2−xCoxIn3 (x = 0.450) + α-Co1−xNix (x = 0.200) |
| II | No. 28 | Co8Ni22In70 | Co1−xNixIn2 (x = 0.612) + Ni2−xCoxIn3 (x = 0.450) + In (Liquid) |
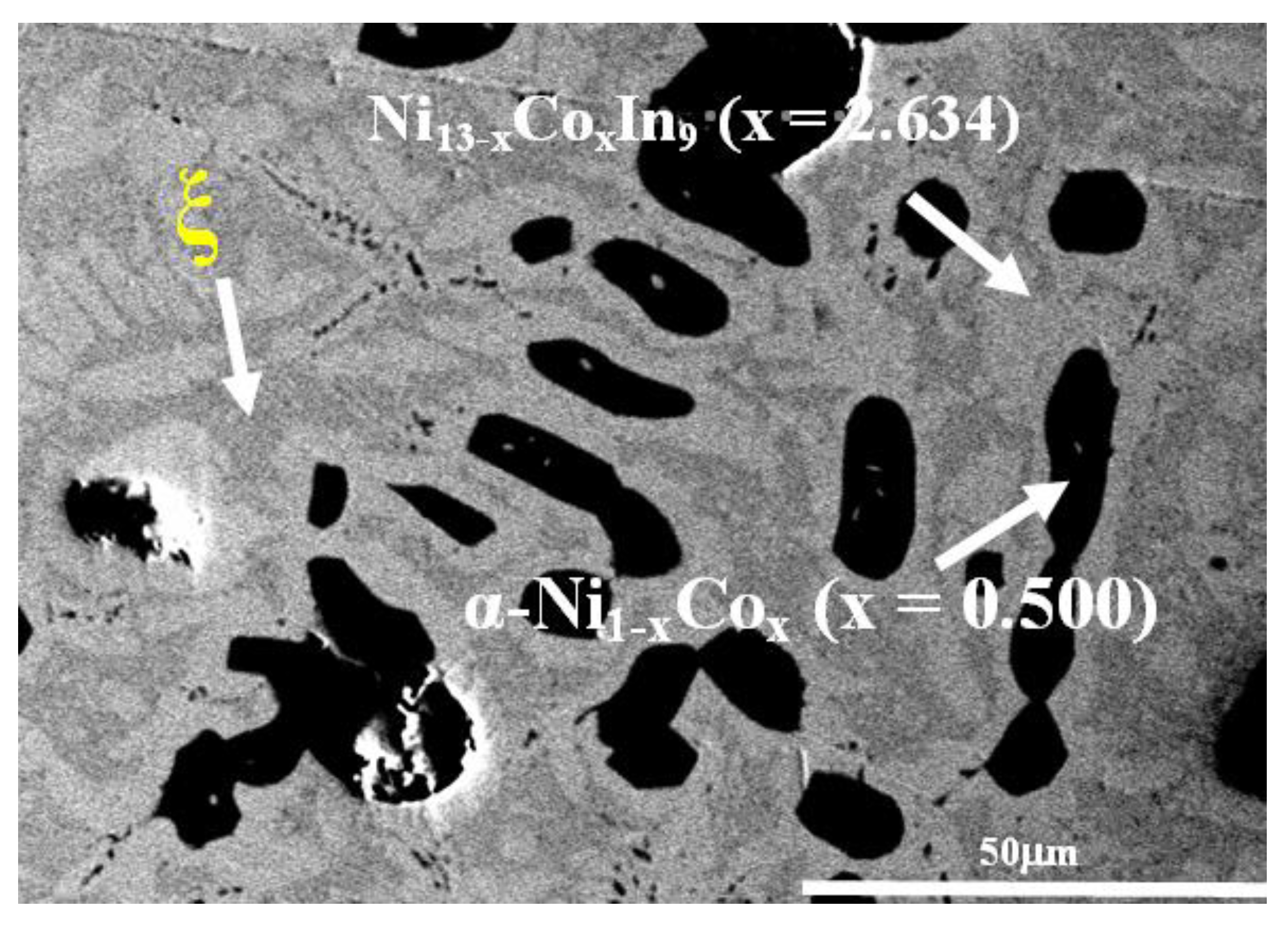
| III | No. 28 | Co18Ni52In30 | ξ + Ni13 − xCoxIn9 (x = 2.634) + α-Ni1−xCox (x = 0.500) |
| IV | No. 30 | Co20Ni45In35 | Ni13 − xCoxIn9 (x = 2.634)+ Ni1−xCoxIn (x = 0.160) + α-Ni1−xCox (x = 0.600) |
| V | No. 31 | Co14Ni66In20 | Ni3In + Ni2−xCoxIn (x = 0.181) + α-Ni1−xCox (x = 0.400) |
| VI | No. 32 | Co12Ni58In30 | Ni2−xCoxIn (x = 0.181) + ξ + α-Ni1−xCox (x = 0.500) |
| VII | No. 33 | Co20Ni34In46 | Ni1−xCoxIn (x = 0.160) + Ni2−xCoxIn3 (x = 0.450) + α-Co1−xNix (x = 0.200) |
| VIII | Co1−xNixIn2 (x = 0.091) + α-Co + In (Liquid) |
| Compound | Phase | Structure Type | Space Group | Lattice Parameters (nm) | Atomic Parameters | Reliability Factors | Goodness of Fit | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | Atoms | Wyck (x, y, z) | Occ | R p(%) | R exp(%) | S (%) | ||||
| Ni2−xCoxIn3(x = 0.200) | Ni2−xCoxIn3 (x = 0.200) | Al2Ni3 | Pm1 (164) | 0.43959(5) | 0.43959(5) | 0.53121(1) | In1 | 1a (0,0,0) | 1 | 10.46 | 14.15 | 3.2 |
| In2 | 2d (1/3, 2/3, 0.6469(3)) | 1 | ||||||||||
| M1 = (Co, Ni) | 2d (0, 0, 0.1381(2)) | 0.1Co + 0.9 Ni | ||||||||||
| Co1−xNixIn2(x = 0.540) | Co1−xNixIn2(x = 0.540) | CuMg2 | Fddd (70) | 0.9424(3) | 0.5288(4) | 1.7742(5) | In1 | 16e (0.9648(2), 1/4,1/4) | 1 | 10.29 | 13.26 | 2.9 |
| M2 = (Co, Ni) | 16g (1/4, 0.9963(7), 1/4) | 0.466Co + 0.534Ni | ||||||||||
| In2 | 16g (1/4, 0.7120(5), 1/4) | 1 | ||||||||||
| Co1−xNixIn2 (x = 0.580) | Co1−xNixIn2(x = 0.565) | CuMg2 | Fddd (70) | 0.9421(2) | 0.5282(3) | 1.7739(3) | In1 | 16e (0.9642(3), 1/4,1/4) | 1 | 9.89 | 12.58 | 2.7 |
| M3 = (Co, Ni) | 16g (1/4, 0.9962(2), 1/4) | 0.438Co + 0.562Ni | ||||||||||
| In2 | 16g (1/4, 0.7131(4), 1/4) | 1 | ||||||||||
| Ni2−xCoxIn3 (x = 0.400) | Al3Ni2 | Pm1 (164) | 0.4397(1) | 0.4397(1) | 0.5319(3) | In1 | 1a (0 0 0) | 1 | 9.89 | 12.58 | 2.7 | |
| In2 | 2d (1/3, 2/3, 0.6412(2)) | 1 | ||||||||||
| M4 = (Co, Ni) | 2d (0, 0, 0.1350) | 0.8Co + 0.2Ni | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; He, W.; Chen, G.; Zeng, W.; Wang, J.; Zeng, L.; Liang, J. The Phase Relations of the Co-Ni-In Ternary System at 673 K and 873 K and Magnetic Properties of Their Compounds. Materials 2020, 13, 3990. https://doi.org/10.3390/ma13183990
Yang T, He W, Chen G, Zeng W, Wang J, Zeng L, Liang J. The Phase Relations of the Co-Ni-In Ternary System at 673 K and 873 K and Magnetic Properties of Their Compounds. Materials. 2020; 13(18):3990. https://doi.org/10.3390/ma13183990
Chicago/Turabian StyleYang, Tonghan, Wei He, Guojian Chen, Weijing Zeng, Jinzhi Wang, Lingmin Zeng, and Jianlie Liang. 2020. "The Phase Relations of the Co-Ni-In Ternary System at 673 K and 873 K and Magnetic Properties of Their Compounds" Materials 13, no. 18: 3990. https://doi.org/10.3390/ma13183990
APA StyleYang, T., He, W., Chen, G., Zeng, W., Wang, J., Zeng, L., & Liang, J. (2020). The Phase Relations of the Co-Ni-In Ternary System at 673 K and 873 K and Magnetic Properties of Their Compounds. Materials, 13(18), 3990. https://doi.org/10.3390/ma13183990




