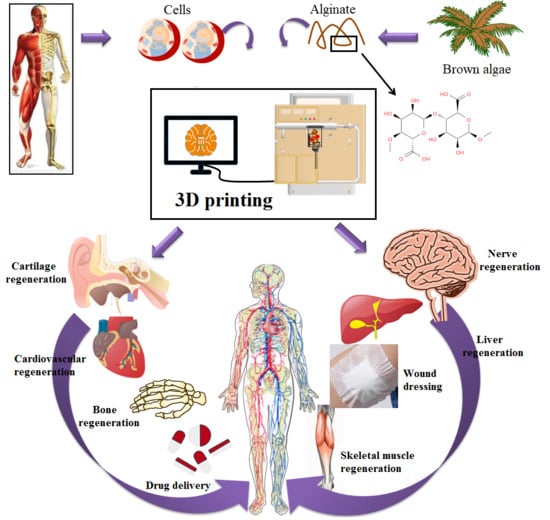
Recent Trends in Three-Dimensional Bioinks Based on Alginate for Biomedical Applications
Abstract
1. Introduction
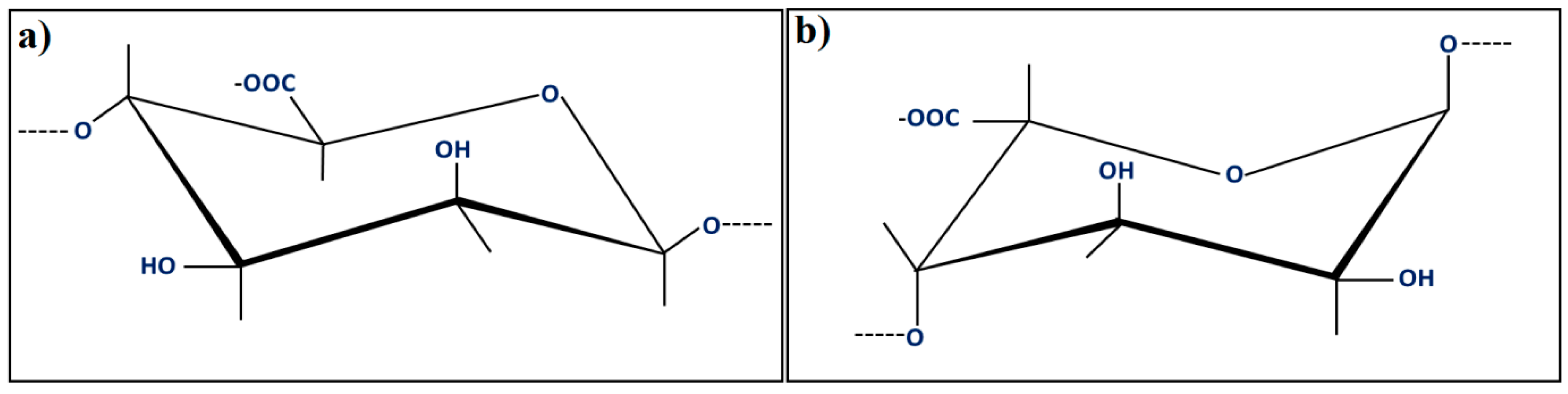
2. Alginate (Alg) as a Printable Material
3. Applications in Biomedical Field
3.1. Bone Regeneration
3.2. Cartilage Regeneration
3.3. Cardiovascular System Formation
3.4. Regeneration of Other Tissues
4. Wound Dressing
5. Drug Delivery
6. Limitations, Advantages and Future Prospects of Alg 3D Printing
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAlg | Aminated alginate |
| AlgDA | Alginate dialdehyde |
| AG | Agarose |
| Alg | Alginate |
| AlgGO | Alginate gel bead containing vegetable oil |
| ALP | Alkaline phosphatase |
| AM | Additive manufacturing |
| BFP1 | Bone formation peptide-1 |
| BLM | Bilayer membrane |
| BMCs | Bone marrow cells |
| BSA | Bovine serum albumin |
| BTE | Bone tissue engineering |
| CAlg | Calcium alginate |
| CaGlu | Calcium gluconate |
| CMC | Carboxymethyl cellulose |
| COL | Collagen |
| CP | Calcium phosphate |
| CPC | Calcium phosphate cement |
| CS | Chitosan |
| CTE | Cardiac tissue engineering |
| CTTE | Cartilage tissue engineering |
| ECM | Extracellular matrix |
| FDM | Fused deposition modeling |
| GelMA | Gelatin methacryloyl |
| GL | Gelatin |
| GO | Graphene oxide |
| GRGDSP | Glycine–arginine–glycine–aspartic acid–serine–proline |
| HA | Hyaluronic acid |
| HAp | Hydroxyapatite |
| hBMSCs | Human bone mesenchymal stem cells |
| HDF | Primary human dermal fibroblast cells |
| HEK | Human embryonic kidney |
| HOS | Human osteosarcoma cell lines |
| HNTs | Halloysite nanotube |
| HPMC | Hydroxypropylmethylcellulose |
| HSFs | Human skin fibroblasts |
| HUCMSCs | Human umbilical cord mesenchymal stem cells |
| HUVSMCs | Human umbilical vein smooth muscle cells |
| MSCs | Mesenchymal stem cells |
| MTX | Methotrexate |
| MZ | Metronidazole |
| NFC | Nano fibrillated cellulose |
| NHDF | Normal human dermal fibroblasts |
| NPCs | Neural progenitor cells |
| PC | Pectin |
| PEO | Poly(ethylene oxide) |
| PEGTA | Poly (ethylene glycol)-tetra-acrylate |
| PGRN | Progranulin |
| PLGA | Poly (lactide-co-glycolide) |
| PNIPAAm | Poly (N-isopropylacrylamide) |
| PVA | Poly (vinyl alcohol) |
| PVP | Polyvinyl pyrrolidone |
| rGO | reduced graphene oxide |
| Na-Alg | Sodium alginate |
| SIM | Simvastatin |
| SLS | Selective laser sintering |
| SMC | Smooth muscle cells |
| SMHs | Shape memory hydrogel |
| 5-FU | 5-fluorouracil |
| TE | Tissue engineering |
| TGF-1 | Transforming growth factor 1 |
| TIJ | Thermal inkjet |
| (TNF)-α | Tumor necrosis factor |
| UV light | Ultraviolet light |
| VIC | Valve leaflet interstitial cells |
| 3D | Three-dimensional |
References
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Oyen, M.L. Applications of Alg-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.C.; Smith, D.K. Multicomponent polysaccharide alginate-based bioinks. J. Mater. Chem B 2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Nam, S.Y. Assessment of coaxial printability for extrusion-based bioprinting of alginate-based tubular constructs. Bioprinting 2020, 4, e00092. [Google Scholar] [CrossRef]
- Schubert, C.; Van Langeveld, M.C.; Donoso, L.A. Innovations in 3D printing: A 3D overview from optics to organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef]
- Banks, J. Adding value in additive manufacturing: Researchers in the United Kingdom and Europe look to 3D printing for customization. IEEE Pulse 2013, 4, 22–26. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical applications for 3D printing: Current and projected uses. Pharm. Ther. 2014, 39, 704. [Google Scholar]
- Marga, F.; Jakab, K.; Khatiwala, C.; Shepherd, B.; Dorfman, S.; Hubbard, B.; Colbert, S.; Forgacs, G. Toward engineering functional organ modules by additive manufacturing. Biofabrication 2012, 4, 022001. [Google Scholar] [CrossRef]
- Chung, J.H.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef]
- Cantley, W.L.; Du, C.; Lomoio, S.; DePalma, T.; Peirent, E.; Kleinknecht, D.; Hunter, M.; Tang-Schomer, M.D.; Tesco, G.; Kaplan, D.L. Functional and sustainable 3D human neural network models from pluripotent stem cells. Acs Biomater. Sci. Eng. 2018, 4, 4278–4288. [Google Scholar] [CrossRef]
- Song, S.J.; Choi, J.; Park, Y.D.; Hong, S.; Lee, J.J.; Ahn, C.B.; Choi, H.; Sun, K. Sodium alginate hydrogel-based bioprinting using a novel multinozzle bioprinting system. Artif. Organs. 2011, 35, 1132–1136. [Google Scholar] [CrossRef]
- Wasko, J.; Fraczyk, J.; Becht, A.; Kaminski, Z.J.; Flinčec Grgac, S.; Tarbuk, A.; Kaminska, M.; Dudek, M.; Gliscinska, E.; Draczynski, Z.; et al. Conjugates of chitosan and calcium alginate with oligoproline and oligohydroxyproline derivatives for potential use in regenerative medicine. Materials 2020, 13, 3079. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B. Improving sodium alginate films properties by phenolic acid addition. Materials 2020, 13, 2895. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, A.; Karewicz, A.; Karnas, K.; Wolski, K.; Zając, M.; Kamiński, K.; Szczubiałka, K.; Zapotoczny, S.; Nowakowska, M. Pioglitazone-loaded nanostructured hybrid material for skin ulcer treatment. Materials 2020, 13, 2050. [Google Scholar] [CrossRef]
- Elsayed, E.M.S.; Elnouby, M.; Gouda, M.H.; Elessawy, N.A.; Santos, D.M.F. Effect of the morphology of tungsten oxide embedded in sodium alginate/polyvinylpyrrolidone composite beads on the photocatalytic degradation of methylene blue dye solution. Materials 2020, 13, 1905. [Google Scholar] [CrossRef]
- Parmentier, L.; Riffault, M.; Hoey, D.A. Utilizing osteocyte derived factors to enhance cell viability and osteogenic matrix deposition within IPN hydrogels. Materials 2020, 13, 1690. [Google Scholar] [CrossRef] [PubMed]
- Vasile, B.S.; Birca, A.C.; Musat, M.C.; Holban, A.M. Wound dressings coated with silver nanoparticles and essential oils for the management of wound infections. Materials 2020, 13, 1682. [Google Scholar] [CrossRef]
- Norambuena-Contreras, J.; Arteaga-Perez, L.E.; Guadarrama-Lezama, A.Y.; Briones, R.; Vivanco, J.F.; Gonzalez-Torre, I. Microencapsulated bio-based rejuvenators for the self-healing of bituminous materials. Materials 2020, 13, 1446. [Google Scholar] [CrossRef]
- Larraza, I.; Ugarte, L.; Fayanas, A.; Gabilondo, N.; Arbelaiz, A.; Corcuera, M.A.; Eceiza, A. Influence of process parameters in graphene oxide obtention on the properties of mechanically strong alginate nanocomposites. Materials 2020, 13, 1081. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Mochane, M.J.; Mtibe, A.; John, M.J.; Sadiku, E.R.; Sefadi, J.S. Electrospun alginate nanofibers toward various applications: A review. Materials 2020, 13, 934. [Google Scholar] [CrossRef]
- Araiza-Verduzco, F.; Rodríguez-Velázquez, E.; Cruz, H.; Rivero, I.A.; Acosta-Martínez, D.R.; Pina-Luis, G.; Alatorre-Meda, M. Photocrosslinked alginate-methacrylate hydrogels with modulable mechanical properties: Effect of the molecular conformation and electron density of the methacrylate reactive group. Materials 2020, 13, 534. [Google Scholar] [CrossRef] [PubMed]
- Carrow, J.K.; Kerativitayanan, P.; Jaiswal, M.K.; Lokhande, G.; Gaharwar, A.K. Polymers for bioprinting. In Essentials of 3D Biofabrication and Translation; Academic Press: Winston-Salem, NC, USA, 2015; pp. 229–248. [Google Scholar]
- Das, D.; Zhang, S.; Noh, I. Synthesis and characterizations of alginate-α-tricalcium phosphate microparticle hybrid film with flexibility and high mechanical property as a biomaterial. Biomed. Mater. 2018, 13, 025008. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R.; et al. Engineering Alg as bioink for bioprinting. Acta Biomater. 2014, 10, 4323–4331. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Y.; Qin, X.; Wa, Q. 3D printing of concentrated Alg/gelatin scaffolds with homogeneous nano apatite coating for bone tissue engineering. Mater. Des. 2018, 146, 12–19. [Google Scholar] [CrossRef]
- Egorov, A.A.; Fedotov, A.Y.; Mironov, A.V.; Komlev, V.S.; Popov, V.K.; Zobkov, Y.V. 3D printing of mineral–polymer bone substitutes based on sodium alginate and calcium phosphate. Beilstein J. Nanotechnol. 2016, 7, 1794–1799. [Google Scholar] [CrossRef]
- Luo, G.; Ma, Y.; Cui, X.; Jiang, L.; Wu, M.; Hu, Y.; Luo, Y.; Pan, H.; Ruan, C. 13–93 bioactive glass/alginate composite scaffolds 3D printed under mild conditions for bone regeneration. Rsc Adv. 2017, 7, 11880–11889. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef]
- Fraczyk, J.; Wasko, J.; Walczak, M.; Kaminski, Z.J.; Puchowicz, D.; Kaminska, I.; Bogun, M.; Kolasa, M.; Stodolak-Zych, E.; Scislowska-Czarnecka, A.; et al. Conjugates of copper alginate with arginine-glycine-aspartic acid (RGD) for potential use in regenerative medicine. Materials 2020, 13, 337. [Google Scholar] [CrossRef]
- Lovskaya, D.; Menshutina, N. Alginate-based aerogel particles as drug delivery systems: Investigation of the supercritical adsorption and in vitro evaluations. Materials 2020, 13, 329. [Google Scholar] [CrossRef]
- Moreno Rivas, S.C.; Armenta Corral, R.I.; Frasquillo Félix, M.C.; Islas Rubio, A.R.; Vázquez Moreno, L.; Ramos-Clamont Montfort, G. Removal of cadmium from aqueous solutions by saccharomyces cerevisiae–alginate system. Materials 2019, 12, 4128. [Google Scholar] [CrossRef] [PubMed]
- Pavli, F.; Argyri, A.A.; Skandamis, P.; Nychas, G.-J.; Tassou, C.; Chorianopoulos, N. Antimicrobial activity of oregano essential oil incorporated in sodium alginate edible films: Control of listeria monocytogenes and spoilage in ham slices treated with high pressure processing. Materials 2019, 12, 3726. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Park, H.; Park, Y.; Kang, H.; Lee, D.; Luo, Y.; Liu, H.; Yang, S.; Zeng, J.; Wu, Z. Sodium alginate-based green packaging films functionalized by guava leaf extracts and their bioactivities. Materials 2019, 12, 2923. [Google Scholar]
- Sabater ISerra, R.; Molina-Mateo, J.; Torregrosa-Cabanilles, C.; Andrio-Balado, A.; Meseguer Dueñas, J.M.; Serrano-Aroca, Á.; Ma, X.; He, L.; Wan, X.; Xiang, S.; et al. Reversible mechanical regulation and splicing ability of alginate-based gel based on photo-responsiveness of molecular-level conformation. Materials 2019, 12, 2919. [Google Scholar]
- Bociaga, D.; Bartniak, M.; Grabarczyk, J.; Przybyszewska, K. Sodium alginate/gelatine hydrogels for direct bioprinting—The effect of composition selection and applied solvents on the bioink properties. Materials 2019, 12, 2669. [Google Scholar] [CrossRef]
- Kotroni, E.; Simirioti, E.; Kikionis, S.; Sfiniadakis, I.; Siamidi, A.; Karalis, V.; Vitsos, A.; Vlachou, M.; Ioannou, E.; Roussis, V.; et al. In Vivo evaluation of the anti-inflammatory activity of electrospun micro/nanofibrous patches loaded with pinus halepensis bark extract on hairless mice skin. Materials 2019, 12, 2596. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Dalla Rosa, M.; Siracusa, V. Characterization of composite edible films based on pectin/alginate/whey protein concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef]
- Dedloff, M.R.; Effler, C.S.; Holban, A.M.; Gestal, M.C. Use of Biopolymers in Mucosally-Administered Vaccinations for Respiratory Disease. Materials 2019, 12, 2445. [Google Scholar] [CrossRef]
- Skaugrud, O.; Hagen, A.; Borgersen, B.; Dornish, M. Biomedical and pharmaceutical applications of alginate and chitosan. Biotechnol. Genet. Eng. 1999, 16, 23–40. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019, 207, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Weir, M.D.; Xu, H.H. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010, 31, 6502–6510. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, A.; Ansari, S.; Chen, C.; Xu, X.; Akiyama, K.; Snead, M.L.; Zadeh, H.H.; Shi, S. Co-encapsulation of anti-BMP2 monoclonal antibody and mesenchymal stem cells in alginate microspheres for bone tissue engineering. Biomaterials 2013, 34, 6572–6579. [Google Scholar] [CrossRef]
- Alsberg, E.; Anderson, K.W.; Albeiruti, A.; Franceschi, R.T.; Mooney, D.J. Cell-interactive alginate hydrogels for bone tissue engineering. J. Dent. Res. 2001, 80, 2025–2029. [Google Scholar] [CrossRef]
- Wang, L.; Shelton, R.M.; Cooper, P.R.; Lawson, M.; Triffitt, J.T.; Barralet, J.E. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials 2003, 24, 3475–3481. [Google Scholar] [CrossRef]
- Jin, H.H.; Kim, D.H.; Kim, T.W.; Shin, K.K.; Jung, J.S.; Park, H.C.; Yoon, S.Y. In vivo evaluation of porous hydroxyapatite/chitosan–alginate composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2012, 51, 1079–1085. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, H.H. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials 2011, 32, 7503–7513. [Google Scholar] [CrossRef]
- Kim, H.L.; Jung, G.Y.; Yoon, J.H.; Han, J.S.; Park, Y.J.; Kim, D.G.; Zhang, M.; Kim, D.J. Preparation and characterization of nano-sized hydroxyapatite/alginate/chitosan composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 54, 20–25. [Google Scholar] [CrossRef]
- Sharma, C.; Dinda, A.K.; Potdar, P.D.; Chou, C.F.; Mishra, N.C. Fabrication and characterization of novel nano-biocomposite scaffold of chitosan–gelatin–alginate–hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C. 2016, 64, 416–427. [Google Scholar] [CrossRef]
- Sowjanya, J.A.; Singh, J.; Mohita, T.; Sarvanan, S.; Moorthi, A.; Srinivasan, N.; Selvamurugan, N. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Colloids Surf. B 2013, 109, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M.; Qanadilo, H.F.; Langer, R.; Shastri, V.P. A rapid-curing alginate gel system: Utility in periosteum-derived cartilage tissue engineering. Biomaterials 2004, 25, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Yang, K.C.; Lin, K.H.; Liu, H.C.; Lin, F.H. A highly organized three-dimensional alginate scaffold for cartilage tissue engineering prepared by microfluidic technology. Biomaterials 2011, 32, 7118–7126. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, E.; Cristallini, C.; Barbani, N.; Vozzi, G.; Giusti, P. Preparation and characterization of alginate/gelatin blend films for cardiac tissue engineering. J. Biomed. Mater. Res. A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 91, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Shachar, M.; Tsur-Gang, O.; Dvir, T.; Leor, J.; Cohen, S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2011, 7, 152–162. [Google Scholar] [CrossRef]
- Dvir-Ginzberg, M.; Gamlieli-Bonshtein, I.; Agbaria, R.; Cohen, S. Liver tissue engineering within alginate scaffolds: Effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue Eng. 2003, 9, 757–766. [Google Scholar] [CrossRef]
- Majima, T.; Funakosi, T.; Iwasaki, N.; Yamane, S.T.; Harada, K.; Nonaka, S.; Minami, A.; Nishimura, S.I. Alginate and chitosan polyion complex hybrid fibers for scaffolds in ligament and tendon tissue engineering. J. Orthop. Sci. 2005, 10, 302–307. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Ma, G.; Fang, D.; Liu, Y.; Zhu, X.; Nie, J. Electrospun sodium alginate/poly (ethylene oxide) core–shell nanofibers scaffolds potential for tissue engineering applications. Carbohydr. Polym. 2012, 87, 737–743. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Maintaining dimensions and mechanical properties of ionically crosslinked alginate hydrogel scaffolds in vitro. J. Biomed. Mater. Res. A An. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 84, 899–907. [Google Scholar]
- Tan, R.; She, Z.; Wang, M.; Fang, Z.; Liu, Y.; Feng, Q. Thermo-sensitive alginate-based injectable hydrogel for tissue engineering. Carbohydr. Polym. 2012, 87, 1515–1521. [Google Scholar] [CrossRef]
- Jeong, S.I.; Krebs, M.D.; Bonino, C.A.; Samorezov, J.E.; Khan, S.A.; Alsberg, E. Electrospun chitosan–alginate nanofibers with in situ polyelectrolyte complexation for use as tissue engineering scaffolds. Tissue Eng. Part A 2011, 17, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Jeong, S.I.; Krebs, M.D.; Bonino, C.A.; Khan, S.A.; Alsberg, E. Electrospun alginate nanofibers with controlled cell adhesion for tissue engineering. Macromol. Biosci. 2010, 10, 934–943. [Google Scholar] [CrossRef]
- Liu, M.; Dai, L.; Shi, H.; Xiong, S.; Zhou, C. In Vitro evaluation of alginate/halloysite nanotube composite scaffolds for tissue engineering. Mater. Sci. Eng. C 2015, 49, 700–712. [Google Scholar] [CrossRef]
- Ashton, R.S.; Banerjee, A.; Punyani, S.; Schaffer, D.V.; Kane, R.S. Scaffolds based on degradable alginate hydrogels and poly (lactide-co-glycolide) microspheres for stem cell culture. Biomaterials 2007, 28, 5518–5525. [Google Scholar] [CrossRef]
- Dobie, K.; Smith, G.; Sloan, A.J.; Smith, A.J. Effects of alginate hydrogels and TGF-β1 on human dental pulp repair in vitro. Connect. Tissue Res. 2002, 43, 387–390. [Google Scholar] [CrossRef]
- Lee, G.S.; Park, J.H.; Shin, U.S.; Kim, H.W. Direct deposited porous scaffolds of calcium phosphate cement with alginate for drug delivery and bone tissue engineering. Acta Biomater. 2011, 7, 3178–3186. [Google Scholar] [CrossRef]
- Chan, A.W.; Whitney, R.A.; Neufeld, R.J. Semisynthesis of a controlled stimuli-responsive alginate hydrogel. Biomacromolecules 2009, 10, 609–616. [Google Scholar] [CrossRef]
- Rastogi, R.; Sultana, Y.; Aqil, M.; Ali, A.; Kumar, S.; Chuttani, K.; Mishra, A.K. Alginate microspheres of isoniazid for oral sustained drug delivery. Int. J. Pharm. 2007, 334, 71–77. [Google Scholar] [CrossRef]
- Xu, Y.; Zhan, C.; Fan, L.; Wang, L.; Zheng, H. Preparation of dual crosslinked alginate–chitosan blend gel beads and in vitro controlled release in oral site-specific drug delivery system. Int. J. Pharm. 2007, 336, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Narayana, S.G.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [CrossRef]
- Murata, Y.; Sasaki, N.; Miyamoto, E.; Kawashima, S. Use of floating alginate gel beads for stomach-specific drug delivery. Eur. J. Pharm. Biopharm. 2000, 50, 221–226. [Google Scholar] [CrossRef]
- Moebus, K.; Siepmann, J.; Bodmeier, R. Alginate–poloxamer microparticles for controlled drug delivery to mucosal tissue. Eur. J. Pharm. Biopharm. 2009, 72, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Zhang, X.C.; Zhou, F.Z.; Zhang, X.Z.; Cheng, S.X.; Zhuo, R.X. Sustained release of antineoplastic drugs from chitosan-reinforced alginate microparticle drug delivery systems. Int. J. Pharm. 2008, 357, 15–21. [Google Scholar] [CrossRef]
- Dai, Y.N.; Li, P.; Zhang, J.P.; Wang, A.Q.; Wei, Q. A novel pH sensitive N-succinyl chitosan/ alginate hydrogel bead for nifedipine delivery. Biopharm. Drug Dispos. 2008, 29, 173–184. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Gao, Q.; Liu, X.; Tong, Z. Fabrication of novel core-shell hybrid alginate hydrogel beads. Int. J. Pharm. 2008, 351, 104–112. [Google Scholar] [CrossRef]
- Giunchedi, P.; Gavini, E.; Moretti, M.D.; Pirisino, G. Evaluation of alginate compressed matrices as prolonged drug delivery systems. Aaps Pharm. Sci. Tech. 2000, 1, 31–36. [Google Scholar] [CrossRef]
- Hashimoto, T.; Suzuki, Y.; Tanihara, M.; Kakimaru, Y.; Suzuki, K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials 2004, 25, 1407–1414. [Google Scholar] [CrossRef]
- Singh, R.; Singh, D. Radiation synthesis of PVP/Alg hydrogel containing nanosilver as wound dressing. J. Mater. Sci. Mater. Med. 2012, 23, 2649–2658. [Google Scholar] [CrossRef]
- Öztürk, E.; Ağalar, C.; Keçeci, K.; Denkbaş, E.B. Preparation and characterization of ciprofloxacin-loaded alginate/chitosan sponge as a wound dressing material. J. Appl. Polym. Sci. 2006, 101, 1602–1609. [Google Scholar] [CrossRef]
- Rezvanian, M.; Amin, M.C.; Ng, S.F. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.E.; Zulfakar, M.H.; Ng, S.F. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int. J. Pharm. 2012, 434, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate/poly (vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.H.; Takeuchi, S. Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv. Mater. 2007, 19, 2696–2701. [Google Scholar] [CrossRef]
- Martinez, C.J.; Kim, J.W.; Ye, C.; Ortiz, I.; Rowat, A.C.; Marquez, M.; Weitz, D. A microfluidic approach to encapsulate living cells in uniform alginate hydrogel microparticles. Macromol. Biosci. 2012, 12, 946–951. [Google Scholar] [CrossRef]
- Rouillard, A.D.; Berglund, C.M.; Lee, J.Y.; Polacheck, W.J.; Tsui, Y.; Bonassar, L.J.; Kirby, B.J. Methods for photocrosslinking alginate hydrogel scaffolds with high cell viability. Tissue Eng. Part. C Methods 2011, 17, 173–179. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Wüst, S.; Godla, M.E.; Müller, R.; Hofmann, S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014, 10, 630–640. [Google Scholar] [CrossRef]
- Shim, J.H.; Lee, J.S.; Kim, J.Y.; Cho, D.W. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J. Micromech. Microeng. 2012, 22, 085014. [Google Scholar] [CrossRef]
- WuGH, H.S. Review: Polymeric-Based 3D Printing for Tissue Engineering. J. Med. Biol. Eng. 2015, 35, 285–292. [Google Scholar]
- Pahlevanzadeh, F.; Emadi, R.; Valiani, A.; Kharaziha, M.; Poursamar, S.A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Three-dimensional printing constructs based on the chitosan for tissue regeneration: State of the art, developing directions and prospect trends. Materials 2020, 13, 2663. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. Part. A. 2014, 20, 1342–1351. [Google Scholar] [CrossRef]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Bertassoni, L.E. Three-dimensional bioprinting for regenerative dentistry and craniofacial tissue engineering. J. Dent. Res. 2015, 94, 143S–152S. [Google Scholar] [CrossRef]
- Wang, X.F.; Lu, P.J.; Song, Y.; Sun, Y.C.; Wang, Y.G.; Wang, Y. Nano hydroxyapatite particles promote osteogenesis in a three-dimensional bio-printing construct consisting of alginate/gelatin/hASCs. RSC Adv. 2016, 6, 6832–6842. [Google Scholar] [CrossRef]
- Gkioni, K.; Leeuwenburgh, S.C.; Douglas, T.E.; Mikos, A.G.; Jansen, J.A. Mineralization of hydrogels for bone regeneration. Tissue Eng. Part B Rev. 2010, 16, 577–585. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Alblas, J.; Hennink, W.E.; Öner, F.C.; Dhert, W.J. Organ printing: The future of bone regeneration? Trends Biotechnol. 2011, 29, 601–606. [Google Scholar] [CrossRef]
- Detsch, R.; Sarker, B.; Zehnder, T.; Boccaccini, A.R.; Douglas, T.E. Additive manufacturing of cell-loaded alginate enriched with alkaline phosphatase for bone tissue engineering application. Bionanomaterials 2014, 15, 79–87. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.J.; Lee, H.; Park, S.A.; Lee, J.Y. Three dimensional cell printing with sulfated alginate for improved bone morphogenetic protein-2 delivery and osteogenesis in bone tissue engineering. Carbohydr. Polym. 2018, 196, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Oh, S.; Seok, J.M.; Park, S.A.; Lee, J.Y. Graphene oxide/alginate composites as novel bioinks for three-dimensional mesenchymal stem cell printing and bone regeneration applications. Nanoscale 2019, 11, 23275–23285. [Google Scholar] [CrossRef] [PubMed]
- Heo, E.Y.; Ko, N.R.; Bae, M.S.; Lee, S.J.; Choi, B.J.; Kim, J.H.; Kim, H.K.; Park, S.A.; Kwon, I.K. Novel 3D printed alginate–BFP1 hybrid scaffolds for enhanced bone regeneration. J. Ind. Eng. Chem. 2017, 45, 61–67. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, Q.; Wu, Y.; Zhang, X.; Wen, F.; Chen, X.; Zhang, S.; Heng, B.C.; He, Y.; Ouyang, H.W. 3D-printed atsttrin-incorporated alginate/hydroxyapatite scaffold promotes bone defect regeneration with TNF/TNFR signaling involvement. Adv. Healthc. Mater. 2015, 4, 1701–1708. [Google Scholar] [CrossRef]
- Ye, K.; Felimban, R.; Traianedes, K.; Moulton, S.E.; Wallace, G.G.; Chung, J.; Quigley, A.; Choong, P.F.; Myers, D.E. Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed chitosan scaffold. PLoS ONE 2014, 9, e102638. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hagg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Kundu, J.; Shim, J.H.; Jang, J.; Kim, S.W.; Cho, D.W. An additive manufacturing-based PCL–alginate–chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen M 2015, 9, 1286–1297. [Google Scholar] [CrossRef]
- Liberski, A.; Latif, N.; Raynaud, C.; Bollensdorff, C.; Yacoub, M. Alginate for cardiac regeneration: From seaweed to clinical trials. Glob. Cardiol. Sci. Pract. 2016, 4, e201604. [Google Scholar] [CrossRef]
- Duan, B. State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann. Biomed. Eng. 2017, 45, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the nature of biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lam, M.T. Alginate Application for Heart and Cardiovascular Diseases. Alginates and Their Biomedical Applications; Springer: Singapore, 2018; pp. 185–212. [Google Scholar]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Hochberg, M. Self-regulation of growth in three dimensions. J. Exp. Med. 1973, 138, 745–753. [Google Scholar] [CrossRef]
- Auger, F.A.; Gibot, L.; Lacroix, D. The pivotal role of vascularization in tissue engineering. Annu. Rev. Biomed. Eng. 2013, 15, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; He, Y.; Fu, J.Z.; Liu, A.; Ma, L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Akkouch, A.; Dababneh, A.; Dolati, F.; Ozbolat, I.T. In vitro study of directly bioprinted perfusable vasculature conduits. Biomater. Sci. 2015, 3, 134–143. [Google Scholar] [CrossRef]
- Xu, C.; Chai, W.; Huang, Y.; Markwald, R.R. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol. Bioeng. 2012, 109, 3152–3160. [Google Scholar] [CrossRef]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef]
- Ramiah, P.; du Toit, L.C.; Choonara, Y.E.; Kondiah, P.P.; Pillay, V. Hydrogel-based Bioinks for 3D Bioprinting in Tissue Regeneration. Front. Mater. 2020, 7, 76. [Google Scholar] [CrossRef]
- Duin, S.; Schütz, K.; Ahlfeld, T.; Lehmann, S.; Lode, A.; Ludwig, B.; Gelinsky, M. 3D Bioprinting of functional islets of langerhans in an alginate/methylcellulose hydrogel blend. Adv. Healthc. Mater. 2019, 8, 1801631. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Emami, K.; Wu, H.; Sun, W. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication 2010, 2, 045004. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Xu, Y.; Chen, X.; Schreyer, D.J. Influence of mechanical properties of alginate-based substrates on the performance of Schwann cells in culture. J. Biomater. Sci. Polym. Ed. 2016, 27, 898–915. [Google Scholar] [CrossRef]
- Ning, L.; Sun, H.; Lelong, T.; Guilloteau, R.; Zhu, N.; Schreyer, D.J.; Chen, X. 3D bioprinting of scaffolds with living Schwann cells for potential nerve tissue engineering applications. Biofabrication 2018, 10, 035014. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef]
- Mozetic, P.; Giannitelli, S.M.; Gori, M.; Trombetta, M.; Rainer, A. Engineering muscle cell alignment through 3D bioprinting. J. Biomed. Mater. Res. A. 2017, 105, 2582–2588. [Google Scholar] [CrossRef]
- Berg, J.; Hiller, T.; Kissner, M.S.; Qazi, T.H.; Duda, G.N.; Hocke, A.C.; Hippenstiel, S.; Elomaa, L.; Weinhart, M.; Fahrenson, C.; et al. Optimization of cell-laden bioinks for 3D bioprinting and efficient infection with influenza A virus. Sci. Rep. 2018, 8, 1–3. [Google Scholar] [CrossRef]
- Pourchet, L.J.; Thepot, A.; Albouy, M.; Courtial, E.J.; Boher, A.; Blum, L.J.; Marquette, C.A. Human skin 3D bioprinting using scaffold-free approach. Adv. Healthc. Mater. 2017, 6, 1601101. [Google Scholar] [CrossRef]
- Shi, L.; Xiong, L.; Hu, Y.; Li, W.; Chen, Z.; Liu, K.; Zhang, X. Three-dimensional printing alginate/gelatin scaffolds as dermal substitutes for skin tissue engineering. Polym. Eng. Sci. 2018, 58, 1782–1790. [Google Scholar] [CrossRef]
- Kogelenberg, S.V.; Yue, Z.; Dinoro, J.N.; Baker, C.S.; Wallace, G.G. Three-dimensional printing and cell therapy for wound repair. Adv. Wound Care 2018, 7, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Ismail, A.F.; Aziz, M.; Akbari, M.; Berto, F.; Chen, X.B. In Vitro and in vivo evaluation of chitosan-alginate/gentamicin wound dressing nanofibrous with high antibacterial performance. Polym. Test 2020, 82, 106298. [Google Scholar] [CrossRef]
- He, P.; Zhao, J.; Zhang, J.; Li, B.; Gou, Z.; Gou, M.; Li, X. Bioprinting of skin constructs for wound healing. Burns Trauma 2018, 6. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Kashani, E.; Fazljou, S.M.; Torbati, M.; Akbarzadeh, A. Alginate -based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 1–22. [Google Scholar] [CrossRef]
- Pereira, R.F.; Barrias, C.C.; Granja, P.L.; Bartolo, P.J. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine 2013, 8, 603–621. [Google Scholar] [CrossRef]
- Liu, J.; Chi, J.; Wang, K.; Liu, X.; Liu, J.; Gu, F. Full-thickness wound healing using 3D bioprinted gelatin-alginate scaffolds in mice: A histopathological study. Int. J. Clin. Exp. Pathol. 2016, 9, 11197–11205. [Google Scholar]
- Yang, J.; Wang, S.; Xiong, Y.; Chen, J.; Ghanem, A.; Wang, Y.; Sun, B. Three dimensional printing bilayer membrane scaffold promotes wound healing. Front. Bioeng. Biotechnol. 2019, 7, 348. [Google Scholar]
- Moulton, S.E.; Wallace, G.G. 3-dimensional (3D) fabricated polymer based drug delivery systems. J. Control. Release 2014, 193, 27–34. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing pharmaceuticals: Drug development to frontline care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.C. 3D printing of a wearable personalized oral delivery device: A first-in-human study. Sci. Adv. 2018, 4, eaat2544. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shansky, J.; Borselli, C.; Mooney, D.; Vandenburgh, H. Design and fabrication of a biodegradable, covalently crosslinked shape-memory alginate scaffold for cell and growth factor delivery. Tissue Eng. Part A. 2012, 18, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S.; et al. Size-and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef]
- Bansal, M.; Sharma, V.; Singh, G.; Harikumar, S.L. 3d Printing for The Future of Pharmaceuticals Dosages Forms. Int. J. App. Pharm. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Katakam, P.; Dey, B.; Assaleh, F.H.; Hwisa, N.T.; Adiki, S.K.; Chandu, B.R.; Mitra, A. Top-down and bottom-up approaches in 3D printing technologies for drug delivery challenges. Crit. Rev. Drug Carrier Syst. 2015, 32, 61–87. [Google Scholar] [CrossRef]
- Jain, D.; Bar-Shalom, D. Alginate drug delivery systems: Application in context of pharmaceutical and biomedical research. Drug Dev. Ind. Pharm. 2014, 40, 1576–1584. [Google Scholar] [CrossRef]
- Do, A.V.; Akkouch, A.; Green, B.; Ozbolat, I.; Debabneh, A.; Geary, S.; Salem, A.K. Controlled and sequential delivery of fluorophores from 3D printed Alg-PLGA tubes. Ann. Biomed. Eng. 2017, 45, 297–305. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, Y.; Zhang, J.; Wu, J.P.; Kirk, T.B.; Xu, J.; Ma, D.; Xue, W. Three-dimensional printing of shape memory hydrogels with internal structure for drug delivery. Mater. Sci. Eng. C 2018, 84, 44–51. [Google Scholar] [CrossRef]
- Satpathy, A.; Datta, P.; Wu, Y.; Ayan, B.; Bayram, E.; Ozbolat, I.T. Developments with 3D bioprinting for novel drug discovery. Expert Opin. Drug Discov. 2018, 13, 1115–1129. [Google Scholar] [CrossRef]
- Dai, X.; Ma, C.; Lan, Q.; Xu, T. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 2016, 8, 045005. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773. [Google Scholar] [CrossRef]
- Xu, R.; Su, C.; Cui, L.; Zhang, K.; Li, J. Preparing sodium alginate/polyethyleneimine spheres for potential application of killing tumor cells by reducing the concentration of copper ions in the lesions of colon cancer. Materials 2019, 12, 1570. [Google Scholar] [CrossRef] [PubMed]
- Cidade, M.T.; Ramos, D.J.; Santos, J.; Carrelo, H.; Calero, N.; Borges, J.P. Injectable hydrogels based on pluronic/water systems filled with alginate microparticles for biomedical applications. Materials 2019, 12, 1083. [Google Scholar] [CrossRef] [PubMed]
- Torres-Caban, R.; Vega-Olivencia, C.A.; Alamo-Nole, L.; Morales-Irizarry, D.; Roman-Velazquez, F.; Mina-Camilde, N. Removal of copper from water by adsorption with calcium-alginate/spent-coffee-grounds composite beads. Materials 2019, 12, 395. [Google Scholar] [CrossRef]
- Xu, S.; Liu, X.; Tabaković, A.; Schlangen, E. Investigation of the potential use of calcium alginate capsules for self-healing in porous asphalt concrete. Materials 2019, 12, 168. [Google Scholar] [CrossRef]
- Menshutina, N.; Tsygankov, P.; Ivanov, S. Synthesis and properties of silica and alginate hybrid aerogel particles with embedded carbon nanotubes (CNTs) for selective sorption. Materials 2019, 12, 52. [Google Scholar] [CrossRef]
- Siracusa, V.; Romani, S.; Gigli, M.; Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Lotti, N. Characterization of active edible films based on citral essential oil, alginate and pectin. Materials 2018, 11, 1980. [Google Scholar] [CrossRef]
- Rottensteiner-Brandl, U.; Detsch, R.; Sarker, B.; Lingens, L.; Köhn, K.; Kneser, U.; Bosserhoff, A.K.; Horch, R.E.; Boccaccini, A.R.; Arkudas, A. Encapsulation of rat bone marrow derived mesenchymal stem cells in alginate dialdehyde/gelatin microbeads with and without nanoscaled bioactive glass for in vivo bone tissue engineering. Materials 2018, 11, 1880. [Google Scholar] [CrossRef]
- Szekalska, M.; Sosnowska, K.; Czajkowska-Kośnik, A.; Winnicka, K. Calcium chloride modified alginate microparticles formulated by the spray drying process: A strategy to prolong the release of freely soluble drugs. Materials 2018, 11, 1522. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Wu, S.; Dong, L.; Wang, Q.; Liu, Q. Microfluidic synthesis of ca-alginate microcapsules for self-healing of bituminous binder. Materials 2018, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Sathish, V.; Mallik, S.; Khoda, B. 3D Printability of alginate-carboxymethyl cellulose hydrogel. Materials 2018, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, J.; Li, S.; Tong, Y.; Ye, B. Synthesis of magnetic microspheres with sodium alginate and activated carbon for removal of methylene blue. Materials 2017, 10, 84. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Kashefian, M. Drug delivery and cytocompatibility of ciprofloxacin loaded gelatin nanofibers-coated Mg alloy. Mater. Lett. 2017, 207, 179–182. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Razzaghi, M.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Polymethyl methacrylate-based bone cements containing carbon nanotubes and graphene oxide: An overview of physical, mechanical, and biological properties. Polymers 2020, 12, 1469. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Daroonparvar, M.; Chen, X. Antibacterial activity and in vivo wound healing evaluation of polycaprolactone-gelatin methacryloyl-cephalexin electrospun nanofibrous. Mater. Lett. 2019, 256, 126618. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Shuang, C.P.; Berto, F. Preparation of poly (ε-caprolactone)-hydroxyapatite composite coating for improvement of corrosion performance of biodegradable magnesium. Mater. Des. Process. Commun. 2020, 2, e170. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Chen, X.; Ismail, A.F.; Aziz, M.; Abdolahi, E.; Mahmoodiyan, F. Improved antibacterial properties of an Mg-Zn-Ca alloy coated with chitosan nanofibers incorporating silver sulfadiazine multiwall carbon nanotubes for bone implants. Polym. Adv. Technol. 2019, 30, 1333–1339. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun nano-fibers for biomedical and tissue engineering applications: A comprehensive review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide Core–Shell Electrospun Dressing for Burn Wound Management. Macromol. Biosci. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Akbari, M.; Ismail, A.F.; Aziz, M.; Hadisi, Z.; Pagan, E.; Daroonparvar, M.; Chen, X. Coating biodegradable magnesium alloys with electrospun poly-L-lactic acid-åkermanite-doxycycline nanofibers for enhanced biocompatibility, antibacterial activity, and corrosion resistance. Surf. Coat. Technol. 2019, 377, 124898. [Google Scholar] [CrossRef]
- Saberi, A.; Bakhsheshi-Rad, H.R.; Karamian, E.; Kasiri, M.; Ghomi, H. A study on the corrosion behavior and biological properties of polycaprolactone/bredigite composite coating on biodegradable Mg-Zn-Ca-GNP nanocomposite. Prog. Org. Coat. 2020, 147, 105822. [Google Scholar] [CrossRef]
- Sprio, S.; Dapporto, M.; Montesi, M.; Panseri, S.; Lattanzi, W.; Pola, E.; Logroscino, G.; Tampieri, A. Novel Osteointegrative Sr-substituted apatitic cements enriched with alginate. Materials 2016, 9, 763. [Google Scholar] [CrossRef]
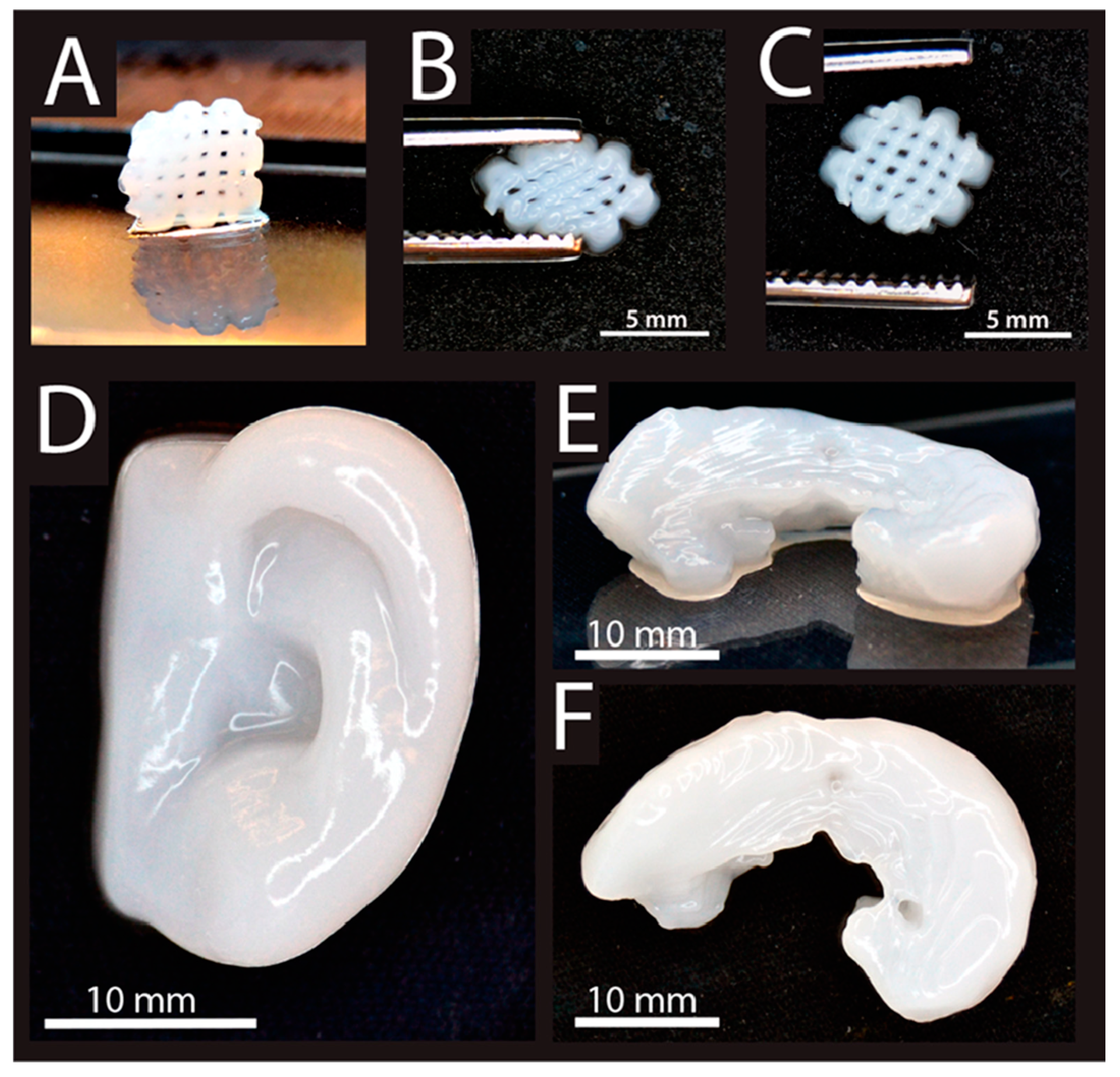
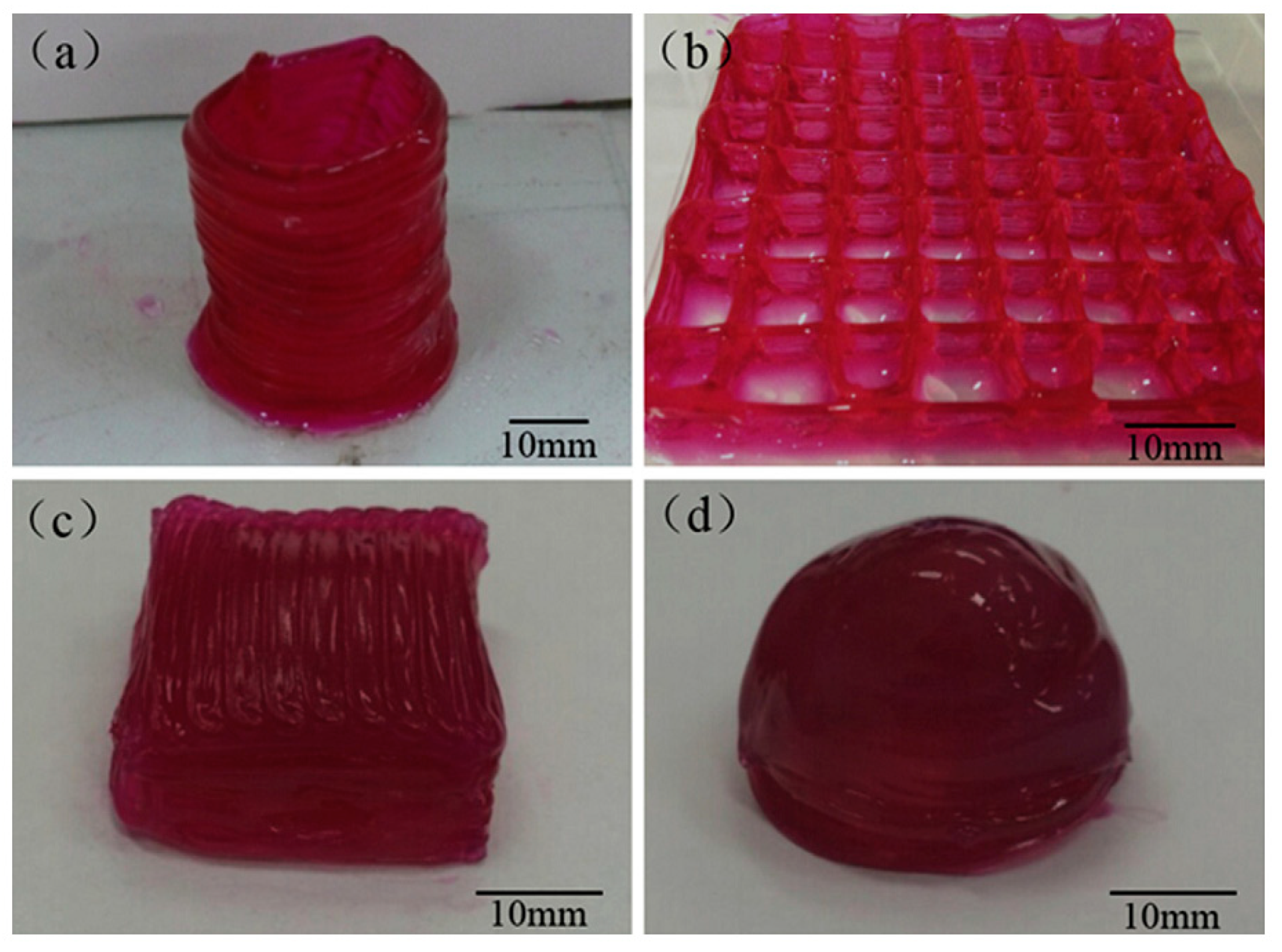
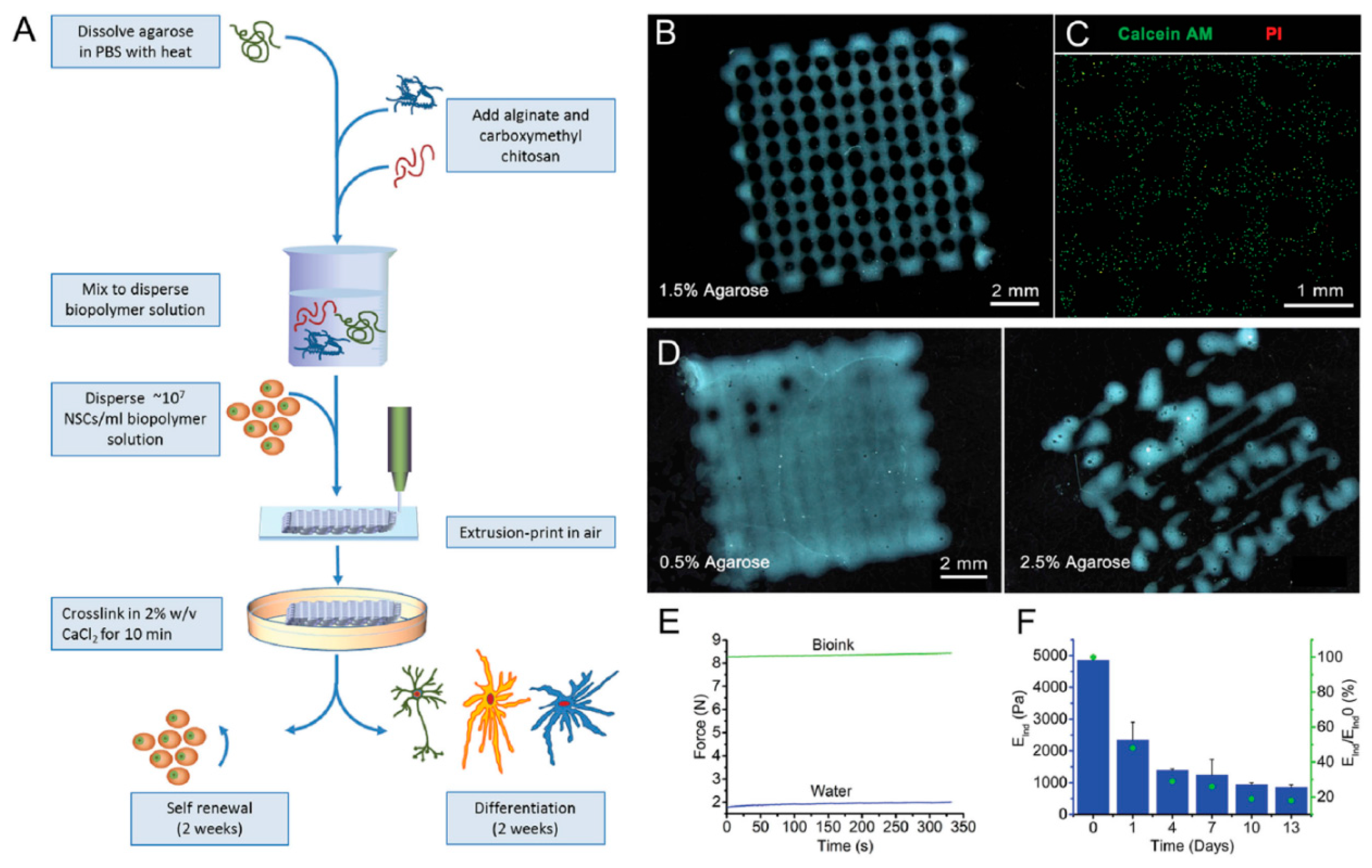
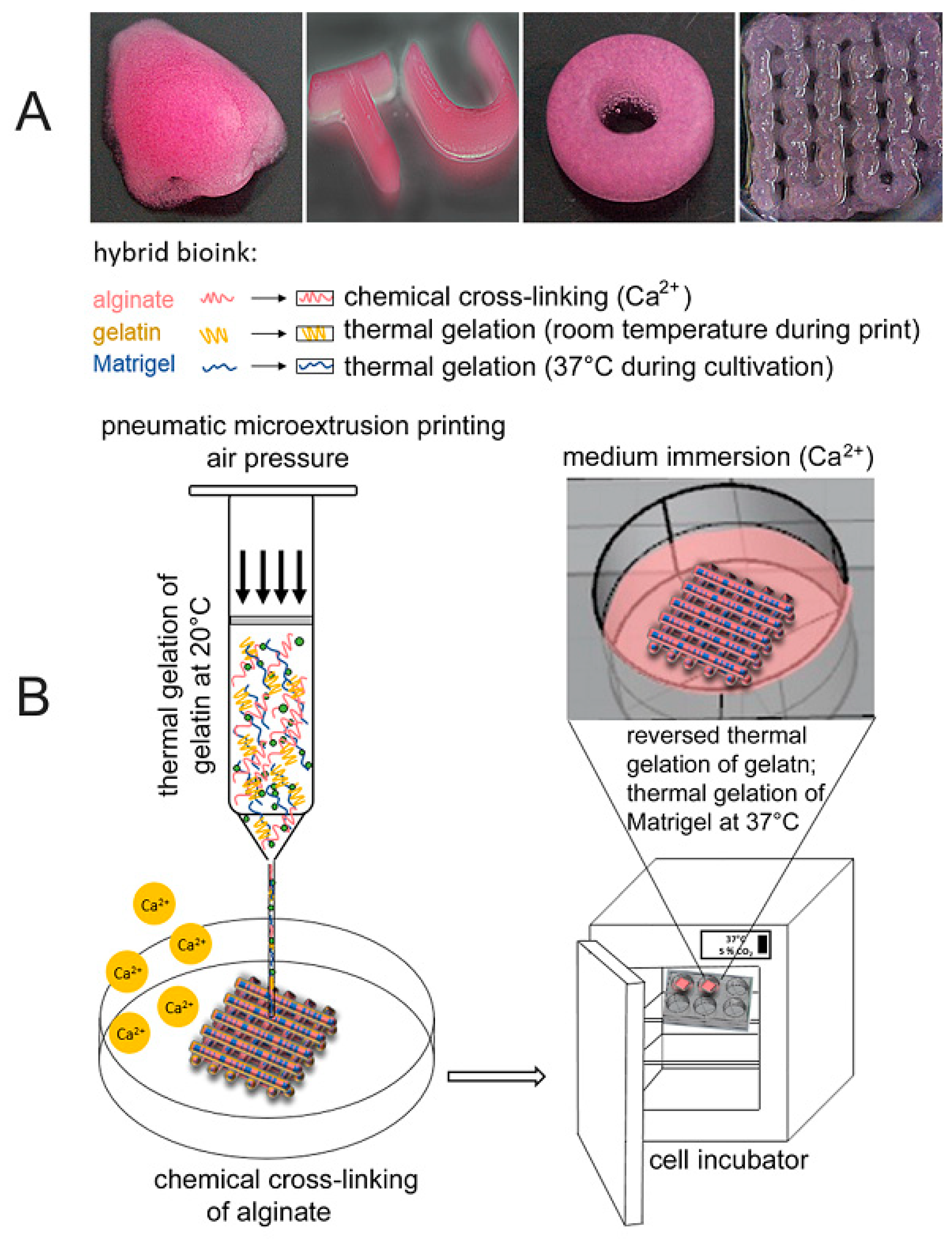
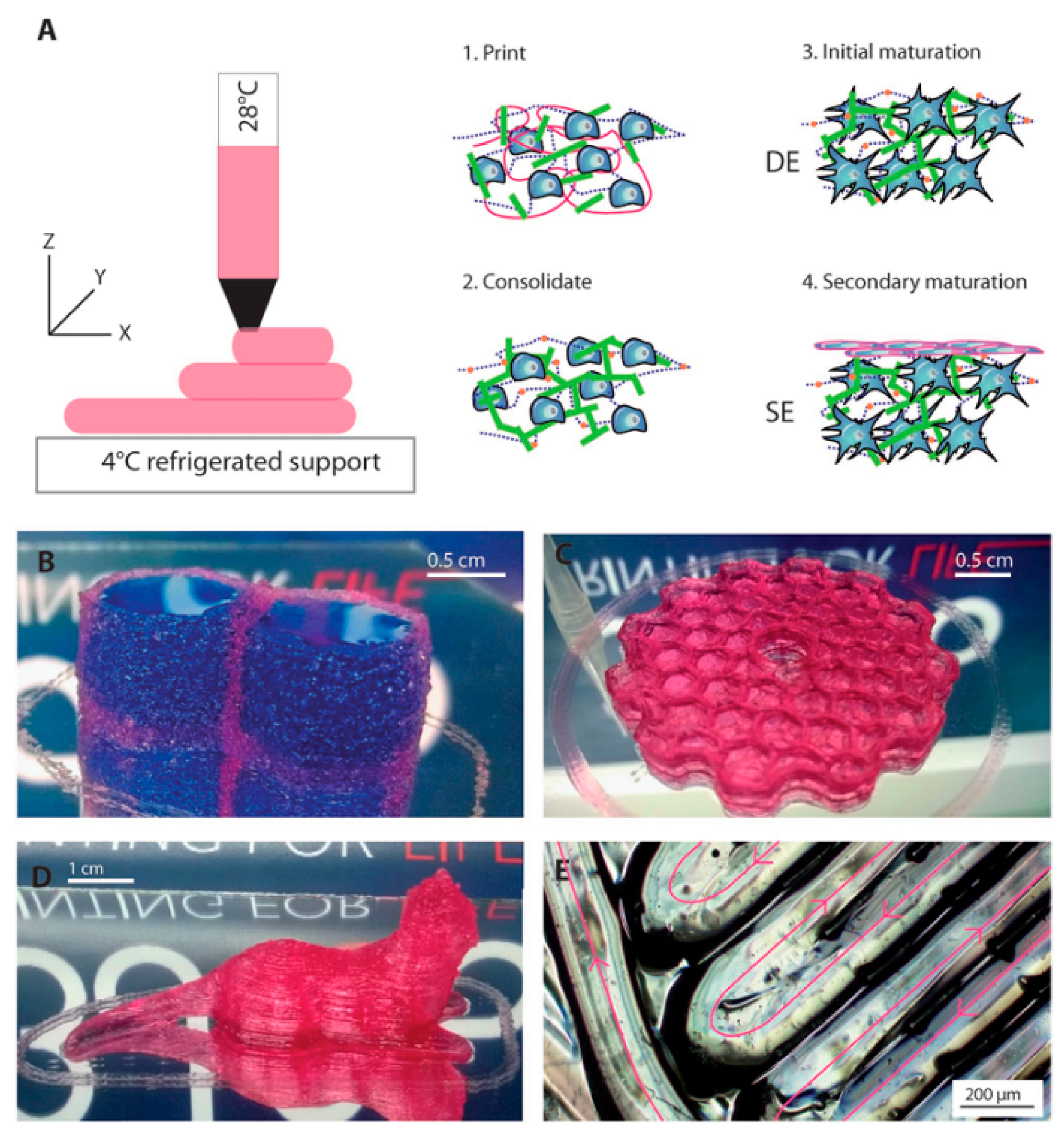
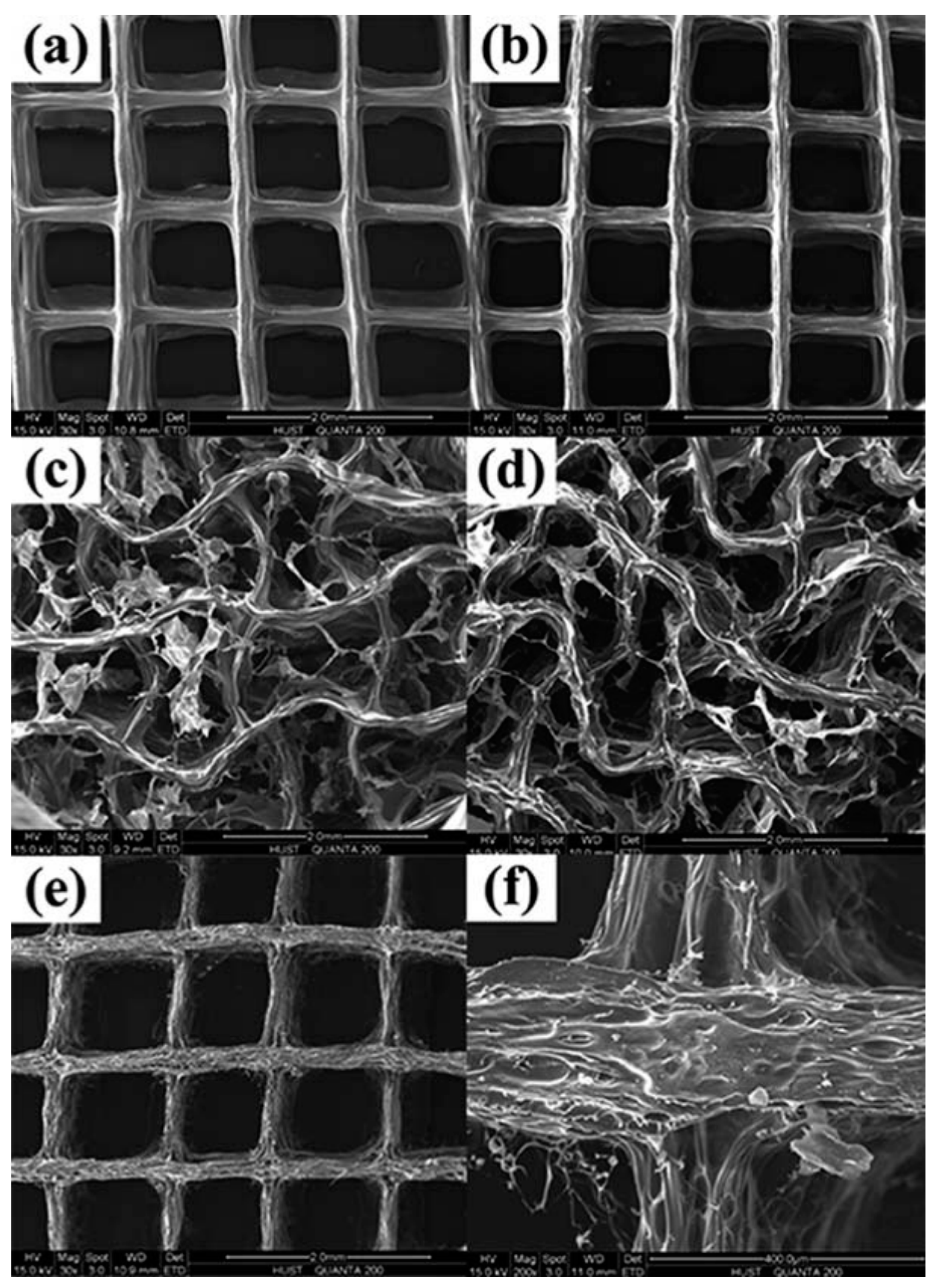
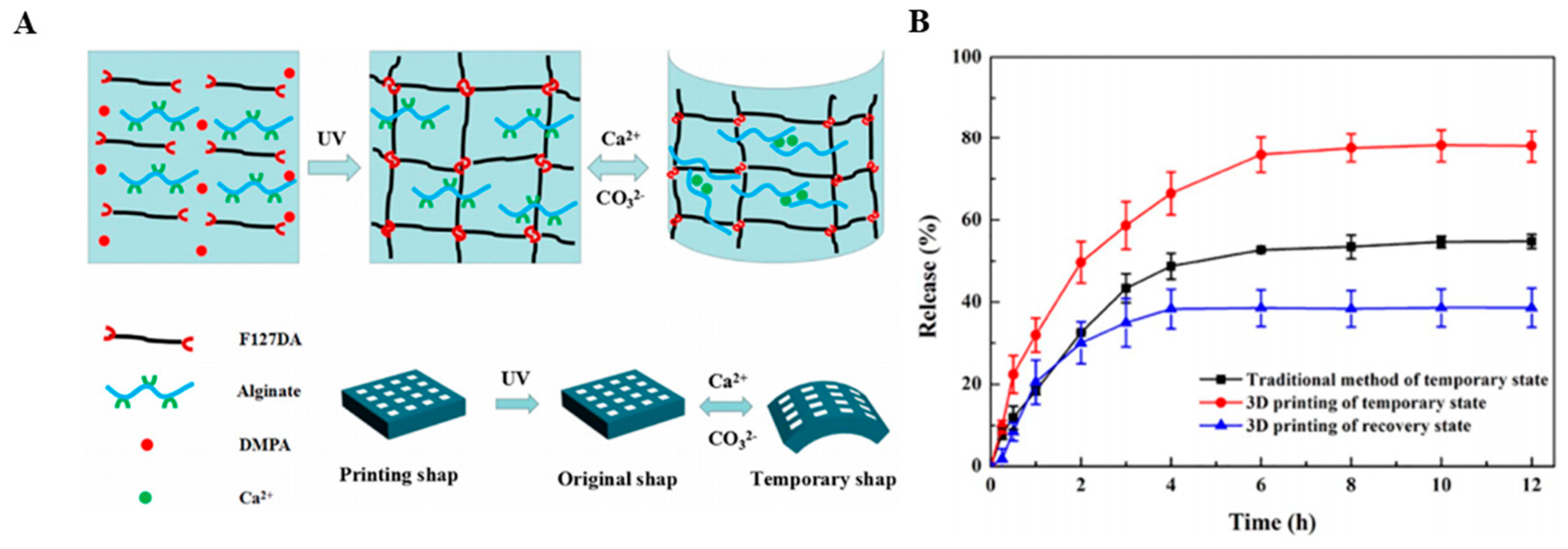
| Material | Fabrication Method | Crosslinking Type | Structure | Cells Viability and Attachments Support | Cell Type | Mechanical Results | Application | Reference |
|---|---|---|---|---|---|---|---|---|
| Alg/CPC | Extruding Alg-cell droplets into wells containing calcium chloride | Ionically with calcium chloride | Injectable hydrogel | Well hUCMSCs viability, osteo differentiation, and formation of bone minerals | hUCMSCs | Constructed mechanical properties were similar to cancellous bone. The flexural strength of CPC-chitosan-fiber-microbead ≈ 4 MPa | BTE | [44] |
| Alg/anti-BMP2 monoclonal antibodies | Extruding of Alg droplets through a syringe into calcium chloride solution | Ionically with calcium chloride | Microspheres | Well osteo differentiation (in vitro), new bone formation (in vivo) | MSC | - | BTE | [45] |
| Alg/RGD peptide | Reacting with peptide and cross linking | Ionically with CaSO4 | Hydrogels | High osteoblast attachment and spreading | MC3T3-E1 osteoblast | - | BTE | [46] |
| Na-Alg | Gelation | Ionically with CaCl2 | Gel | Proliferation of BMCs were faster on MVG gels than LVM | BMCs | Tensile strength for MVG ≈ 240 KPa and LVM ≈ 280 KPa | BTE | [47] |
| HAp/Alg/CS | In situ coprecipitation, freeze-drying | Ionically with CaCl2 | Scaffolds | No cytotoxic and (in vitro), new bone generation (in vivo) | MG-63 cells | - | BTE | [48] |
| Alg/fibrin | Loading in a syringe which was equipped with device of bead generating | Ionically with calcium chloride | Microbeads | Highly expressions of bone markers such as ALP, OC, collagen I, and Runx2 | hUCMSCs | - | BTE | [49] |
| Alg/CS/HAp | Distribution and freeze drying | Ionically with CaCl2 | Scaffolds | High differentiation and mineralization | MC3T3-E1 | Compressive strength = 0.68 MPa and elastic modulus = 13.35 MPa | BTE | [50] |
| Alg/CS/Gl/HAp | Simple foaming | Glutaraldehyde (for CS) | Scaffolds | Well Cell attachment, viability, proliferation | Osteoblast | - | BTE | [51] |
| Alg/CS/silica | Blending and freeze drying | CaCl2 | Scaffolds | No significant toxicity | Osteoprogenitor, MG63, HOS | Compressive stress = 0.59 ± 0.405 Mpa, Young’s Modulus = 8.16 ± 0.567 MPa | BTE | [52] |
| Na-Alg | Mixing with syringe | CaSO4/CaCl2 | Gel system | Chondrocytes viability for several weeks | Chondrocytes | Young’s Modulus ≈ 0.17 ± 0.01 MPa | CTTE | [53] |
| Alg | Using microfluidic device | Ionically with calcium chloride | Scaffolds | Nontoxic, well cells proliferation and maintaining cells phenotypes | Chondrocytes | - | CTTE | [54] |
| Alg/GL | Casting | Ionically with Ca2+ | Film | Suitable cell proliferation, cell differentiation for same blend specimen | C2C12 myoblasts | - | CTE | [55] |
| Alg/RGD peptide | Freeze drying | Ionically with calcium ions | Scaffolds | High cell adherence to the matrix, acceleration of cardiac tissue regeneration | Neonatal rat cardiac cells | - | CTE | [56] |
| Alg | Freeze drying | - | Scaffolds | Favorable cells viability, urea and albumin secretion | Hepatocyte | - | Liver TE | [57] |
| Na-Alg/CS | Spinning of Alg into a coagulation system | - | Hybrid fibers | Well fibroblast adhesion, Fibroblast produce collagen I fibers | Fibroblast | Tensile Strength > 200 MPa. | Ligament and tendon TE | [58] |
| Alg | Injection of oxidized Alg into a Teflon mold. | Ionically cross-linked with calcium ions | Hydrogels | Improvement of cartilage-like tissue formation (in vivo) | Chondrocyte | - | TE | [59] |
| Na-Alg/PEO | Electrospinning | Ionically with CaCl2 | Core–shell nanofibers | Nontoxic | Fibroblasts cells | - | TE | [60] |
| Alg | Gelation and casting in a Teflon mold. | Ionically with Ca2+ | Scaffolds | - | - | In high Ca content: compressive modulus ≈ 70 KPa and compressive strength ≈ 590 KPa | TE | [61] |
| AAlg/PNIPAAm | Mixing solutions, dialyzing and freeze drying | - | Injectable hydrogel | Nontoxic | hBMSCs | - | TE | [62] |
| Alg/CS/GRGDSP peptide/PEO | Electrospinning | Mixing and lyophilization | Nanofibers | Favorable cells attachment and proliferation | MC3T3 | - | TE | [63] |
| Alg/lignin/CaCO3 | Gelation with Pressurized carbon dioxide | CaCO3 (releasing Ca2+) | Aerogels | Non-cytotoxic, well cell adhesion | L929 | Young’s Modulus = 1.36 ± 0.24 MPa | TE | [64] |
| Alg/PEO/GRGDSP | Electrospinning | Ionically with CaCl2 | Nanofibers | Good adhesion, spread, and proliferation | HDF | - | TE | [65] |
| Alg/HNTs | Mixing of solutions and freeze drying | Ionically with calcium chloride | Scaffolds | Good cell attachment and proliferation | NIH3T3 | - | TE | [66] |
| Alg/PLGA | Water/oil emulsion, lyophilization | Ionically with calcium chloride | Microspheres | Increase of NPCs expansion rate | NPCs | - | TE | [67] |
| Alg/TGF-1 | Mixing and gelation | Ionically with calcium chloride | Hydrogel | Induction of odontoblast-like cell differentiation | Odontoblast-like cell | - | Repair human dental pulp | [68] |
| Alg/CPC | Directly injection into a fibrous structure in a Ca2+ containing bath | Ionically with Ca2+ | Scaffolds | Favorable cells proliferation and osteo differentiation, new bone tissue formation and the defect closing (in vivo) | MSCs | - | Drug delivery and BTE | [69] |
| Alg | Reacting with glutaraldehyde + carboxylate moieties as stimuli responsive sensors | Glutaraldehyde | Stimuli-responsive hydroge | - | - | - | Drug delivery and TE | [70] |
| Na-Alg/isoniazid | Emulsification | Ionically with calcium chloride | Spherical microspheres | - | - | - | Oral sustained drug delivery | [71] |
| Alg/CS | Alg/chitosan solution was dripped via needle | Dual crosslinking with Ca2+ and SO42− | Beads | - | - | - | Oral drug delivery | [72] |
| CAlg/CMC/5-FU | Ionic gelation, extruding solutions via syringe | Ionically with calcium chloride | Beads | Significant reduction of cells viability for all formulations after 48 h | Colon adenocarcinoma cells (HT-29) | - | Colon drug delivery | [73] |
| Alg/CS, AlgGO/MZ | Solutions dropped into calcium pantothenate and left at room temperature | - | Beads | - | - | - | Stomach drug delivery | [74] |
| Terbutaline sulfate and (BSA)-loaded Alg–poloxamer | Emulsification + external gelation | Ionically with CaCl2 | Microparticle | - | - | - | Drug delivery to mucosal tissue | [75] |
| Alg/CS/5-FU and tegafur | Extruding of solutions through a syringe into calcium chloride solution | Ionically with calcium chloride | Microparticle | - | - | - | Drug delivery | [76] |
| Alg/N-Succinyl CS/nife- dipine | Ionic gelation | Ionically with calcium chloride | Beads | - | - | - | Drug delivery | [77] |
| Alg/CaCO3 | Templating water-in-oil emulsion and subsequent in situ gelation | Ionically with Ca2+ | Core-shell hydrogel beads | - | - | - | Drug delivery | [78] |
| Na-Alg/HPMC/CaGlu | Direct compression | Ionically with calcium ions | Matrices | - | - | - | Drug delivery | [79] |
| Alg/peptides derived from laminin and elastin | Using commercial calcium Alg dressing and cross linking with the peptide | Hybrid peptides derived from laminin and elastin | Fibrous dressings | Enhancement of NHDF proliferation (in vitro), significantly greater epithelialization and a larger volume of regenerated tissue (in vivo) | NHDF | - | Wound dressings | [80] |
| Alg/PVP/nano-silver | Mixing Al and PVP, heating and irradiation | Gamma irradiation | Hydrogel network | - | - | - | Wound dressings | [81] |
| Alg/CS | Gelation and lyophilization | Ionically with calcium chloride | Sponge | - | - | - | Wound dressings | [82] |
| Na-Alg/PC/GL/SIM | Solvent casting | - | Film | Nontoxic | HDF | Tensile strength SA–PC–SIM ≈ 3.32 ± 0.58, N/mm2 SA–GL–SIM ≈ 1.78 ± 0.02 N/mm2 | Wound dressings | [83] |
| Na-Alg/ibuprofen) | Casting and pouring in petri dish | - | Hydrocolloid films | The bilayer films treated wound faster than single layer SA films (in vivo) | - | Tensile strength ≈ 27.22 ± 0.95 MPa | Wound dressings | [84] |
| Oxidized Alg/GL | Via syringe fibrin glue applicator | AlgDA | Hydrogel | Acceleration of epithelialization | - | - | Wound dressings | [85] |
| Na-Alg/PVA/ZnO | Electrospinning | Glutaraldehyde, CaCl2 | Nanofibers | Less toxic in 0.5 and 1% ZnO | L929 | - | Wound dressings | [86] |
| Na-Alg | Syringe pumps infused solutions into the microfluidic device | Ionically with Ca2+ | Microbeads | Cells viability enhanced from 19.3% to 74.3% with the increase of CaCO3 concentration from 1.14 to 9.10 mg∙mL−1 | Mammalian cells (Jurkat) | - | Cell Encapsulation | [87] |
| Alg | Microfluidic technique | Ionically with Ca2+ | Hydrogel microparticles | Cells viability over 98% | Yeast cells | - | Cell encapsulation | [88] |
| Alg | Mixing and pouring molds and using UV | Photoinitiator VA-086 | Hydrogel scaffolds | Noncytotoxic | Chondrocyte | 10–20 kPa modulus range | Cell encapsulation | [89] |
| Material | Printing Method | Cell Type | Target Tissue | Results | Reference |
|---|---|---|---|---|---|
| Alg/gelatin scaffolds with nano apatite coating | Bioscaffolder 3.1, Gesim | Rat bone marrow stem cells | Bone | Alg/gelatin scaffolds with nano apatite coating had 2-fold higher Young’s Modulus compared with the scaffolds without coating, stimulated the proliferation and osteogenic differentiation of rat bone marrow stem cells | [26] |
| Na-Alg and calcium phosphate | A custom-designed 3D printer | - | Bone | The compressive strength of composite materials increased with Alg concentration from 0.45 Mpa up to about 1.0 MPa | [27] |
| Bioactive glass/Alg | Bioscaffolder 2.1 platform (GeSiM) (extrusion based) | BMSCs | Bone | Shrinkage ratios decreased by half, 13–93 BG incorporation improved the compressive strength and modulus of the SA scaffold, achieving highest values of 16.74 ± 1.78 MPa and 79.49 ± 7.38 MPa | [28] |
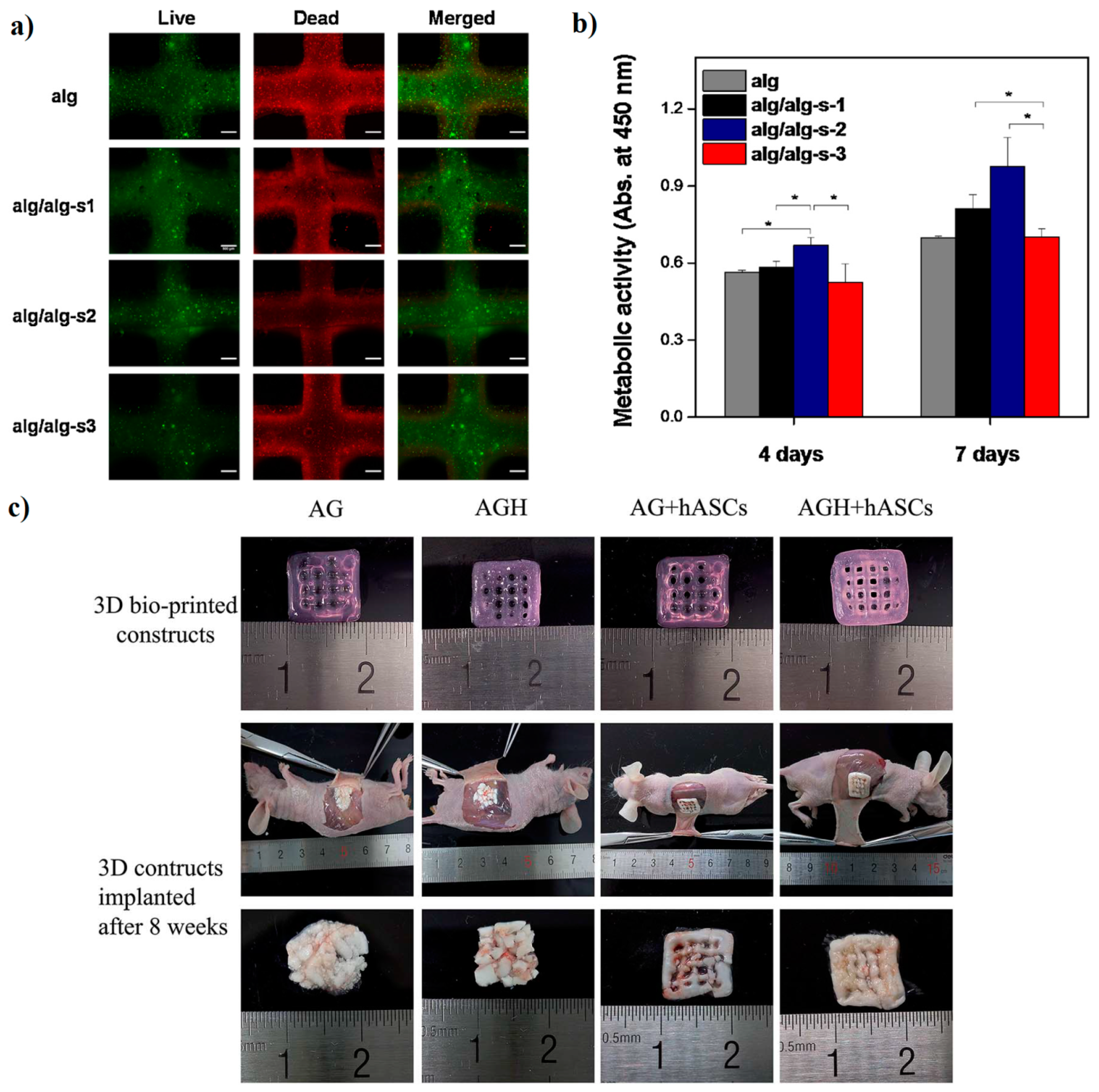
| Na-Alg/gelatin/nano hydroxyapatite/hASCs | 3D Bioplotter | Human adipose-derived stem cells (hASCs) | Bone | New formation of bone in existence of HA was higher than in the Alg/gelatin | [98] |
| Alg and alkaline phosphatase (ALP) | 3D Bioplotter | MG-63 osteoblast-like cells | Bone | Mineralization was observed in all scaffolds containing ALP | [101] |
| Alg/Alg-sulfate/bone morphogenetic protein 2 | Custom-made 3D bioprinting system (extrusion based) | MC3T3-E1 osteoblasts | Bone | Improve retention of bone morphogenetic protein 2, promote osteoblastic proliferation and differentiation | [102] |
| Alg/graphene oxide | Custom-made 3D bioprinting system | Mesenchymal stem cells (MSCs) | Bone | Improve printability, structural stability, and osteogenic activities | [103] |
| Alg/bone formation peptide-1(BFP1) | 3D printer | human adipose-derived stem cells (hADSCs) | Bone | Good cell viability and proliferation, enhancement bone regeneration activities | [104] |
| Alg/Hydroxyapatite/Atsttrin | 3D bioprinter | C3H cells | Bone | Promote bone defect repair | [105] |
| Alg/Nanocellulose | Stereolithography (for ear painting) | Human chondrocytes, L929 | Cartilage | Excellent printability at room temperature, high shape fidelity | [107] |
| Alg Sulfate/Nanocellulose | BioFactory, regenHU | Bovine chondrocytes | Cartilage | Very good printability, promote cell spreading, proliferation, and collagen II synthesis | [108] |
| Na-Alg/Collagen (Col) or agarose (AG) | A 3D–Bioplotter | Chondrocytes | Cartilage | The mechanical strength was improved in both SA/COL and SA/AG groups compared to SA alone, incorporation of COL could distinctly enhance cell adhesion, proliferation and the expression of cartilage specific genes such as Acan, Col2al and Sox9 | [109] |
| Alg/PCL | Additive manufacturing (AM) with a multihead deposition system (MHDS) | Chondrocyte | Cartilage | PCL–Alg gels containing transforming growth factor-b (TGFb) showed higher ECM formation. Enhancement of cartilage tissue and collagen II | [110] |
| formation in the PCL–Alg gel (+TGFb) hybrid scaffold | |||||
| Alg/gelatin | Dual-syringe Fab@Home bioprinter | Aortic root sinus smooth muscle cells (SMC) and aortic valve leaflet interstitial cells (VIC) | Cardiac | High cells viability, anatomically complex, heterogeneously encapsulated aortic valve hydrogel conduits could fabricate with 3D bioprinting | [115] |
| Calcium Alg | Novel 3D bioprinting method by using a coaxial nozzle | L929 | Vascular | High-strength structures, good cell viability | [118] |
| Na-Alg | Coaxial deposition system | Human umbilical vein smooth muscle cells (HUVSMCs) | Vascular | Good proliferation, deposition of smooth muscle matrix and collagen | [119] |
| Na-Alg | Inkjet Printing | Fibroblast (3T3 cell) | Vascular | Cell viability of printed cellular tubes has been found above 82% | [120] |
| Na-Alg | Inkjet Printing | Mouse fibroblast | Vascular | Fibroblast cell viability of printed cellular tubes was found to be above 90%, successfully print of vascular-like structures | [121] |
| Gelatin methacryloyl (GelMA), sodium Alg, and 4-arm poly(ethylene glycol)-tetra-acrylate (PEGTA) | Multilayered coaxial extrusion systems | Endothelial and stem cells | Vascular | Favorable physicochemical characteristics, support of the proliferation and early maturation of vascular cells, bioprinting of complex 3D vasculature | [122] |
| Alg | Extrusion-based | HepG2 | Liver | Enhancement of cell viability, control of cellular level differentiation, control of tissue level function | [125] |
| Alg/Hyaluronic Acid (HA) | Extrusion-based | Schwann | Nerve | Scaffolds created from this bioprinting process can support the performance of encapsulated Schwann cell viability, proliferation and cellular protein expression | [127] |
| Alg/Agarose/carboxymethyl-chitosan (CMC) | Extrusion-based | Human neural stem cells | Nerve | Formation of stable cells encapsulated 3D structures | [128] |
| Pluronic/Alg | Extrusion-based | Myoblast | Muscle | Improved viability of cells compared to the normal 2D cultures | [129] |
| Alg/Gelatin | Extrusion-based | A549 | Lung | The first attempt to generate 3D bioprinted humanized models for infection studies | [130] |
| Gelatin/Alg | Bio-extrusion | Primary human skin cells | Skin | Cells spreading induced a rapid differentiation of the dermis, leading to a stiff tissue on which epidermis can be rapidly seeded | [131] |
| Gelatin/Alg | Extrusion free-forming system | Human skin fibroblasts (HSFs) | Skin | Dual crosslinking process can make dermal substitute scaffolds with physicochemical properties that match with human skin tissue | [132] |
| Gelatin/Alg | Extrusion-based | - | Skin | Enhanced the detachment of the crusts, decreased the agglomeration of keratinized substances | [140] |
| Alg/PLGA | Extruded type | - | Skin | Promoted cell adhesion and proliferation promote neovascularization and collagen I/III deposition | [141] |
| Alg/PLGA | Coaxial extrusion-based system | Human embryonic kidney (HEK293) | Kidney | Sequential release and retention of drug lack of cytotoxicity improving mechanical strength | [150] |
| Alg/pluronic F127 diacrylate macromer (F127DA) | Extruded type | 3T3 cells | Drug delivery in vascular tissue | Rapid drug release high recovery ratio in a short time the low cytotoxicity | [151] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pahlevanzadeh, F.; Mokhtari, H.; Bakhsheshi-Rad, H.R.; Emadi, R.; Kharaziha, M.; Valiani, A.; Poursamar, S.A.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Recent Trends in Three-Dimensional Bioinks Based on Alginate for Biomedical Applications. Materials 2020, 13, 3980. https://doi.org/10.3390/ma13183980
Pahlevanzadeh F, Mokhtari H, Bakhsheshi-Rad HR, Emadi R, Kharaziha M, Valiani A, Poursamar SA, Ismail AF, RamaKrishna S, Berto F. Recent Trends in Three-Dimensional Bioinks Based on Alginate for Biomedical Applications. Materials. 2020; 13(18):3980. https://doi.org/10.3390/ma13183980
Chicago/Turabian StylePahlevanzadeh, Farnoosh, Hamidreza Mokhtari, Hamid Reza Bakhsheshi-Rad, Rahmatollah Emadi, Mahshid Kharaziha, Ali Valiani, S. Ali Poursamar, Ahmad Fauzi Ismail, Seeram RamaKrishna, and Filippo Berto. 2020. "Recent Trends in Three-Dimensional Bioinks Based on Alginate for Biomedical Applications" Materials 13, no. 18: 3980. https://doi.org/10.3390/ma13183980
APA StylePahlevanzadeh, F., Mokhtari, H., Bakhsheshi-Rad, H. R., Emadi, R., Kharaziha, M., Valiani, A., Poursamar, S. A., Ismail, A. F., RamaKrishna, S., & Berto, F. (2020). Recent Trends in Three-Dimensional Bioinks Based on Alginate for Biomedical Applications. Materials, 13(18), 3980. https://doi.org/10.3390/ma13183980