Formation of a Bacteriostatic Surface on ZrNb Alloy via Anodization in a Solution Containing Cu Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
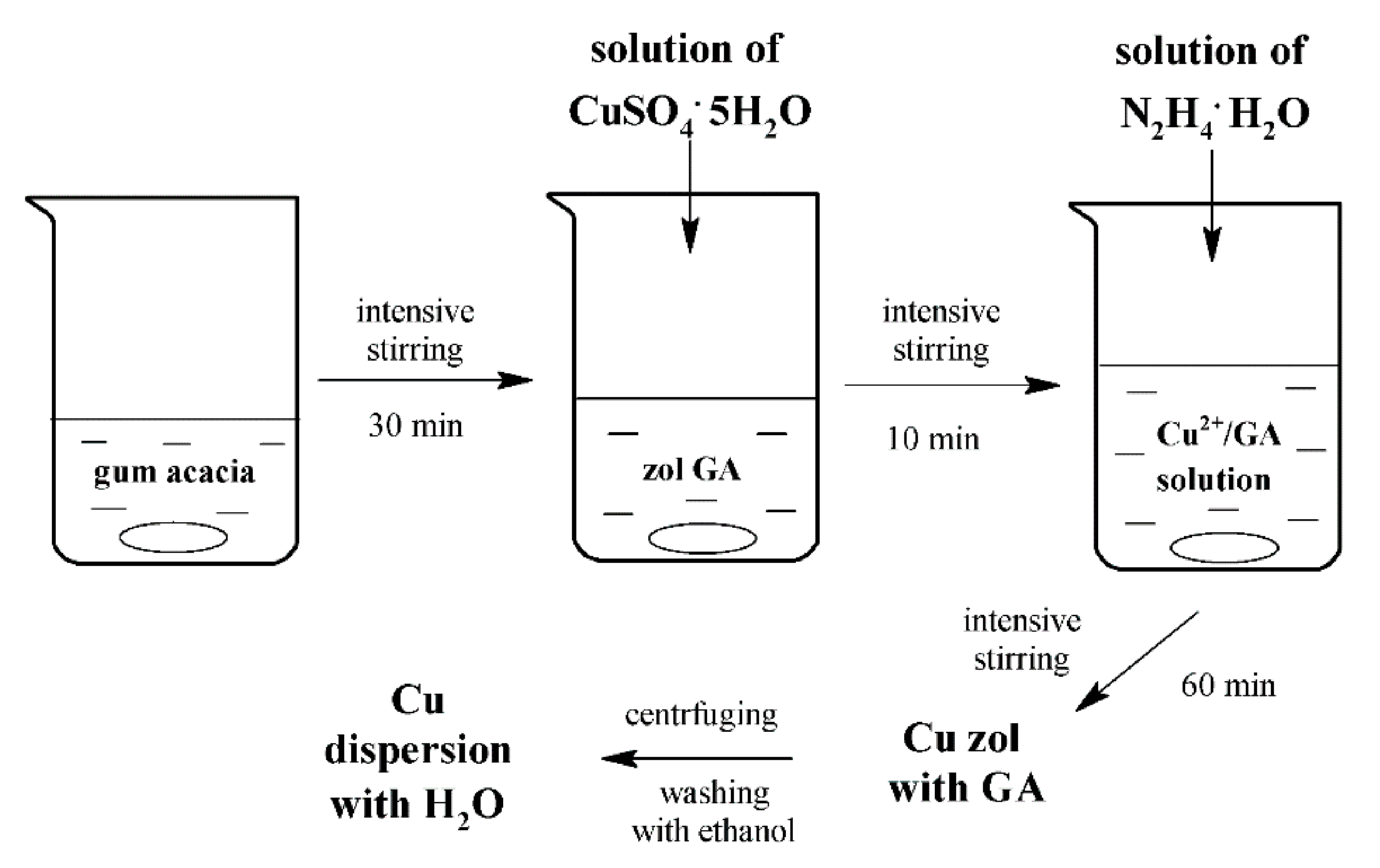
2.2. Synthesis of Cu Nanocrystals
2.3. Nanoparticle Characterization
2.4. Plasma Electrolytic Oxidation (PEO)
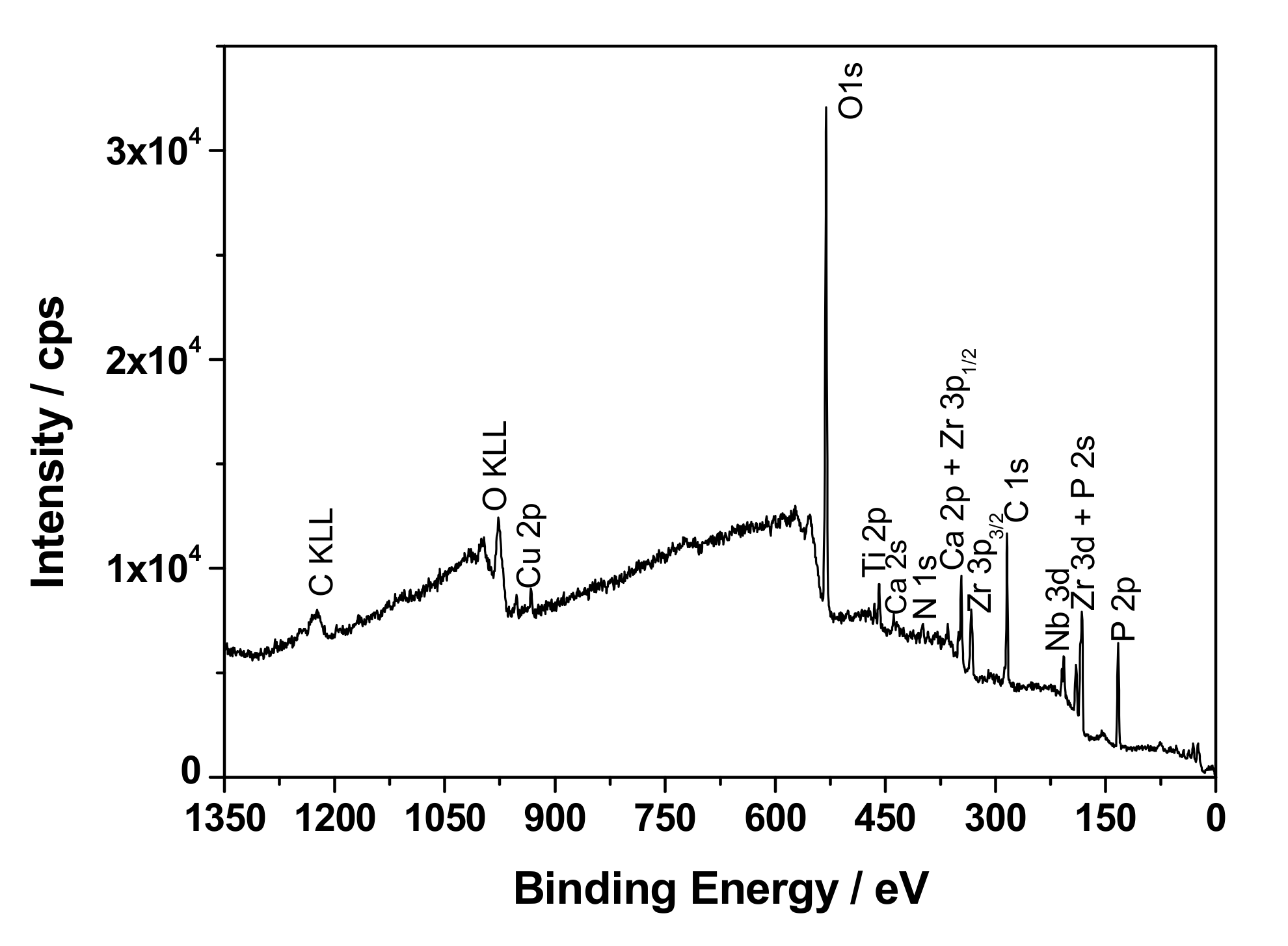
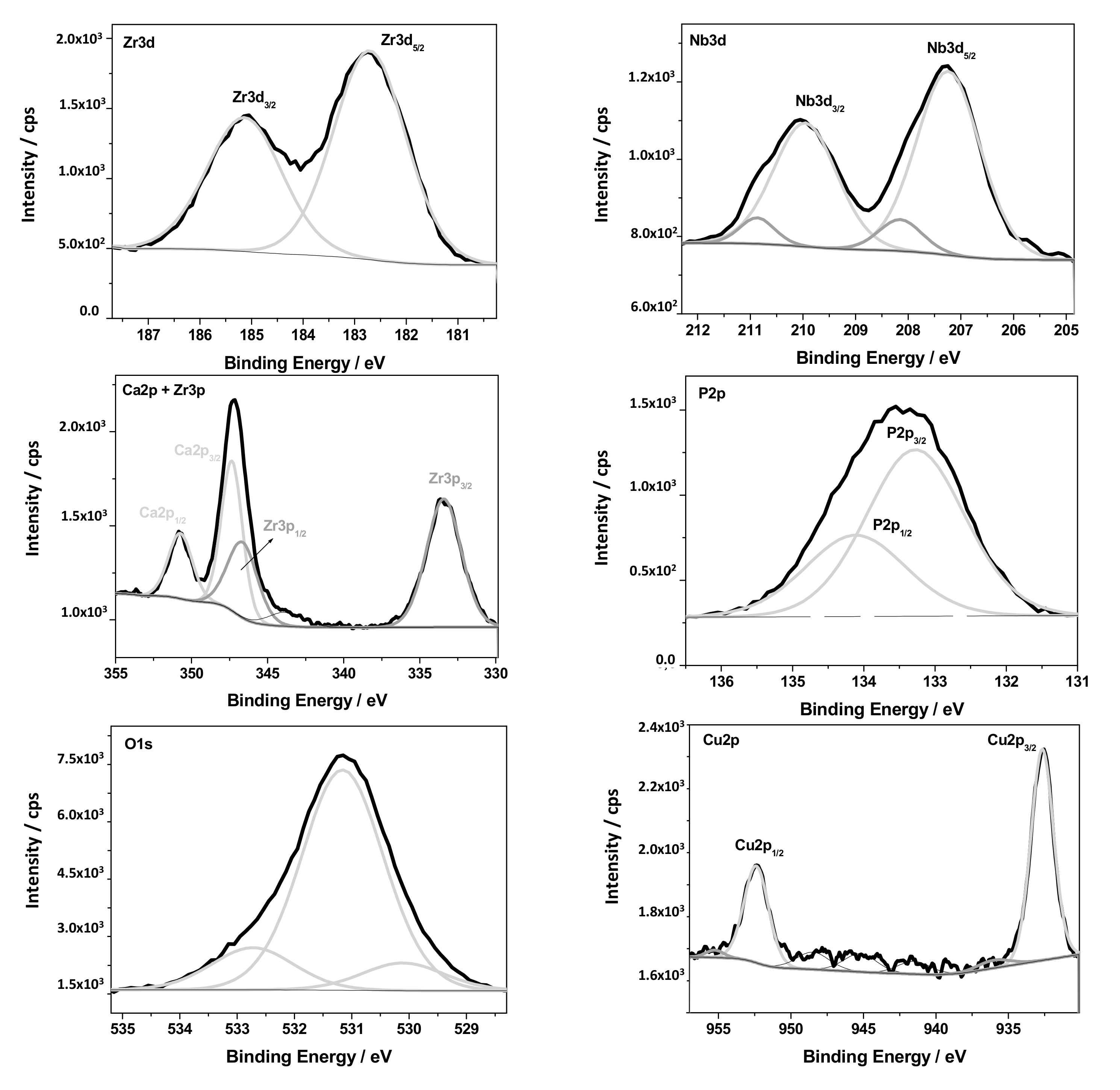
2.5. Surface Analysis
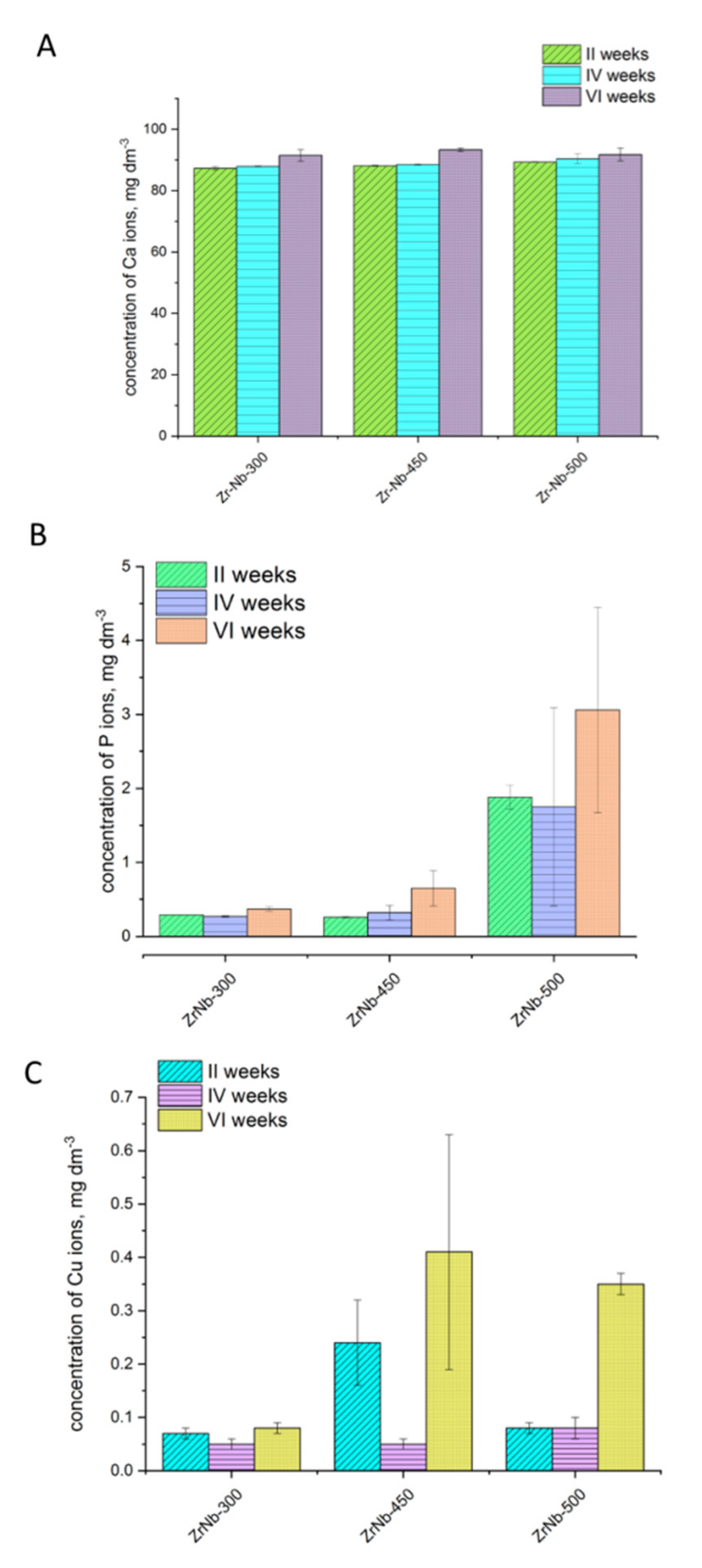
2.6. Analysis of Ion Release from Oxide Coatings
2.7. Cell Culture
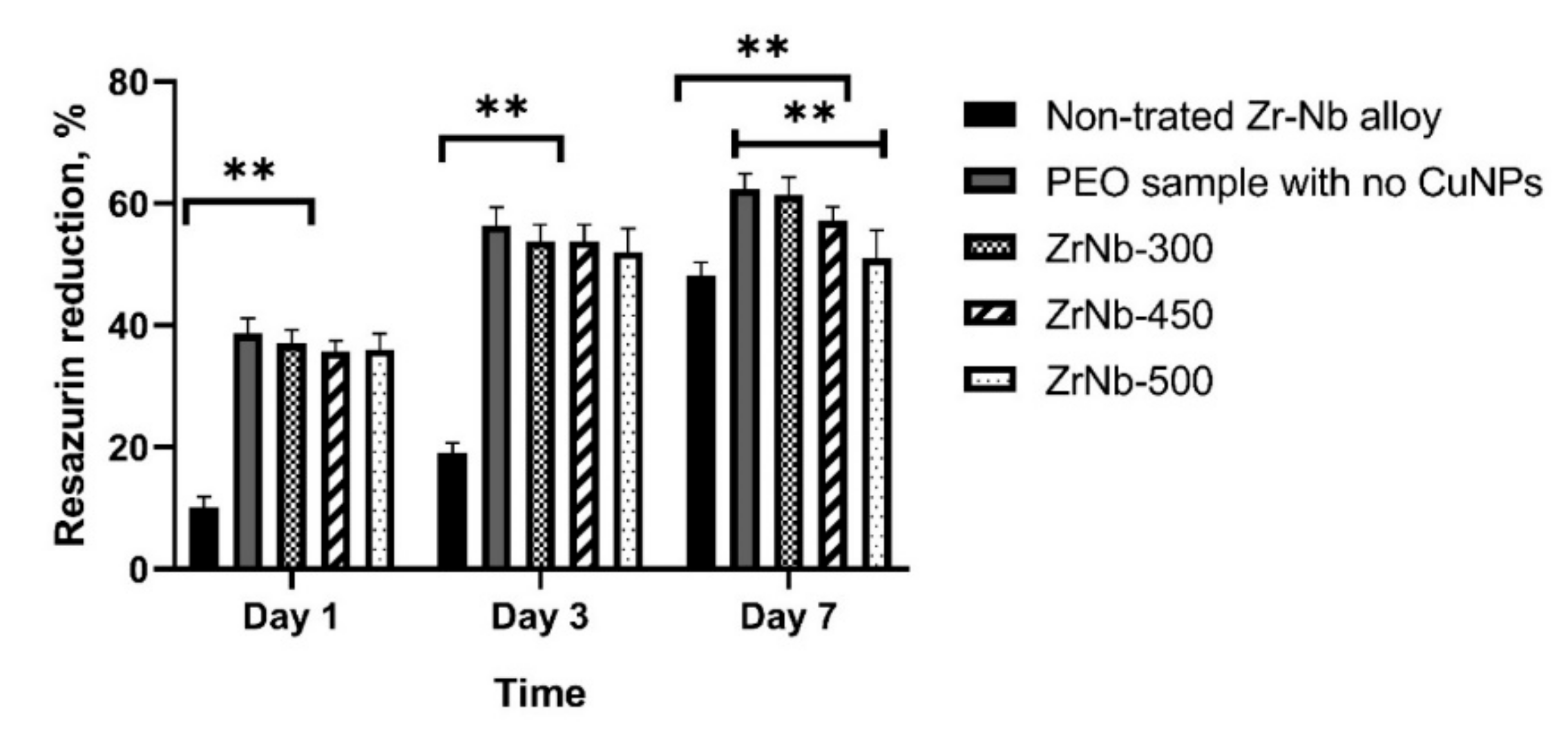
2.8. Bacteria Adhesion
2.9. Statistics
3. Results and Discussion
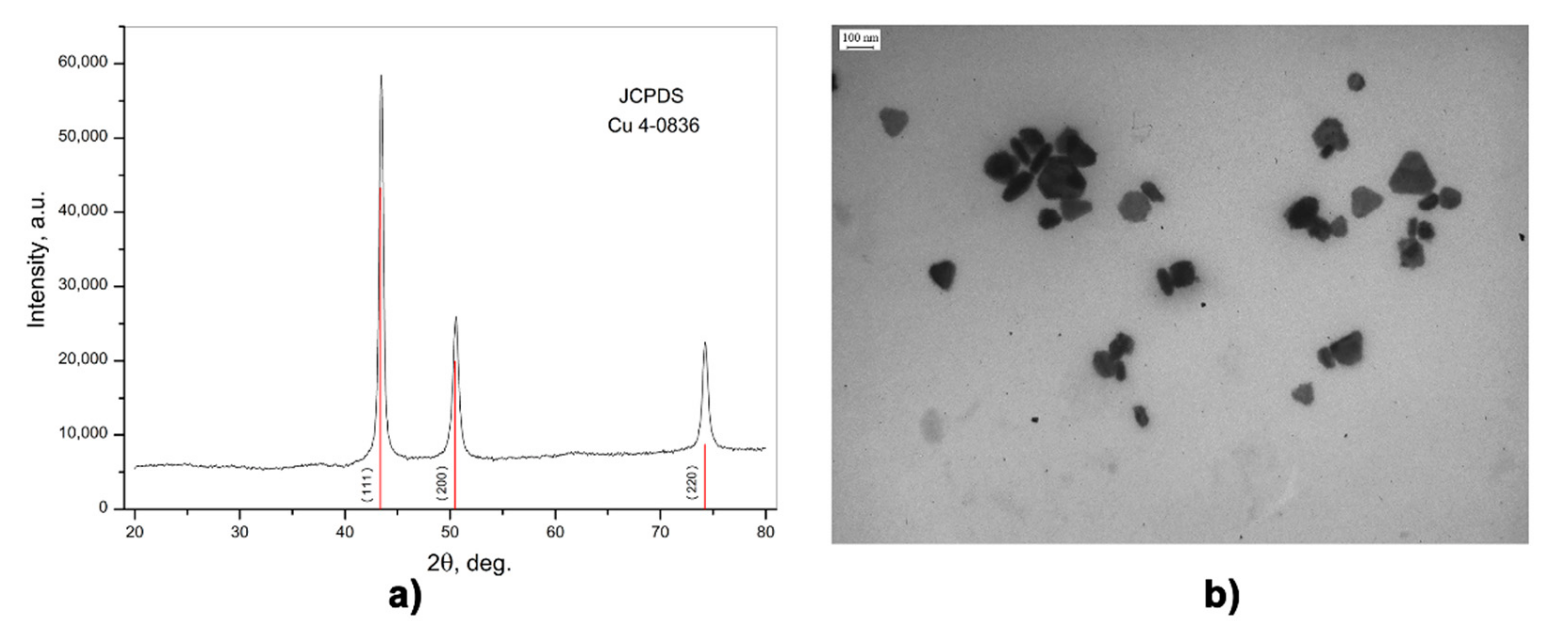
3.1. CuNPs Characterization
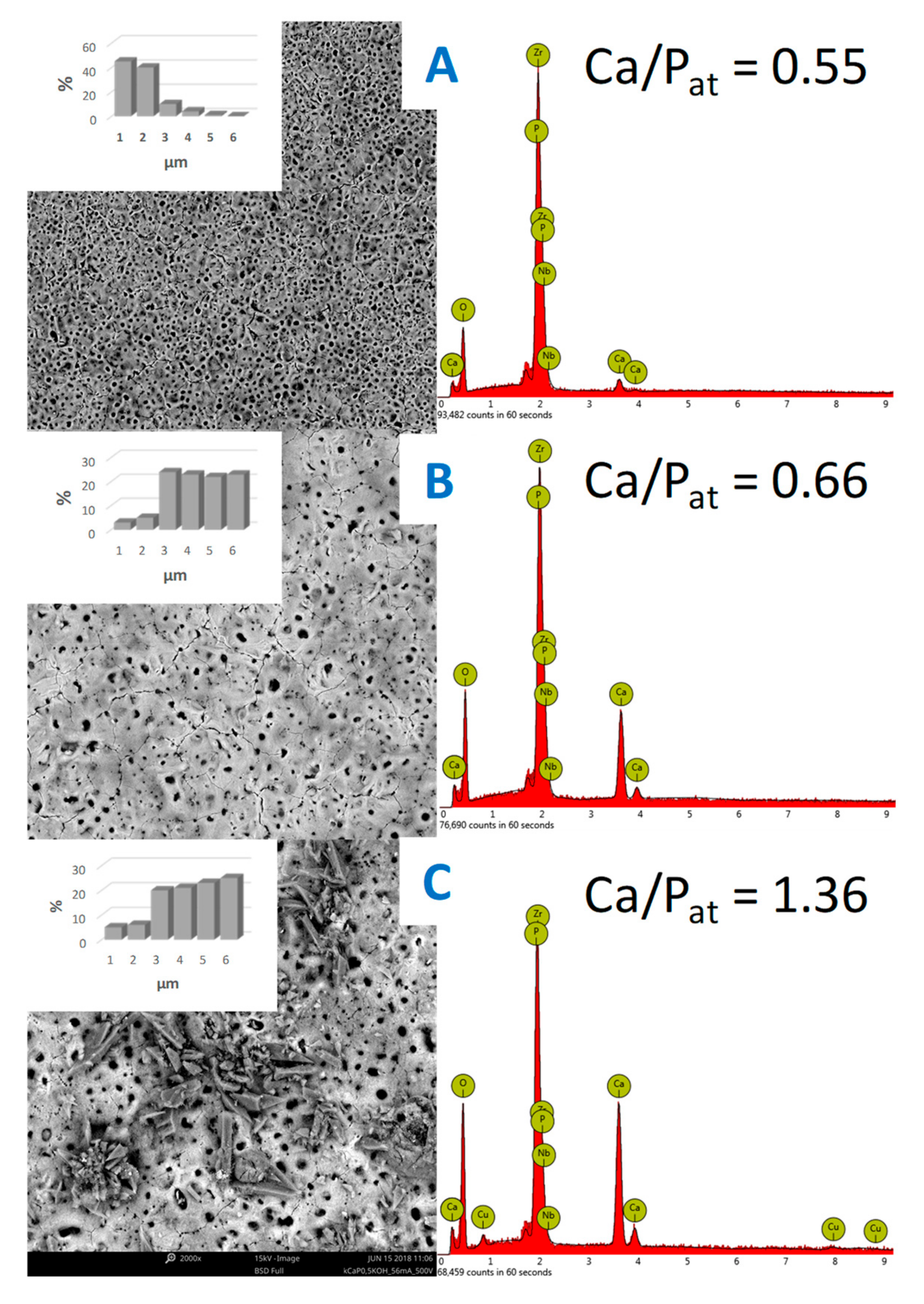
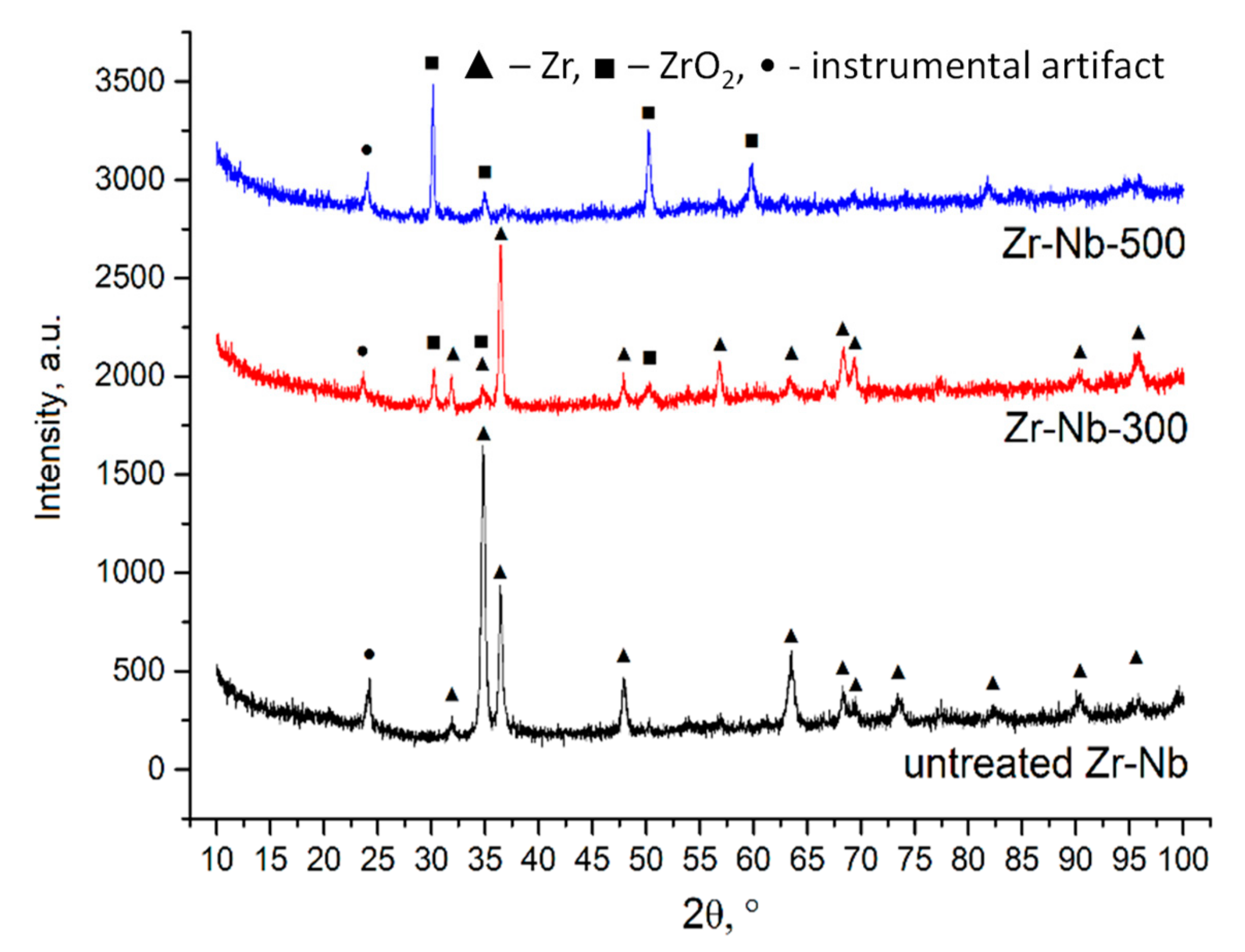
3.2. Structural Characterization
3.3. Analysis of Ion Release
3.4. Cell Culture
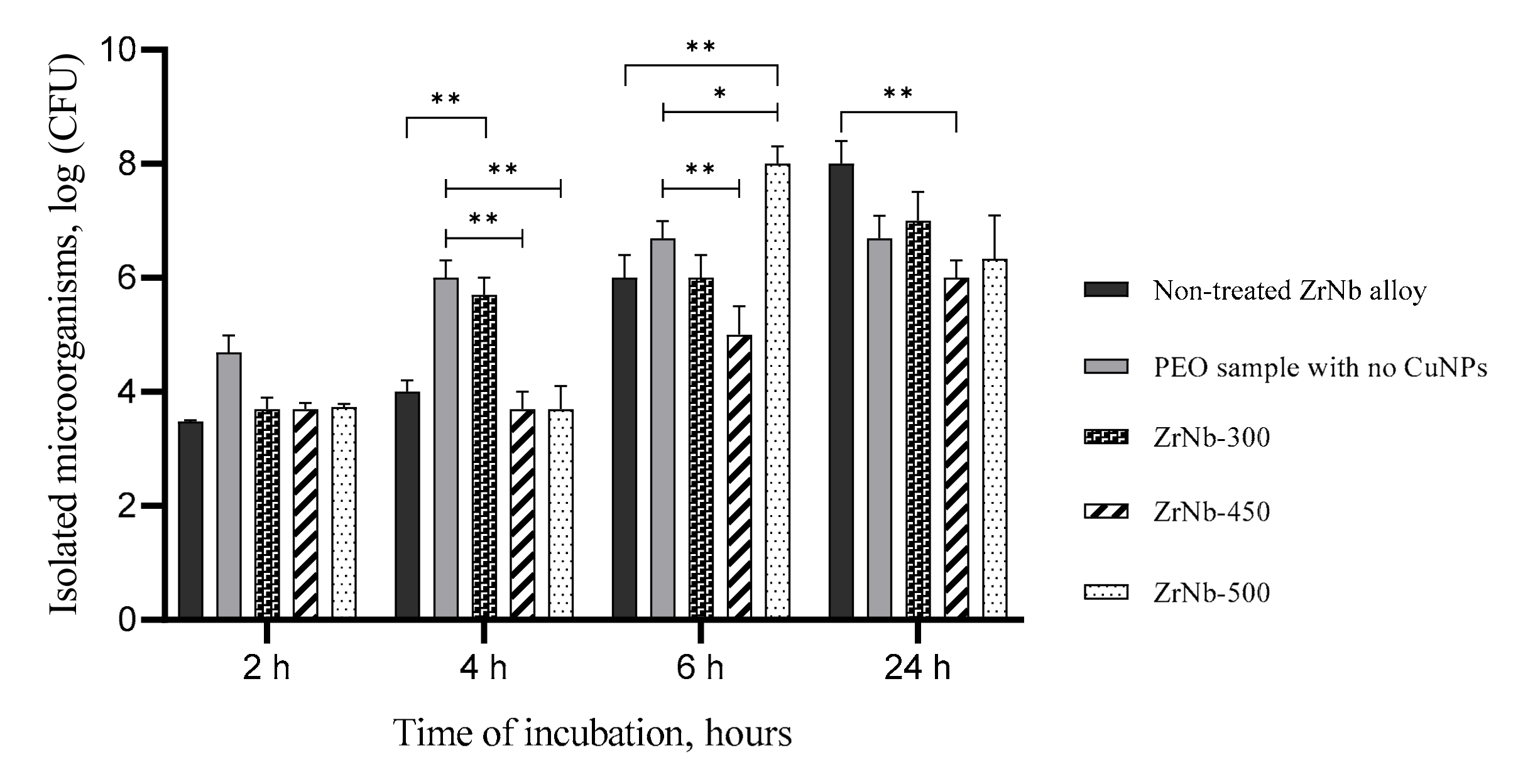
3.5. Bacterial Adhesion Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Insua, A.; Monje, A.; Wang, H.L.; Miron, R.J. Basis of bone metabolism around dental implants during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. Part A 2017, 105, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Liaw, K.; Delfini, R.H.; Abrahams, J.J. Dental Implant Complications. Semin. Ultrasound CT MRI 2015, 36, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kirmanidou, Y.; Sidira, M.; Drosou, M.E.; Bennani, V.; Bakopoulou, A.; Tsouknidas, A.; Michailidis, N.; Michalakis, K. New Ti-Alloys and Surface Modifications to Improve the Mechanical Properties and the Biological Response to Orthopedic and Dental Implants: A Review. BioMed Res. Int. 2016, 2016, 2908570. [Google Scholar] [CrossRef] [PubMed]
- Al-Radha, A.S.D.; Dymock, D.; Younes, C.; O’Sullivan, D. Surface properties of titanium and zirconia dental implant materials and their effect on bacterial adhesion. J. Dent. 2012, 40, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, H.A.; Ayatollahi, M.R.; Asnafi, A. To reduce the maximum stress and the stress shielding effect around a dental implant–bone interface using radial functionally graded biomaterials. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.R.; Zhang, X.P.; He, Z.M.; Liu, Y.H.; Liu, Z.H. Effects of Ce on the short-term biocompatibility of Ti-Fe-Mo-Mn-Nb-Zr alloy for dental materials. J. Mater. Sci. Mater. Med. 2004, 15, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef]
- Zhao, X.L.; Li, L.; Niinomi, M.; Nakai, M.; Zhang, D.L.; Suryanarayana, C. Metastable Zr–Nb alloys for spinal fixation rods with tunable Young’s modulus and low magnetic resonance susceptibility. Acta Biomater. 2017, 62, 372–384. [Google Scholar] [CrossRef]
- Mishchenko, O.; Ovchynnykov, O.; Kapustian, O.; Pogorielov, M. New Zr-Ti-Nb alloy for medical application: Development, chemical and mechanical properties, and biocompatibility. Materials 2020, 13, 1306. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of dental prosthesis and restorative materials interface on oral biofilms. Int. J. Mol. Sci. 2018, 19, 3157. [Google Scholar] [CrossRef]
- Patel, A.R.; Patra, F.; Shah, N.P.; Shukla, D. Biological control of mycotoxins by probiotic lactic acid bacteria. Dyn. Dairy Ind. Consum. Demands 2017, 2015, 2–4. [Google Scholar] [CrossRef]
- Ehlers, M.R.; Todd, R.M. Genesis and Maintenance of Attentional Biases: The Role of the Locus Coeruleus-Noradrenaline System. Neural Plast. 2017, 1, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ge, Y.; Wu, Y.; Chen, J.; Xu, H.H.; Yang, M.; Li, M.; Ren, B.; Feng, M.; Weir, M.D.; et al. Anti-Bacterial and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate. Molecules 2017, 22, 2013. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Ortega, E.; Ortiz-García, I.; Jiménez-Guerra, A.; Monsalve-Guil, L.; Muñoz-Guzón, F.; Perez, R.A.; Gil, F.J. Comparison between sandblasted acid-etched and oxidized titanium dental implants: In vivo study. Int. J. Mol. Sci. 2019, 20, 3267. [Google Scholar] [CrossRef] [PubMed]
- Michalska, J.; Sowa, M.; Piotrowska, M.; Widziołek, M.; Tylko, G.; Dercz, G.; Socha, R.P.; Osyczka, A.M.; Simka, W. Incorporation of Ca ions into anodic oxide coatings on the Ti-13Nb-13Zr alloy by plasma electrolytic oxidation. Mater. Sci. Eng. C 2019, 104, 109957. [Google Scholar] [CrossRef]
- Polo, T.O.B.; da Silva, W.P.; Momesso, G.A.C.; Lima-Neto, T.J.; Barbosa, S.; Cordeiro, J.M.; Hassumi, J.S.; da Cruz, N.C.; Okamoto, R.; Barão, V.A.; et al. Plasma Electrolytic Oxidation as a Feasible Surface Treatment for Biomedical Applications: An in vivo study. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Yeung, W.K.; Reilly, G.C.; Matthews, A.; Yerokhin, A. In vitro biological response of plasma electrolytically oxidized and plasma-sprayed hydroxyapatite coatings on Ti-6Al-4V alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 939–949. [Google Scholar] [CrossRef]
- Chung, C.J.; Su, R.T.; Chu, H.J.; Chen, H.T.; Tsou, H.K.; He, J.L. Plasma electrolytic oxidation of titanium and improvement in osseointegration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1023–1030. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vazquez-Rodríguez, A.; Montelongo-Peralta, L.Z.; Treviño-Gonzalez, M.T.; Castro, E.D.B.; Saucedo-Salazar, E.M.; Morales, R.C.; Soto, D.R.; González, F.T.; et al. In vivo antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using acacia rigidula as a reducing and capping agent. Int. J. Nanomed. 2018, 13, 2349–2363. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Zaręba, T.; Szaraniec, B.; Pałka, K.; Mielczarek, A.; Menaszek, E.; Tyski, S. In vitro studies of nanosilver-doped titanium implants for oral and maxillofacial surgery. Int. J. Nanomed. 2017, 12, 4285–4297. [Google Scholar] [CrossRef]
- Molteni, C.; Abicht, H.K.; Solioz, M. Killing of bacteria by copper surfaces involves dissolved copper. Appl. Environ. Microbiol. 2010, 76, 4099–4101. [Google Scholar] [CrossRef]
- Oleshko, O.; Deineka, V.V.; Husak, Y.; Korniienko, V.; Mishchenko, O.; Holubnycha, V.; Pisarek, M.; Michalska, J.; Kazek-Kęsik, A.; Jakóbik-Kolon, A.; et al. Ag nanoparticle-decorated oxide coatings formed via plasma electrolytic oxidation on ZrNb alloy. Materials 2019, 12, 3742. [Google Scholar] [CrossRef] [PubMed]
- Chawla, P.; Kumar, N.; Bains, A.; Dhull, S.B.; Kumar, M.; Kaushik, R.; Punia, S. Gum arabic capped copper nanoparticles: Synthesis, characterization, and applications. Int. J. Biol. Macromol. 2020, 146, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Leśniak-Ziółkowska, K.; Kazek-Kęsik, A.; Rokosz, K.; Raaen, S.; Stolarczyk, A.; Krok-Borkowicz, M.; Pamuła, E.; Gołda-Cępa, M.; Brzychczy-Włoch, M.; Simka, W. Electrochemical modification of the Ti-15Mo alloy surface in solutions containing ZnO and Zn3(PO4)2 particles. Mater. Sci. Eng. C 2020, 115, 111098. [Google Scholar] [CrossRef]
- Penido, M.G.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatric Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Martínez-Campos, E.; Mohedano, M.; Martínez-Corriá, R.; Ramos, V.; Arrabal, R.; Matykina, E. In vitro and in vivo evaluation of PEO-modified titanium for bone implant applications. Surf. Coat. Technol. 2018, 347, 358–368. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Kalemba-Rec, I.; Simka, W. Anodization of a Medical-Grade Ti-6Al-7Nb Alloy in a Ca(H2PO2)2-Hydroxyapatite Suspension. Materials 2019, 12, 3002. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D. 1.111–Bioactive Ceramics: Physical Chemistry. Compr. Biomater. 2011, 1, 187–281. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- Simka, W.; Socha, R.P.; Dercz, G.; Michalska, J.; Maciej, A.; Krząkała, A. Anodic oxidation of Ti–13Nb–13Zr alloy in silicate solutions. Appl. Surf. Sci. 2013, 279, 317–323. [Google Scholar] [CrossRef]
- Rafieerad, A.R.; Ashra, M.R.; Mahmoodian, R.; Bushroa, A.R. Surface characterization and corrosion behavior of calcium phosphate-base composite layer on titanium and its alloys via plasma electrolytic oxidation: A review paper. Mater. Sci. Eng. C 2015, 57, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Bigham, A.; Saudi, A.; Rafienia, M.; Rahmati, S.; Bakhtiyari, H.; Salahshouri, F.; Sattary, M.; Hassanzadeh-Tabrizi, S.A. Electrophoretically deposited mesoporous magnesium silicate with ordered nanopores as an antibiotic-loaded coating on surface-modified titanium. Mater. Sci. Eng. C 2019, 96, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Holubnycha, V.; Pogorielov, M.; Korniienko, V.; Kalinkevych, O.; Ivashchenko, O.; Peplinska, B.; Jarek, M. Antibacterial activity of the new copper nanoparticles and Cu NPs/chitosan solution. In Proceedings of the IEEE 7th International Conference on Nanomaterials: Applications and Properties, NAP 2017, Zatoka, Ukraine, 10–15 September 2017. [Google Scholar] [CrossRef]
- Sowa, M.; Simka, W. Effect of DC plasma electrolytic oxidation on surface characteristics and corrosion resistance of zirconium. Materials 2018, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.H.K.; Weir, M.D.; Ge, Y.; Li, M.; Wang, S.; Li, Y.; Xu, X.; et al. Effect of antibacterial dental adhesive on multispecies biofilms formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef]
- Grischke, J.; Eberhard, J.; Stiesch, M. Antimicrobial dental implant functionalization strategies—A systematic review. Dent. Mater. J. 2016, 35, 545–558. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Necula, B.S.; van Leeuwen, J.P.T.M.; Fratila-Apachitei, L.E.; Zaat, S.A.J.; Apachitei, I.; Duszczyk, J. In vitro cytotoxicity evaluation of porous TiO2-Ag antibacterial coatings for human fetal osteoblasts. Acta Biomater. 2012, 8, 4191–4197. [Google Scholar] [CrossRef]
| Sample | Contact Angle, ° |
|---|---|
| ZrNb-300 | 57.4 ± 1.9 |
| ZrNb-450 | 40.7 ± 4.3 |
| ZrNb-500 | 28.7 ± 1.4 |
| Binding Energy/eV High Resolution Spectra | Chemical Bonds/States | Chemical Composition/at.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nb3d5/2 | Zr3d5/2 | Ti2p3/2 | Cu2p3/2 | P2p3/2 | Ca2p3/2 | O1s | C1s | N1s | ||
| 207.2 | 182.7 | 458.7 | 932.7 | 530.1 | Me-O | Nb—1.2 | ||||
| C—28.4 | ||||||||||
| 133.3 | 347.4 | 531.2 | 288.9 | PO43−, CO32− | Ca—3.0 | |||||
| P—14.5 | ||||||||||
| Zr—3.9 | ||||||||||
| 284.5 | C-C | N—1.5 | ||||||||
| 285.7 | 399.6 | C-O/C-N | Ti—2.0 | |||||||
| 532.7 | 287.4 | C=O | O—44.7 | |||||||
| 401.3 | NHx | Cu—1.0 | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korniienko, V.; Oleshko, O.; Husak, Y.; Deineka, V.; Holubnycha, V.; Mishchenko, O.; Kazek-Kęsik, A.; Jakóbik-Kolon, A.; Pshenychnyi, R.; Leśniak-Ziółkowska, K.; et al. Formation of a Bacteriostatic Surface on ZrNb Alloy via Anodization in a Solution Containing Cu Nanoparticles. Materials 2020, 13, 3913. https://doi.org/10.3390/ma13183913
Korniienko V, Oleshko O, Husak Y, Deineka V, Holubnycha V, Mishchenko O, Kazek-Kęsik A, Jakóbik-Kolon A, Pshenychnyi R, Leśniak-Ziółkowska K, et al. Formation of a Bacteriostatic Surface on ZrNb Alloy via Anodization in a Solution Containing Cu Nanoparticles. Materials. 2020; 13(18):3913. https://doi.org/10.3390/ma13183913
Chicago/Turabian StyleKorniienko, Viktoriia, Oleksandr Oleshko, Yevheniia Husak, Volodymyr Deineka, Viktoriia Holubnycha, Oleg Mishchenko, Alicja Kazek-Kęsik, Agata Jakóbik-Kolon, Roman Pshenychnyi, Katarzyna Leśniak-Ziółkowska, and et al. 2020. "Formation of a Bacteriostatic Surface on ZrNb Alloy via Anodization in a Solution Containing Cu Nanoparticles" Materials 13, no. 18: 3913. https://doi.org/10.3390/ma13183913
APA StyleKorniienko, V., Oleshko, O., Husak, Y., Deineka, V., Holubnycha, V., Mishchenko, O., Kazek-Kęsik, A., Jakóbik-Kolon, A., Pshenychnyi, R., Leśniak-Ziółkowska, K., Kalinkevich, O., Kalinkevich, A., Pisarek, M., Simka, W., & Pogorielov, M. (2020). Formation of a Bacteriostatic Surface on ZrNb Alloy via Anodization in a Solution Containing Cu Nanoparticles. Materials, 13(18), 3913. https://doi.org/10.3390/ma13183913








