Study on Hydrogen Diffusion Behavior during Welding of Heavy Plate
Abstract
1. Introduction
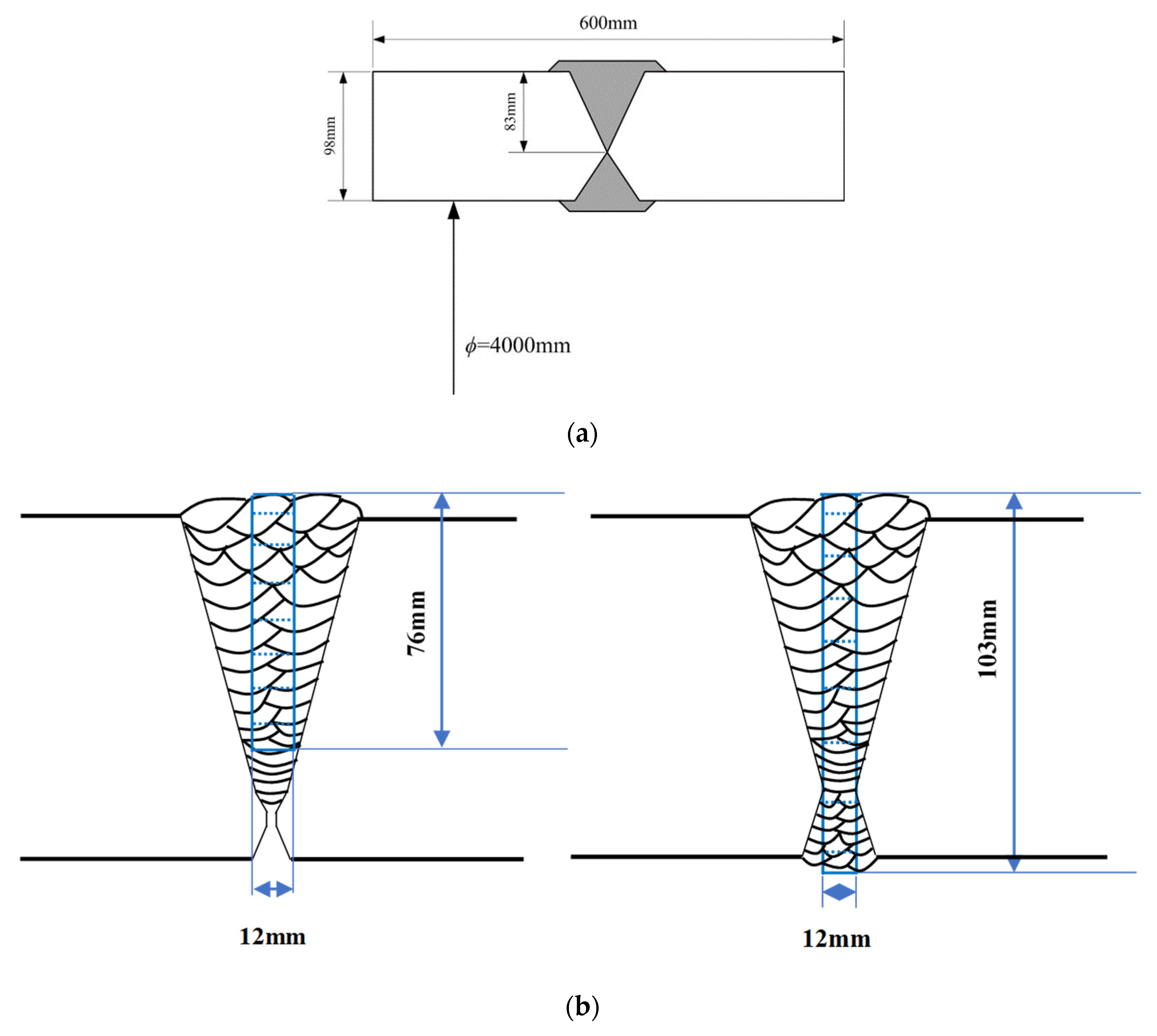
2. Materials and Experiment
3. Numerical Modelling
3.1. Numerical Approach
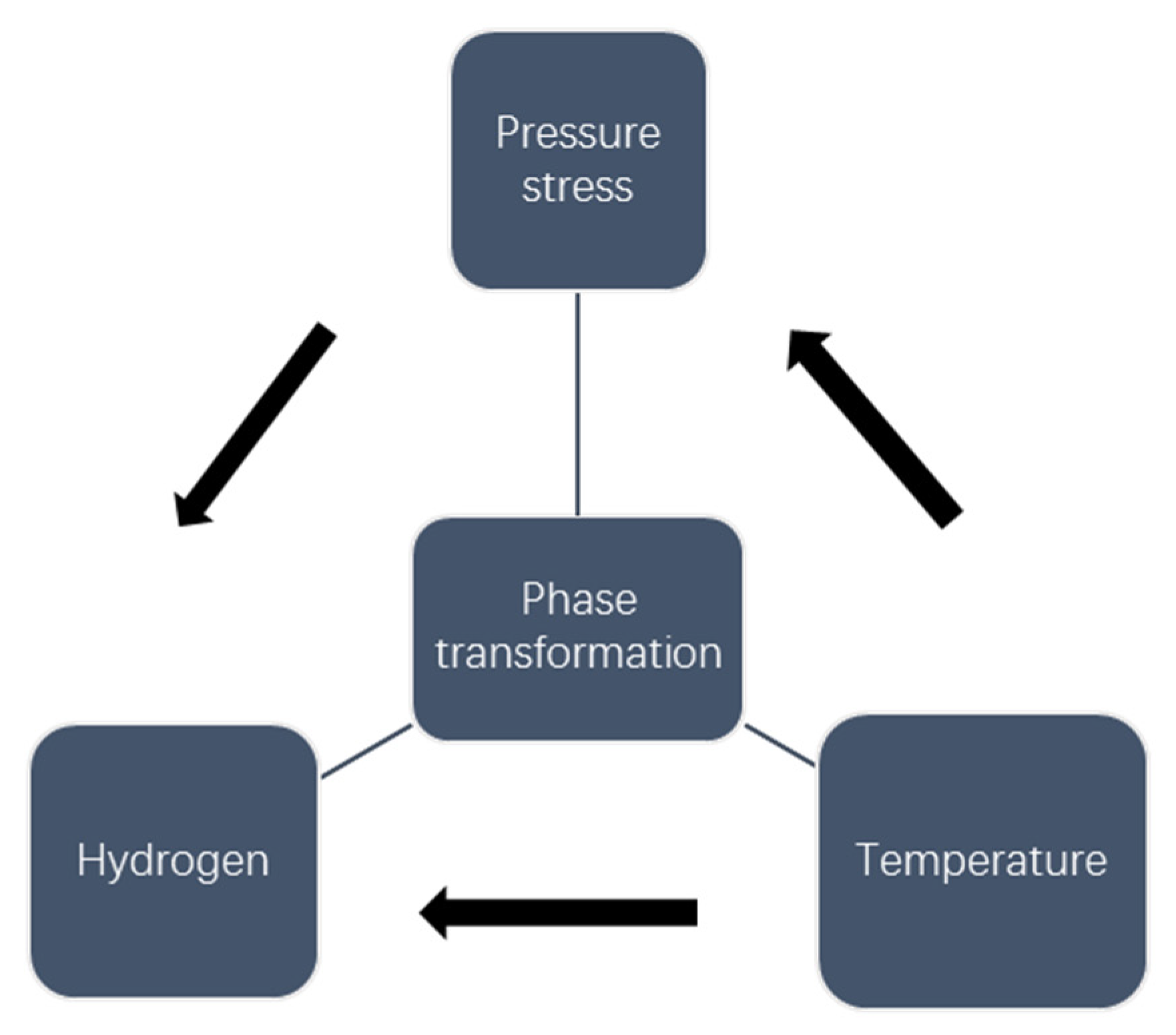
3.2. Phase Transformation
3.3. Simulation Procedure
3.3.1. Thermal Analysis
3.3.2. Mechanical Analysis
3.3.3. Hydrogen Diffusion Analysis
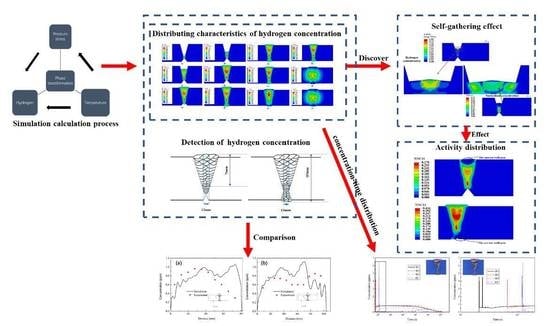
4. Results
4.1. Simulation Results of Multi-Pass Welding of Heavy Plate
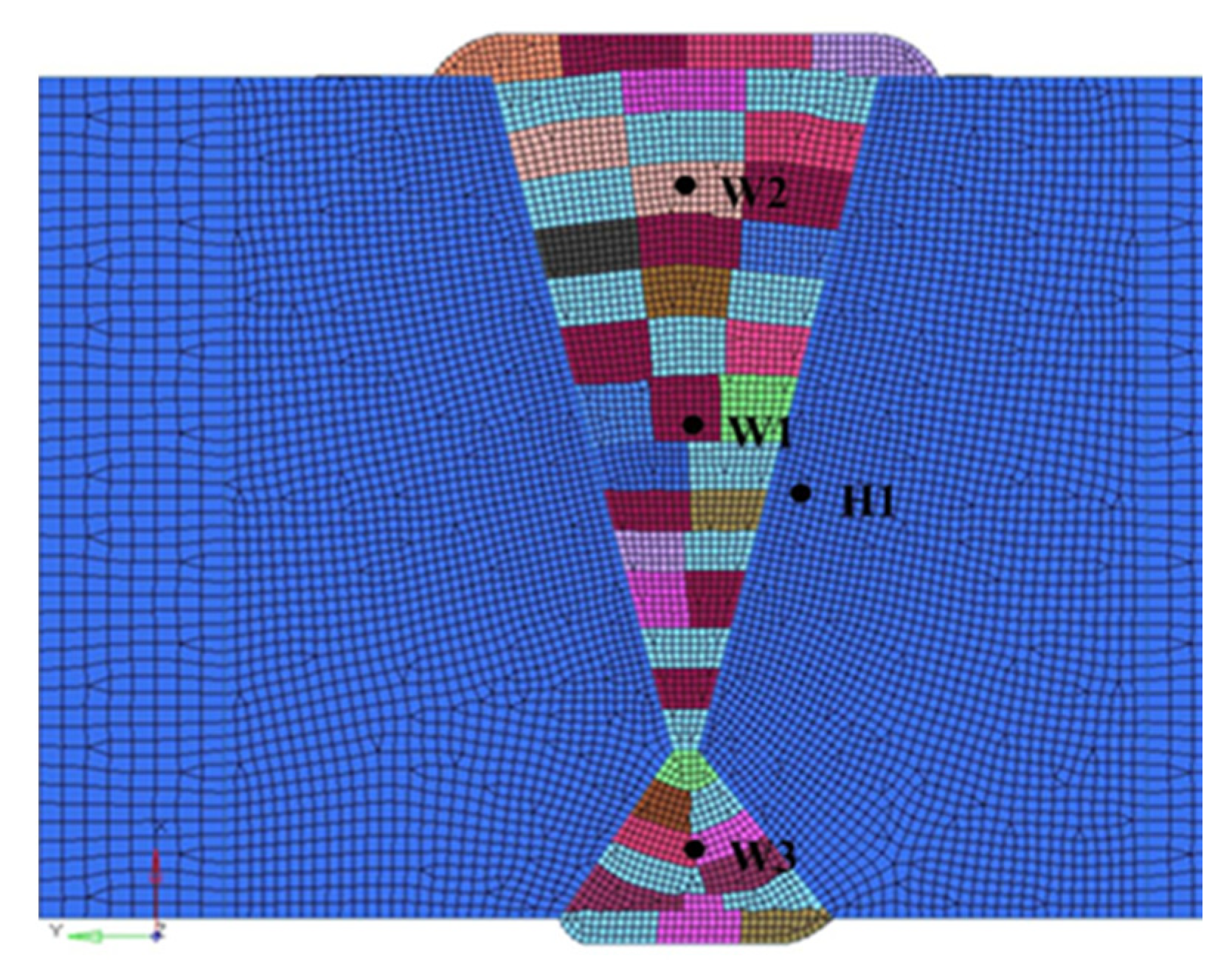
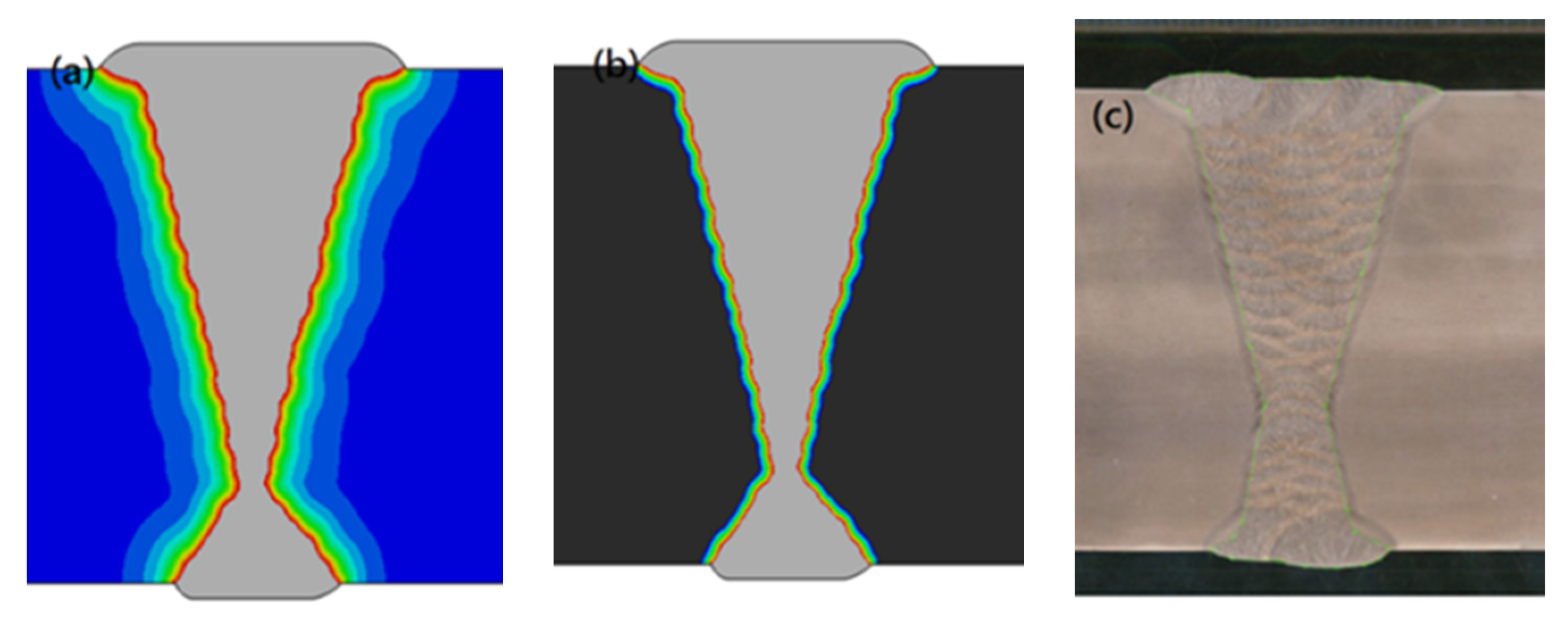
4.1.1. Welding Temperature Field
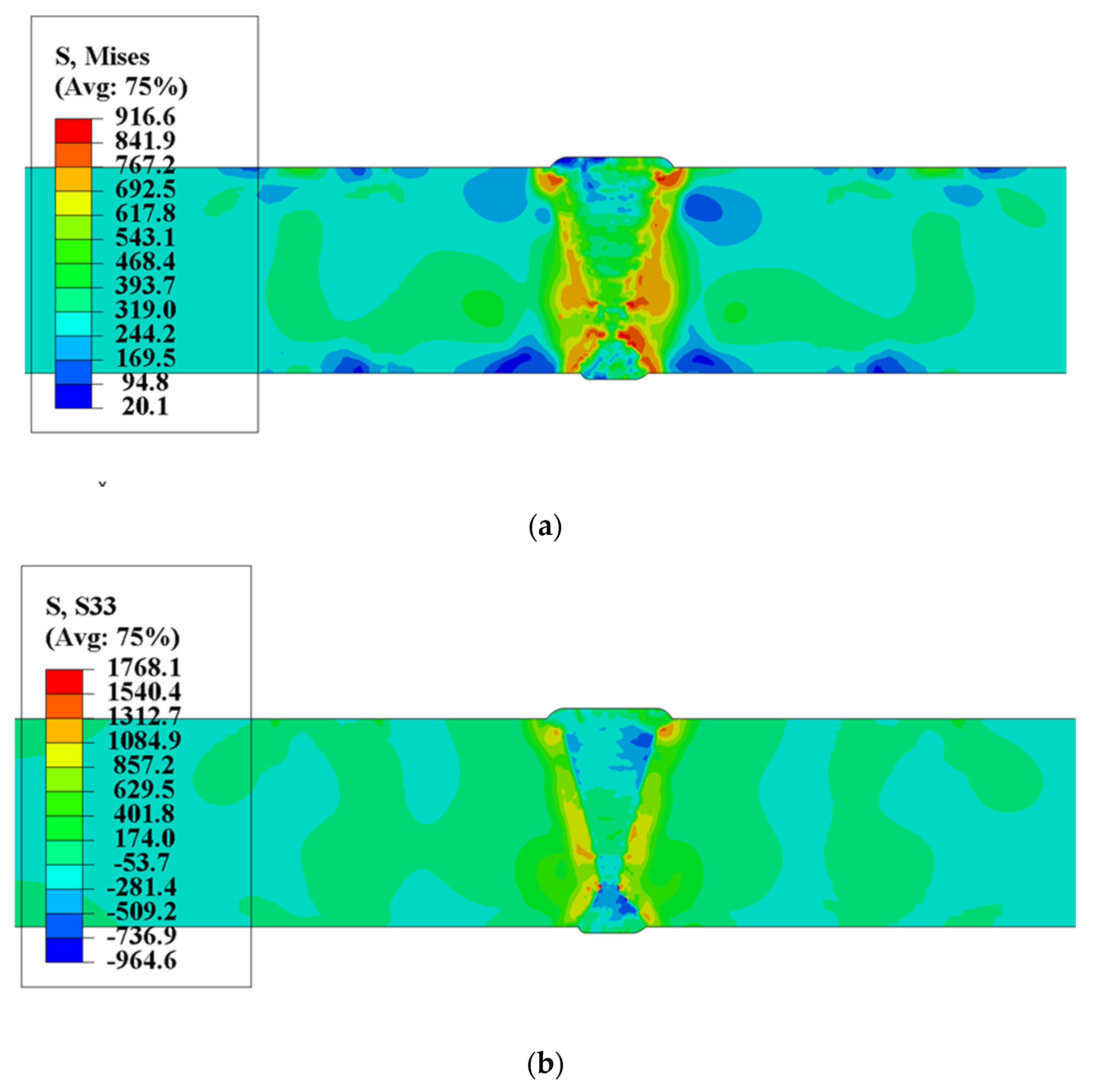
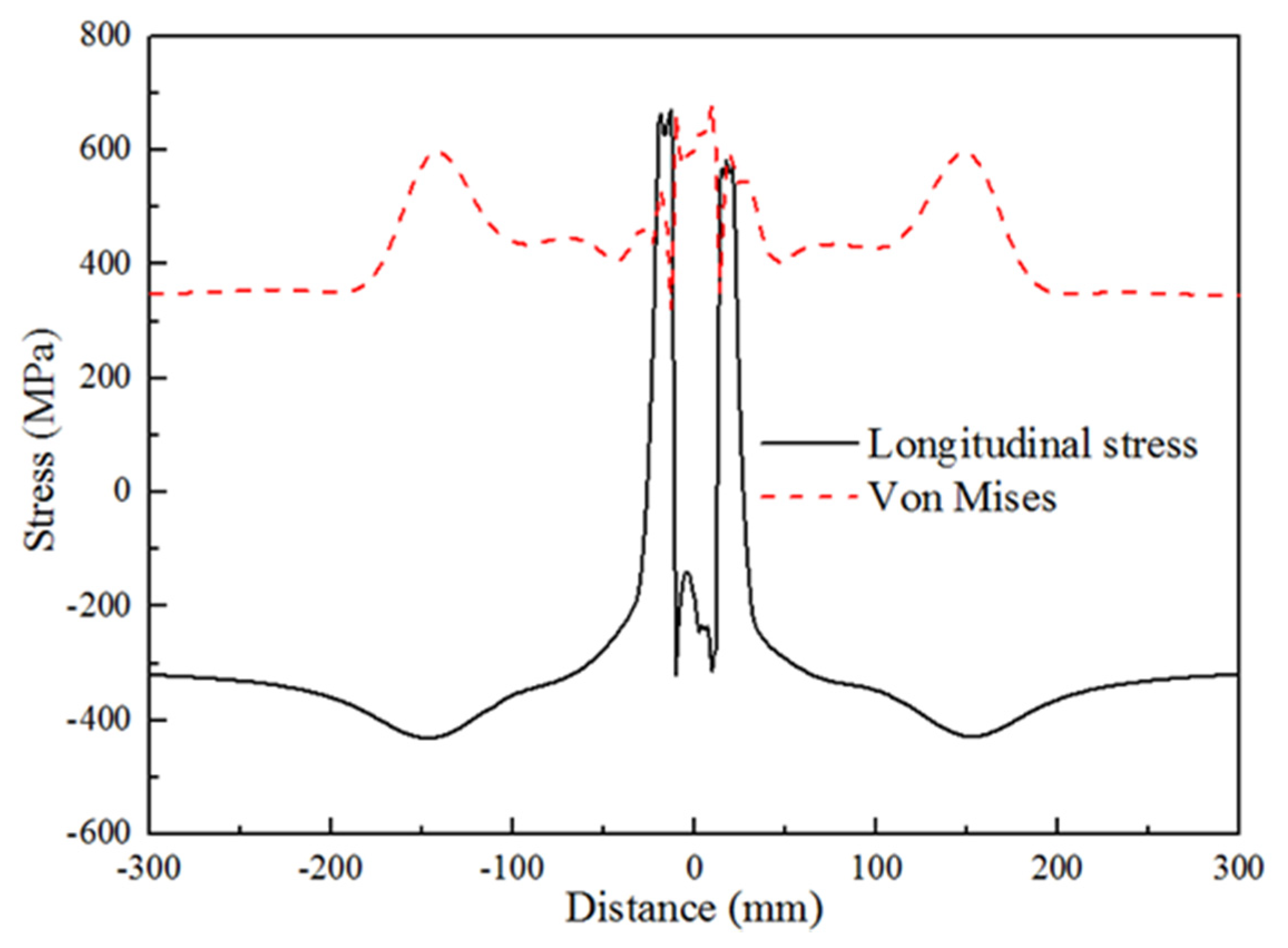
4.1.2. Residual Stress Distribution
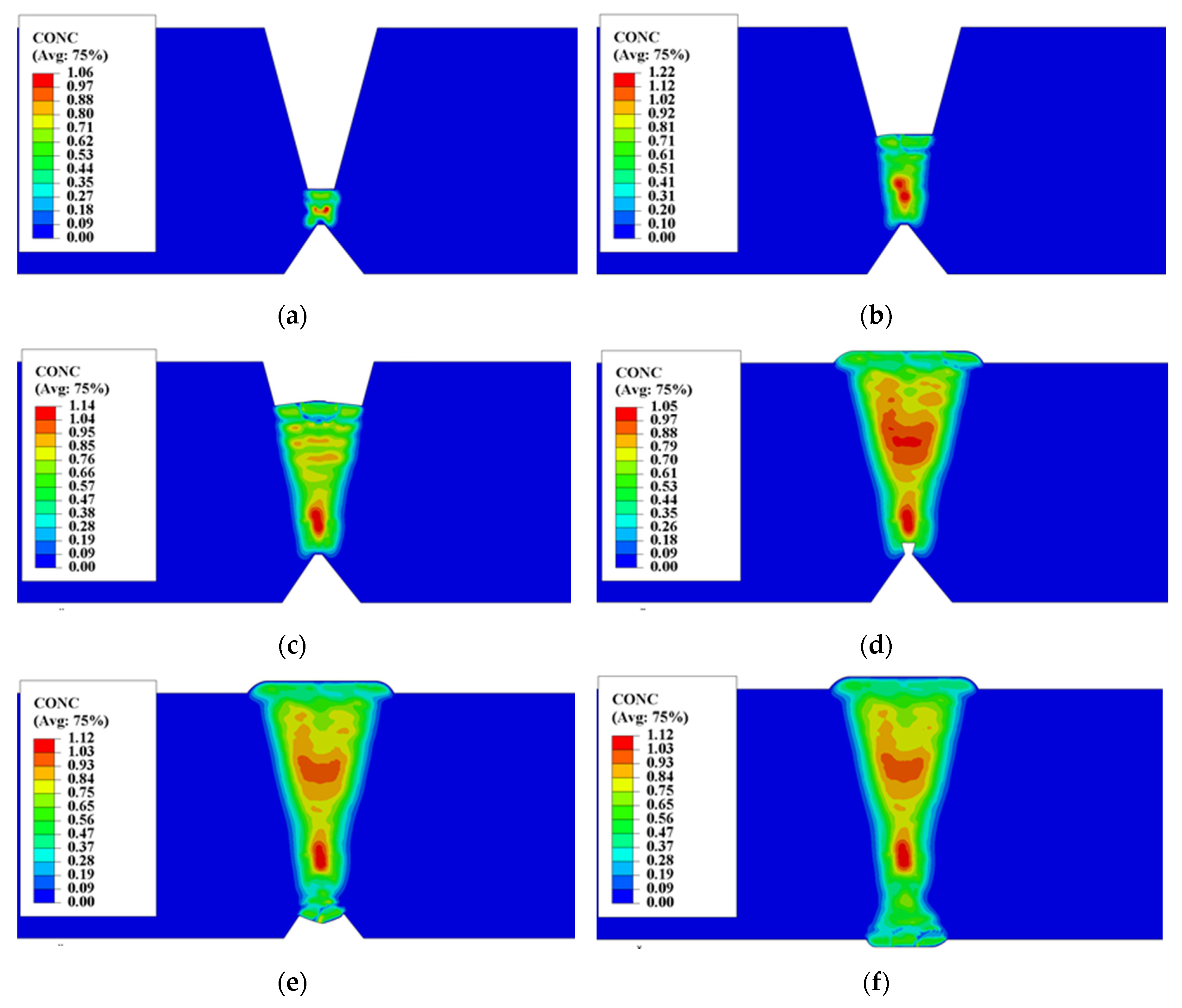
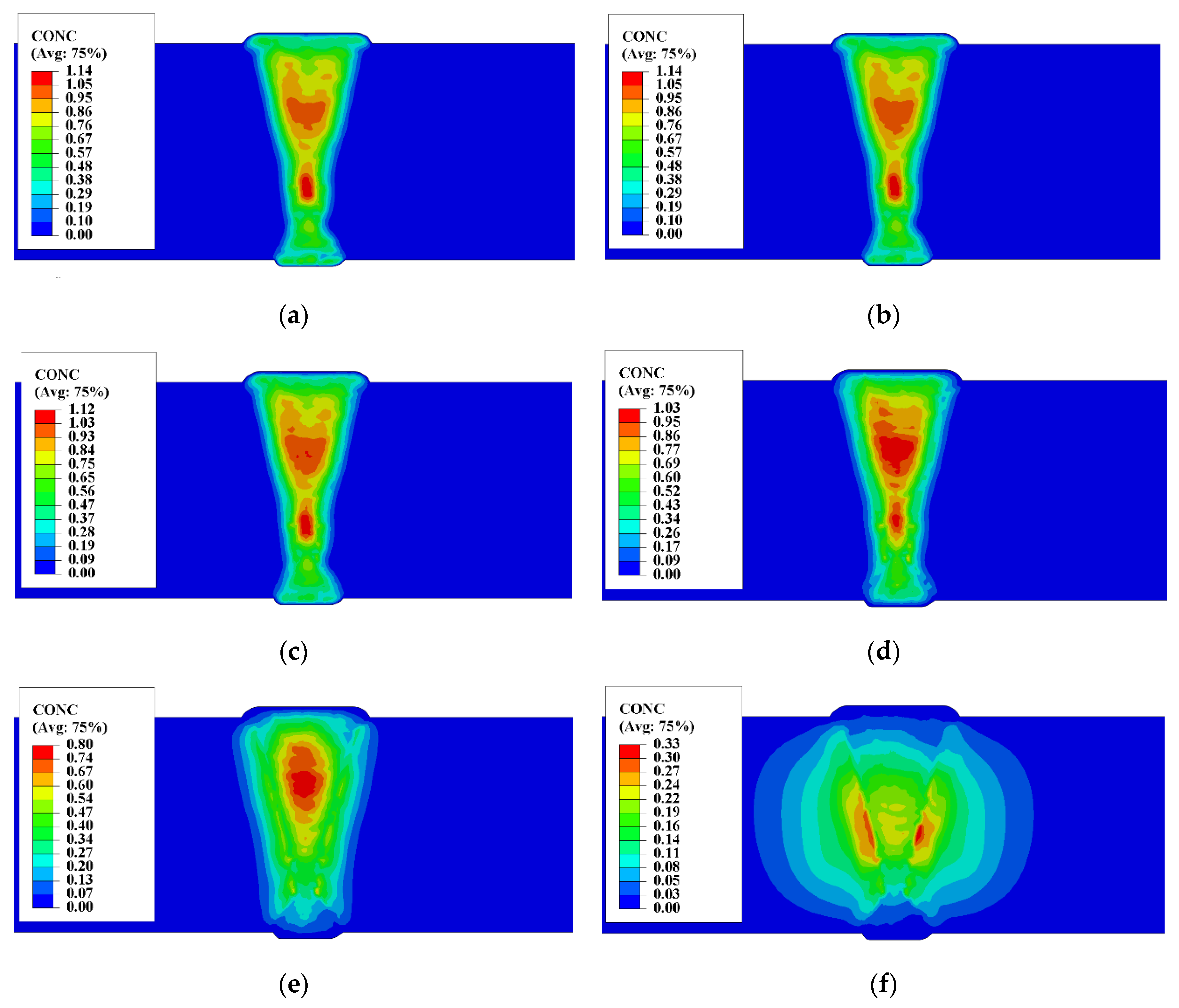
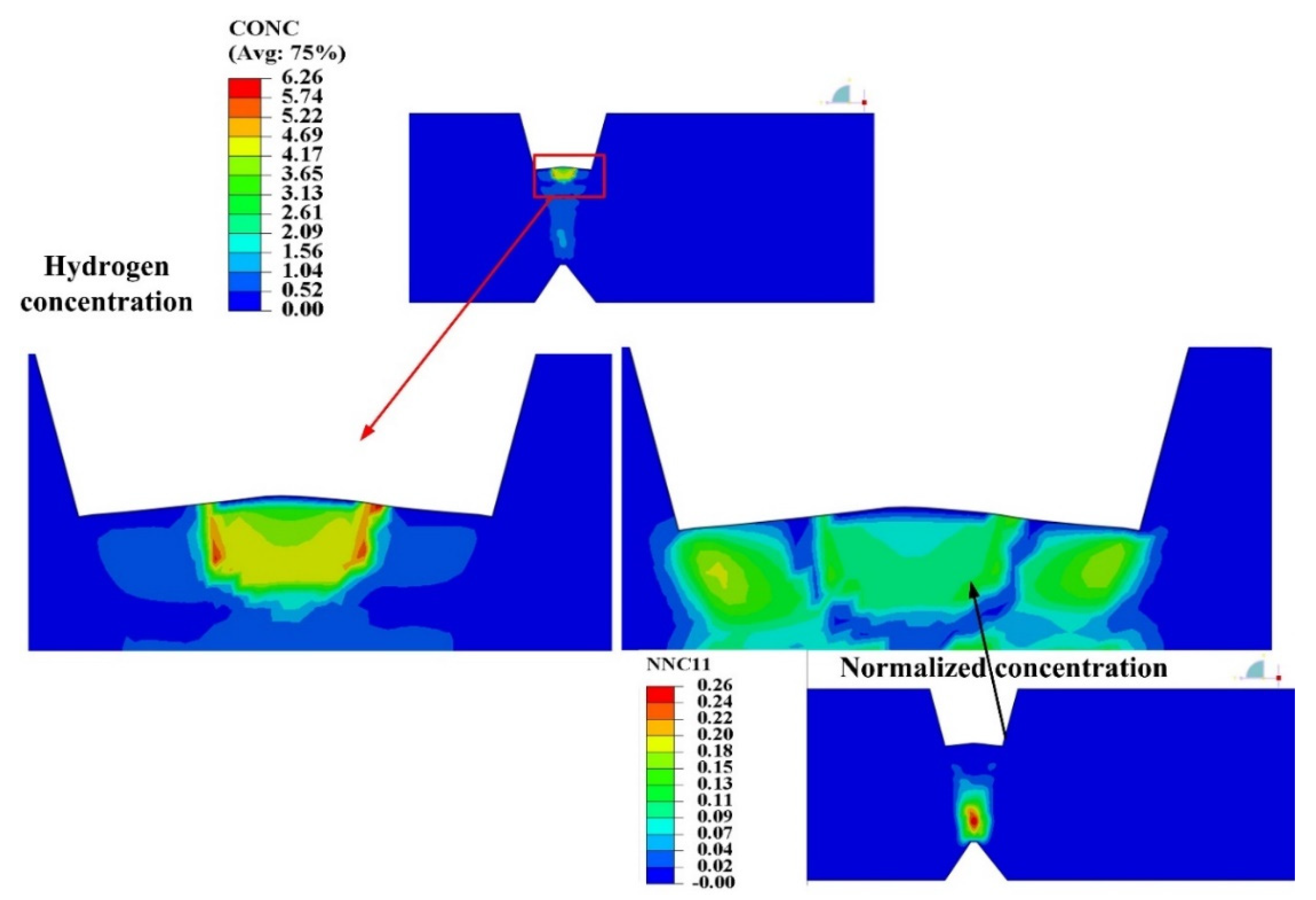
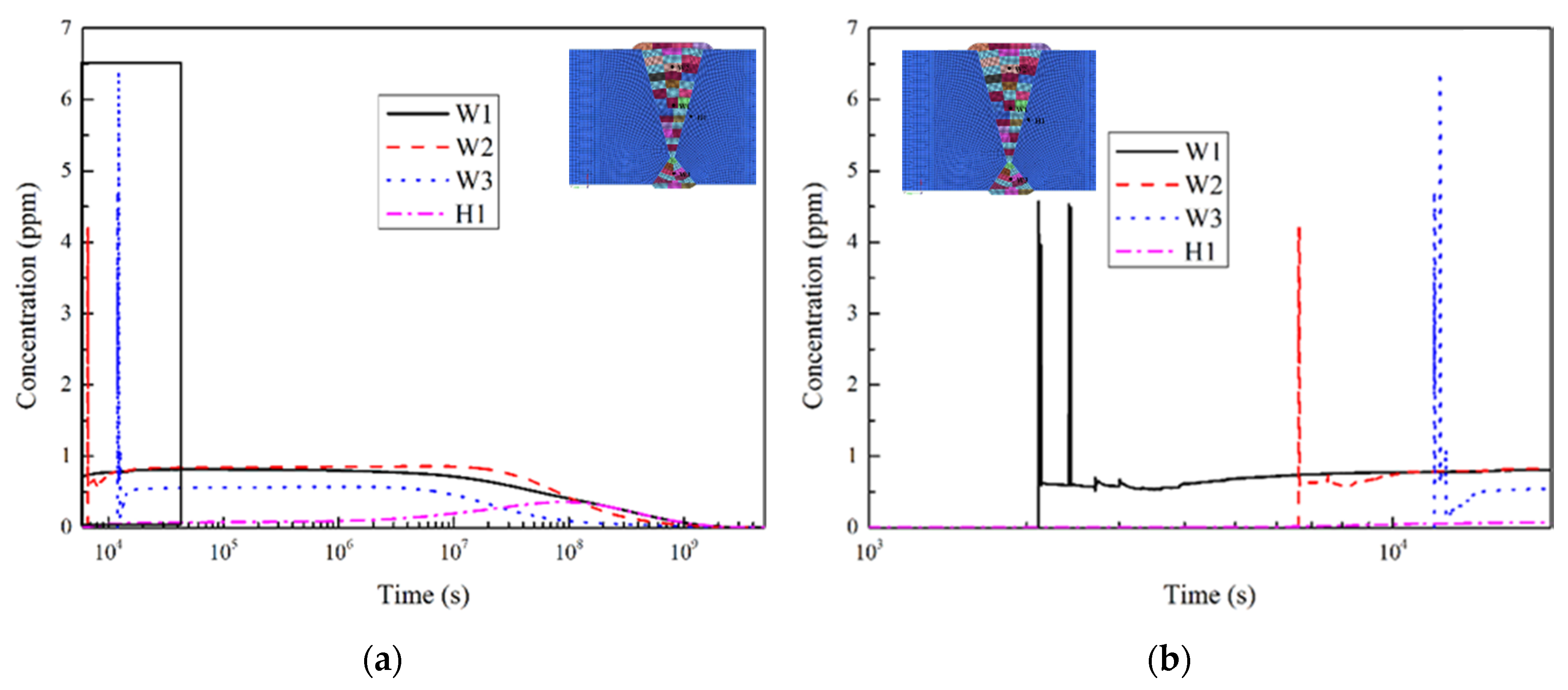
4.1.3. Hydrogen Diffusion Behavior
5. Discussion
5.1. Comparison of Results
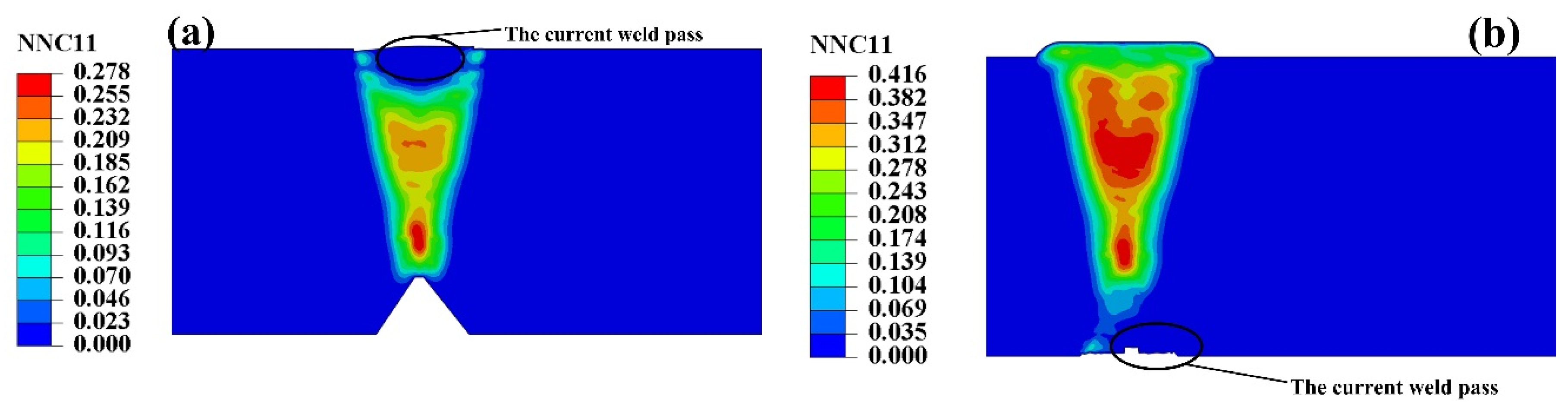
5.2. Self-Gathering Effect of Welding of Heavy Plate
5.3. The Effect of Self-Gathering Effect between Passes in the Thickness of Heavy Plate
6. Conclusions
- (1)
- The simulation results of distribution trend of hydrogen concentration along the thickness direction of the welded joint of heavy plate were in good agreement with the experimental results, and there were certain deviations in the results of local position.
- (2)
- The size effect and large number of welded passes of heavy plate results in the total introduced amount of hydrogen from the outside being large. So, the time required for natural dehydrogenation after heavy plate welding was much longer than that of middle and thin plates.
- (3)
- The simulation results and experimental results show that the position of the weld pass with a 20–30 mm distance from the surface in WM has the largest hydrogen content, and it was maintained at a high level for a long time. Thus, the position has the largest sensitivity of cold cracking. This phenomenon is consistent with existing research results.
- (4)
- During the welding of heavy plate, the self-gathering effect between the weld passes was effective in the short-range and in the long-range, invalid.
Author Contributions
Funding
Conflicts of Interest
References
- Tomkow, J.; Fydrych, D.; Rogalski, G. Role of Bead Sequence in Underwater Welding. Materials 2019, 12, 3372. [Google Scholar] [CrossRef]
- Pfeil, L. The Effect of Occluded Hydrogen on the Tensile Strength of Iron. Proc. R. Soc. Lond. Ser. A Contain. Pap. A Math. Phys. Character (1905–1934) 1926, 112, 182–195. [Google Scholar] [CrossRef]
- Troiano, A.R. The Role of Hydrogen and Other Interstitials in the Mechanical Behavior of Metals. Metallogr. Microstruct. Anal. 2016, 5, 557–569. [Google Scholar] [CrossRef]
- Oriani, R. A Mechanistic Theory of Hydrogen Embrittlement of Steels. Ber. Bunsenges. für Phys. Chem. 2010, 76, 848–857. [Google Scholar] [CrossRef]
- Gerberich, W.; Marsh, P.G.; Hoehn, J.W. Hydrogen Induced Cracking Mechanisms—Are There Critical Experiments? Hydrog. Eff. Mater. 2013, 539–554. [Google Scholar] [CrossRef]
- Hydrogen-induced intergranular fracture of steels. Eng. Fract. Mech. 2001, 68, 773–788. [CrossRef]
- Lynch, S. Hydrogen embrittlement (HE) phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Świerczyńska, A.; Fydrych, D.; Landowski, M.; Rogalski, G.; Łabanowski, J. Hydrogen embrittlement of X2CrNiMoCuN25-6-3 super duplex stainless steel welded joints under cathodic protection. Constr. Build. Mater. 2020, 238, 117697. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B. Hydrogen embrittlement of low carbon structural steel at macro-, micro- and nano-levels. Int. J. Hydrog. Energy 2020, 45, 2145–2156. [Google Scholar] [CrossRef]
- Beachem, C. A new model for hydrogen-assisted cracking. Metall. Trans. 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Deng, D. FEM prediction of welding residual stress and distortion in carbon steel considering phase transformation effects. Mater. Des. 2009, 30, 359–366. [Google Scholar] [CrossRef]
- Cheon, J.; Na, S.-J. Prediction of welding residual stress with real-time phase transformation by CFD thermal analysis. Int. J. Mech. Sci. 2017, 131, 37–51. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, X.S.; Wang, P.; Xiong, X.; Fang, H.Y. Numerical simulation of residual stress in 10Ni5CrMoV steel weldments. J. Mater. Process. Technol. 2017, 240, 77–86. [Google Scholar] [CrossRef]
- Yazdipour, N.; Haq, A.J.; Muzaka, K.; Pereloma, E.V. 2D modelling of the effect of grain size on hydrogen diffusion in X70 steel. Comput. Mater. Sci. 2012, 56, 49–57. [Google Scholar] [CrossRef]
- Olden, V.; Saai, A.; Jemblie, L.; Johnsen, R. FE simulation of hydrogen diffusion in duplex stainless steel. Int. J. Hydrog. Energy 2014, 39, 1156–1163. [Google Scholar] [CrossRef]
- Ilin, D.N.; Saintier, N.; Olive, J.-M.; Abgrall, R.; Aubert, I. Simulation of hydrogen diffusion affected by stress-strain heterogeneity in polycrystalline stainless steel. Int. J. Hydrog. Energy 2014, 39, 2418–2422. [Google Scholar] [CrossRef]
- Świerczyńska, A.; Fydrych, D.; Rogalski, G. Diffusible hydrogen management in underwater wet self-shielded flux cored arc welding. Int. J. Hydrog. Energy 2017, 42, 24532–24540. [Google Scholar] [CrossRef]
- Wang, Y.F.; Hu, S.Y.; Li, Y.; Cheng, G.X. Improved hydrogen embrittlement resistance after quenching-tempering treatment for a Cr-Mo-V high strength steel. Int. J. Hydrog. Energy 2019, 44, 29017–29026. [Google Scholar] [CrossRef]
- Rhode, M.; Mente, T.; Steppan, E.; Steger, J.; Kannengiesser, T. Hydrogen trapping in T24 Cr-Mo-V steel weld joints-microstructure effect vs. experimental influence on activation energy for diffusion. Weld. World 2018, 62, 277–287. [Google Scholar] [CrossRef]
- Abe, M.; Nakatani, M.; Namatame, N.; Terasaki, T. Influence of dehydrogenation heat treatment on hydrogen distribution in multi-layer welds of Cr-Mo-V steel. Weld. World 2012, 56, 114–123. [Google Scholar] [CrossRef]
- Koyama, M.; Rohwerder, M.; Tasan, C.C.; Bashir, A.; Akiyama, E.; Takai, K.; Raabe, D.; Tsuzaki, K. Recent progress in microstructural hydrogen mapping in steels: Quantification, kinetic analysis, and multi-scale characterisation. Mater. Sci. Technol. 2017, 33, 1481–1496. [Google Scholar] [CrossRef]
- Saini, N.; Pandey, C.; Mahapatra, M.M. Effect of diffusible hydrogen content on embrittlement of P92 steel. Int. J. Hydrog. Energy 2017, 42, 17328–17338. [Google Scholar] [CrossRef]
- Węglowski, M.S.; Zeman, M. Prevention of cold cracking in ultra-high strength steel Weldox 1300. Arch. Civ. Mech. Eng. 2014, 14, 417–424. [Google Scholar] [CrossRef]
- Kannengiesser, T.; Boellinghaus, T. Cold cracking tests-an overview of present technologies and applications. Weld. World 2013, 57, 3–37. [Google Scholar] [CrossRef]
- Chakraborty, G.; Rejeesh, R.; Albert, S.K. Study on hydrogen assisted cracking susceptibility of HSLA steel by implant test. Def. Technol. 2016, 12, 490–495. [Google Scholar] [CrossRef]
- Rogne, B.R.S.; Kheradmand, N.; Deng, Y.; Barnoush, A. In situ micromechanical testing in environmental scanning electron microscope: A new insight into hydrogen-assisted cracking. Acta Mater. 2018, 144, 257–268. [Google Scholar] [CrossRef]
- Deng, Y.; Hajilou, T.; Wan, D.; Kheradmand, N.; Barnoush, A. In-situ micro-cantilever bending test in environmental scanning electron microscope: Real time observation of hydrogen enhanced cracking. Scr. Mater. 2017, 127, 19–23. [Google Scholar] [CrossRef]
- Hajilou, T.; Deng, Y.; Rogne, B.R.; Kheradmand, N.; Barnoush, A. In situ electrochemical microcantilever bending test: A new insight into hydrogen enhanced cracking. Scr. Mater. 2017, 132, 17–21. [Google Scholar] [CrossRef]
- Echebarria, B.; Folch, R.; Karma, A.; Plapp, M. Quantitative phase-field model of alloy solidification. Phys. Rev. E 2004, 70, 061604. [Google Scholar] [CrossRef]
- Karma, A.; Rappel, W.-J. Quantitative phase-field modeling of dendritic growth in two and three dimensions. Phys. Rev. E 1998, 57, 4323–4349. [Google Scholar] [CrossRef]
- Zheng, W.J.; Dong, Z.B.; Wei, Y.H.; Song, K.J.; Guo, J.L.; Wang, Y. Phase field investigation of dendrite growth in the welding pool of aluminum alloy 2A14 under transient conditions. Comput. Mater. Sci. 2014, 82, 525–530. [Google Scholar] [CrossRef]
- Qin, M.; Cheng, G.X.; Zhang, Z.X.; Li, Q.; Zhang, J.X. Numerical simulation of temperature field and residual stress in multi-pass welds in 2.25cr-1mo-0.25v steel plate and comparison with experimental measurements. In Pressure Vessels and Piping Conference; American Society of Mechanical Engineers: New York, NY, USA, 2017; Volume 57915, p. V01BT01A033. [Google Scholar]
- Xiong, Z.L.; Zheng, W.J.; Tang, L.P.; Yang, J.G. Self-Gathering Effect of the Hydrogen Diffusion in Welding Induced by the Solid-State Phase Transformation. Materials 2019, 12, 2897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Li, R.B.; Sun, H.; Huang, L.Q.; Li, J.X. Phase transition behavior of low alloy heat resistant steel of 2.25Cr-1Mo-0.25V. J. Shanghai Univ. Electr. Eng. 2012, 15, 119–122. (In Chinese) [Google Scholar]
- Liu, C.; Zhang, J.X.; Xue, C.B. Numerical investigation on residual stress distribution and evolution during multipass narrow gap welding of thick-walled stainless steel pipes. Fusion Eng. Des. 2011, 86, 288–295. [Google Scholar] [CrossRef]
- Xu, S.; Wei, R.; Wang, W.; Chen, X. Residual stresses in the welding joint of the nozzle-to-head area of a layered high-pressure hydrogen storage tank. Int. J. Hydrog. Energy 2014, 39, 11061–11070. [Google Scholar] [CrossRef]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Metall. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Toribio, J.; Lorenzo, M.; Vergara, D.; Kharin, V. Effects of manufacturing-induced residual stresses and strains on hydrogen embrittlement of cold drawn steels. Procedia Eng. 2011, 10, 3540–3545. [Google Scholar] [CrossRef]
- Lee, H.W. Weld metal hydrogen-assisted cracking in thick steel plate weldments. Mater. Sci. Eng. A 2007, 445, 328–335. [Google Scholar] [CrossRef]
- Jiang, W.C.; Gong, J.M.; Tang, J.Q.; Chen, H.; Tu, S.D. Finite element simulation of the effect of welding residual stress on hydrogen diffusion. Acta Metall. Sin. 2006, 42, 1221–1226. [Google Scholar]
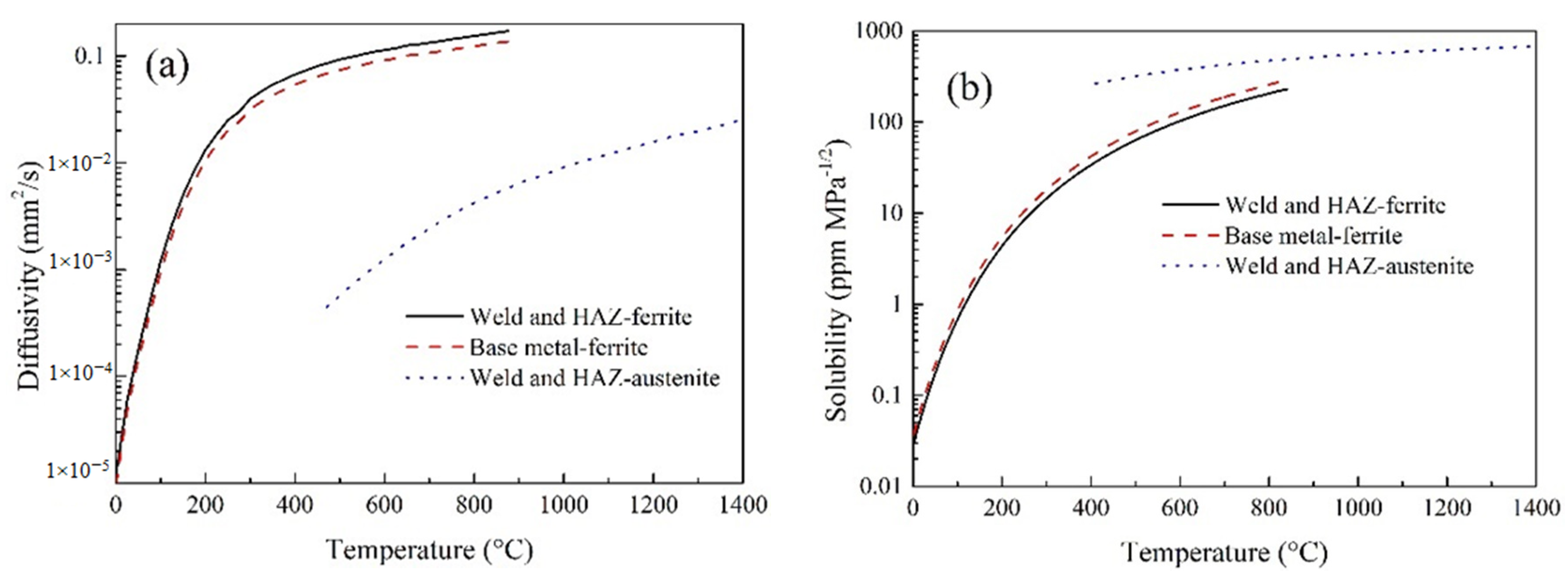
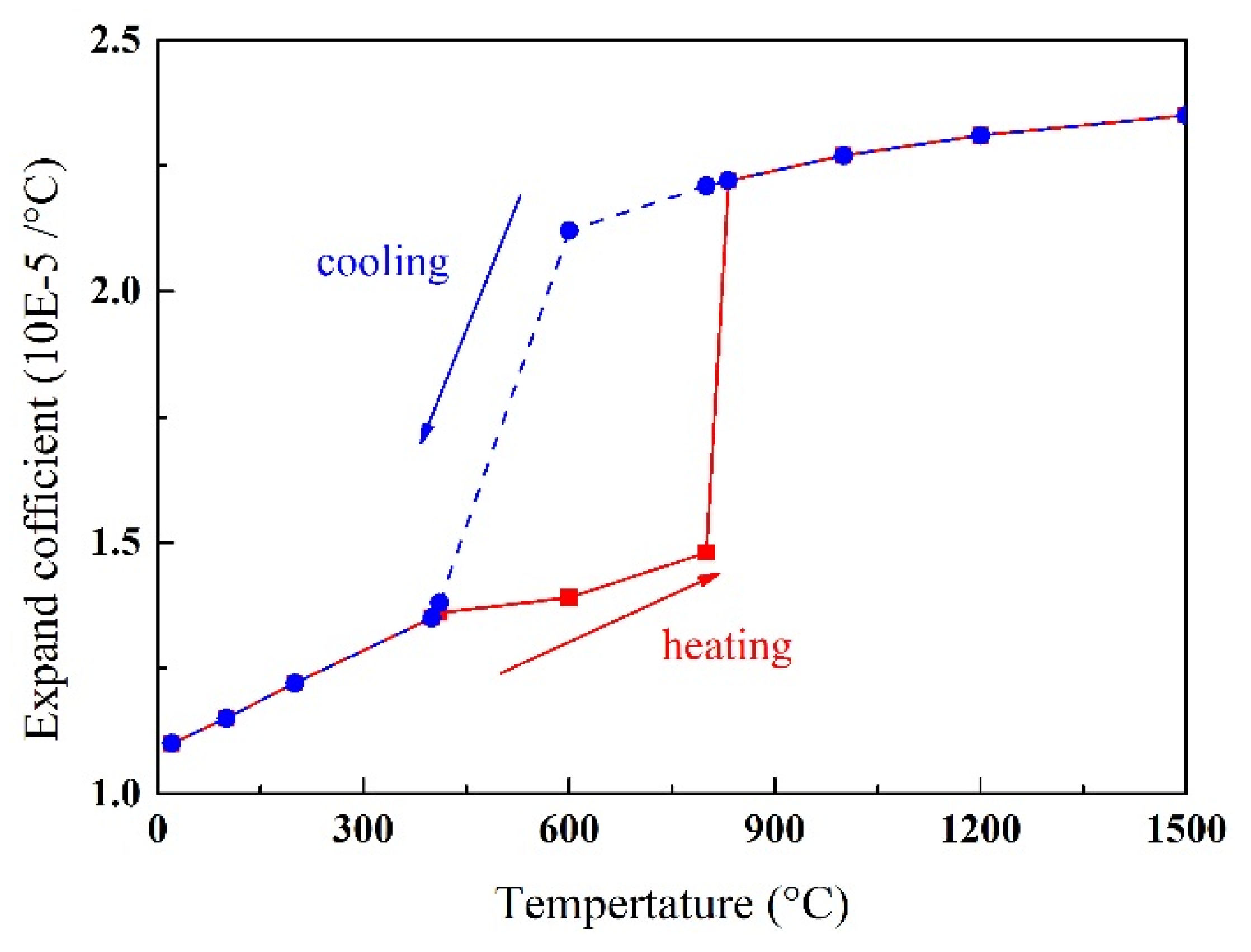
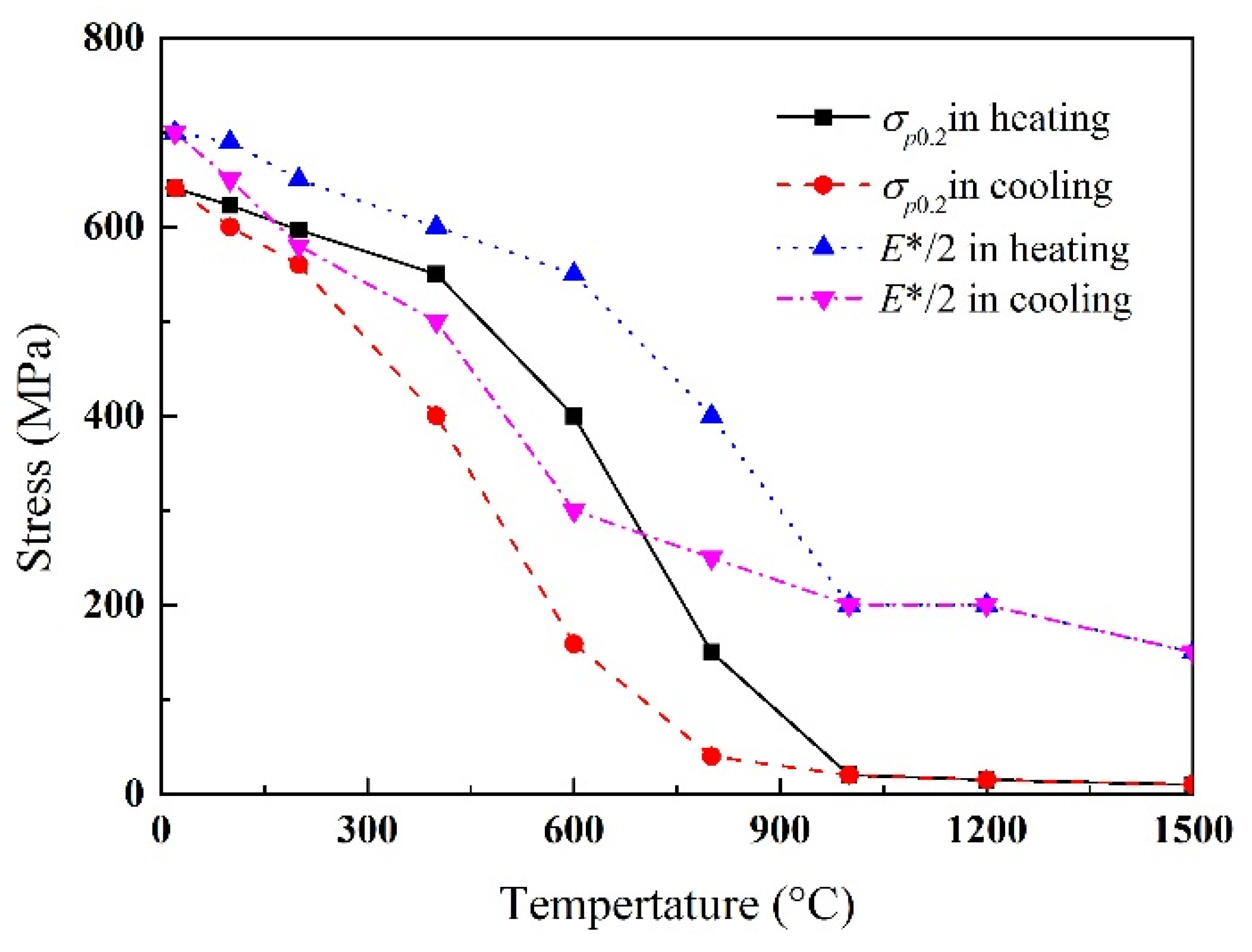
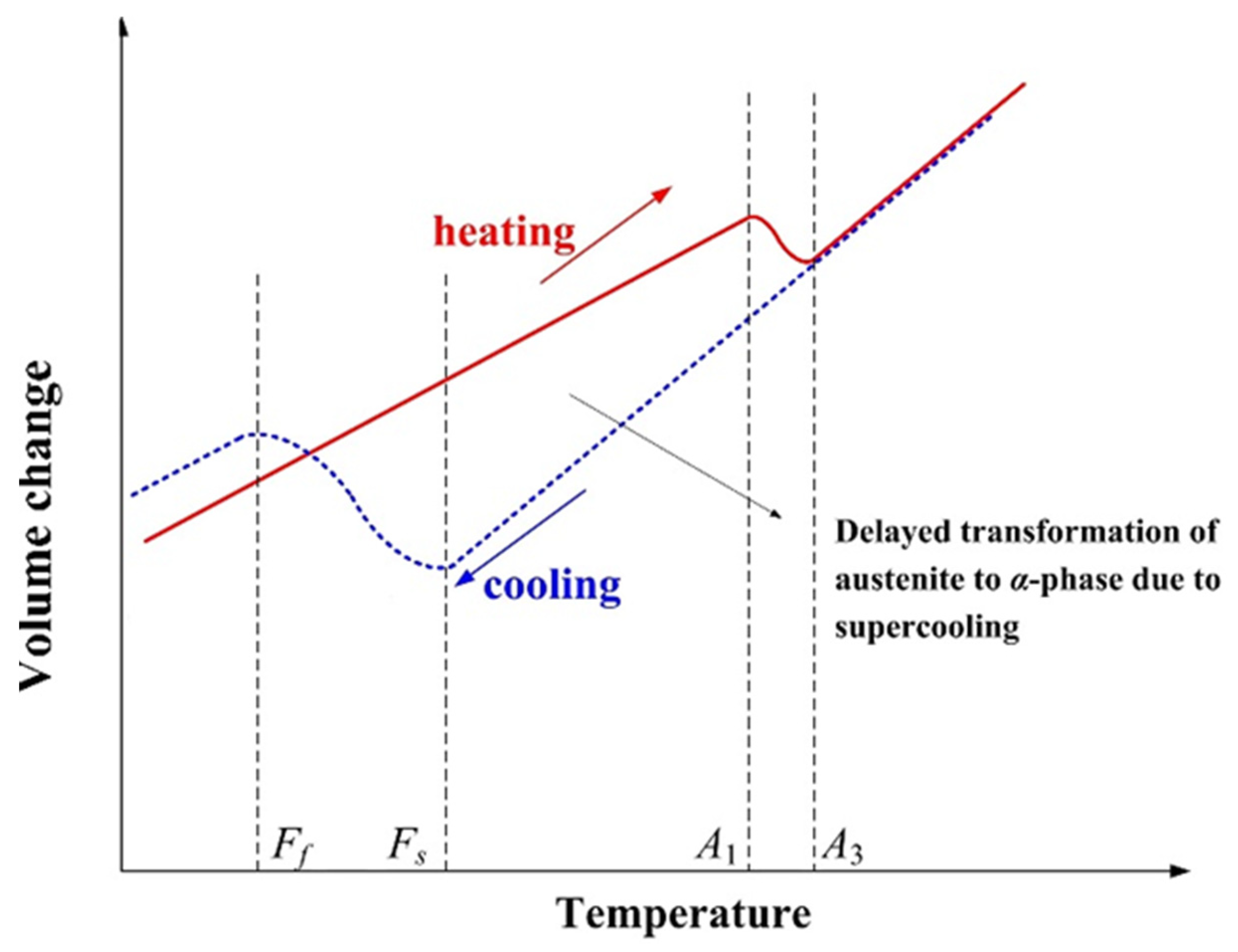


| Material Properties | Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|
| 20 | 100 | 200 | 400 | 600 | 700 | 850 | |
| Conductivity (W/m°C) | 54.4 | 54.0 | 52.8 | 47.7 | 40.0 | 33.4 | 27.3 |
| Specific heat (J/kg°C) | 423 | 473 | 478 | 492 | 530 | 650 | 682 |
| Young’s modulus (GPa) | 215 | 207 | 204 | 185 | 166 | 134 | 118 |
| Harden modulus (GPa) | 1.4 | 1.3 | 1.3 | 1.2 | 1.1 | 1.1 | 1.0 |
| Yield stress (MPa) | 641 | 623 | 597 | 550 | 451 | 275 | 98 |
| Thermal expansion coefficient (1 × 10−5) | 1.10 | 1.15 | 1.22 | 1.37 | 1.42 | 1.46 | 1.49 |
| Density (g/cm3) | 7.81 | 7.79 | 7.77 | 7.72 | 7.69 | 7.66 | 7.61 |
| Possion’s ratio | 0.29 | 0.30 | 0.30 | 0.30 | 0.31 | 0.32 | 0.32 |
| Material Properties | Temperature (°C) | ||||||
|---|---|---|---|---|---|---|---|
| 400 | 600 | 700 | 800 | 900 | 1200 | 1400 | |
| Conductivity (W/m°C) | 18.0 | 19.2 | 22.5 | 24.0 | 28.1 | 32.2 | 34.0 |
| Specific heat (J/kg°C) | 540 | 570 | 584 | 608 | 632 | 676 | 700 |
| Young’s modulus (GPa) | 167 | 163 | 156 | 145 | 132 | 60 | 10 |
| Harden modulus (GPa) | 1.4 | 1.3 | 1.1 | 0.9 | 0.8 | 0.5 | 0.3 |
| Yield stress (MPa) | 160 | 141 | 123 | 101 | 60 | 25 | 10 |
| Thermal expansion coefficient (1 × 10−5) | 1.8 | 1.9 | 2.0 | 2.0 | 2.0 | 2.1 | 2.2 |
| Density (g/cm3) | 7.75 | 7.66 | 7.61 | 7.56 | 7.46 | 7.37 | 7.32 |
| Possion’s ratio | 0.32 | 0.32 | 0.33 | 0.33 | 0.33 | 0.34 | 0.39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Liu, G.; Zheng, W. Study on Hydrogen Diffusion Behavior during Welding of Heavy Plate. Materials 2020, 13, 3887. https://doi.org/10.3390/ma13173887
Yang J, Liu G, Zheng W. Study on Hydrogen Diffusion Behavior during Welding of Heavy Plate. Materials. 2020; 13(17):3887. https://doi.org/10.3390/ma13173887
Chicago/Turabian StyleYang, Jianguo, Guohao Liu, and Wenjian Zheng. 2020. "Study on Hydrogen Diffusion Behavior during Welding of Heavy Plate" Materials 13, no. 17: 3887. https://doi.org/10.3390/ma13173887
APA StyleYang, J., Liu, G., & Zheng, W. (2020). Study on Hydrogen Diffusion Behavior during Welding of Heavy Plate. Materials, 13(17), 3887. https://doi.org/10.3390/ma13173887