Fast-Versus Slow-Resorbable Calcium Phosphate Bone Substitute Materials—Texture Analysis after 12 Months of Observation
Abstract
:1. Introduction
2. Materials and Methods
- High-rate resorbable: β tricalcium phosphate (Curasan: Cerasorb M, Wake Forest, NC, USA);
- Low-rate resorbable: hydroxyapatite (Zimmer Biomet Dental: Endobone, Palm Beach Gardens, FL, USA).
Statistical Analysis
3. Results
- Sum average;
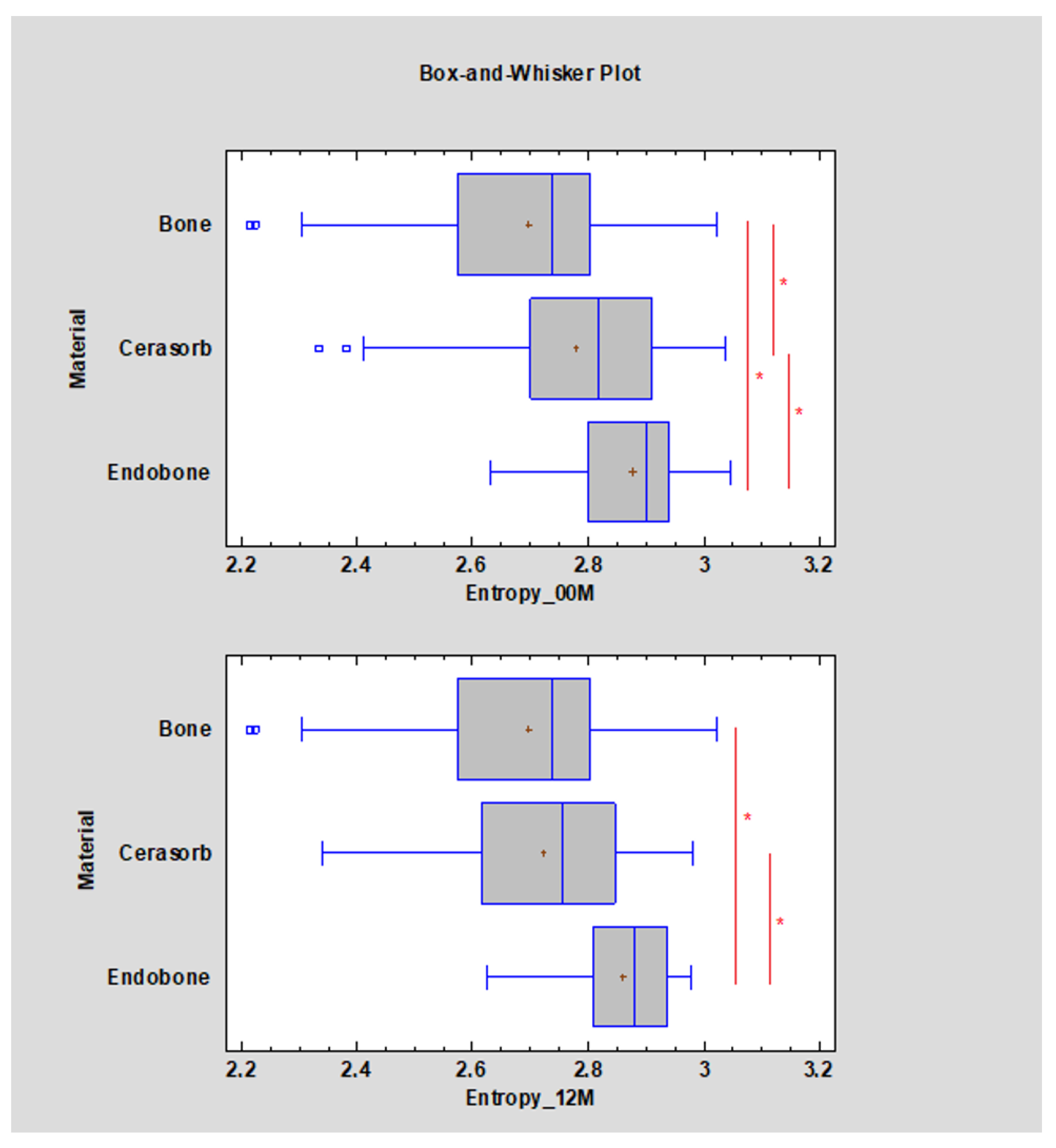
- Entropy
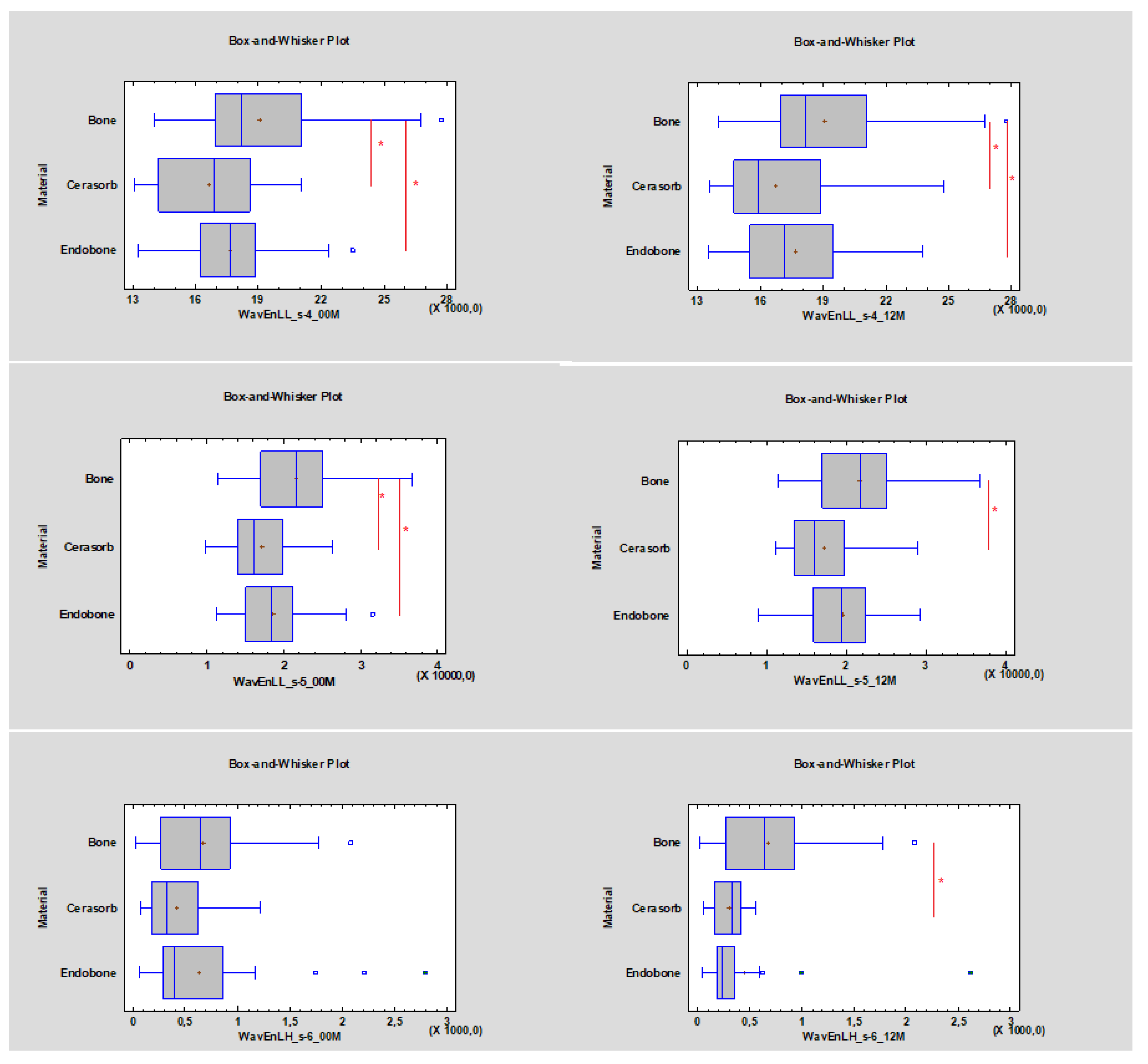
- Wavelet energy (LL_s-4, LL_s-5, LH_s-6).
- ROI values lower than 17,910 (approximately 10,674) for WavEnLL_s-4 and lower than 18,577 (approximately 6485.85) for WavEnLL_s-5 represent cortical bone;
- WavEnLH_s-6 indicates bone tissue that is better than other wavelets, while ROI values between 484.04 and 523.24 indicate bone tissue instead of soft tissue (Table 2).
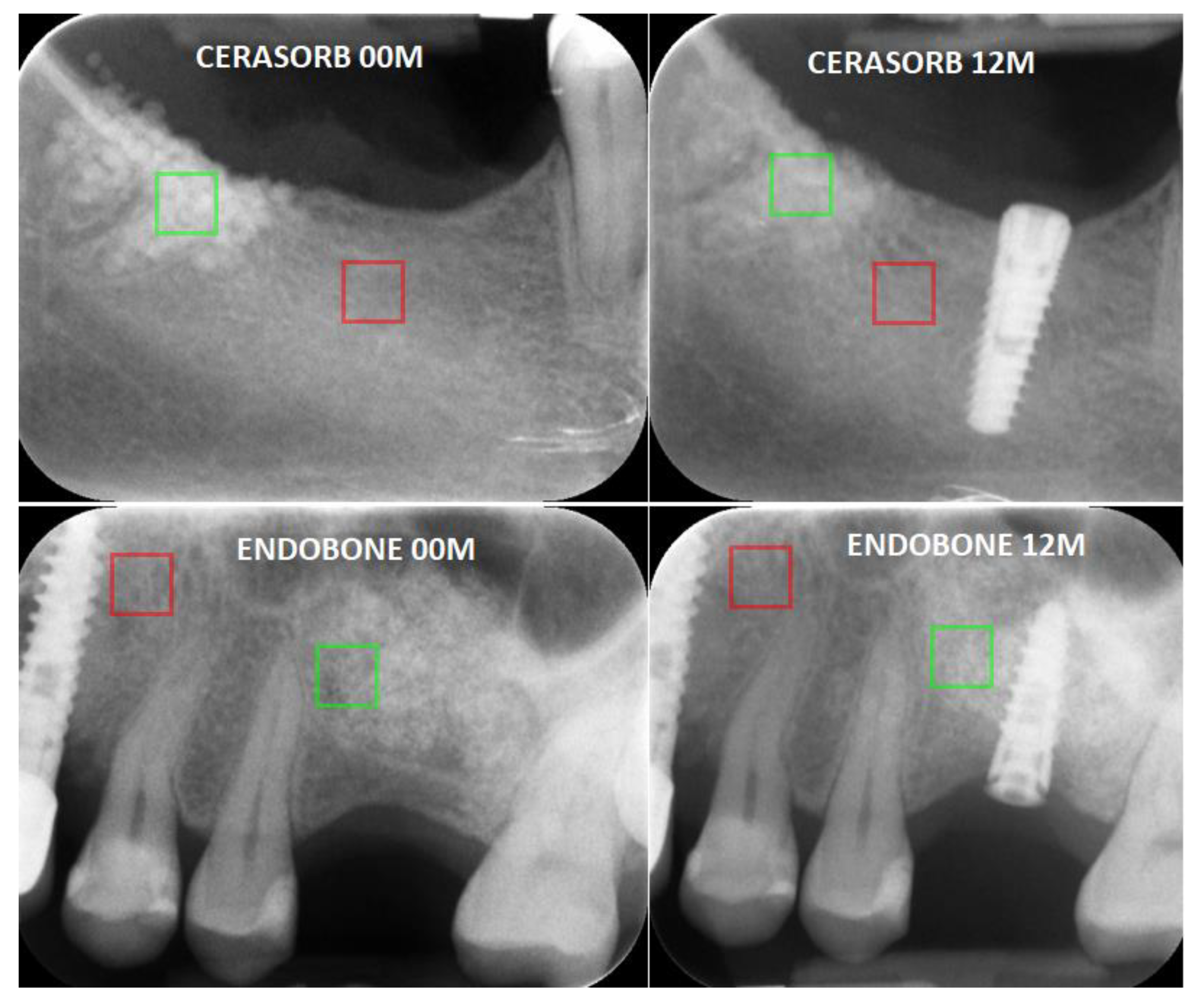
- The WavEnLL_s-4 value at 00 M for Cerasorb was 16,885, while the value for Endobone was 17,657. After 12 months the WavEnLL_s-4 value for Cerasorb was 15,876 (it decreased p < 0.05), while that for Endobone was 17,166 (p < 0.05);
- The WavEnLL_s-5 (p < 0.05) value at 00 M for Cerasorb was 16,073, while that for Endobone was 18,690. After 12 months the WavEnLL_s-5 value for Cerasorb was 15,907, while that for Endobone was 19,292 (p < 0.05);
- The WavEnLH_s-6 values after 12 months were 338.47 for Cerasorb and 231.82 for Endobone (p < 0.05).
4. Discussion
- Co-occurrence matrix derived: angular second moment, contrast, correlation, sum of squares, inverse difference moment, sum average, sum variance, sum of entropies, entropy, difference variance, and difference entropy;
- Run-length matrix derived: run length nonuniformity, grey level nonuniformity, long run emphasis, short run emphasis, and fraction of image in runs.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kalfas, I.H. Principles of bone healing. Neurosurg. Focus 2001, 10, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakhmatia, Y.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of Bone Defects by Guided Tissue Regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Al-Nawas, B.; Schiegnitz, E. Augmentation procedures using bone substitute materials or autogenous bone—a systematic review and meta-analysis. Eur. J. Oral Implant. 2014, 7, S219–S234. [Google Scholar]
- Scarano, A.; Carinci, F.; Assenza, B.; Piattelli, M.; Murmura, G.; Piattelli, A. Vertical ridge augmentation of atrophic posterior mandible using an inlay technique with a xenograft without miniscrews and miniplates: case series. Clin. Oral Implant. Res. 2011, 22, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Beauvais, S.; Drevelle, O.; Jann, J.; Lauzon, M.A.; Foruzanmehr, M.; Grenier, G.; Roux, S.; Faucheux, N. Interactions between bone cells and biomaterials an update. Front. Biosci. 2016, 8, 227–263. [Google Scholar] [CrossRef] [Green Version]
- Samavedi, S.; Whittington, A.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Koźlik, M.; Wójcicki, P.; Rychlik, D. Preparaty kościozastępcze. Dent. Med. Probl. 2011, 48, 547–553. [Google Scholar]
- Horowitz, R.A.; Mazor, Z.; Foitzik, C.; Prasad, H.; Rohrer, M.; Palti, A. β-Tricalcium phosphate as bone substitute material: Properties and clinical applications. J. Osseointegr. 2010, 2, 61–68. [Google Scholar]
- Louis, P.J. Vertical Ridge Augmentation Using Titanium Mesh. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 353–368. [Google Scholar] [CrossRef]
- Strzelecki, M.; Szczypinski, P.; Materka, A.; Klepaczko, A. A software tool for automatic classification and segmentation of 2D/3D medical images. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2013, 702, 137–140. [Google Scholar] [CrossRef]
- Szczypinski, P.; Strzelecki, M.; Materka, A.; Klepaczko, A. MaZda—A software package for image texture analysis. Comput. Methods Progr. Biomed. 2009, 94, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Szczypiński, P.; Strzelecki, M. MaZda—A Software for Texture Analysis. In Proceedings of the International Symposium on Information Technology Convergence, Jeonju, Korea, 23–24 November 2007; pp. 245–249. [Google Scholar]
- Materka, A.; Strzelecki, M.; Lerski, R.; Schad, L.; Pietikäinen, M.K. Feature evaluation of texture test objects for magnetic resonance imaging. In Workshop on Texture Analysis and Machine Vision; World Scientific: Singapore, 2000; Volume 40. [Google Scholar]
- Materka, A.; Strzelecki, M. Texture Analysis Methods—A Review; COST B11 Report; Institute of Electronics, Technical University of Lodz: Lodz, Poland, 1998. [Google Scholar]
- Misch, C.M. Autogenous Bone: Is it Still the Gold Standard? Implant. Dent. 2010, 19, 361. [Google Scholar] [CrossRef] [PubMed]
- Mittal, Y.; Jindal, G.; Garg, S. Bone manipulation procedures in dental implants. Indian J. Dent. 2016, 7, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Checchi, V.; Savarino, L.; Montevecchi, M.; Felice, P.; Checchi, L. Clinical-radiographic and histological evaluation of two hydroxyapatites in human extraction sockets: A pilot study. Int. J. Oral Maxillofac. Surg. 2011, 40, 526–532. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I.H. Textural Features for Image Classification. IEEE Trans. Syst. Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Szczypiński, P.; Kociołek, M.; Materka, A.; Strzelecki, M. Computer program for image textureanalysis in PhD students laboratory. In Proceedings of the International Conference on Signals and Electronic Systems, Łódź, Poland, 18–21 September 2001; pp. 225–262. [Google Scholar]
- Kołaciński, M.; Kozakiewicz, M.; Materka, A. Textural entropy as a potential feature for quantitative assessment of jaw bone healing process. Arch. Med. Sci. 2015, 11, 78–84. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Gurzawska, K. Zastosowanie dyskretnej transformacji falkowej do matematycznego opisu radiotekstury kości żuchwy po zabiegach implantologicznych. Mag. Stomatol. 2009, 19, 90–93. [Google Scholar]
- Horch, H.-H.; Sader, R.; Pautke, C.; Neff, A.; Deppe, H.; Kolk, A. Synthetic, pure-phase beta-tricalcium phosphate ceramic granules (Cerasorb®) for bone regeneration in the reconstructive surgery of the jaws. Int. J. Oral Maxillofac. Surg. 2006, 35, 708–713. [Google Scholar] [CrossRef]
- Blokhuis, T.J. Materials that allow resorption. In Bone Substitute Biomaterials; Mallick, K., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 80–92. [Google Scholar]
- Hannink, G.; Arts, J.J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42 (Suppl. 2), S22–S25. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.P.R.; Calvo-Guirado, J.L.; Ruiz, R.A.D.; De Val, J.E.M.S.; Vicente-Ortega, V.; Meseguer-Olmo, L. Retracted: Bone response to hydroxyapatites with open porosity of animal origin (porcine [OsteoBiol® mp3] and bovine [Endobon®]): A radiological and histomorphometric study. Clin. Oral Implant. Res. 2011, 22, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Palm, F. CERASORB® M—A new synthetic pure-phase ß-TCP ceramic material in oral and maxillofacial surgery. Implantol. J. 2006, 4, 6–12. [Google Scholar]
- Sponer, P.; Urban, K.; Kucera, T. Comparison of apatite-wollastonite glass-ceramic and b-tricalcium phosphate uses as bone graft substitutes after curettage of bone cysts. In Advances in Ceramics Electric and Magnetic Ceramics, Bioceramics, Ceramics and Environment; InTech: London, UK, 2011; pp. 473–482. [Google Scholar]
- Jensen, S.S.; Broggini, N.; Hjorting-Hansen, E.; Schenk, R.; Buser, D. Bone healing and graft resorption of autograft, anorganic bovine bone and β-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin. Oral Implant. Res. 2006, 17, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Low, K.L.; Tan, S.H.; Zein, S.H.S.; Roether, J.A.; Mouriño, V.; Boccaccini, A.R. Calcium phosphate-based composites as injectable bone substitute materials: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 273–286. [Google Scholar] [CrossRef]
| Reference Region | 00 M | 12 M | Significance |
|---|---|---|---|
| Cancellous bone | 2.69 ± 0.18 | 2.71 ± 0.18 | p = 0.585 |
| Cortical bone | 2.55 ± 0.10 | 2.48 ± 0.21 | p = 0.542 |
| Texture Feature | Value | p Value | Reference |
|---|---|---|---|
| Entropy | <2.61 | p < 0.05 | Bone tissue (cortical and cancellous) |
| SumAverg | >63.14 | p < 0.05 | Cortical bone |
| Wavelet LL_s-4 | <17,910 (approximately 10,674) | p < 0.05 | Cortical bone |
| Wavelet LL_s-5 | <18,577 (approximately 6485) | p < 0.05 | Cortical bone |
| Wavelet LH_s-6 | 484–523 | p < 0.05 | Bone tissue (cortical and cancellous) |
| Fisher’s Coefficient | FOLLOW-UP | |
|---|---|---|
| Material | 00 M | 12 M |
| Endobone | S(0,5)SumAverg 1.1 | S(0,5)SumAverg 1.6 |
| S(0,5)Entropy 1.4 | S(0,5)Entropy 1.2 | |
| S(5,5)Entropy 1.2 | S(5,5)Entropy 1.0 | |
| S(5,5)Entropy 1.2 | S(5,5)Entropy 0.9 | |
| S(5,0)Entropy 1.1 | S(5,0)Entropy 0.9 | |
| Cerasorb | S(0,5)SumAverg 1.8 | S(0,5)SumAverg 1.4 |
| S(0,5)Entropy 0.9 | S(0,5)Entropy 0.6 | |
| S(5,5)Entropy 0.8 | S(5,5)Entropy 0.5 | |
| S(5,5)Entropy 0.8 | S(5,5)Entropy 0.5 | |
| S(5,0)Entropy 0.7 | S(5,0)Entropy 0.4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wach, T.; Kozakiewicz, M. Fast-Versus Slow-Resorbable Calcium Phosphate Bone Substitute Materials—Texture Analysis after 12 Months of Observation. Materials 2020, 13, 3854. https://doi.org/10.3390/ma13173854
Wach T, Kozakiewicz M. Fast-Versus Slow-Resorbable Calcium Phosphate Bone Substitute Materials—Texture Analysis after 12 Months of Observation. Materials. 2020; 13(17):3854. https://doi.org/10.3390/ma13173854
Chicago/Turabian StyleWach, Tomasz, and Marcin Kozakiewicz. 2020. "Fast-Versus Slow-Resorbable Calcium Phosphate Bone Substitute Materials—Texture Analysis after 12 Months of Observation" Materials 13, no. 17: 3854. https://doi.org/10.3390/ma13173854
APA StyleWach, T., & Kozakiewicz, M. (2020). Fast-Versus Slow-Resorbable Calcium Phosphate Bone Substitute Materials—Texture Analysis after 12 Months of Observation. Materials, 13(17), 3854. https://doi.org/10.3390/ma13173854






