Laser Texturing as a Way of Influencing the Micromechanical and Biological Properties of the Poly(L-Lactide) Surface
Abstract
1. Introduction
2. Material and Methods
2.1. Material and Surface Modification
2.2. Roughness and Wettability
2.3. In Vitro Cytotoxicity
2.4. Micromechanical Properties
2.5. Statistical Analysis
3. Results
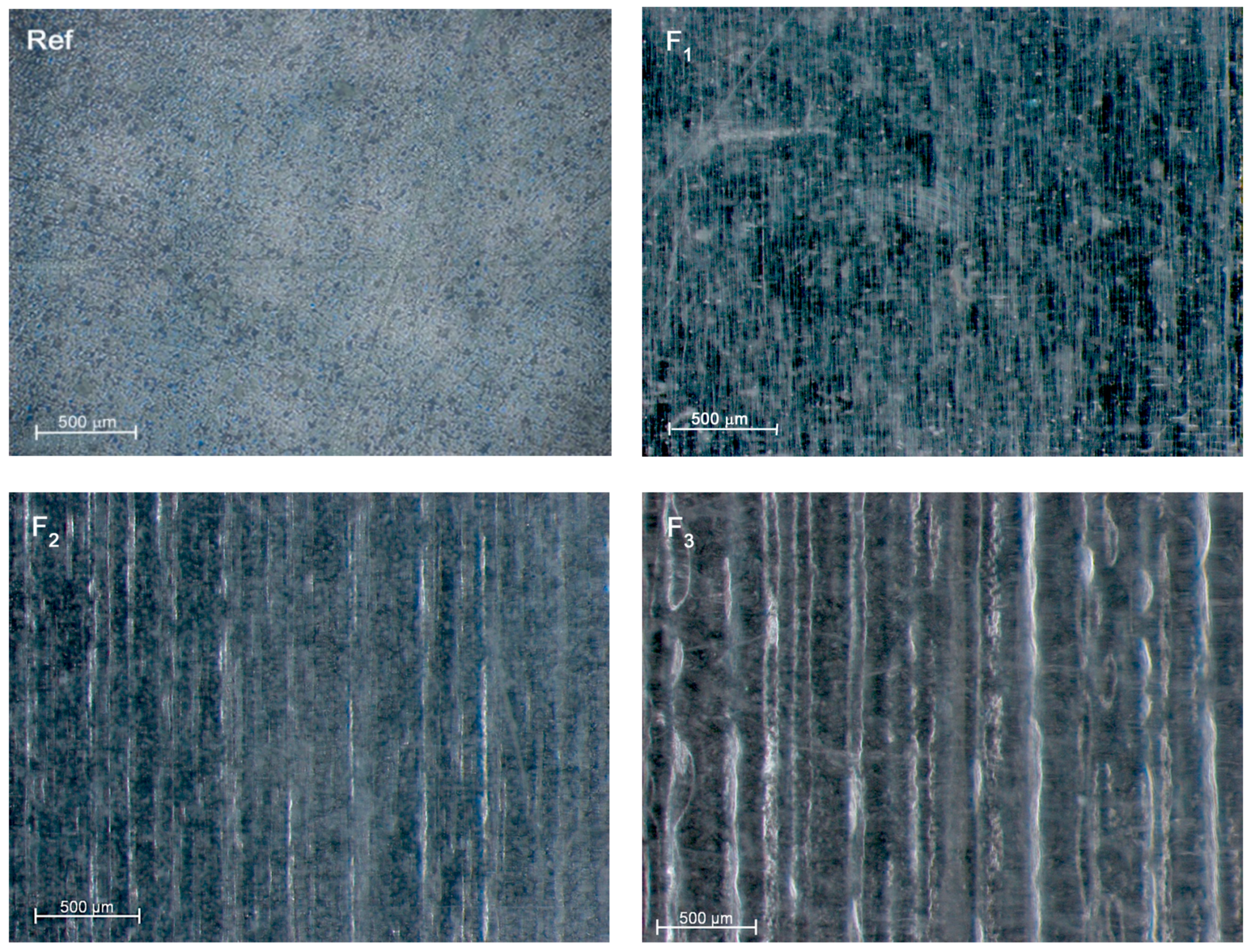
3.1. Roughness and Wettability
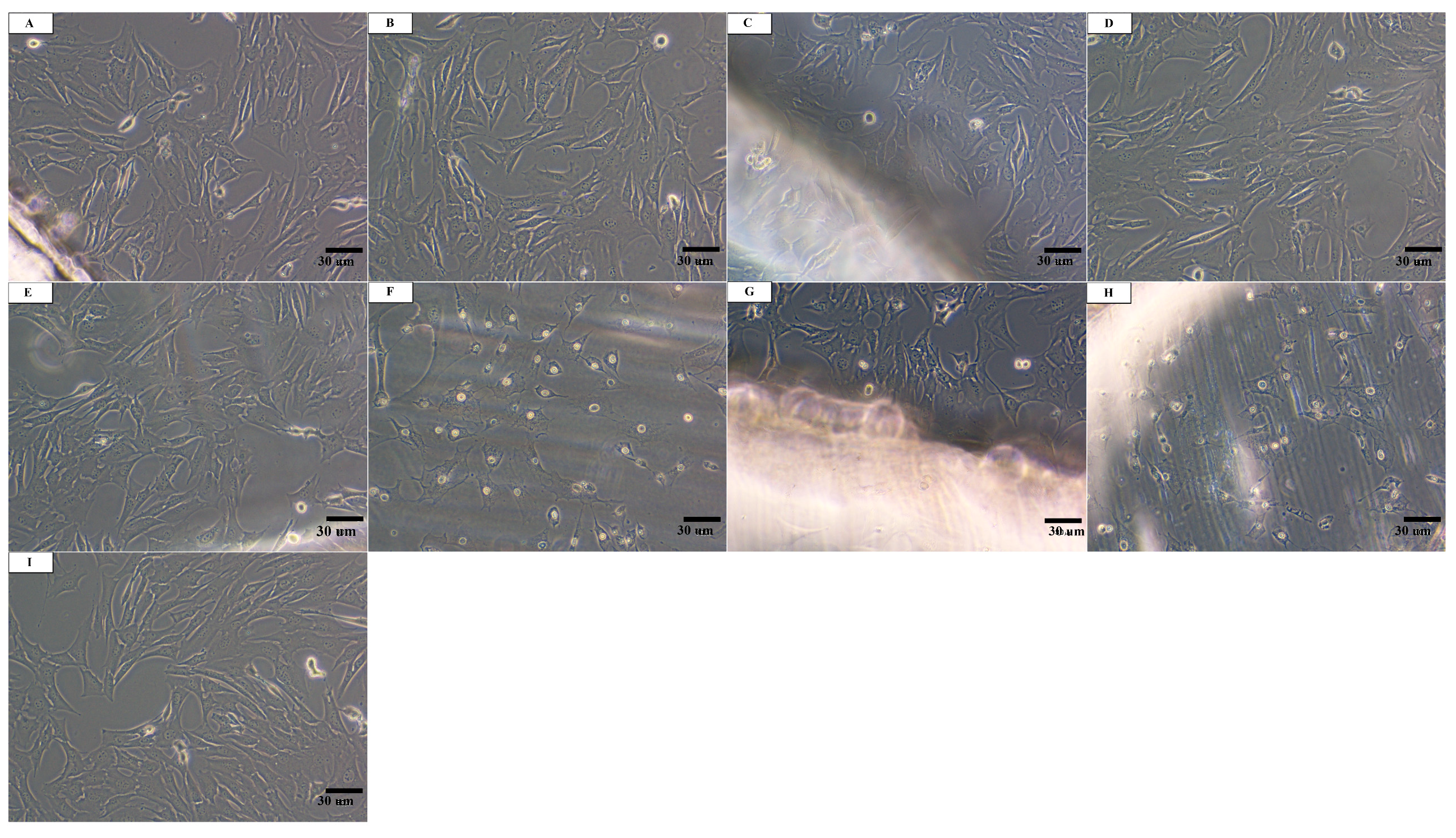
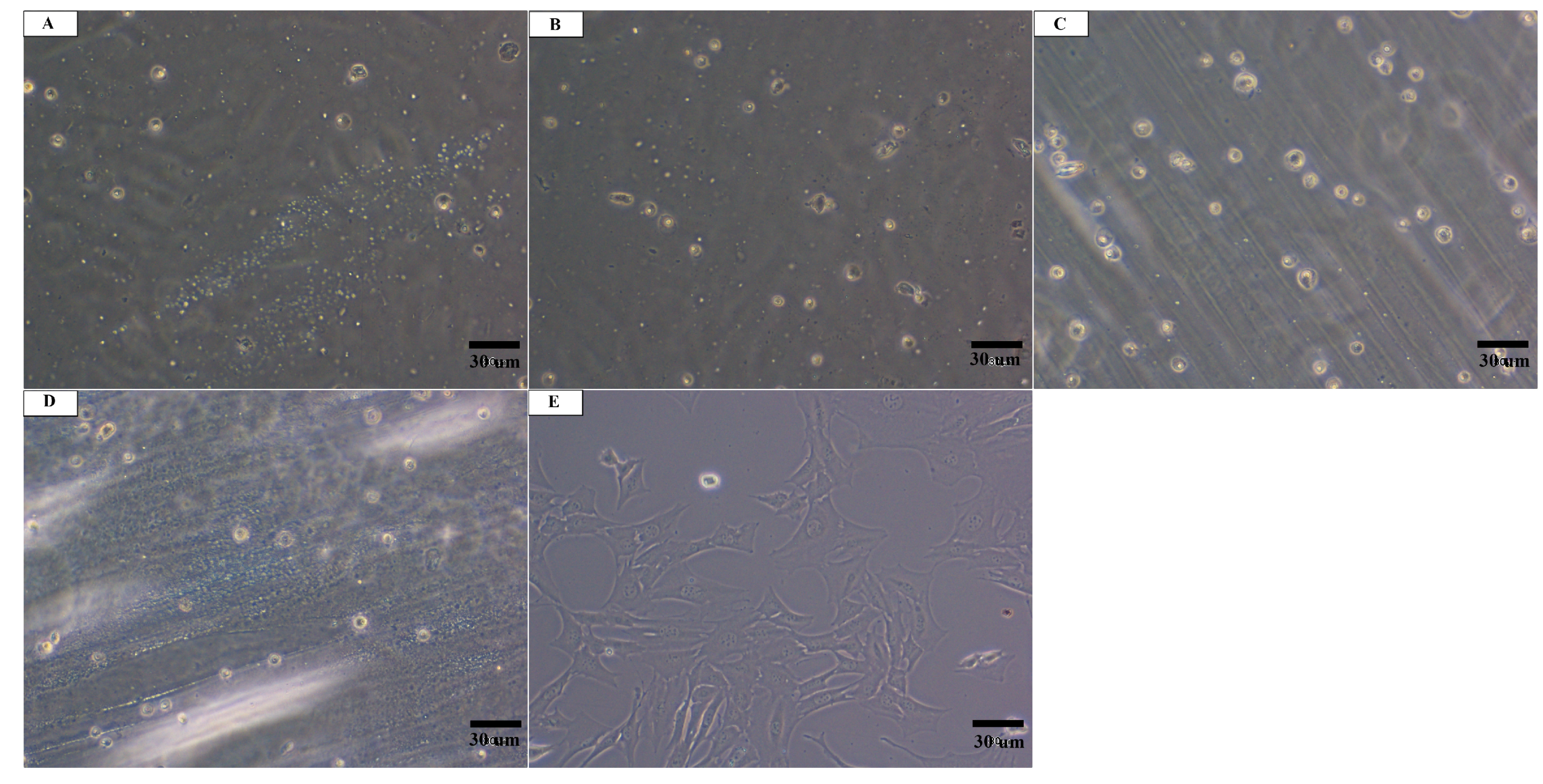
3.2. In Vitro Cytotoxicity
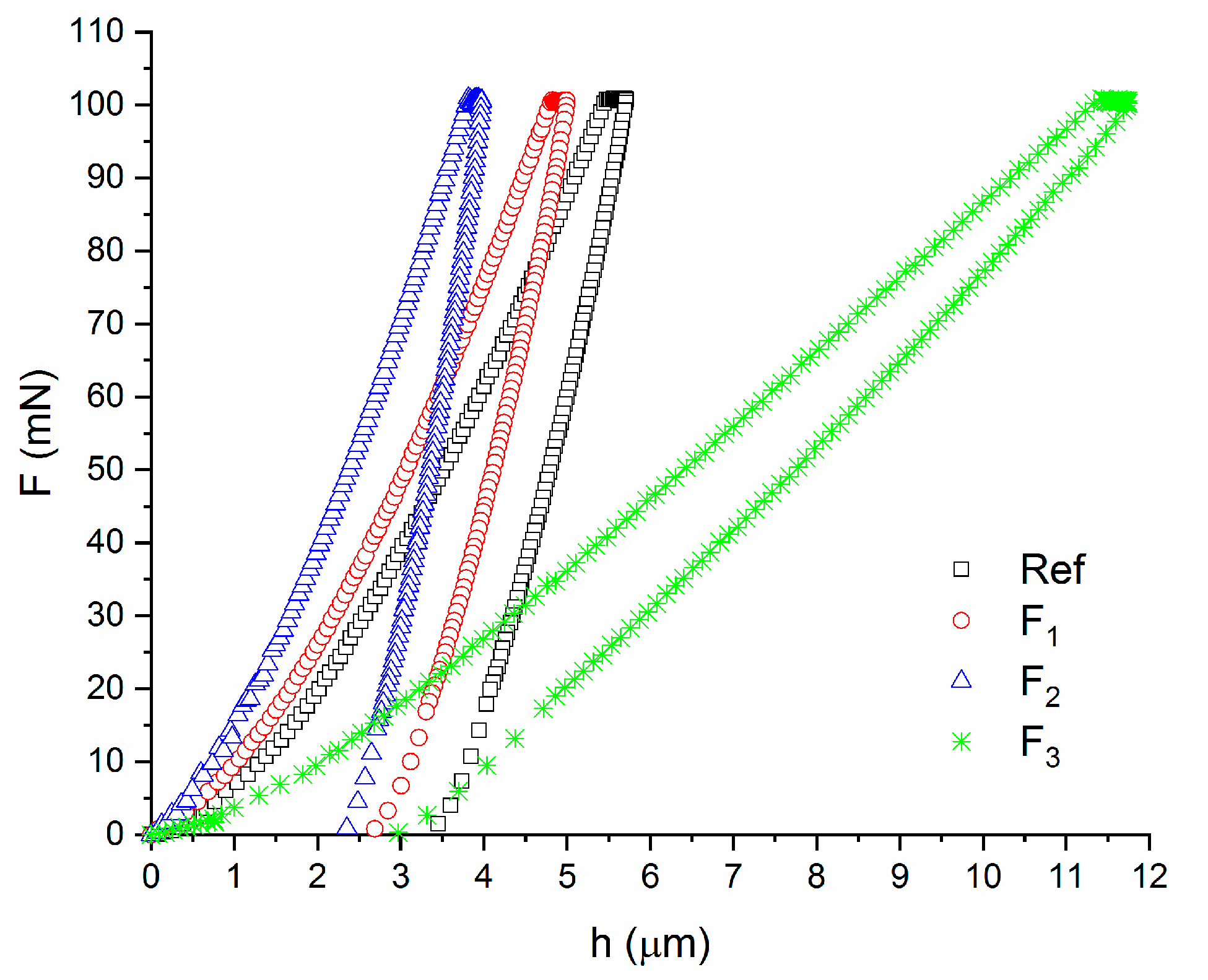
3.3. Micromechanical Properties
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Jardini, A.L.; Filho, R.M. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Wiebe, J.; Nef, H.M.; Hamm, C.W. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J. Am. Coll. Cardiol. 2014, 64, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Altomare, L.; Gadegaard, N.; Visai, L.; Tanzi, M.C.; Farè, S. Biodegradable microgrooved polymeric surfaces obtained by photolithography for skeletal muscle cell orientation and myotube development. Acta Biomater. 2010, 6, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical aspects of cell culture substrates: Topography, roughness, and elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Alves, A.T.N.N.; Resende, R.F.B.; Calasans-Maia, J.; Granjeiro, J.M.; Calasans-Maia, M.D. Early osseointegration driven by the surface chemistry and wettability of dental implants. J. Appl. Oral Sci. 2015, 23, 279–287. [Google Scholar] [CrossRef]
- Bastekova, K.; Guselnikova, O.; Postnikov, P.; Elashnikov, R.; Kunes, M.; Kolska, Z.; Švorčík, V.; Lyutakov, O. Spatially selective modification of PLLA surface: From hydrophobic to hydrophilic or to repellent. Appl. Surf. Sci. 2017, 397, 226–234. [Google Scholar] [CrossRef]
- Guo, C.; Cai, N.; Dong, Y. Duplex surface modification of porous poly (lactic acid) scaffold. Mater. Lett. 2013, 94, 11–14. [Google Scholar] [CrossRef]
- Kiss, É.; Bertóti, I.; Vargha-Butler, E.I. XPS and wettability characterization of modified poly(lactic acid) and poly(lactic/glycolic acid) films. J. Colloid Interface Sci. 2002, 245, 91–98. [Google Scholar] [CrossRef]
- Riveiro, A.; Maçon, A.L.B.; del Val, J.; Comesaña, R.; Pou, J. Laser surface texturing of polymers for biomedical applications. Front. Phys. 2018, 6. [Google Scholar] [CrossRef]
- Fisher, J.P.; Reddi, A.H. Functional tissue engineering of bone: Signals and scaffolds. In Topics in Tissue Engineering; University of Oulu: Oulu, Finland, 2003. [Google Scholar]
- Bhatla, A.; Yao, Y.L. Effect of laser surface modification on the crystallinity of poly(L-lactic acid). J. Manuf. Sci. Eng. Trans. ASME 2009, 131, 051004. [Google Scholar] [CrossRef]
- Rytlewski, P.; Mróz, W.; Zenkiewicz, M.; Czwartos, J.; Budner, B. Laser induced surface modification of polylactide. J. Mater. Process. Technol. 2012, 212, 1700–1704. [Google Scholar] [CrossRef]
- Kancharla, V.V.; Chen, S. Fabrication of biodegradable polymeric micro-devices using laser micromachining. Biomed. Microdevices 2002, 4, 105–109. [Google Scholar] [CrossRef]
- Stepak, B.; Antończak, A.J.; Bartkowiak-Jowsa, M.; Filipiak, J.; Pezowicz, C.; Abramski, K.M. Fabrication of a polymer-based biodegradable stent using a CO2 laser. Arch. Civil Mech. Eng. 2014, 14, 317–326. [Google Scholar] [CrossRef]
- Antończak, A.J.; Stȩpak, B.D.; Szustakiewicz, K.; Wójcik, M.R.; Abramski, K.M. Degradation of poly(l-lactide) under CO2 laser treatment above the ablation threshold. Polym. Degrad. Stab. 2014, 109, 97–105. [Google Scholar] [CrossRef]
- Dadbin, S. Surface modification of LDPE film by CO2 pulsed laser irradiation. Eur. Polym. J. 2002, 38, 2489–2495. [Google Scholar] [CrossRef]
- Stępak, B.D.; Antończak, A.J.; Abramski, K.M. Rapid fabrication of microdevices by controlling the PDMS curing conditions during replication of a laser-prototyped mould. J. Micromech. Microeng. 2015, 25, 10. [Google Scholar] [CrossRef]
- Waugh, D.G.; Lawrence, J.; Morgan, D.J.; Thomas, C.L. Interaction of CO2 laser-modified nylon with osteoblast cells in relation to wettability. Mater. Sci. Eng. C 2009, 29, 2514–2524. [Google Scholar] [CrossRef][Green Version]
- Zheng, Y.; Xiong, C.; Wang, Z.; Li, X.; Zhang, L. A combination of CO2 laser and plasma surface modification of poly(etheretherketone) to enhance osteoblast response. Appl. Surf. Sci. 2015, 344, 79–88. [Google Scholar] [CrossRef]
- Kobielarz, M.; Tomanik, M.; Mroczkowska, K.; Szustakiewicz, K.; Oryszczak, M.; Mazur, A.; Antończak, A.; Filipiak, J. Laser-modified PLGA for implants: In vitro degradation and mechanical properties. Acta Bioeng. Biomech. 2020, 22, 179–197. [Google Scholar] [CrossRef]
- Kobielarz, M.; Gazińska, M.; Tomanik, M.; Stępak, B.; Szustakiewicz, K.; Filipiak, J.; Antończak, A.; Pezowicz, C. Physicochemical and mechanical properties of CO2 laser-modified biodegradable polymers for medical applications. Polym. Degrad. Stab. 2019, 165, 182–195. [Google Scholar] [CrossRef]
- Fan, Y.; Nishida, H.; Shirai, Y.; Tokiwa, Y.; Endo, T. Thermal degradation behaviour of poly(lactic acid) stereocomplex. Polym. Degrad. Stab. 2004, 86, 197–208. [Google Scholar] [CrossRef]
- Jia, W.; Luo, Y.; Yu, J.; Liu, B.; Hu, M.; Chai, L.; Wang, C. Effects of high-repetition-rate femtosecond laser micromachining on the physical and chemical properties of polylactide (PLA). Opt. Express 2015, 23, 26932–26939. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Michaljaničová, I.; Sajdl, P.; Fitl, P.; Švorčík, V. Surface ablation of PLLA induced by KrF excimer laser. Appl. Surf. Sci. 2013, 283, 438–444. [Google Scholar] [CrossRef]
- Michaljanicová, I.; Slepicka, P.; Heitz, J.; Barb, R.A.; Sajdlc, P.; Svorcíka, V. Comparison of KrF and ArF excimer laser treatment of biopolymer surface. Appl. Surf. Sci. 2015, 339, 144–150. [Google Scholar] [CrossRef]
- Antończak, A.J.; Stępak, B.; Szustakiewicz, K.; Wójcik, M.; Kozioł, P.E.; Łazarek, Ł.; Abramski, K.M. Effect of CO2 laser micromachining on physicochemical properties of poly(L-lactide). In Proceedings of the SPIE 2014—International Conference on Applications of Optics and Photonics, Aveiro, Portugal, 22 August 2014; Volume 9286, pp. 92860Z-1–92860Z-10. [Google Scholar]
- ISO. Surface Texture. Profile Method. Terms, Definitions and Surface Texture Parameters; BS EN ISO 4287: Geometrical Product Specification (GPS); ISO: Geneva, Switzerland, 2000. [Google Scholar]
- ISO. ISO 10993-5: Biological Evaluation of Medical Devices—Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicity; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Castillejo, M.; Rebollar, E.; Oujja, M.; Sanz, M.; Selimis, A.; Sigletou, M.; Psycharakis, S.; Ranella, A.; Fotakis, C. Fabrication of porous biopolymer substrates for cell growth by UV laser: The role of pulse duration. Appl. Surf. Sci. 2012, 258, 8919–8927. [Google Scholar] [CrossRef]
- Rodrigues, N.; Benning, M.; Ferreira, A.M.; Dixon, L.; Dalgarno, K. Manufacture and characterisation of porous PLA scaffolds. Procedia CIRP 2016, 49, 33–38. [Google Scholar] [CrossRef]
- Kryszak, B.; Szustakiewicz, K.; Stępak, B.; Gazińska, M.; Antończak, A.J. Structural, thermal and mechanical changes in poly(L-lactide)/hydroxyapatite composite extruded foils modified by CO2 laser irradiation. Eur. Polym. J. 2019, 114, 57–65. [Google Scholar] [CrossRef]
- Riveiro, A.; Soto, R.; Comesaña, R.; Boutinguiza, M.; Del Val, J.; Quintero, F.; Lusquiños, F.; Pou, J. Laser surface modification of PEEK. Appl. Surf. Sci. 2012, 258, 9437–9442. [Google Scholar] [CrossRef]
- Aflori, M.; Butnaru, M.; Tihauan, B.-M.; Doroftei, F. Eco-friendly method for tailoring biocompatible and antimicrobial surfaces of poly-L-lactic acid. Nanomaterials 2019, 9, 428. [Google Scholar] [CrossRef]
- Slepickova-Kasalkova, N.; Slepicka, P.; Kolska, Z.; Svorcik, V. Wettability and other surface properties of modified polymers. Wetting Wettability 2015. [Google Scholar] [CrossRef]
- Correia, D.M.; Ribeiro, C.; Botelho, G.; Borges, J.; Lopes, C.; Vaz, F.; Carabineiro, S.A.C.; Machado, A.V.; Lanceros-Méndez, S. Superhydrophilic poly(L-lactic acid) electrospun membranes for biomedical applications obtained by argon and oxygen plasma treatment. Appl. Surf. Sci. 2016, 371, 74–82. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 259–271. [Google Scholar] [CrossRef]
- Slepičková Kasálková, N.; Slepička, P.; Bačáková, L.; Sajdl, P.; Švorčík, V. Biocompatibility of plasma nanostructured biopolymers. Nucl. Instrum. Methods Phys. Res. B 2013, 307, 642–646. [Google Scholar] [CrossRef]
- Vesel, A.; Junkar, I.; Cvelbar, U.; Kovač, J.; Mozetič, M. Surface modification of polyester by oxygen- and nitrogen-plasma treatment. Surf. Interface Anal. 2008, 40, 1444–1453. [Google Scholar] [CrossRef]
- Chan, C.M.; Ko, T.M.; Hiraoka, H. Polymer surface modification by plasmas and photons. Surf. Sci. Rep. 1996, 24, 1–54. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, B.; Wang, Y.; Liu, C.; Shen, C. Effect of different sterilization methods on the properties of commercial biodegradable polyesters for single-use, disposable medical devices. Mater. Sci. Eng. C 2019, 105, 110041–110049. [Google Scholar] [CrossRef]
- Washburn, N.R.; Yamada, K.M.; Simon, C.G.; Kennedy, S.B.; Amis, E.J. High-throughput investigation of osteoblast response to polymer crystallinity: Influence of nanometer-scale roughness on proliferation. Biomaterials 2004, 25, 1215–1224. [Google Scholar] [CrossRef]
- Slepička, P.; Siegel, J.; Lyutakov, O.; Slepičková-Kasálková, N.; Kolská, Z.; Bačáková, L.; Švorčík, V. Polymer nanostructures for bioapplications induced by laser treatment. Biotechnol. Adv. 2018, 36, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Terada, C.; Imamura, T.; Ohshima, T.; Maeda, N.; Tatehara, S.; Tokuyama-Toda, R.; Yamachika, S.; Toyoda, N.; Satomura, K. The effect of irradiation with a 405 nm blue-violet laser on the bacterial adhesion on the osteosynthetic biomaterials. Int. J. Photoenergy 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Rapacz-Kmita, A.; Szaraniec, B.; Mikołajczyk, M.; Stodolak-Zych, E.; Dzierzkowska, E.; Gajek, M.; Dudek, P. Multifunctional biodegradable polymer/clay nanocomposites with antibacterial properties in drug delivery systems. Acta Bioeng. Biomech. 2020, 22, 1–19. [Google Scholar] [CrossRef]
- Kubies, D.; Himmlová, L.; Riedel, T.; Chánová, E.; Balík, K.; Douděrová, M.; Bártová, J.; Pešáková, V. The interaction of osteoblasts with bone-implant materials: 1. The effect of physicochemical surface properties of implant materials. Physiol. Res. 2011, 60, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Ajala, O.; Werther, C.; Nikaeen, P.; Singh, R.P.; Depan, D. Influence of graphene nanoscrolls on the crystallization behavior and nano-mechanical properties of polylactic acid. Polym. Adv. Technol. 2019, 30, 1825–1835. [Google Scholar] [CrossRef]
- Wright-Charlesworth, D.D.; Miller, D.M.; Miskioglu, I.; King, J.A. Nanoindentation of injection molded PLA and self-reinforced composite PLA after in vitro conditioning for three months. J. Biomed. Mater. Res. Part A 2005, 74, 388–396. [Google Scholar] [CrossRef]

| Specimen | Optical Power P (W) | Scanning Speed V (cm/s) | Hatching (Line-to-Line Space) h (μm) | Pulse Repetition Rate (PRR) (Hz) | Pulse Energy Ei (mJ) | Accumulated Fluence FA (J/cm2) |
|---|---|---|---|---|---|---|
| Reference | - | - | - | - | - | - |
| F1 | 0.447 | 7.1 | 25.4 | 2.8 | 15.5 | 24 |
| F2 | 0.867 | 31.0 | 48 | |||
| F3 | 1.286 | 45.8 | 71 |
| Roughness Parameters | Reference | F1 | F2 | F3 |
|---|---|---|---|---|
| Rz (µm) | 0.10 ± 0.04 | 0.21 ± 0.05 | 2.87 ± 0.31 | 6.67 ± 0.66 |
| Ra * (µm) | 0.02 | 0.04 | 0.57 | 1.33 |
| Specimen Type | EIT (GPa) | HIT (MPa) | HV | hm (μm) | We (nJ) | Wp (nJ) |
|---|---|---|---|---|---|---|
| Ref | 2.1 ± 0.9 | 200.5 ± 53.2 | 18.9 ± 5.0 | 6.1 ± 0.9 | 116.0 ± 46.7 | 145.7 ± 27.3 |
| F1 | 3.4 ± 0.8 | 300.2 ± 54.9 | 28.3 ± 5.2 | 4.7 ± 0.5 | 80.6 ± 13.0 | 113.4 ± 5.6 |
| F2 | 4.1 ± 1.0 | 429.6 ± 205.8 | 40.5 ± 19.4 | 4.3 ± 0.7 | 73.6 ± 7.3 | 117.3 ± 9.3 |
| F3 | 0.4 ± 0.03 | 115.9 ± 17.7 | 10.9 ± 1.7 | 1.2 ± 0.4 | 376.6 ± 40.2 | 166.7 ± 25.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomanik, M.; Kobielarz, M.; Filipiak, J.; Szymonowicz, M.; Rusak, A.; Mroczkowska, K.; Antończak, A.; Pezowicz, C. Laser Texturing as a Way of Influencing the Micromechanical and Biological Properties of the Poly(L-Lactide) Surface. Materials 2020, 13, 3786. https://doi.org/10.3390/ma13173786
Tomanik M, Kobielarz M, Filipiak J, Szymonowicz M, Rusak A, Mroczkowska K, Antończak A, Pezowicz C. Laser Texturing as a Way of Influencing the Micromechanical and Biological Properties of the Poly(L-Lactide) Surface. Materials. 2020; 13(17):3786. https://doi.org/10.3390/ma13173786
Chicago/Turabian StyleTomanik, Magdalena, Magdalena Kobielarz, Jarosław Filipiak, Maria Szymonowicz, Agnieszka Rusak, Katarzyna Mroczkowska, Arkadiusz Antończak, and Celina Pezowicz. 2020. "Laser Texturing as a Way of Influencing the Micromechanical and Biological Properties of the Poly(L-Lactide) Surface" Materials 13, no. 17: 3786. https://doi.org/10.3390/ma13173786
APA StyleTomanik, M., Kobielarz, M., Filipiak, J., Szymonowicz, M., Rusak, A., Mroczkowska, K., Antończak, A., & Pezowicz, C. (2020). Laser Texturing as a Way of Influencing the Micromechanical and Biological Properties of the Poly(L-Lactide) Surface. Materials, 13(17), 3786. https://doi.org/10.3390/ma13173786








