A Nanosensor for Naked-Eye Identification and Adsorption of Cadmium Ion Based on Core–Shell Magnetic Nanospheres
Abstract
1. Introduction
2. Experimental
2.1. Materials
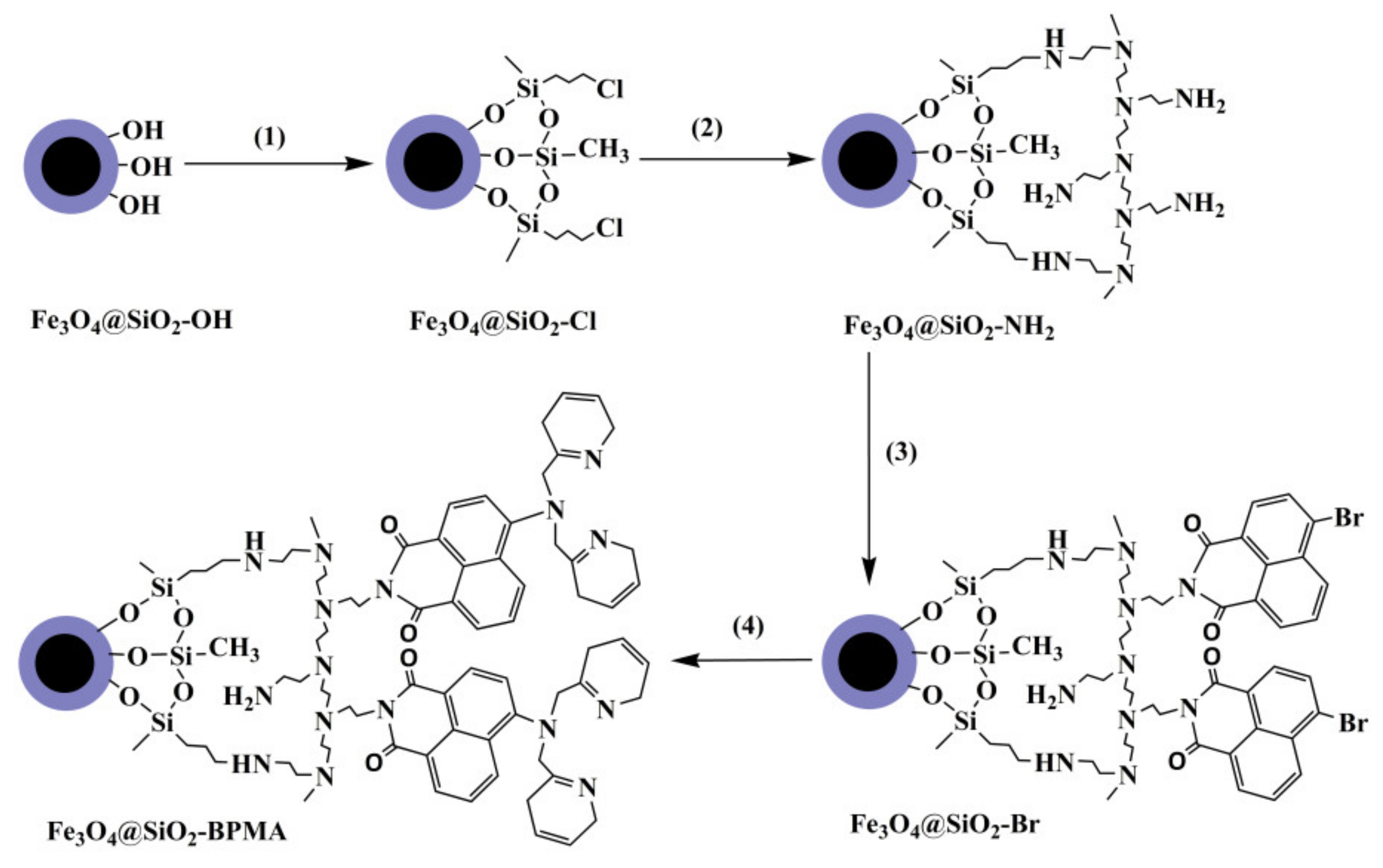
2.2. Synthesis of Fe3O4@SiO2 Nanospheres
2.3. Fe3O4@SiO2 Functionalized with BPMA
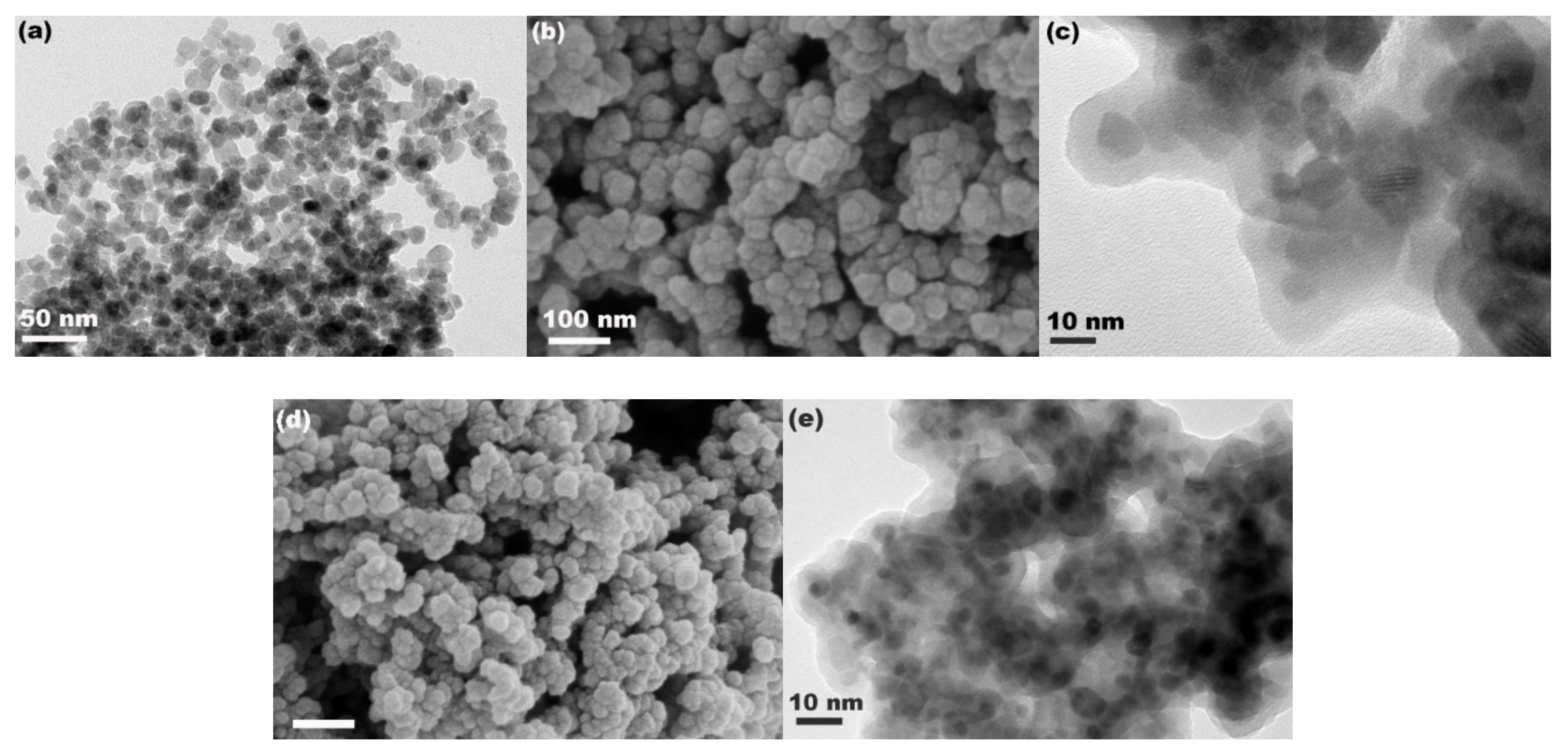
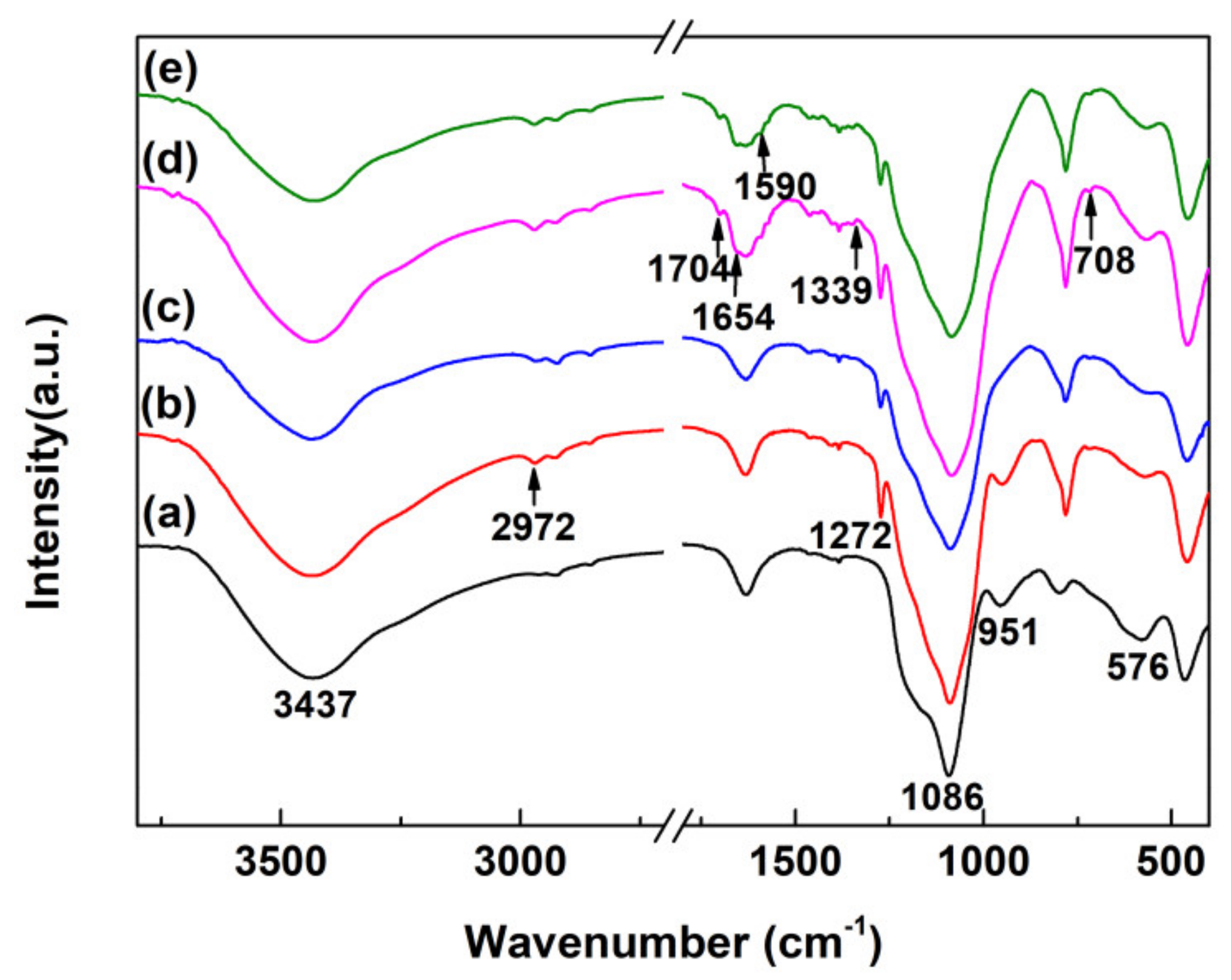
2.4. Characterization
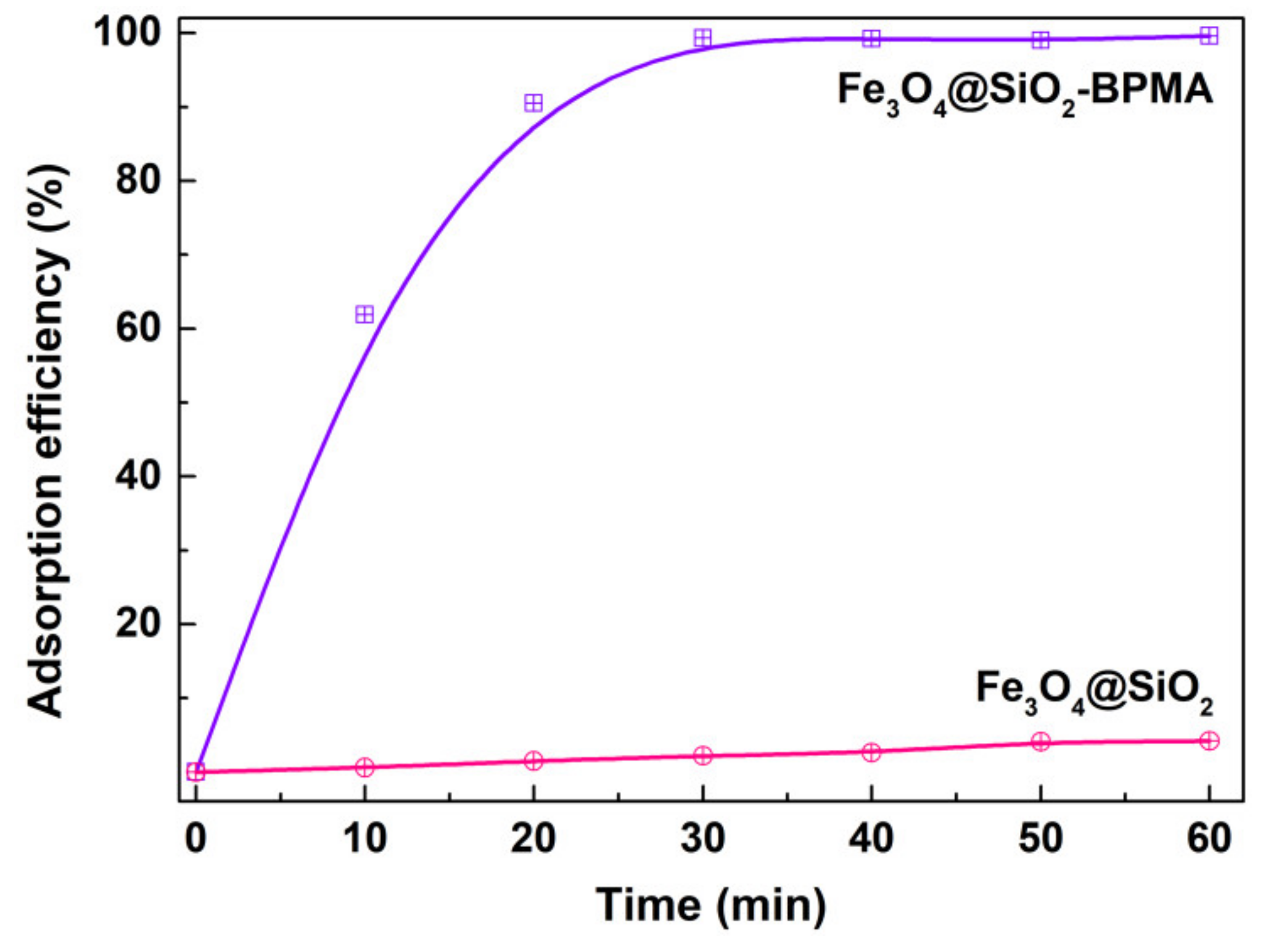
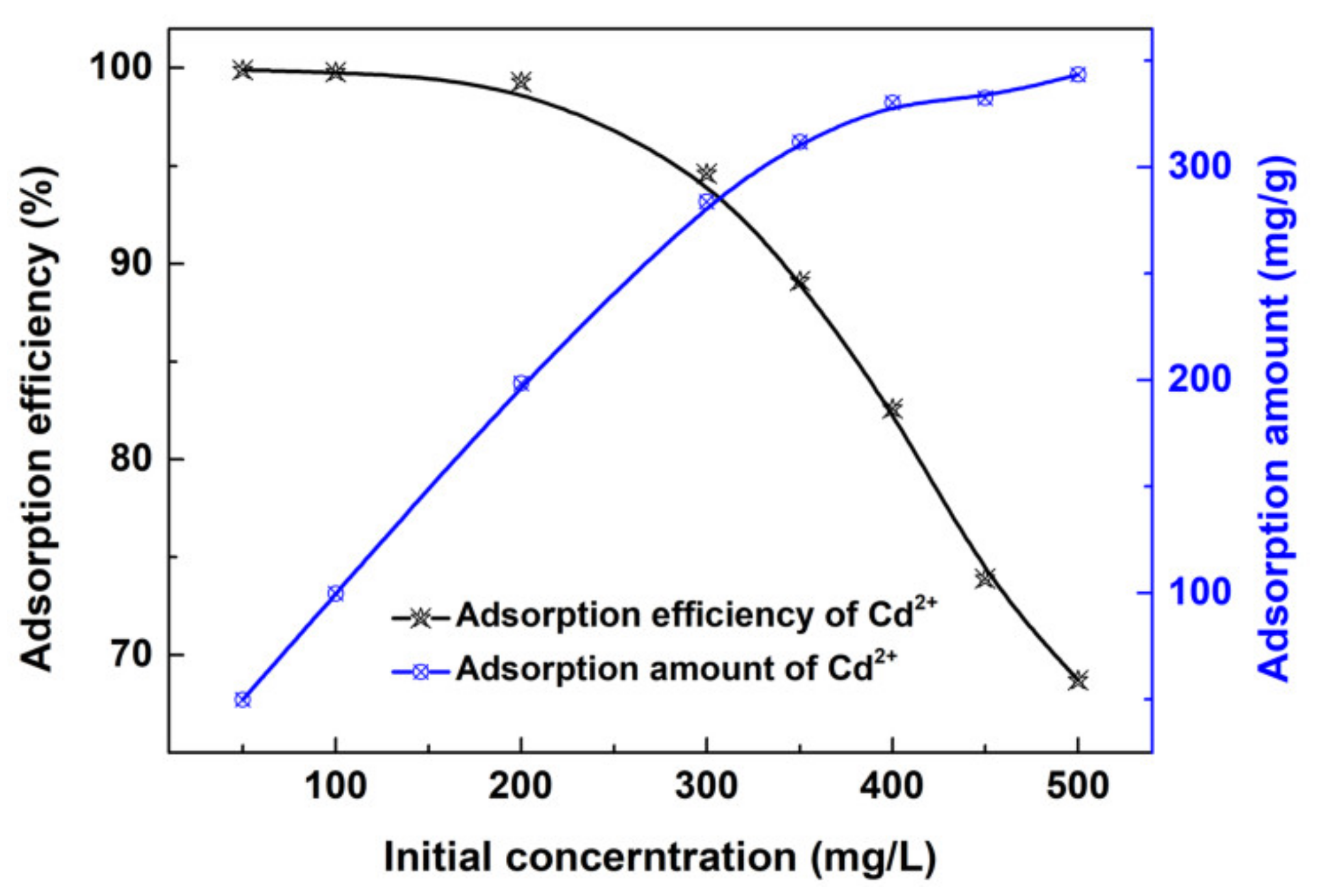
2.5. Adsorption Study
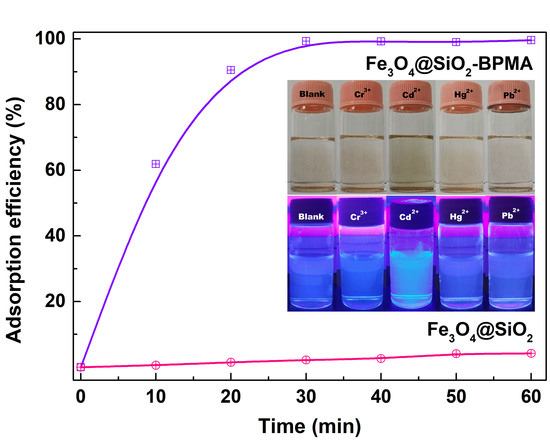
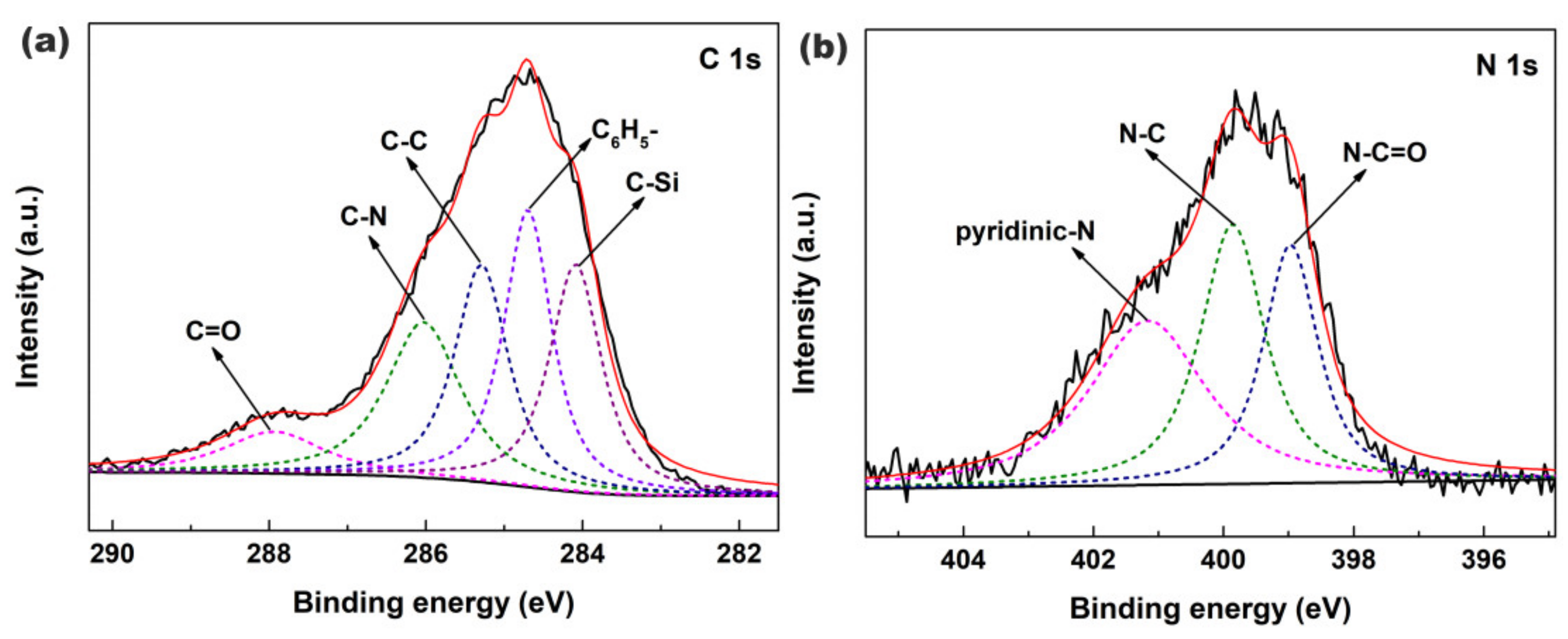
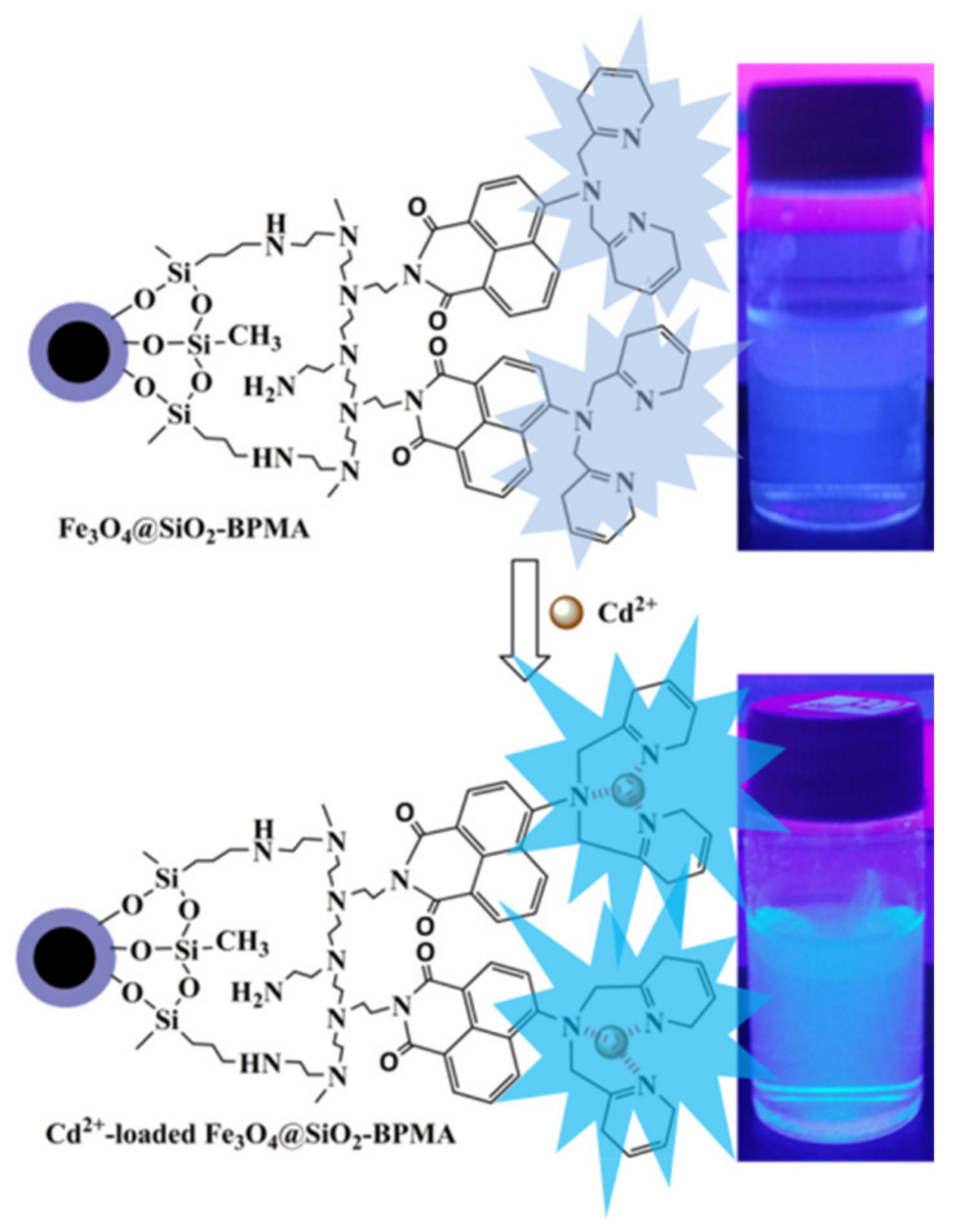
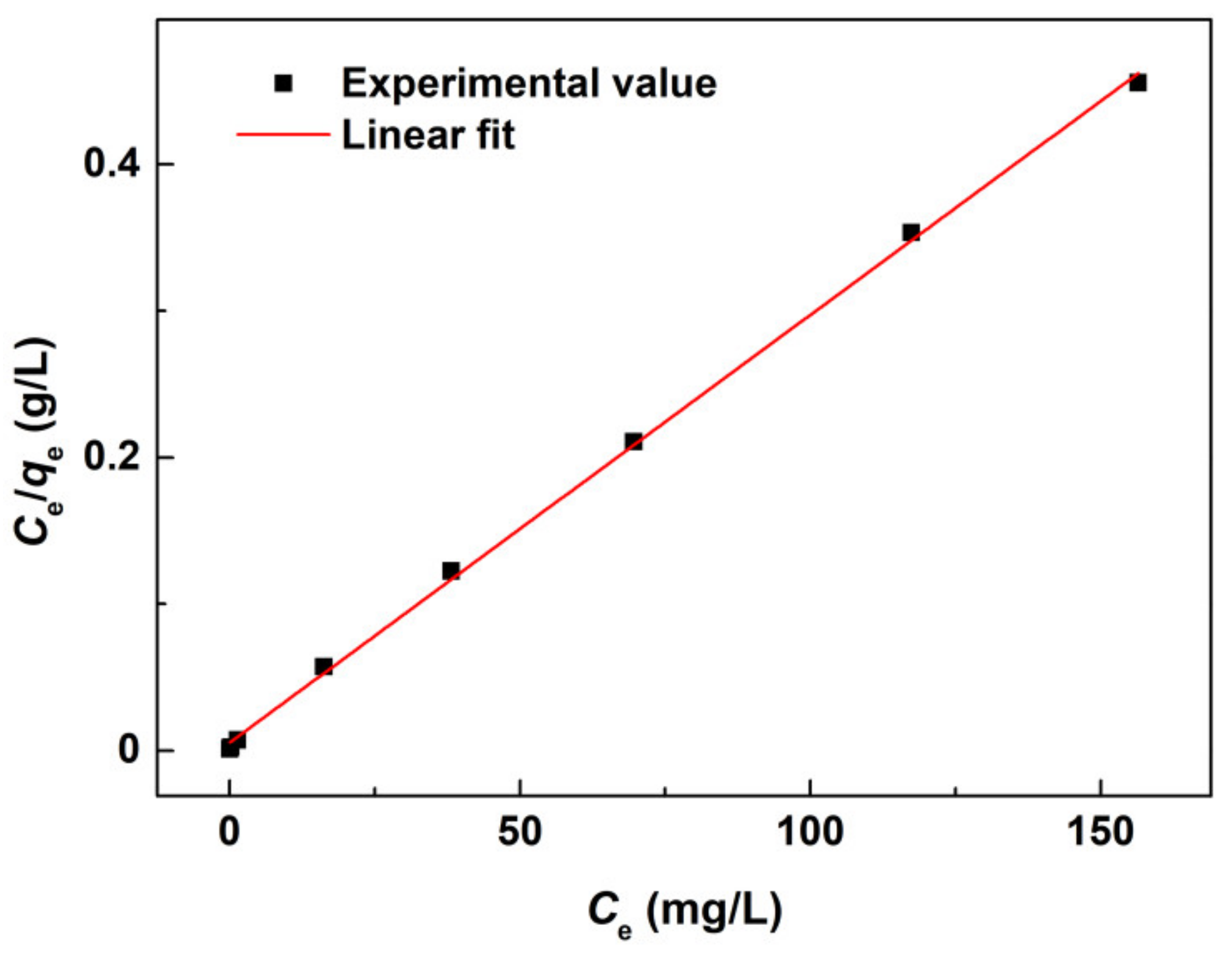
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Makarewicz, E.; Cysewski, P.; Michalik, A.; Ziółkowska, D. Properties of Acid or Alkali Treated Cadmium Pigments. Dyes Pigment. 2013, 96, 338–348. [Google Scholar] [CrossRef]
- İlhan, M.; Keskin, İ.Ç. Photoluminescence, Radioluminescence and Thermoluminescence Properties of Eu3+ Doped Cadmium Tantalate Phosphor. Dalton Trans. 2018, 47, 13939–13948. [Google Scholar] [CrossRef]
- Ma, J.; Ni, S.; Zhang, J.; Yang, X.; Zhang, L. The Electrochemical Performance of Nickel Chromium Oxide as a New Anode Material for Lithium Ion Batteries. Electrochim. Acta 2015, 176, 1420–1426. [Google Scholar] [CrossRef]
- Vizuete, J.; Pérez-López, M.; Míguez-Santiyán, M.P.; Hernández-Moreno, D. Mercury (Hg), Lead (Pb), Cadmium (Cd), Selenium (Se), and Arsenic (As) in Liver, Kidney, and Feathers of Gulls: A Review. In Reviews of Environmental Contamination and Toxicology Volume 247; Springer International Publishing: Cham, Switzerland, 2008; Volume 247, pp. 85–146. [Google Scholar] [CrossRef]
- Ur Rehman, M.Z.; Rizwan, M.; Hussain, A.; Saqib, M.; Ali, S.; Sohail, M.I.; Shafiq, M.; Hafeez, F. Alleviation of Cadmium (Cd) Toxicity and Minimizing its Uptake in Wheat (Triticum Aestivum) by Using Organic Carbon Sources in Cd-Spiked Soil. Environ. Pollut. 2018, 241, 557–565. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, P.C.; Couttenye, M.M.; Lamberts, L.V.; Elseviers, M.M.; Goodman, W.G.; Schrooten, I.; Cabrera, W.E.; De Broe, M.E. Aluminum, Iron, Lead, Cadmium, Copper, Zinc, Chromium, Magnesium, Strontium, and Calcium Content in Bone of End-Stage Renal Failure Patients. Clin. Chem. 1999, 45, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.S.; Li, Y.H.; Cui, G.H.; Dong, G.Y. New Two-Dimensional Cd(II) Coordination Networks Bearing Benzimidazolyl-Based Linkers as Bifunctional Chemosensors for Detection of Acetylacetone and Fe3+. CrystEngComm 2020, 22, 905–914. [Google Scholar] [CrossRef]
- Quan, C.; Liu, J.; Sun, W.; Cheng, X. Highly Sensitive and Selective Fluorescence Chemosensors Containing Phenanthroline Moieties for Detection of Zn2+ and Cd2+ Ions. Chem. Pap. 2019, 74, 485–497. [Google Scholar] [CrossRef]
- Eseola, A.O.; Görls, H.; Bangesh, M.; Plass, W. ESIPT-capable 2,6-di(1H-imidazol-2-yl)phenols with Very Strong Fluorescent Sensing Signals towards Cr(III), Zn(II) and Cd(II): Molecular Variation Effects on Turn-On Efficiency. New J. Chem. 2018, 42, 7884–7900. [Google Scholar] [CrossRef]
- Vera, M.; Santacruz Ortega, H.; Inoue, M.; Machi, L. Bichromophoric Naphthalene Derivatives of Diethylenetriaminepentaacetate (DTPA): Fluorescent Response to H+, Ca2+, Zn2+, and Cd2+. Supramol. Chem. 2019, 31, 336–348. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, A.; Wang, D.; Wang, J.; Chen, P.; Sun, H. Cube-like Fe3O4@SiO2@Au@Ag Magnetic Nanoparticles: A Highly Efficient SERS Substrate for Pesticide Detection. Nanotechnology 2018, 29, 165302. [Google Scholar] [CrossRef]
- Yuan, Z.; Xu, R.; Li, J.; Chen, Y.; Wu, B.; Feng, J.; Chen, Z. Biological Responses to Core-Shell-Structured Fe3O4@SiO2-NH2 Nanoparticles in Rats by a Nuclear Magnetic Resonance-Based Metabonomic Strategy. Int. J. Nanomed. 2018, 13, 2447–2462. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, J.; Zhu, X. Solid Phase Extraction of Bisphenol A Using Magnetic Core-Shell (Fe3O4@SiO2) Nanoparticles Coated with an Ionic liquid, and its Quantitation by HPLC. Microchim. Acta 2016, 183, 1315–1321. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, Y.; Luo, W.; Wang, Y.; Tang, X.; Dou, W.; Cui, Y.; Liu, W. A Resumable Two-Photon Fluorescent Probe for Cu2+ And S2− Based on Magnetic Silica Core-Shell Fe3O4@SiO2 Nanoparticles and its Application in Bioimaging. Anal. Chim. Acta 2018, 1014, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Cheng, C.; Shi, H.; Yang, H.; Yuan, H.; Ni, C. Rational Design of GO-Modified Fe3O4/SiO2 Nanoparticles with Combined Rhenium-188 and Gambogic Acid for Magnetic Target Therapy. ACS Appl. Mater. Interfaces 2017, 9, 28195–28208. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Shaw, L.L. Enhancement of Hydrogen Desorption from Nano-Composite Prepared by Ball-Milling MgH2 with in-situ Aerosol-Spraying LiBH4. ACS Sustain. Chem. Eng. 2019, 7, 15064–15072. [Google Scholar] [CrossRef]
- Morita, Y.; Sakurai, R.; Wakimoto, T.; Kobayashi, K.; Xu, B.; Toku, Y.; Song, G.; Luo, Q.; Ju, Y. tLyP-1-Conjugated Core-Shell Nanoparticles, Fe3O4NPs@mSiO2, for Tumor-Targeted Drug Delivery. Appl. Surf. Sci. 2019, 474, 17–24. [Google Scholar] [CrossRef]
- Ding, Z.; Li, H.; Shaw, L. New Insights into the Solid-State Hydrogen Storage of Nanostructured LiBH4-MgH2 System. Chem. Eng. J. 2019, 385, 123856. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, W.; Ma, H.; Zheng, J.; Zhou, B.; Li, Y. Immobilized Palladium on Surface-Modified Fe3O4/SiO2 Nanoparticles: As a Magnetically Separable and Stable Recyclable High-Performance Catalyst for Suzuki and Heck Cross-Coupling Reactions. Tetrahedron 2012, 68, 3577–3584. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, Y.; Li, L.; Shaw, L. High Reversible Capacity Hydrogen Storage Through Nano-LiBH4 + Nano-MgH2 System. Energy Storage Mater. 2019, 20, 24–35. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Y.; Nguyen, L.; Zhao, Y.; Wu, Z.; Goh, T.W.; Liu, J.J.; Li, Y.; Zhu, T.; Huang, W.; et al. Catalysis on Singly Dispersed Rh Atoms Anchored on an Inert Support. ACS Catal. 2017, 8, 110–121. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, Z.; Ma, T.; Lu, C.-T.; Ma, W.; Shaw, L. Predicting the Hydrogen Release Ability of LiBH4-based Mixtures by Ensemble Machine Learning. Energy Storage Mater. 2019. [Google Scholar] [CrossRef]
- Peralta-Perez, M.R.; Saucedo-Castañeda, G.; Gutierrez-Rojas, M.; Campero, A. SiO2 Xerogel: A Suitable Inert Support for Microbial Growth. J. Sol-Gel Sci. Technol. 2001, 20, 105–110. [Google Scholar] [CrossRef]
- Ding, Z.; Ma, W.; Wei, K.; Wu, J.; Zhou, Y.; Xie, K. Boron Removal from Metallurgical-Grade Silicon Using Lithium Containing Slag. J. Non-Cryst. Solids 2012, 358, 2708–2712. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, J.; Liu, C.; Yan, H. Surface Modification Design for Improving the Strength and Water Vapor Permeability of Waterborne Polymer/SiO2 Composites: Molecular Simulation and Experimental Analyses. Polymers 2020, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Eom, G.H.; Kim, J.H.; Jo, Y.D.; Kim, E.Y.; Bae, J.M.; Kim, C.; Kim, S.J.; Kim, Y. Anion Effects on Construction of Cadmium(II) Compounds with A Chelating Ligand Bis(2-Pyridylmethyl)Amine: Their Photoluminescence and Catalytic Activities. Inorg. Chim. Acta 2012, 387, 106–116. [Google Scholar] [CrossRef]
- Davies, C.J.; Solan, G.A.; Fawcett, J. Synthesis and Structural Characterisation of Cobalt(II) and Iron(II) Chloride Complexes Containing Bis(2-Pyridylmethyl)Amine and Tris(2-Pyridylmethyl)Amine Ligands. Polyhedron 2004, 23, 3105–3114. [Google Scholar] [CrossRef]
- Park, B.K.; Lee, S.H.; Lee, E.Y.; Kwak, H.; Lee, Y.M.; Lee, Y.J.; Jun, J.Y.; Kim, C.; Kim, S.J.; Kim, Y. Zinc(II) polymeric compounds with a chelating ligand bis(2-pyridylmethyl)amine (bispicam) directed by intermolecular C/N/O–H…X (X=Cl, Br, I) interactions: Catalytic Activities. J. Mol. Struct. 2008, 890, 123–129. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Deng, C.; Xiao, Z.; Chen, C.; Luo, X.; Zhou, Y.; Jiang, Q. A Nanosensor for Naked-Eye Identification and Adsorption of Cadmium Ion Based on Core–Shell Magnetic Nanospheres. Materials 2020, 13, 3678. https://doi.org/10.3390/ma13173678
Xu Y, Deng C, Xiao Z, Chen C, Luo X, Zhou Y, Jiang Q. A Nanosensor for Naked-Eye Identification and Adsorption of Cadmium Ion Based on Core–Shell Magnetic Nanospheres. Materials. 2020; 13(17):3678. https://doi.org/10.3390/ma13173678
Chicago/Turabian StyleXu, Yaohui, Chi Deng, Zhigang Xiao, Chang Chen, Xufeng Luo, Yang Zhou, and Qiang Jiang. 2020. "A Nanosensor for Naked-Eye Identification and Adsorption of Cadmium Ion Based on Core–Shell Magnetic Nanospheres" Materials 13, no. 17: 3678. https://doi.org/10.3390/ma13173678
APA StyleXu, Y., Deng, C., Xiao, Z., Chen, C., Luo, X., Zhou, Y., & Jiang, Q. (2020). A Nanosensor for Naked-Eye Identification and Adsorption of Cadmium Ion Based on Core–Shell Magnetic Nanospheres. Materials, 13(17), 3678. https://doi.org/10.3390/ma13173678