A New Type of Composite Membrane PVA-NaY/PA-6 for Separation of Industrially Valuable Mixture Ethanol/Ethyl Tert-Butyl Ether by Pervaporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Preparation of Composite Membranes
2.3. Material Characterization of NaY and Composite Membranes
2.4. Pervaporation Experiments
2.5. Gas Chromatography
3. Results and Discussion
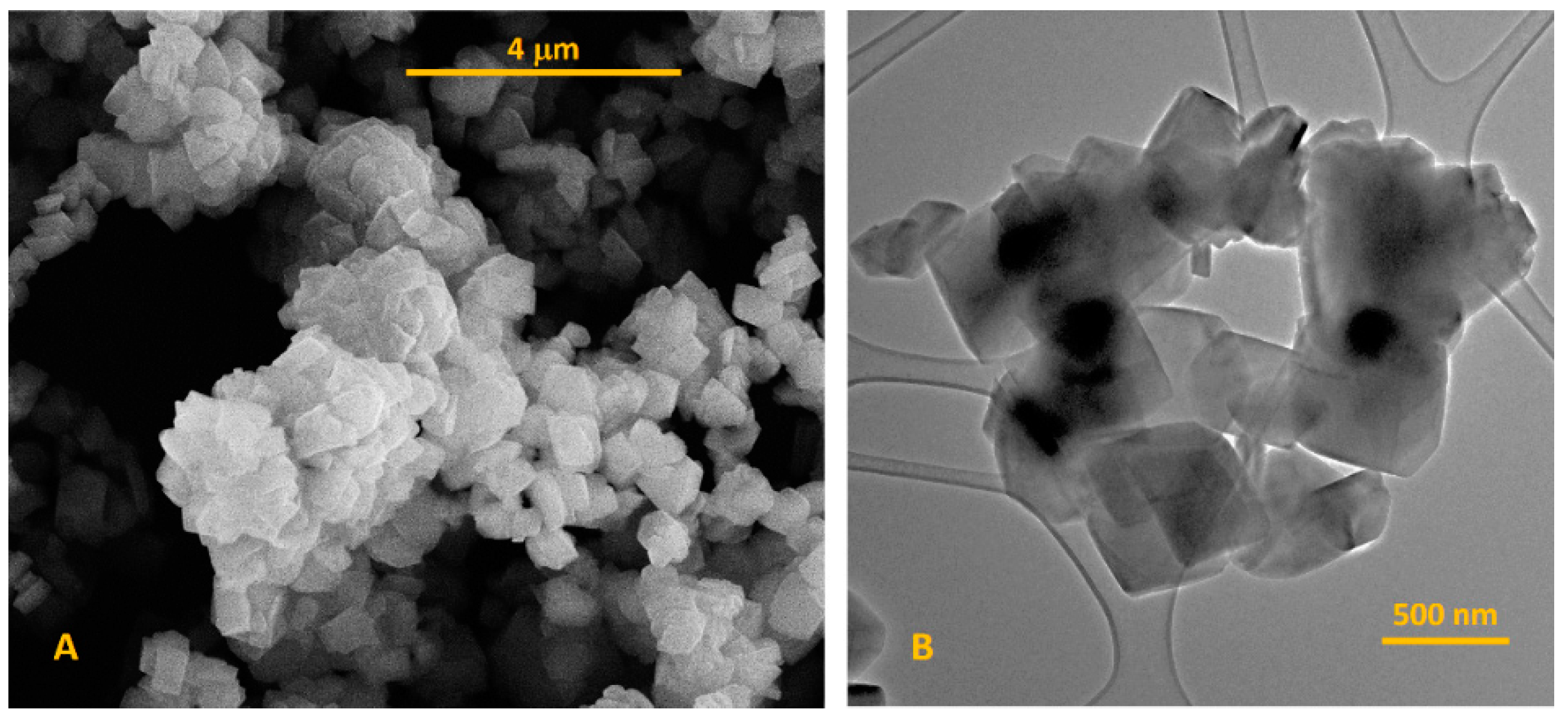
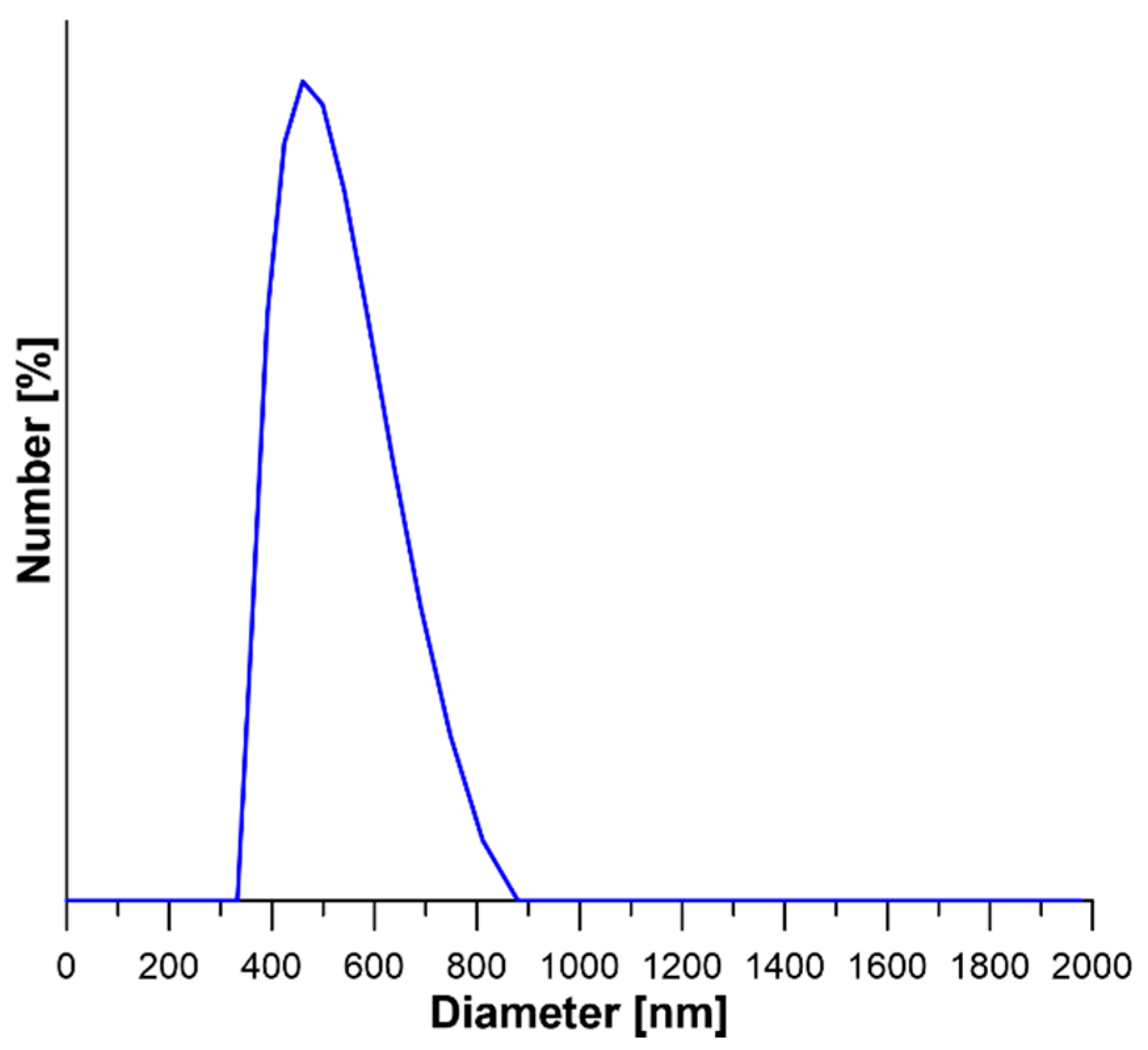
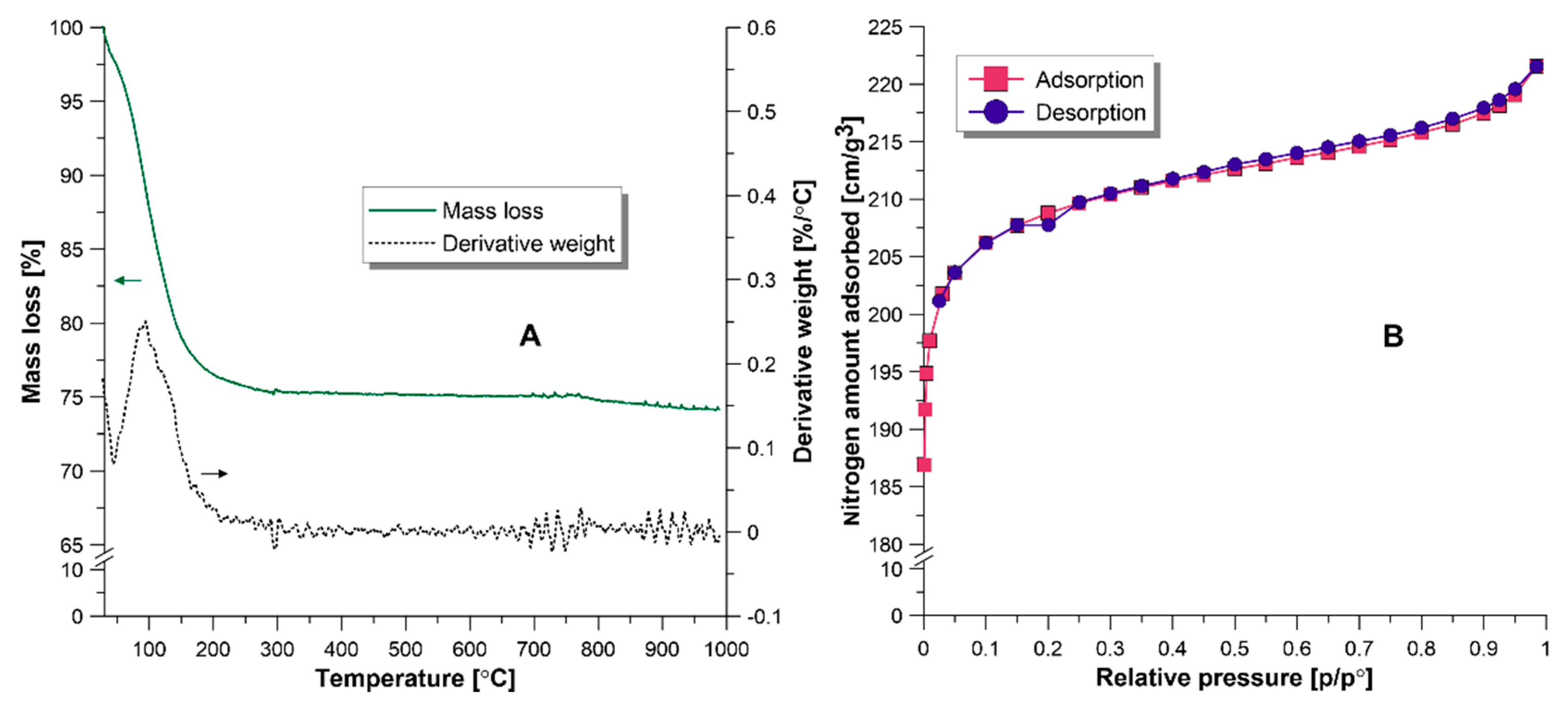
3.1. Characterization of NaY Zeolite
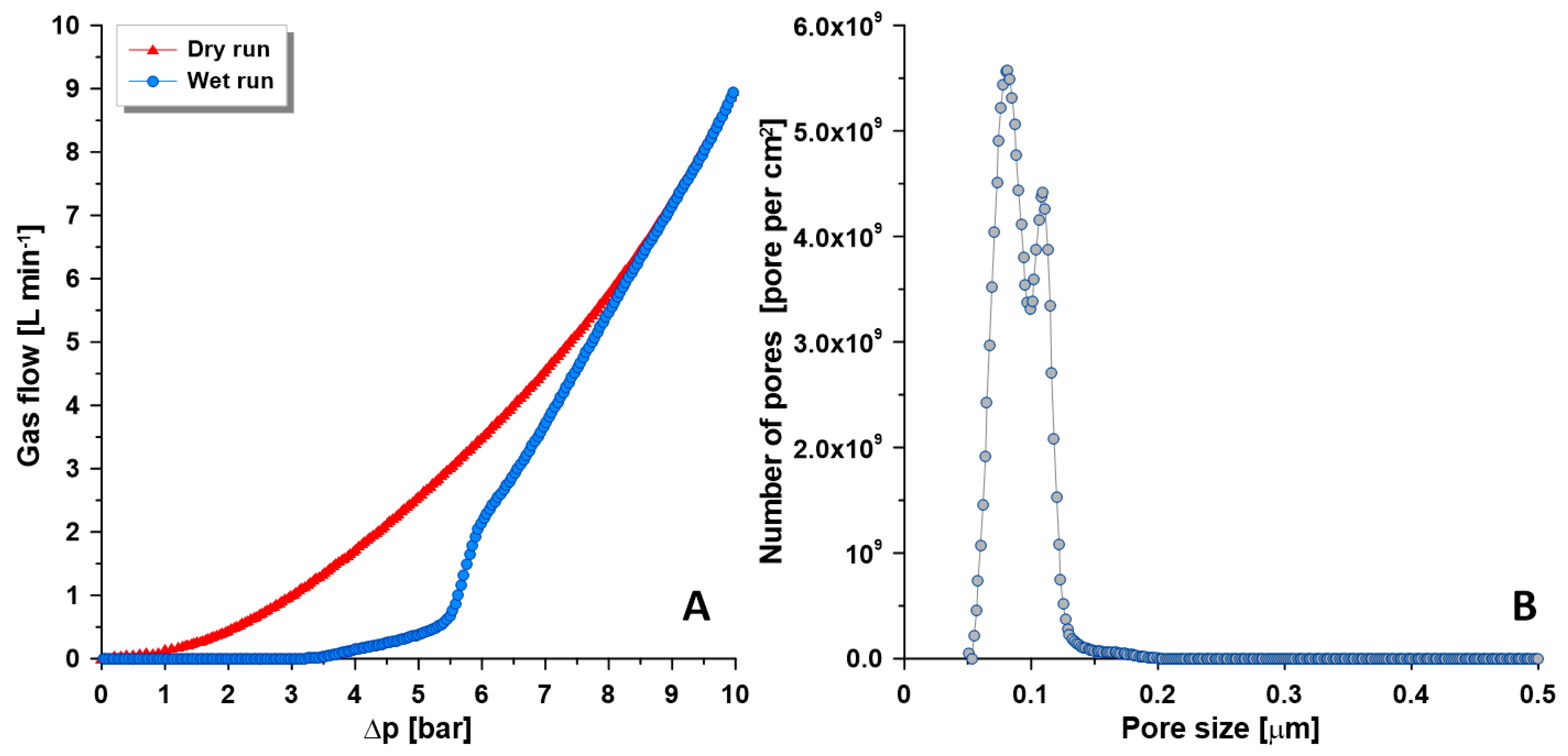
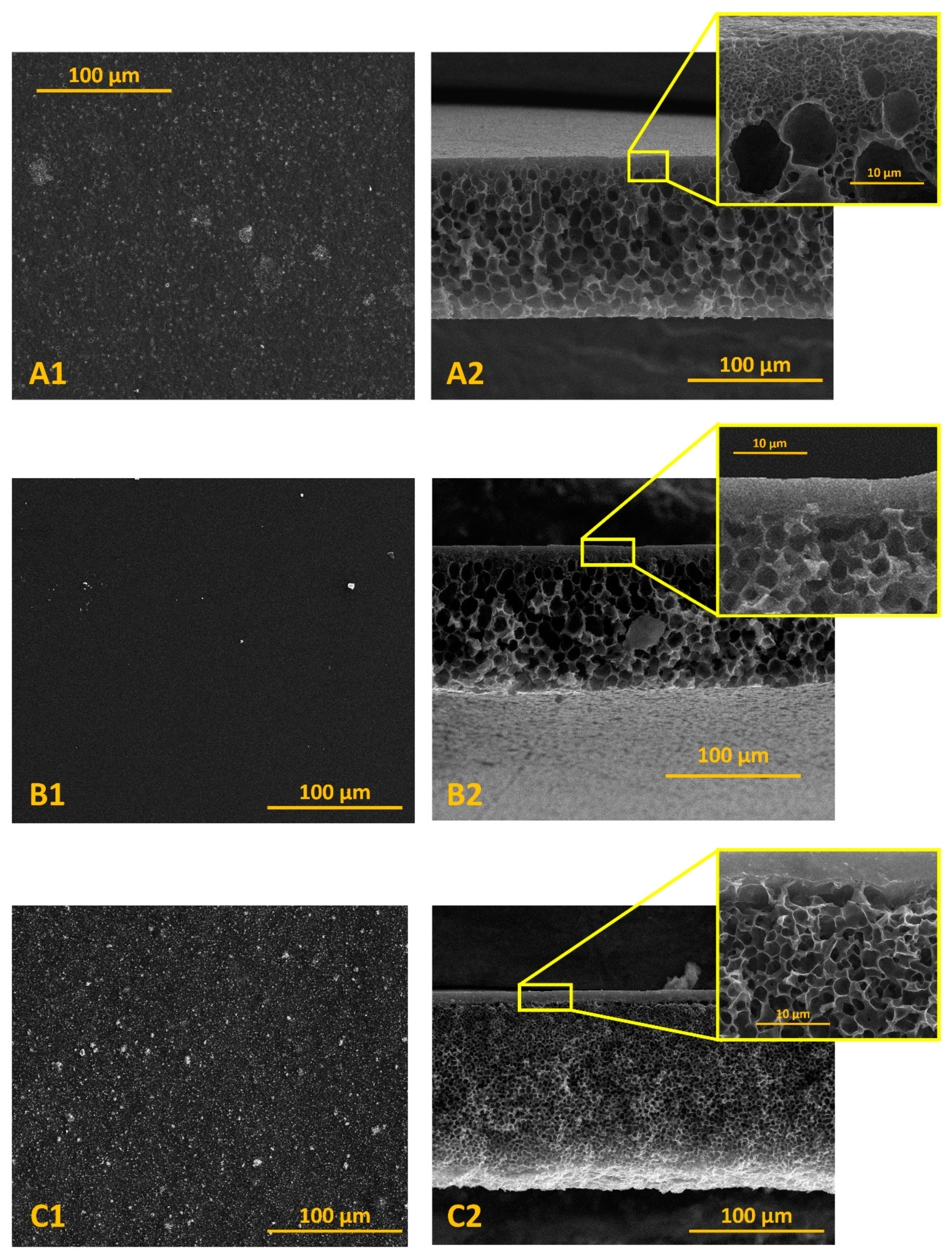
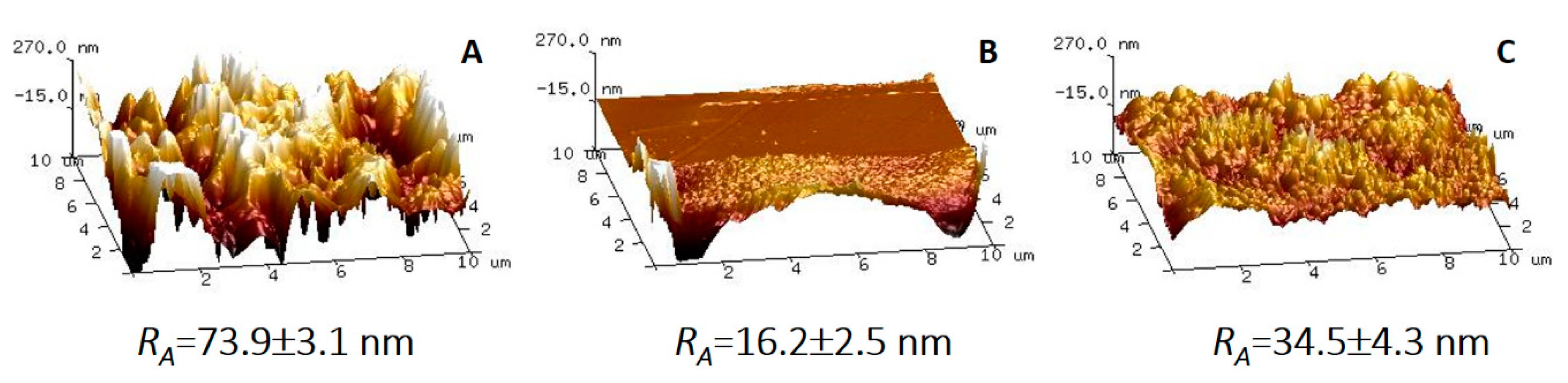
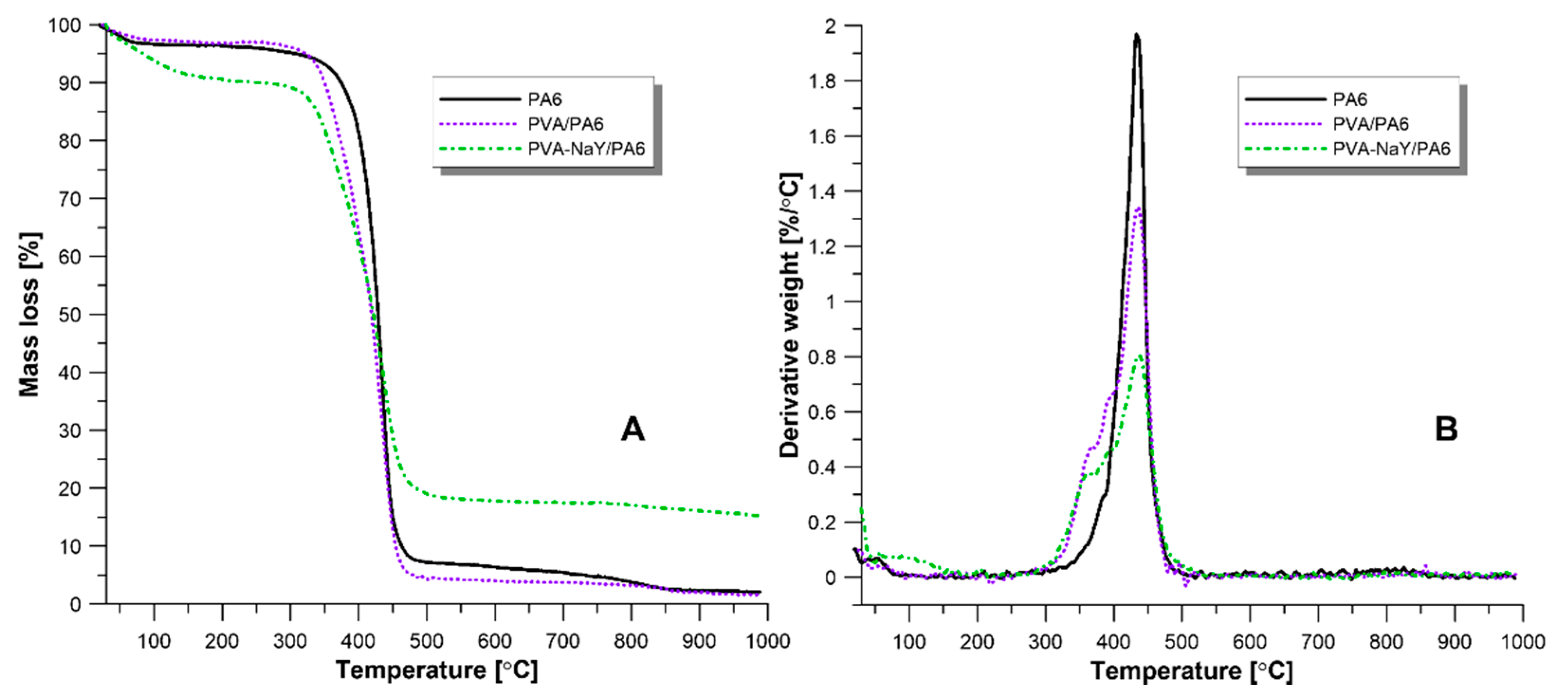
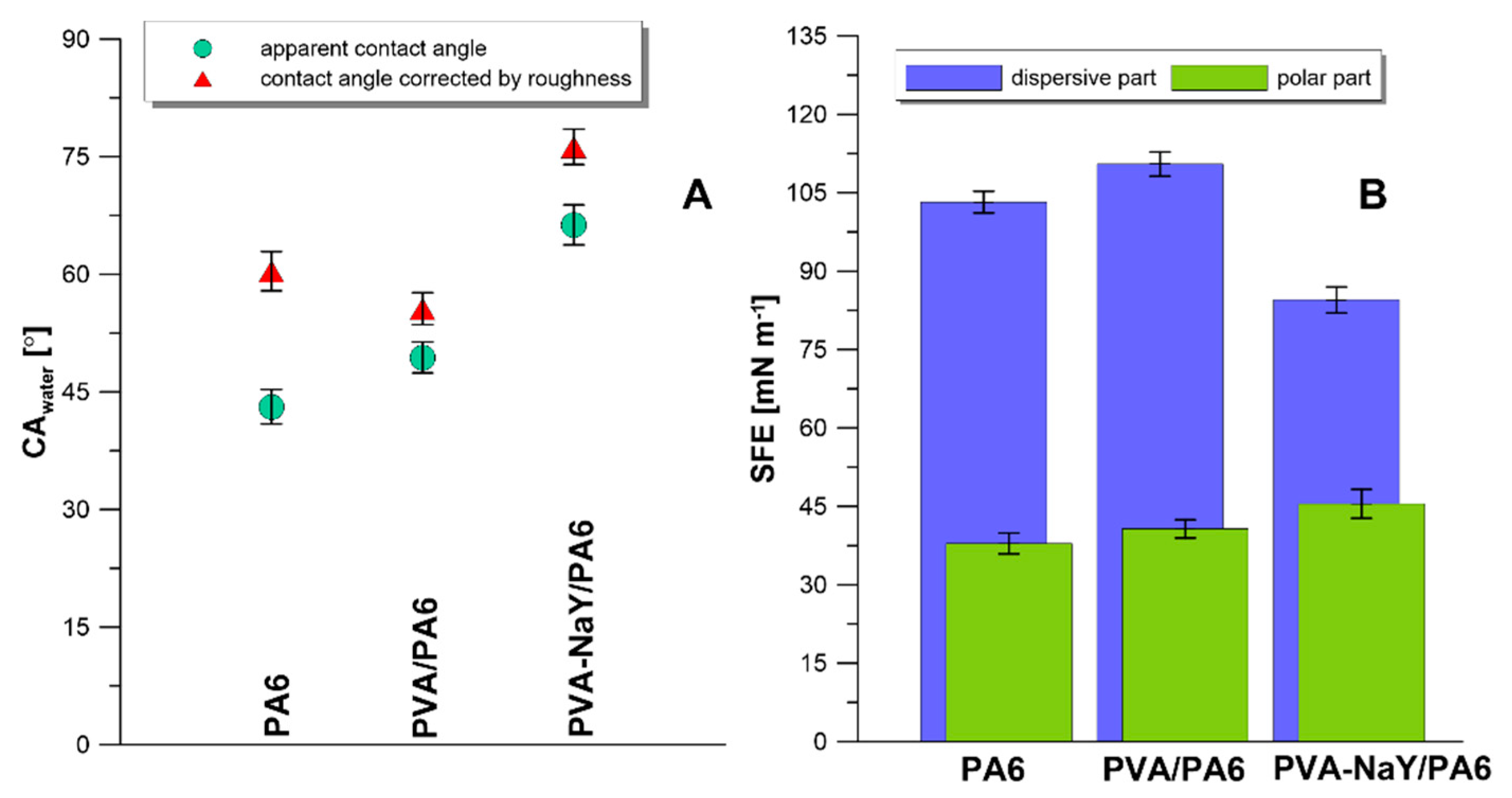
3.2. Characteristics of Composite Membranes
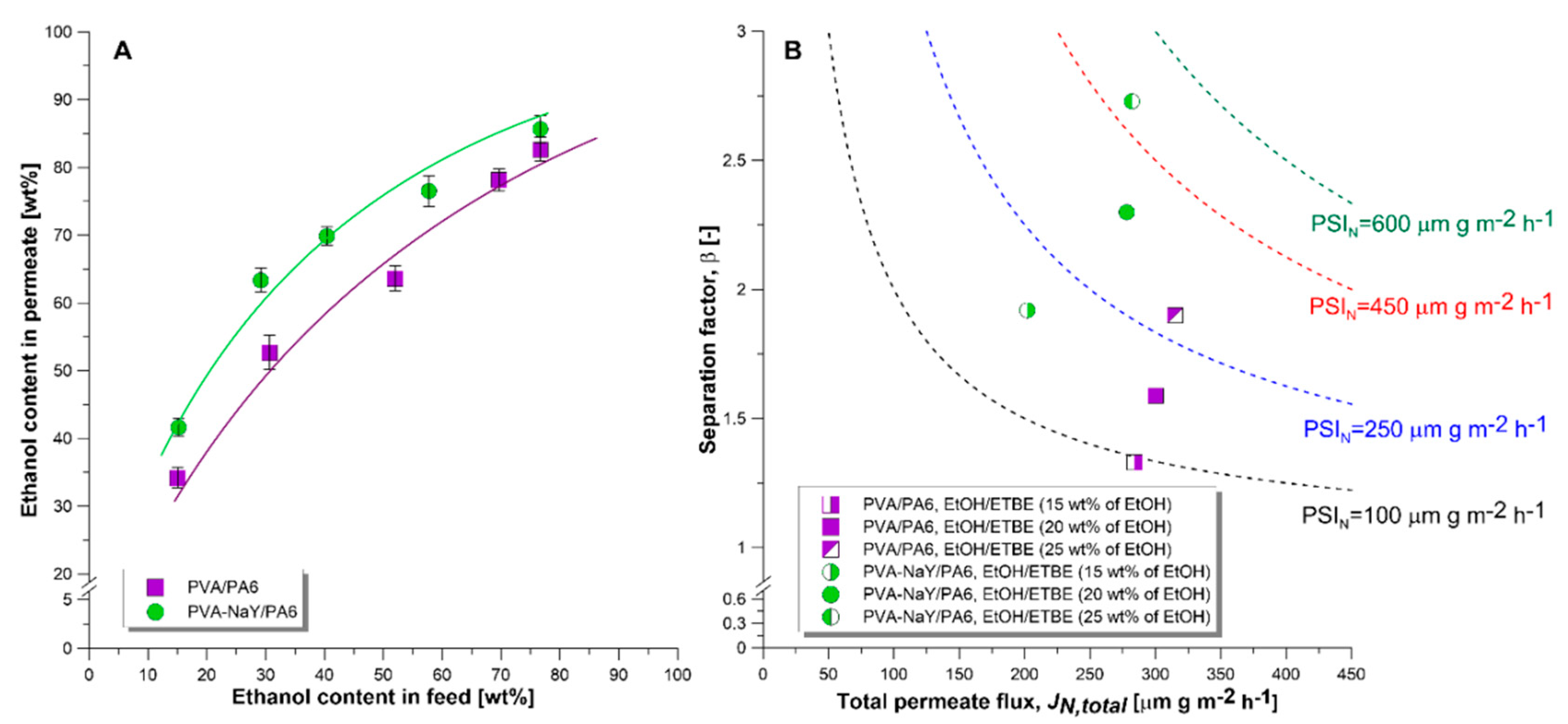
3.3. Pervaporation Experiments
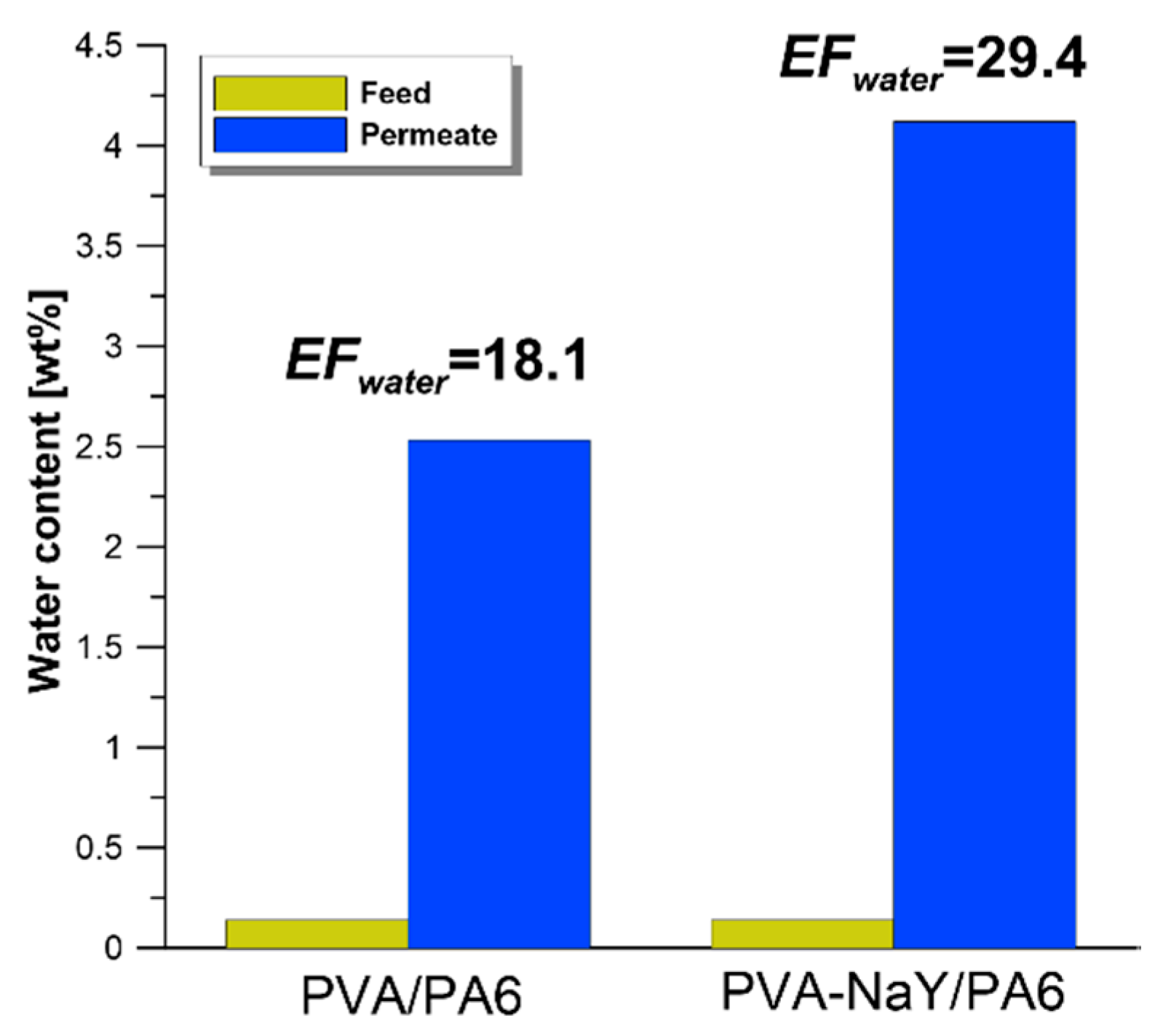
3.4. Influence of Water Presence on Separation Effectiveness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Touchal, S.; Roizard, D.; Perrin, L. Pervaporation properties of polypyrrolidinone-based membranes for EtOH/ETBE mixtures separation. J. Appl. Polym. Sci. 2006, 99, 3622–3630. [Google Scholar] [CrossRef]
- Williams, P.R.D. MTBE in California Drinking Water: An Analysis of Patterns and Trends. Environ. Forensics 2001, 2, 75–85. [Google Scholar] [CrossRef]
- Galán, G.; Martín, M.; Grossmann, I. Integrated Renewable Production of ETBE from Switchgrass. ACS Sustain. Chem. Eng. 2019, 7, 8943–8953. [Google Scholar] [CrossRef]
- Yee, K.F.; Mohamed, A.R.; Tan, S.H. A review on the evolution of ethyl tert-butyl ether (ETBE) and its future prospects. Renew. Sustain. Energy Rev. 2013, 22, 604–620. [Google Scholar] [CrossRef]
- Nicholls, H.C.G.; Mallinson, H.E.H.; Rolfe, S.A.; Hjort, M.; Spence, M.J.; Thornton, S.F. Influence of contaminant exposure on the development of aerobic ETBE biodegradation potential in microbial communities from a gasoline-impacted aquifer. J. Hazard. Mater. 2020, 388, 122022. [Google Scholar] [CrossRef]
- de Menezes, E.W.; Cataluña, R. Optimization of the ETBE (ethyl tert-butyl ether) production process. Fuel Process. Technol. 2008, 89, 1148–1152. [Google Scholar] [CrossRef]
- Hassan Hassan Abdellatif, F.; Babin, J.; Arnal-Herault, C.; Nouvel, C.; Six, J.-L.; Jonquieres, A. Bio-based membranes for ethyl tert-butyl ether (ETBE) bio-fuel purification by pervaporation. J. Membr. Sci. 2017, 524, 449–459. [Google Scholar] [CrossRef]
- Volkov, V.V. Separation of liquids by pervaporation through polymeric membranes. Russ. Chem. Bull. 1994, 43, 187–198. [Google Scholar] [CrossRef]
- Luis, P. Chapter 3—Pervaporation. In Fundamental Modelling of Membrane Systems, 1st ed.; Luis, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 71–102. [Google Scholar] [CrossRef]
- Baker, R. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2004. [Google Scholar] [CrossRef]
- Číhal, P.; Vopička, O.; Lanč, M.; Kludský, M.; Velas, J.; Hrdlička, Z.; Michalcová, A.; Dendisová, M.; Friess, K. Poly(butylene succinate)-cellulose triacetate blends: Permeation, pervaporation, sorption and physical structure. Polym. Test. 2018, 65, 468–479. [Google Scholar] [CrossRef]
- Dutta, B.K.; Sikdar, S.K. Separation of azeotropic organic liquid mixtures by pervaporation. Aiche J. 1991, 37, 581–588. [Google Scholar] [CrossRef]
- Olsson, J.; Trägårdh, G.; Lipnizki, F. The influence of permeant and membrane properties on mass transfer in pervaporation of volatile organic compounds from dilute aqueous solutions. Sep. Sci. Technol. 2002, 37, 1199–1223. [Google Scholar] [CrossRef]
- Wang, M.; Arnal-Herault, C.; Rousseau, C.; Palenzuela, A.; Babin, J.; David, L.; Jonquieres, A. Grafting of multi-block copolymers: A new strategy for improving membrane separation performance for ethyl tert-butyl (ETBE) bio-fuel purification by pervaporation. J. Membr. Sci. 2014, 469, 31–42. [Google Scholar] [CrossRef]
- Hassan Hassan Abdellatif, F.; Babin, J.; Arnal-Herault, C.; David, L.; Jonquieres, A. Grafting of cellulose acetate with ionic liquids for biofuel purification by a membrane process: Influence of the cation. Carbohydr. Polym. 2016, 147, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Zereshki, S.; Figoli, A.; Madaeni, S.S.; Galiano, F.; Drioli, E. Pervaporation separation of ethanol/ETBE mixture using poly(lactic acid)/poly(vinyl pyrrolidone) blend membranes. J. Membr. Sci. 2011, 373, 29–35. [Google Scholar] [CrossRef]
- Jonquières, A.; Clément, R.; Lochon, P. New film-forming poly(urethane-amide-imide) block copolymers: Influence of soft block on membrane properties for the purification of a fuel octane enhancer by pervaporation. Eur. Polym. J. 2005, 41, 783–795. [Google Scholar] [CrossRef]
- Assabumrungrat, S.; Kiatkittipong, W.; Praserthdam, P.; Goto, S. Simulation of pervaporation membrane reactors for liquid phase synthesis of ethyl tert-butyl ether from tert-butyl alcohol and ethanol. Catal. Today 2003, 79–80, 249–257. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, S.; Gong, Y.; Zhou, Y.; Chen, X.; Liu, Y.; Hu, N.; Zhang, F.; Chen, X.; Kita, H. Effect of flouride on preparation and pervaporation performance of NaY zeolite membrane for EtOH/ETBE mixture. Microporous Mesoporous Mater. 2019, 282, 48–52. [Google Scholar] [CrossRef]
- Ramezani, H.; Azizi, S.N.; Hosseini, S.R. NaY zeolite as a platform for preparation of Ag nanoparticles arrays in order to construction of H2O2 sensor. Sens. Actuators B 2017, 248, 571–579. [Google Scholar] [CrossRef]
- Broach, R.W. Zeolite Types and Structures. In Zeolites in Industrial Separation and Catalysis, 1st ed.; Kulprathipanja, S., Ed.; John Wiley & Sons: Glasgow, UK, 2010; pp. 27–60. [Google Scholar] [CrossRef]
- Singleton, N.L.; Huddersman, K.D.; Needham, M.I. The adsorption properties of NaY zeolite for separation of aromatic triazoles. J. Chem. Soc. Faraday Trans. 1998, 94, 3777–3780. [Google Scholar] [CrossRef]
- Sawamura, K.-i.; Furuhata, T.; Sekine, Y.; Kikuchi, E.; Subramanian, B.; Matsukata, M. Zeolite Membrane for Dehydration of Isopropylalcohol—Water Mixture by Vapor Permeation. ACS Appl. Mater. Interfaces 2015, 7, 13728–13730. [Google Scholar] [CrossRef]
- Kita, H.; Fuchida, K.; Horita, T.; Asamura, H.; Okamoto, K. Preparation of Faujasite membranes and their permeation properties. Sep. Purif. Technol. 2001, 25, 261–268. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Kim, Y.-K. Pervaporation separation of MTBE—Methanol mixtures using cross-linked PVA membranes. J. Appl. Polym. Sci. 2000, 75, 1699–1707. [Google Scholar] [CrossRef]
- Chrzanowska, E.; Gierszewska, M.; Kujawa, J.; Raszkowska-Kaczor, A.; Kujawski, W. Development and Characterization of Polyamide—Supported Chitosan Nanocomposite Membranes for Hydrophilic Pervaporation. Polymers 2018, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Ceynowa, J.; Adamczak, P. Enzyme membrane based upon polyamide-6 for oil hydrolysis. J. Appl. Polym. Sci. 1992, 46, 749–755. [Google Scholar] [CrossRef]
- Kujawski, W.; Adamczak, P.; Narebska, A. A Fully Automated System for the Determination of Pore Size Distribution in Microfiltration and Ultrafiltration Membranes. Sep. Sci. Technol. 1989, 24, 495–506. [Google Scholar] [CrossRef]
- Hernández, A.; Calvo, J.I.; Prádanos, P.; Tejerina, F. Pore size distributions in microporous membranes. A critical analysis of the bubble point extended method. J. Membr. Sci. 1996, 112, 1–12. [Google Scholar] [CrossRef]
- Szczerbińska, J.; Kujawski, W.; Arszyńska, J.M.; Kujawa, J. Assessment of air-gap membrane distillation with hydrophobic porous membranes utilized for damaged paintings humidification. J. Membr. Sci. 2017, 538, 1–8. [Google Scholar] [CrossRef]
- Kujawska, A.; Knozowska, K.; Kujawa, J.; Kujawski, W. Influence of downstream pressure on pervaporation properties of PDMS and POMS based membranes. Sep. Purif. Technol. 2016, 159, 68–80. [Google Scholar] [CrossRef]
- Sivakumar, K.; Santhanam, A.; Natarajan, M.; Velauthapillai, D.; Rangasamy, B. Seed-Free Synthesis and Characterization of Zeolite Faujasite Aluminosilicate Coating on α-Alumina Supports. Int. J. Appl. Ceram. Technol. 2016, 13, 1182–1189. [Google Scholar] [CrossRef]
- Mu, L.; Feng, W.; Zhang, H.; Hu, X.; Cui, Q. Synthesis and catalytic performance of a small crystal NaY zeolite with high SiO2/Al2O3 ratio. RSC Adv. 2019, 9, 20528–20535. [Google Scholar] [CrossRef]
- Ramezani, H.; Azizi, S.N.; Cravotto, G. Improved removal of methylene blue on modified hierarchical zeolite Y: Achieved by a “destructive-constructive” method. Green Process. Synth. 2019, 8, 730–741. [Google Scholar] [CrossRef]
- Cele, M.; Friedrich, H.; Bala, M. A study of Fe (III) TPPCl encapsulated in zeolite NaY and Fe (III) NaY in the oxidation of n-octane, cyclohexane, 1-octene and 4-octene. React. Kinet., Mech. Catal. 2013, 111. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas./Solid Systems With Special Reference to the Determination of Surface Area and Porosity; International Union of Pure and Applied Chemistry: Zürich, Switzerland, 1985; pp. 603–619. [Google Scholar]
- Rynkowska, E.; Fatyeyeva, K.; Marais, S.; Kujawa, J.; Kujawski, W. Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior. Polymers 2019, 11, 1799. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.M.A.; Carvalho, R.A.; Moraes, I.C.F.; Luciano, C.G.; Bittante, A.M.Q.B.; Sobral, P.J.A. Development of films based on blends of gelatin and poly(vinyl alcohol) cross linked with glutaraldehyde. Food Hydrocolloids 2011, 25, 1751–1757. [Google Scholar] [CrossRef]
- Williams, P.M. Membrane Roughness. In Encyclopedia of Membranes, 1st ed.; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–2. [Google Scholar] [CrossRef]
- Das, C.; Gebru, K. Response Surface Optimization of Electro-Spun Polyvinyl Alcohol Nano-Fiber Membrane Process Parameters and its Characterization. J. Membr. Sep. Technol. 2017, 5, 140–156. [Google Scholar] [CrossRef]
- Zuo, C.; Feng, S.; Huang, L.; Tao, T.; Yin, W.; Chen, Q. Phase shifting algorithms for fringe projection profilometry: A review. Opt. Lasers. Eng. 2018, 109, 23–59. [Google Scholar] [CrossRef]
- Kujawski, W.; Li, G.; Van der Bruggen, B.; Pedišius, N.; Tonkonogij, J.; Tonkonogovas, A.; Stankevičius, A.; Šereika, J.; Jullok, N.; Kujawa, J. Preparation and Characterization of Polyphenylsulfone (PPSU) Membranes for Biogas Upgrading. Materials 2020, 13, 2847. [Google Scholar] [CrossRef]
- Kim, H.J.; Baek, Y.; Choi, K.; Kim, D.-G.; Kang, H.; Choi, Y.-S.; Yoon, J.; Lee, J.-C. The improvement of antibiofouling properties of a reverse osmosis membrane by oxidized CNTs. RSC Adv. 2014, 4, 32802–32810. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; WILEY-VCH Verlag GmbH & Co.: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- Sankar Ganesh, R.; Navaneethan, M.; Mani, G.K.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Kawasaki, S.; Hayakawa, Y. Influence of Al doping on the structural, morphological, optical, and gas sensing properties of ZnO nanorods. J. Alloys Compd. 2017, 698, 555–564. [Google Scholar] [CrossRef]
- Mehrasa, M.; Anarkoli, A.O.; Rafienia, M.; Ghasemi, N.; Davary, N.; Bonakdar, S.; Naeimi, M.; Agheb, M.; Salamat, M.R. Incorporation of zeolite and silica nanoparticles into electrospun PVA/collagen nanofibrous scaffolds: The influence on the physical, chemical properties and cell behavior. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 457–465. [Google Scholar] [CrossRef]
- Kurşun, F. Application of PVA-b-NaY zeolite mixture membranes in pervaporation method. J. Mol. Struct. 2020, 1201, 127170. [Google Scholar] [CrossRef]
- Knozowska, K.; Kujawski, W.; Zatorska, P.; Kujawa, J. Pervaporative Efficiency of Organic Solvents Separation Employing Hydrophilic and Hydrophobic Commercial Polymeric Membranes. J. Membr. Sci. 2018, 564, 444–455. [Google Scholar] [CrossRef]
- Knozowska, K.; Li, G.; Kujawski, W.; Kujawa, J. Novel heterogeneous membranes for enhanced separation in organic-organic pervaporation. J. Membr. Sci. 2020, 599, 117814. [Google Scholar] [CrossRef]
- Scholes, C.; Kentish, S.; Stevens, G. Carbon Dioxide Separation through Polymeric Membrane Systems for Flue Gas Applications. Recent Pat. Chem. Eng. 2008, 1, 52–66. [Google Scholar] [CrossRef]


| Solvent | LOQ (%) | LOD (%) |
|---|---|---|
| ETBE | 0.16 | 0.20 |
| EtOH | 0.03 | 0.09 |
| H2O | 0.03 | 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knozowska, K.; Kujawa, J.; Lagzdins, R.; Figoli, A.; Kujawski, W. A New Type of Composite Membrane PVA-NaY/PA-6 for Separation of Industrially Valuable Mixture Ethanol/Ethyl Tert-Butyl Ether by Pervaporation. Materials 2020, 13, 3676. https://doi.org/10.3390/ma13173676
Knozowska K, Kujawa J, Lagzdins R, Figoli A, Kujawski W. A New Type of Composite Membrane PVA-NaY/PA-6 for Separation of Industrially Valuable Mixture Ethanol/Ethyl Tert-Butyl Ether by Pervaporation. Materials. 2020; 13(17):3676. https://doi.org/10.3390/ma13173676
Chicago/Turabian StyleKnozowska, Katarzyna, Joanna Kujawa, Renars Lagzdins, Alberto Figoli, and Wojciech Kujawski. 2020. "A New Type of Composite Membrane PVA-NaY/PA-6 for Separation of Industrially Valuable Mixture Ethanol/Ethyl Tert-Butyl Ether by Pervaporation" Materials 13, no. 17: 3676. https://doi.org/10.3390/ma13173676
APA StyleKnozowska, K., Kujawa, J., Lagzdins, R., Figoli, A., & Kujawski, W. (2020). A New Type of Composite Membrane PVA-NaY/PA-6 for Separation of Industrially Valuable Mixture Ethanol/Ethyl Tert-Butyl Ether by Pervaporation. Materials, 13(17), 3676. https://doi.org/10.3390/ma13173676








