An Overview on the Catalytic Materials Proposed for the Simultaneous Removal of NOx and Soot
Abstract
1. Introduction
2. PGM-Free Catalysts for the Simultaneous Removal of Soot and NOx
2.1. Hydrotalcites and Mixed Metal Oxides Catalysts
2.2. Perovskite-Based Catalysts
3. PGM-Based Catalysts for Simultaneous Removal of Soot and NOx
3.1. DPNR Catalysts for the Simultaneous Removal of NOx and Soot
3.2. Pt-Free DPNR Systems
4. SCR/DPF Combined Technologies for the Simultaneous Removal of Soot and NOx
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The State of the Art in Selective Catalytic Reduction of NOx by Ammonia Using Metal-Exchanged Zeolite Catalysts. Catal. Rev. Sci. Eng. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E., II. Overview of the Fundamental Reactions and Degradation Mechanisms of NOx Storage/Reduction Catalysts. Catal. Rev. Sci. Eng. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Resitoglu, I.A.; Keskin, A.; Altinisik, K. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef]
- Jelles, S.J.; Makkee, M.; Moulijn, J.A. Ultra Low Dosage of Platinum and Cerium Fuel Additives in Diesel Particulate Control. Top. Catal. 2001, 16, 269–273. [Google Scholar] [CrossRef]
- Salvat, O.; Marez, P.; Belot, G. Passenger Car Serial Application of a Particulate Filter System on a Common Rail Direct Injection Diesel Engine. SAE Tech. Pap. 2000, 109, 227–239. [Google Scholar]
- Cooper, B.J.; Thoss, J.E. Role of NO in Diesel Particulate Emission Control. SAE Tech. Pap. 1989, 98, 612–624. [Google Scholar]
- Stanmore, B.R.; Brilhac, J.F.; Gilot, P. The oxidation of soot: A review of experiments, mechanisms and models. Carbon 2001, 39, 2247–2268. [Google Scholar] [CrossRef]
- Setiabudi, A.; Makkee, M.; Moulijn, J.A. An optimal usage of NOx in a combined Pt/ceramic foam and a wall-flow monolith filter for an effective NOx-assisted soot oxidation. Top. Catal. 2002, 30, 305–308. [Google Scholar] [CrossRef]
- Yang, R.; Gao, Y.; Wang, J.; Wang, Q. Layered double hydroxide (LDH) derived catalysts for simultaneous catalytic removal of soot and NOx. Dalton Trans. 2014, 43, 10317–10327. [Google Scholar] [CrossRef]
- Dhal, G.C.; Mohana, D.; Prasad, D.R. Preparation and application of effective different catalysts for simultaneous control of diesel soot and NOx emissions: An overview. Catal. Sci. Technol. 2017, 7, 1803–1825. [Google Scholar] [CrossRef]
- Atanda, L.; Al-Yassir, N.; Al-Khattaf, S. Kinetic modeling of ethylbenzene dehydrogenation over hydrotalcite catalysts. Chem. Eng. J. 2011, 171, 1387–1398. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Tsubaki, N.; Li, X.G.; Li, Z.Q.; Xie, Y.N.; Hu, T.D.; Zhang, J. Performance of K-promoted hydrotalcite-derived CoMgAlO catalysts used for soot combustion, NOx storage and simultaneous soot–NOx removal. Appl. Catal. B Environ. 2009, 91, 406–415. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Zou, Z.Q.; Li, X.G.; Zha, Y.Q. Simultaneous soot combustion and nitrogen oxides storage on potassium-promoted hydrotalcite-based CoMgAlO catalysts. J. Hazard. Mater. 2009, 161, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Milt, V.G.; Banús, E.D.; Miró, E.E.; Yates, M.; Martín, J.C.; Rasmussen, S.B.; Ávila, P. Structured catalysts containing Co, Ba and K supported on modified natural sepiolite for the abatement of diesel exhaust pollutants. Chem. Eng. J. 2010, 157, 530–538. [Google Scholar] [CrossRef]
- Aneggi, E.; de Leitenburg, C.; Dolcetti, G. Diesel soot combustion activity of ceria promoted with alkali metals. Catal. Today 2008, 136, 3–10. [Google Scholar] [CrossRef]
- Castoldi, L.; Matarrese, R.; Lietti, L.; Forzatti, P. Intrinsic reactivity of alkaline and alkaline-earth metal oxide catalysts for oxidation of soot. Appl. Catal. B Environ. 2009, 90, 278–285. [Google Scholar] [CrossRef]
- Bao, M.; Ni, M.; Yu, P.; Zhang, Y.; Zhang, Z.; Mou, Z. Diesel vehicle exhaust carbon-smoke combustion and NOx storage-reduction dual-functional catalyst and its preparing method. Chinese Patent CN100398198C, 2 July 2008. [Google Scholar]
- Takahashi, N.; Matsunaga, S.; Tanaka, T.; Sobukawa, H.; Shinjoh, H. New approach to enhance the NOx storage performance at high temperature using basic MgAl2O4 spinel support. Appl. Catal. B Environ. 2007, 77, 73–78. [Google Scholar] [CrossRef]
- Wang, Z.P.; Shangguan, W.F.; Su, J.X.; Jiang, Z. Catalytic oxidation of diesel soot on mixed oxides derived from hydrotalcites. Catal. Lett. 2006, 112, 149–154. [Google Scholar] [CrossRef]
- Becerra, M.E.; Arias, N.P.; Giraldo, O.H.; López Suárez, F.E.; Illán Gómez, M.J.; Bueno López, A. Soot combustion manganese catalysts prepared by thermal decomposition of KMnO4. Appl. Catal. B Environ. 2011, 102, 260–266. [Google Scholar] [CrossRef]
- Ura, B.; Trawczyński, J.; Kotarba, A.; Bieniasz, W.; Illán-Gómez, M.J.; Bueno-López, A.; López-Suárez, F.E. Effect of potassium addition on catalytic activity of SrTiO3 catalyst for diesel soot combustion. Appl. Catal. B Environ. 2011, 101, 169–175. [Google Scholar] [CrossRef]
- Zhang, Z.; Mou, Z.; Yu, P.; Zhang, Y.; Ni, X. Diesel soot combustion on potassium promoted hydrotalcite-based mixed oxide catalysts. Catal. Commun. 2007, 8, 1621–1624. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhang, Y.X.; Wang, Z.P.; Gao, X.Y. Catalytic performance and mechanism of potassium-supported Mg–Al hydrotalcite mixed oxides for soot combustion with O2. J. Catal. 2010, 271, 12–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Q.; Li, Q.; Wang, Z.; Gao, X.; Zhang, Z. Determination of Mechanism for Soot Oxidation with NO on Potassium Supported Mg-Al Hydrotalcite Mixed Oxides. Chem. Eng. Technol. 2011, 34, 1864–1868. [Google Scholar] [CrossRef]
- Wang, Z.P.; Chen, M.X.; Shangguan, W.F. Simultaneous Catalytic Removal of NOx and Diesel Soot over Cu-Containing Hydrotalcite Derived Catalysts. Acta Phys. Chim. Sin. 2009, 25, 79–85. [Google Scholar]
- Wang, Z.; Jiang, Z.; Shangguan, W. Simultaneous catalytic removal of NOx and soot particulate over Co–Al mixed oxide catalysts derived from hydrotalcites. Catal. Commun. 2007, 8, 1659–1664. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Xian, H.; Tsubaki, N.; Li, X.G.; Xie, Y.N.; Hu, T.D.; Zhang, J. Hydrotalcite-Derived MnxMg3−xAlO Catalysts Used for Soot Combustion, NOx Storage and Simultaneous Soot-NOx Removal. Environ. Sci. Technol. 2010, 44, 4747–4752. [Google Scholar] [CrossRef]
- Wang, Z.P.; Li, Q.; Wang, L.G.; Shangguan, W.F. Simultaneous catalytic removal of NOx and soot particulates over CuMgAl hydrotalcites derived mixed metal oxides. Appl. Clay Sci. 2012, 55, 125–130. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Dai, F.; Zha, Y.; Xie, Y.; Hu, T.; Zhang, J. Multifunctional hydrotalcite-derived K/MnMgAlO catalysts used for soot combustion, NOx storage and simultaneous soot–NOx removal. Chem. Eng. J. 2012, 184, 106–112. [Google Scholar] [CrossRef]
- Cui, C.; Ma, J.; Wang, Z.; Liu, W.; Liu, W.; Wang, L. High Performance of Mn-Doped MgAlOx Mixed Oxides for Low Temperature NOx Storage and Release. Catalysts 2019, 9, 677. [Google Scholar] [CrossRef]
- Yu, J.J.; Jiang, Z.; Kang, S.F.; Hao, Z.P. Catalytic combustion of soot over Cu-Mg/Al composite oxides. Acta Phys. Chim. Sin. 2004, 20, 1459–1464. [Google Scholar]
- Toops, T.J.; Smith, D.B.; Partridge, W.P. NOx adsorption on Pt/K/Al2O3. Catal. Today 2006, 114, 112–124. [Google Scholar] [CrossRef]
- Matarrese, R.; Castoldi, L.; Lietti, L.; Forzatti, P. High performances of Pt-K/Al2O3 versus Pt-Ba/Al2O3 LNT catalysts in the simultaneous removal of NOx and soot. Top. Catal. 2007, 42, 293–297. [Google Scholar] [CrossRef]
- Matarrese, R.; Castoldi, L.; Artioli, N.; Finocchio, E.; Busca, G.; Lietti, L. On the activity and stability of Pt-K/Al2O3 LNT catalysts for diesel soot and NOx abatement. Appl. Catal. B Environ. 2014, 144, 783–791. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Mishra, A.; Prasad, R. Preparation and Application of Perovskite Catalysts for Diesel Soot Emissions Control: An Overview. Catal. Rev. 2014, 56, 57–81. [Google Scholar] [CrossRef]
- Dhal, G.C.; Dey, S.; Mohan, D.; Prasad, R. Simultaneous abatement of diesel soot and NOx emissions by effective catalysts at low temperature: An overview. Catal. Rev. 2018, 60, 437–496. [Google Scholar] [CrossRef]
- Teraoka, Y.; Kanada, K.; Kagawa, S. Synthesis of La–K–Mn–O perovskite-type oxides and their catalytic property for simultaneous removal of NOx and diesel soot particulates. Appl. Catal. B Environ. 2001, 34, 73–78. [Google Scholar] [CrossRef]
- Peron, G.; Glisenti, A. Perovskites as Alternatives to Noble Metals in Automotive Exhaust Abatement: Activation of Oxygen on LaCrO3 and LaMnO3. Top. Catal. 2019, 62, 244–251. [Google Scholar] [CrossRef]
- Dhal, G.C.; Dey, S.; Mohan, D.; Prasad, R. Study of Fe, Co, and Mn-based perovskite-type catalysts for the simultaneous control of soot and NOx from diesel engine exhaust. Mat. Discov. 2017, 10, 37–42. [Google Scholar] [CrossRef]
- Dhal, G.C.; Dey, S.; Mohan, D.; Prasad, R. Simultaneous Control of NOx-Soot by Substitutions of Ag and K on Perovskite (LaMnO3) Catalyst. Bull. Chem. React. Eng. Catal. 2018, 13, 144–154. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Liang, P.; Xu, C.; Duan, A.; Jiang, G.; Xu, J.; Liu, J. Highly Active La1-xKxCoO3 Perovskite-type Complex Oxide Catalysts for the Simultaneous Removal of Diesel Soot and Nitrogen Oxides Under Loose Contact Conditions. Catal. Lett. 2008, 124, 91–99. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J.; Ura, B.; Trawczynski, J. Potassium Stability in Soot Combustion Perovskite Catalysts. Top. Catal. 2009, 52, 2097–2100. [Google Scholar] [CrossRef]
- Hernández-Giménez, A.M.; Lozano Castelló, D.; Bueno-López, A. Diesel soot combustion catalysts: Review of active phases. Chem. Pap. 2014, 68, 1154–1168. [Google Scholar] [CrossRef]
- Teraoka, Y.; Nakano, K.; Shangguan, W.F.; Kagawa, S. Simultaneous catalytic removal of nitrogen oxides and diesel soot particulate over perovskite-related oxides. Catal. Today 1996, 27, 107–113. [Google Scholar] [CrossRef]
- Bin, F.; Song, C.; Lv, G.; Song, J.; Gong, C.; Huang, Q. La1–xKxCoO3 and LaCo1–yFeyO3 Perovskite Oxides: Preparation, Characterization, and Catalytic Performance in the Simultaneous Removal of NOx and Diesel Soot. Ind. Eng. Chem. Res. 2011, 50, 6660–6667. [Google Scholar] [CrossRef]
- Fang, S.; Wang, L.; Sun, Z.; Feng, N.; Shen, C.; Lin, P.; Wan, H.; Guan, G. Catalytic removal of diesel soot particulates over K and Mg substituted La1−xKxCo1−yMgyO3 perovskite oxides. Catal. Commun. 2014, 49, 15–19. [Google Scholar] [CrossRef]
- Teraoka, Y.; Nakano, K.; Kagawa, S.; Shangguan, W.F. Simultaneous removal of nitrogen oxides and diesel soot particulates catalyzed by perovskite-type oxides. Appl. Catal. B Environ. 1995, 5, L181–L185. [Google Scholar] [CrossRef]
- Fino, D.; Fino, P.; Saracco, G.; Specchia, V. Studies on kinetics and reactions mechanism of La2−xKxCu1−yVyO4 layered perovskites for the combined removal of diesel particulate and NOx. Appl. Catal. B Environ. 2003, 43, 243–259. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Xua, C.M.; Liu, J. Nanometric La1−xKxMnO3 perovskite-type oxides-highly active catalysts for the combustion of diesel soot particle under loose contact conditions. Catal. Lett. 2005, 102, 251–256. [Google Scholar] [CrossRef]
- Kureti, S.; Weisweiler, W.; Hizbullah, K. Simultaneous conversion of nitrogen oxides and soot into nitrogen and carbon dioxide over iron containing oxide catalysts in diesel exhaust gas. Appl. Catal. B Environ. 2003, 43, 281–291. [Google Scholar] [CrossRef]
- Fino, D.; Russo, N.; Saracco, G.; Specchia, V. The role of suprafacial oxygen in some perovskites for the catalytic combustion of soot. J. Catal. 2003, 217, 367–375. [Google Scholar] [CrossRef]
- Onrubia, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Key factors in Sr-doped LaBO3 (B = Co or Mn) perovskites for NO oxidation in efficient diesel exhaust purification. Appl. Catal. B Environ. 2017, 213, 198–210. [Google Scholar] [CrossRef]
- Onrubia, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Strontium doping and impregnation onto alumina improve the NOx storage and reduction capacity of LaCoO3 perovskites. Catal. Today 2019, 333, 208–218. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Xian, H.; Hernández, W.Y.; Meng, M.; Zou, H.; Ma, A.; Zhang, T.; Jiang, Z.; Tsubaki, N.; et al. De-NOx in alternative lean/rich atmospheres on La1−xSrxCoO3 perovskites. Energy Environ. Sci. 2011, 4, 3351–3354. [Google Scholar] [CrossRef]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-doped perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Si, W.; Luo, J.; Su, W.; Chang, H.; Li, J.; Hao, J.; Crittenden, J. Surface Tuning of La0.5Sr0.5CoO3 Perovskite Catalysts by Acetic Acid for NOx Storage and Reduction. Environ. Sci. Technol. 2016, 50, 6442–6448. [Google Scholar] [CrossRef]
- Fang, F.; Zhao, P.; Feng, N.; Chen, C.; Li, X.; Liu, G.; Wan, H.; Guan, G. Construction of a hollow structure in La0.9K0.1CoO3−δ nanofibers via grain size control by Sr substitution with an enhanced catalytic performance for soot removal. Catal. Sci. Technol. 2019, 9, 4938–4951. [Google Scholar] [CrossRef]
- Zhao, P.; Fang, F.; Feng, N.; Chen, C.; Liu, G.; Chen, L.; Zhu, Z.; Meng, J.; Wan, H.; Guan, G. Self-templating construction of mesopores on three-dimensionally ordered macroporous La0.5Sr0.5MnO3 perovskite with enhanced performance for soot combustion. Catal. Sci. Technol. 2019, 9, 1835–1846. [Google Scholar] [CrossRef]
- Li, Z.; Meng, M.; Li, Q.; Xie, Y.; Hu, T.; Zhang, J. Fe-substituted nanometric La0.9K0.1Co1−xFexO3−δ perovskite catalysts used for soot combustion, NOx storage and simultaneous catalytic removal of soot and NOx. Chem. Eng. J. 2010, 164, 98–105. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, R.; Yang, X. Simultaneous catalytic removal of NOx and diesel soot particulates over La1-xCexNiO3 perovskite oxide catalysts. Catal. Commun. 2009, 10, 1029–1033. [Google Scholar] [CrossRef]
- Bueno-López, A. Diesel soot combustion ceria catalysts. Appl. Catal. B Environ. 2014, 146, 1–11. [Google Scholar] [CrossRef]
- Maffei, N.; Nossova, L.; Turnbull, M.J.; Caravaggio, G.; Burich, R. Doped barium cerate perovskite catalysts for simultaneous NOx storage and soot oxidation. Appl. Catal. A Gen. 2020, 600, 117465. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.-M.; Duan, A.J.; Jiang, G.-Y. The Structures, Adsorption Characteristics of La−Rb−Cu−O Perovskite-like Complex Oxides, and Their Catalytic Performances for the Simultaneous Removal of Nitrogen Oxides and Diesel Soot. J. Phys. Chem. C 2008, 112, 5930–5941. [Google Scholar] [CrossRef]
- Li, X.; Yang, D.; Chang, S.; Wang, P.; Guo, J.; Zhao, Y. Progress in After-treatment Technology for Light-duty Diesel Engine Exhaust. Precious Met. 2015, 36, 70–76. [Google Scholar]
- Zhang, Y.; Wang, X.; Wang, Z.; Li, Q.; Zhang, Z.; Zhou, L. Direct Spectroscopic evidence of CO spillover and subsequent reaction with preadsorbed NOx on Pd and K cosupported Mg−Al mixed oxides. Environ. Sci. Technol. 2012, 46, 9614–9619. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; Weber, M.J.; Thissen, N.F.W.; Garcia-Alonso, D.; Vervuurt, R.H.J.; Assali, S.; Bol, A.A.; Verheijen, M.A.; Kessels, W.M.M. Atomic layer deposition of Pd and Pt nanoparticles for catalysis: On the mechanisms of nanoparticle formation. Nanotechnology 2016, 27, 034001. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Homola, T.; Bryukvin, A.; Cameron, D.C. Catalytic Performance of Ag2O and Ag Doped CeO2 Prepared by Atomic Layer Deposition for Diesel Soot Oxidation. Coatings 2018, 8, 237. [Google Scholar] [CrossRef]
- O’Neill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst Design with Atomic Layer Deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef]
- Park, S.J.; Ahn, H.A.; Heo, I.J.; Nam, I.-S.; Lee, J.H.; Youn, Y.K.; Kim, H.J. Hydrotalcite as a Support for NOx Trap Catalyst. Top. Catal. 2010, 53, 57–63. [Google Scholar] [CrossRef]
- Li, P.; He, C.; Cheng, J.; Hao, Z.P. Catalytic Oxidation of Chlorobenzene on Composite Oxide Pd/M3AlO (M = Mg, Co, Ni, Cu, Zn) Catalysts Derived from Pd-Contained Hydrotalcite Precursors. Acta Phys. Chim. Sin. 2009, 25, 2279–2284. [Google Scholar]
- Nakatani, K.; Hirota, S.; Takeshima, S.; Itoh, K.; Tanaka, T.; Dohmae, K. Simultaneous PM and NOx Reduction System for Diesel Engines. SAE Tech. Pap. 2002, 362–369. [Google Scholar] [CrossRef]
- Mizuno, T.; Suzuki, J. Development of a New DPNR Catalyst. SAE Tech. Pap. 2004. [Google Scholar] [CrossRef]
- Suzuki, J.; Matsumoto, S. Development of Catalysts for Diesel Particulate NOx Reduction. Top. Catal. 2004, 28, 171–176. [Google Scholar]
- Ohashi, N.; Nakatani, K.; Asanuma, T.; Fukuma, T.; Matsubara, H.; Sobue, Y.; Watanabe, M. Development of Next-Generation NOx Reduction System for Diesel Exhaust Emission. SAE Tech. Pap. 2008. [Google Scholar] [CrossRef]
- Tsuzuki, M.; Tahara, J.; Sugiyama, T.; Fujimura, T.; Hirota, S.; Paquet, T. Field Trial for Diesel Passenger Cars with DPNR. ATZ Autotechnol. 2003, 3, 70–74. [Google Scholar]
- Castoldi, L.; Matarrese, R.; Lietti, L.; Forzatti, P. Simultaneous removal of NOx and soot on Pt–Ba/Al2O3 NSR catalysts. Appl. Catal. B Environ. 2006, 64, 25–34. [Google Scholar] [CrossRef]
- Setiabudi, A.; van Setten, B.A.; Makee, M.; Moulijn, J.A. The influence of NOx on soot oxidation rate: Molten salt versus platinum. Appl. Catal. B Environ. 2002, 35, 159–166. [Google Scholar] [CrossRef]
- Jacquot, F.; Logie, V.; Brilhac, J.F.; Gilot, P. Kinetics of the oxidation of carbon black by NO2: Influence of the presence of water and oxygen. Carbon 2002, 40, 335–343. [Google Scholar] [CrossRef]
- Setiabudi, A.; Makkee, M.; Moulijn, J.A. The role of NO2 and O2 in the accelerated combustion of soot in diesel exhaust gases. Appl. Catal. B Environ. 2004, 50, 185–194. [Google Scholar] [CrossRef]
- Corté-Reyes, M.; Herrera, C.; Larrubia, M.Á.; Alemany, J.L. Intrinsic reactivity analysis of soot removal in LNT-catalysts. Appl. Catal. B Environ. 2016, 193, 110–120. [Google Scholar] [CrossRef]
- Jeguirim, M.; Tschamber, V.; Brilhac, J.F.; Ehrburger, P. Interaction mechanism of NO2 with carbon black: Effect of surface oxygen complexes. J. Anal. Appl. Pyrolysis 2004, 72, 171–181. [Google Scholar] [CrossRef]
- Jeguirim, M.; Tschamber, V.; Brilhac, J.F.; Ehrburger, P. Oxidation mechanism of carbon black by NO2: Effect of water vapour. Fuel 2005, 84, 1949–1956. [Google Scholar] [CrossRef]
- Jeguirim, M.; Tschamber, V.; Brilhac, J.F. Kinetics of catalyzed and non-catalyzed soot oxidation with nitrogen dioxide under regeneration particle trap conditions. J. Chem. Technol. Biotechnol. 2009, 84, 770–776. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Keane, O.; Cassidy, A. Beneficial and problematic interactions between NOx trapping materials and carbonaceous particulate matter. Appl. Catal. B Environ. 2007, 75, 102–106. [Google Scholar] [CrossRef][Green Version]
- Castoldi, L.; Artioli, N.; Matarrese, R.; Lietti, L.; Forzatti, P. Study of DPNR catalysts for combined soot oxidation and NOx reduction. Catal. Today 2010, 157, 384–389. [Google Scholar] [CrossRef]
- Artioli, N.; Matarrese, R.; Castoldi, L.; Lietti, L.; Forzatti, P. Effect of soot on the storage-reduction performances of PtBa/Al2O3 LNT catalyst. Catal. Today 2011, 169, 36–44. [Google Scholar] [CrossRef]
- Matarrese, R.; Artioli, N.; Castoldi, L.; Lietti, L.; Forzatti, P. Interaction between soot and stored NOx during operation of LNT Pt–Ba/Al2O3 catalysts. Catal. Today 2012, 184, 271–278. [Google Scholar] [CrossRef]
- Corté-Reyes, M.; Herrera, C.; Pieta, I.S.; Larrubia, M.Á.; Alemany, J.L. In situ TG-MS study of NOx and soot removal over LNT model catalysts. Appl. Catal. A Gen. 2016, 523, 193–199. [Google Scholar]
- Sullivan, J.A.; Dulgheru, P. The effect of C(s) on the trapping of NOx onto Pt/Ba/Al2O3 catalysts. Appl. Catal. B Environ. 2010, 99, 235–241. [Google Scholar] [CrossRef][Green Version]
- Nova, I.; Castoldi, L.; Lietti, L.; Tronconi, E.; Forzatti, P.; Prinetto, F.; Ghiotti, G. NOx adsorption study over Pt–Ba/alumina catalysts: FT-IR and pulse experiments. J. Catal. 2004, 222, 377–388. [Google Scholar] [CrossRef]
- Clayton, R.D.; Harold, M.P.; Balakotaiah, V.; Wan, C.Z. Pt dispersion effects during NOx storage and reduction on Pt/BaO/Al2O3 catalysts. Appl. Catal. B Environ. 2009, 90, 662–676. [Google Scholar] [CrossRef]
- Bhatia, D.; Harold, M.P.; Balakotaiah, V. Modeling the effect of Pt dispersion and temperature during anaerobic regeneration of a lean NOx trap catalyst. Catal. Today 2010, 151, 314–329. [Google Scholar] [CrossRef]
- Klein, J.; Fechete, V.; Bresset, V.; Garin, F.; Tschamber, V. Effect of carbon black combustion on NOx trap catalyst performances. Catal. Today 2012, 189, 60–64. [Google Scholar] [CrossRef]
- Klein, J.; Wu, D.; Tschamber, V.; Fechete, I.; Garin, F. Carbon–NSR catalyst interaction: Impact on catalyst structure and NOx storage efficiency. Appl. Catal. B Environ. 2013, 132, 527–534. [Google Scholar] [CrossRef]
- Wu, D.L.; Tschamber, V.; Limousy, L.; Klein, J.; Westermann, A.; Azambre, B.; Fechete, I.; Garin, F. Simultaneous effect of carbon and water on NOx adsorption on a stabilized Pt–Ba/Al2O3 catalyst. Comptes Rendus Chim. 2014, 17, 687–700. [Google Scholar] [CrossRef]
- Wu, D.L.; Tschamber, V.; Limousy, L.; Fechete, I.; Garin, F. Impact of soot-NSR catalyst contact depending on reactive gas composition on NOx storage. Environ. Prog. Sustain. Energy 2016, 35, 14–19. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Keane, O.; Maguire, L. The influence of SO42- on the catalytic combustion of soot using O2 and NO/O2 mixtures over Na-promoted Al2O3 catalysts. Catal. Commun. 2005, 6, 472–475. [Google Scholar] [CrossRef][Green Version]
- Shuang, L.; Xiaodong, W.; Duan, W.; Rui, R. NOx-Assisted Soot Oxidation on Pt–Mg/Al2O3 Catalysts: Magnesium Precursor, Pt Particle Size, and Pt–Mg Interaction. Ind. Eng. Chem. Res. 2012, 51, 2271–2279. [Google Scholar] [CrossRef]
- Krishna, K.; Makkee, M. Soot oxidation over NOx storage catalysts: Activity and deactivation. Catal. Today 2006, 114, 48–56. [Google Scholar] [CrossRef]
- Kustov, A.L.; Makkee, M. Application of NOx storage/release materials based on alkali-earth oxides supported on Al2O3 for high-temperature diesel soot oxidation. Appl. Catal. B Environ. 2009, 88, 263–271. [Google Scholar] [CrossRef]
- Sanchez, B.S.; Querini, C.A.; Miró, E.E. NOx adsorption and diesel soot combustion over La2O3 supported catalysts containing K.; Rh and Pt. Appl. Catal. A Gen. 2009, 366, 166–175. [Google Scholar] [CrossRef]
- Sanchez, B.S.; Querini, C.A.; Miró, E.E. Potassium effect on the thermal stability and reactivity of NOx species adsorbed on Pt, Rh/La2O3 catalysts. Appl. Catal. A Gen. 2011, 392, 158–165. [Google Scholar] [CrossRef]
- Ito, K.; Kishikawa, K.; Watajima, A.; Ikeue, K.; Machida, M. Soot combustion activity of NOx-sorbing Cs–MnOx–CeO2 catalysts. Catal. Commun. 2007, 8, 2176–2180. [Google Scholar] [CrossRef]
- Li, Y.-J.; Lin, H.; Shangguan, W.-F.; Huang, Z. Properties of BaAl2O4 in the Simultaneous Removal of Soot and NOx. Chem. Eng. Technol. 2007, 30, 1426–1433. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.-J.; Shangguan, W.-F.; Huang, Z. Soot oxidation and NOx reduction over BaAl2O4 catalyst. Combust. Flame 2009, 156, 2063–2070. [Google Scholar] [CrossRef]
- Querini, C.A.; Cornaglia, L.M.; Ulla, M.A.; Miró, E.E. Catalytic combustion of diesel soot on Co,K/MgO catalysts. Effect of the potassium loading on activity and stability. Appl. Catal. B Environ. 1999, 20, 165–177. [Google Scholar] [CrossRef]
- An, H.; McGinn, P.J. Catalytic behavior of potassium containing compounds for diesel soot combustion. Appl. Catal. B Environ. 2006, 62, 46–56. [Google Scholar] [CrossRef]
- Matarrese, R.; Castoldi, L.; Lietti, L.; Forzatti, P. Soot combustion: Reactivity of alkaline and alkaline earth metal oxides in full contact with soot. Catal. Today 2008, 136, 11–17. [Google Scholar] [CrossRef]
- Matarrese, R.; Castoldi, L.; Lietti, L.; Forzatti, P. Simultaneous Removal of NOx and Soot Over Pt–Ba/Al2O3 and Pt–K/Al2O3 DPNR Catalysts. Top. Catal. 2009, 52, 2041–2046. [Google Scholar] [CrossRef]
- Matarrese, R.; Castoldi, L.; Lietti, L. Reaction between soot and stored NOx over K-based LNT catalysts investigated by temperature programmed methods and labeling isotopic experiments. Catal. Today 2012, 197, 228–235. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Ascaso, S.; Moliner, R.; Lázaro, M.J. Me (Cu, Co, V)-K/Al2O3 supported catalysts for the simultaneous removal of soot and nitrogen oxides from diesel exhausts. Chem. Eng. Sci. 2013, 87, 75–90. [Google Scholar] [CrossRef]
- Maricq, M.M. Chemical characterization of particulate emissions from diesel engines: A review. J. Aerosol Sci. 2007, 38, 1079–1118. [Google Scholar] [CrossRef]
- Castoldi, L.; Aneggi, E.; Matarrese, R.; Bonzi, R.; Llorca, J.; Trovarelli, A.; Lietti, L. Silver-based catalytic materials for the simultaneous removal of soot and NOx. Catal. Today 2015, 258, 405–415. [Google Scholar] [CrossRef]
- Matarrese, R.; Aneggi, E.; Castoldi, L.; Llorca, J.; Trovarelli, A.; Lietti, L. Simultaneous removal of soot and NOx over K- and Ba-doped ruthenium supported catalysts. Catal. Today 2016, 267, 119–129. [Google Scholar] [CrossRef]
- Castoldi, L.; Aneggi, E.; Matarrese, R.; Bonzi, R.; Trovarelli, A.; Lietti, L. Simultaneous Removal of Soot and NOx Over Silver and Ruthenium-Based Catalysts. Top. Catal. 2017, 60, 209–213. [Google Scholar] [CrossRef]
- Aneggi, E.; de Leitenburg, C.; Trovarelli, A. Catalysis by Ceria and Related Materials, 2nd ed.; Fornasiero, P., Trovarelli, A., Eds.; Imperial College Press: London, UK, 2013; Volume 12, pp. 565–621. [Google Scholar]
- Matarrese, R.; Morandi, S.; Castoldi, L.; Villa, P.; Lietti, L. Removal of NOx and soot over Ce/Zr/K/Me (Me = Fe, Pt, Ru, Au) oxide catalysts. Appl. Catal. B Environ. 2017, 201, 318–330. [Google Scholar] [CrossRef]
- Guo, M.; Ouyang, F.; Pang, D.; Qiu, L. Highly efficient oxidation of soot over RuOx/SiO2 in fluidized bed reactor. Catal. Commun. 2013, 38, 40–44. [Google Scholar] [CrossRef]
- Aouad, S.; Saab, E.; Aad, E.A.; Aboukais, A. Reactivity of Ru-based catalysts in the oxidation of propene and carbon black. Catal. Today 2007, 119, 273–277. [Google Scholar] [CrossRef]
- Villani, K.; Kirschhock, C.E.A.; Liang, D.; van Tendeloo, G.; Martens, J.A. Catalytic Carbon Oxidation Over Ruthenium-Based Catalysts. Angew. Chem. Int. Ed. 2006, 45, 3106–3109. [Google Scholar] [CrossRef]
- Bueno-López, A.; Lozano-Castelló, D.; McCue, A.J.; Anderson, J.A. NOx storage and reduction over copper-based catalysts. Part 3: Simultaneous NOx and soot removal. Appl. Catal. B Environ. 2016, 198, 266–275. [Google Scholar] [CrossRef]
- Giménez-Mañogil, J.; García- García, A. Identifying the nature of the copper entities over ceria-based supports to promote diesel soot combustion: Synergistic effects. Appl. Catal. A Gen. 2017, 542, 226–239. [Google Scholar] [CrossRef]
- Václavík, M.; Kočí, P.; Novák, V.; Thompsett, D. NOx conversion and selectivity in multi-layer and sequential DOC-LNT automotive exhaust catalysts: Influence of internal transport. Chem. Eng. J. 2017, 329, 128–134. [Google Scholar] [CrossRef]
- Kang, W.; Choi, B.; Jung, S.; Park, S. PM and NOx reduction characteristics of LNT/DPF + SCR/DPF hybrid system. Energy 2018, 143, 439–447. [Google Scholar] [CrossRef]
- Colombo, M.; Koltsakis, G.; Koutoufaris, I. A modeling study of soot and deNOx reaction phenomena in SCRF systems. SAE Tech. Pap. 2011. [Google Scholar] [CrossRef]
- Watling, T.C.; Ravenscroft, M.R.; Avery, G. Development, validation and application of a model for an SCR catalyst coated diesel particulate filter. Catal. Today 2012, 188, 32–41. [Google Scholar] [CrossRef]
- Schrade, F.; Brammer, M.; Schaeffner, J.; Langeheinecke, K.; Kraemer, L. Physico-chemical modeling of an integrated SCR on DPF (SCR/DPF) system. SAE Intern. J. Eng. 2012, 5, 958–974. [Google Scholar] [CrossRef]
- Nova, I.; Tronconi, E. (Eds.) Urea-SCR Technology for de NOx Aftertreatment of Diesel Exhausts; Fundamental and Applied Catalysis; Publisher Springer: New York, NY, USA, 2014. [Google Scholar]
- Mehring, M.; Elsener, M.; Kröcher, O. Selective Catalytic Reduction of NOx with Ammonia over Soot. ACS Catal. 2012, 2, 1507–1518. [Google Scholar] [CrossRef]
- Johansen, K.; Bentzer, H.; Kustov, A.; Larsen, K.; Janssens, T.V.; Barfod, R.G. Integration of Vanadium and Zeolite Type SCR Functionality into DPF in Exhaust Aftertreatment Systems—Advantages and Challenges. SAE Tech. Pap. 2014. [Google Scholar] [CrossRef]
- Kröcher, O.; Devadas, M.; Elsener, M.; Wokaun, A.; Söger, N.; Pfeifer, M.; Demel, Y.; Mussmann, L. Investigation of the selective catalytic reduction of NO by NH3 on Fe-ZSM5 monolith catalysts. Appl. Catal. B Environ. 2006, 66, 208–216. [Google Scholar] [CrossRef]
- Grossale, A.; Nova, I.; Tronconi, E. Study of a Fe–zeolite-based system as NH3SCR catalyst for diesel exhaust aftertreatment. Catal. Today 2008, 136, 18–27. [Google Scholar] [CrossRef]
- Grossale, A.; Nova, I.; Tronconi, E.; Chatterjee, D.; Weibel, M. NH3–NO/NO2 SCR for diesel exhausts aftertreatment: Reactivity, mechanism and kinetic modelling of commercial Fe- and Cu-promoted zeolite catalysts. Top. Catal. 2009, 52, 1837–1841. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E. A comparative study of the NH3-SCR reactions over a Cu-zeolite and a Fe-zeolite catalyst. Catal. Today 2010, 151, 223–230. [Google Scholar] [CrossRef]
- Rappé, K.G. Integrated selective catalytic reduction-diesel particulate filter aftertreatment: Insights into pressure drop, NOx conversion, and passive soot oxidation behavior. Ind. Eng. Chem. Res. 2014, 53, 17547–17557. [Google Scholar] [CrossRef]
- Purfürst, M.; Naumov, S.; Langeheinecke, K.-J.; Gläser, R. Influence of soot on ammonia adsorption and catalytic DeNOx-properties of diesel particulate filters coated with SCR-catalysts. Chem. Eng. Sci. 2017, 168, 423–436. [Google Scholar] [CrossRef]
- Marchitti, F.; Nova, I.; Tronconi, E. Experimental study of the interaction between soot combustion and NH3-SCR reactivity over a Cu–Zeolite SDPF catalyst. Catal. Today 2016, 267, 110–118. [Google Scholar] [CrossRef]
- Yang, Y.; Cho, G.; Rutland, C. Model Based Study of DeNOx Characteristics for Integrated DPF/SCR System over Cu-Zeolite. SAE Tech. Pap. 2015. [Google Scholar] [CrossRef]
- Cavataio, G.; Warner, J.R.; Girard, J.W.; Ura, J.; Dobson, D.; Lambert, C.K. Laboratory study of soot, propylene, and diesel fuel impact on zeolite-based SCR filter catalysts. SAE Int. J. Fuels Lubr. 2009, 2, 342–368. [Google Scholar] [CrossRef]
- Park, S.-Y.; Narayanaswamy, K.; Schmieg, S.J.; Rutland, C.J. A Model Development for Evaluating Soot-NOx Interactions in a Blended 2-Way Diesel Particulate Filter/Selective Catalytic Reduction. Ind. Eng. Chem. Res. 2012, 51, 15582–15592. [Google Scholar] [CrossRef]
- Bissett, E.J. Mathematical model of the thermal regeneration of a wall-flow monolith diesel particulate filter. Chem. Eng. Sci. 1984, 39, 1233–1244. [Google Scholar] [CrossRef]
- Park, S.; Rutland, C.; Narayanaswamy, K.; Schmieg, S.; He, Y.; Brown, D. Development and validation of a model for wall-flow type selective catalytic reduction system. Proc. Inst. Mech. Eng. Part D 2011, 225, 1641–1659. [Google Scholar] [CrossRef]
- Tronconi, E.; Nova, I.; Marchitti, F.; Koltsakis, G.; Karamitros, D.; Maletic, B.; Markert, N.; Chatterjee, D.; Hehle, M. Interaction of NOx Reduction and Soot Oxidation in a DPF with Cu-Zeolite SCR Coating. Emiss. Control Sci. Technol. 2015, 1, 134–151. [Google Scholar] [CrossRef]
- Mihai, O.; Tamm, S.; Stenfeldt, M.; Wang-Hansen, C.; Olsson, L. Evaluation of an integrated selective catalytic reduction-coated particulate filter. Ind. Eng. Chem. Res. 2015, 54, 11779–11791. [Google Scholar] [CrossRef]
- López-De Jesús, Y.M.; Chigada, P.I.; Watling, T.C.; Arulraj, K.; Thorén, A.; Greenham, N.; Markatou, P. NOx and PM Reduction from Diesel Exhaust Using Vanadia SCRF®. SAE Int. J. Engines 2016, 9, 1247–1257. [Google Scholar] [CrossRef]
- Martinovic, F.; Andana, T.; Piumetti, M.; Armandi, M.; Bonelli, B.; Deorsola, F.A.; Bensaid, S.; Pirone, R. Simultaneous improvement of ammonia mediated NOx SCR and soot oxidation for enhanced SCR-on-Filter application. Appl. Catal. A Gen. 2020, 596, 117538. [Google Scholar] [CrossRef]
- Martinovic, F.; Andana, T.; Deorsola, F.A.; Bensaid, S.; Pirone, R. On-Filter Integration of Soot Oxidation and Selective Catalytic Reduction of NOx with NH3 by Selective Two Component Catalysts. Catal. Lett. 2020, 150, 573–585. [Google Scholar] [CrossRef]
- Castoldi, L.; Bonzi, R.; Lietti, L.; Forzatti, P.; Morandi, S.; Ghiotti, G.; Dzwigaj, S. Catalytic behavior of hybrid LNT/SCR systems: Reactivity and in situ FTIR study. J. Catal. 2011, 282, 128–144. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. The reduction of NOx stored on LNT and combined LNT-SCR systems. Catal. Today 2010, 155, 131–139. [Google Scholar] [CrossRef]
- Choi, B.; Lee, K.-S. LNT/CDPF catalysts for simultaneous removal of NOx and PM from diesel vehicle exhaust. Chem. Eng. J. 2014, 240, 476–486. [Google Scholar] [CrossRef]
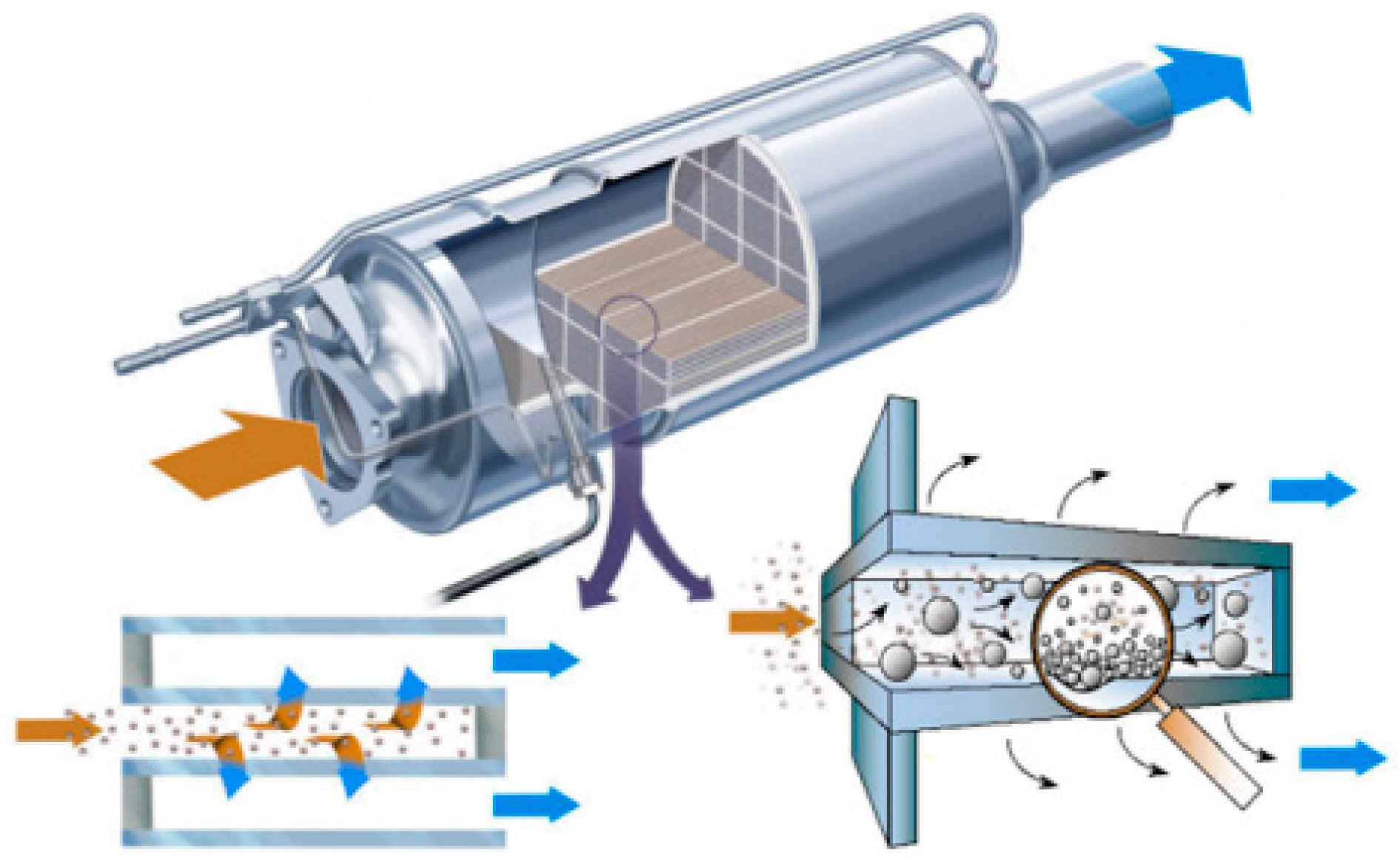
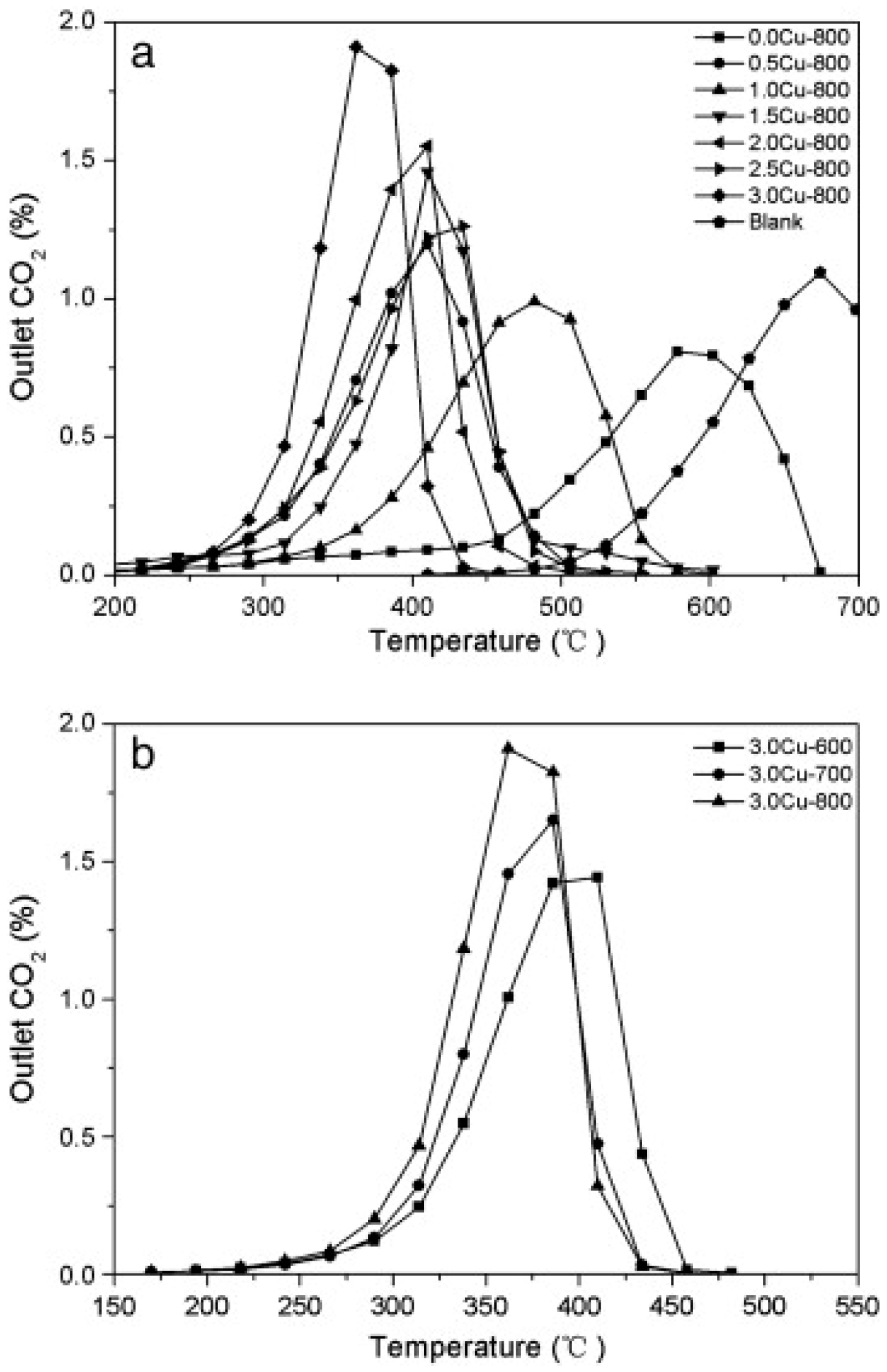

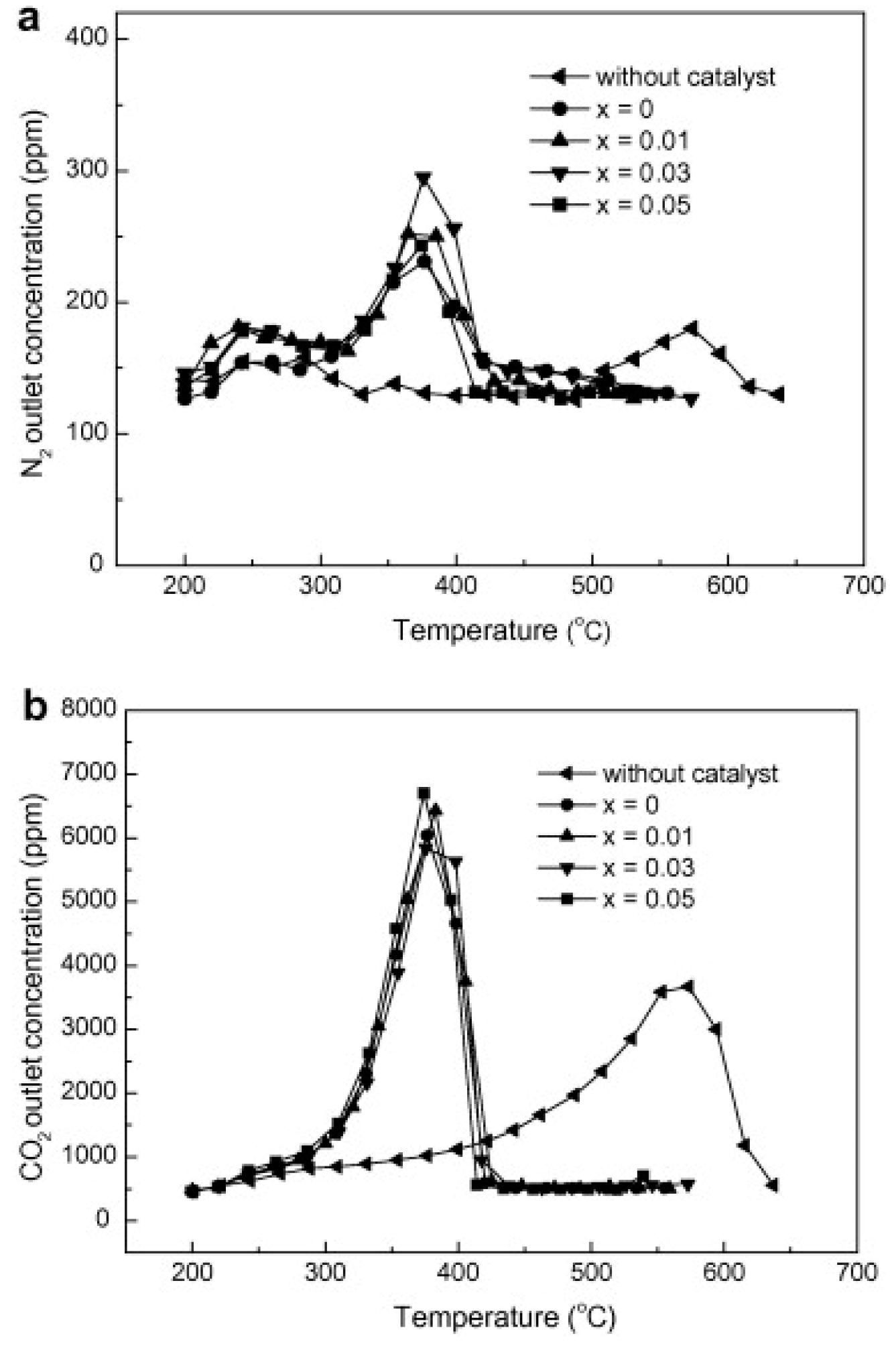
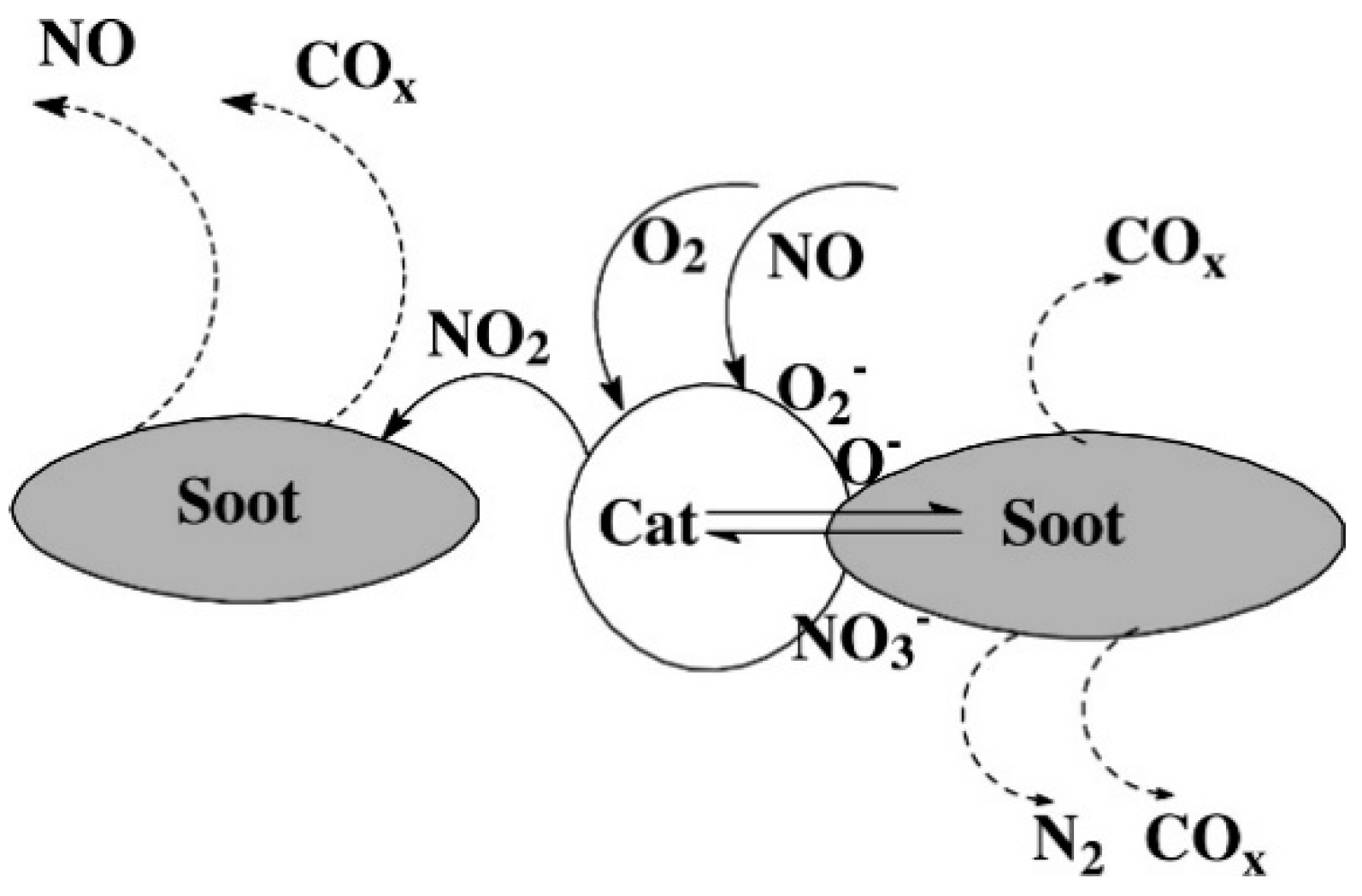
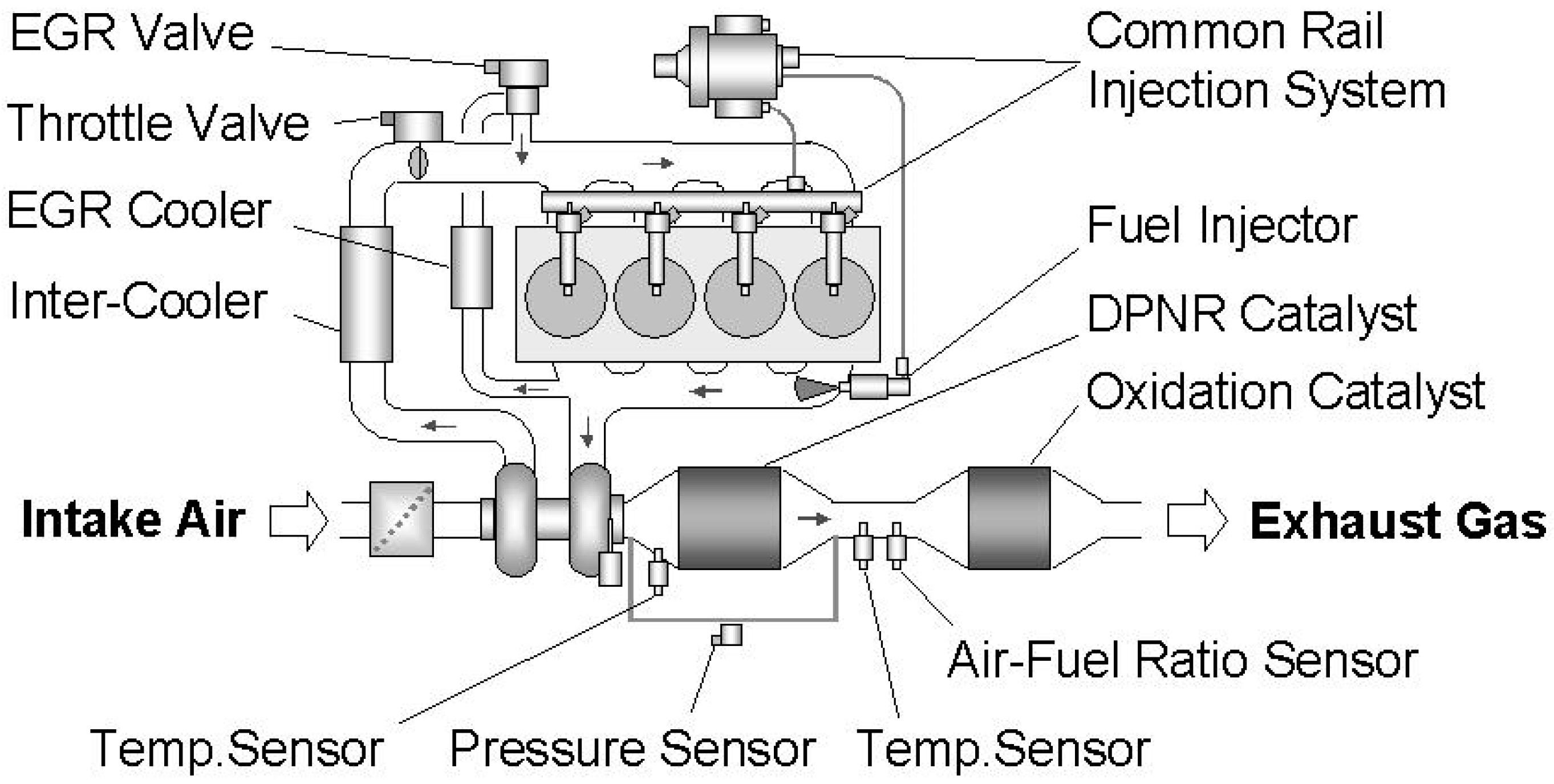

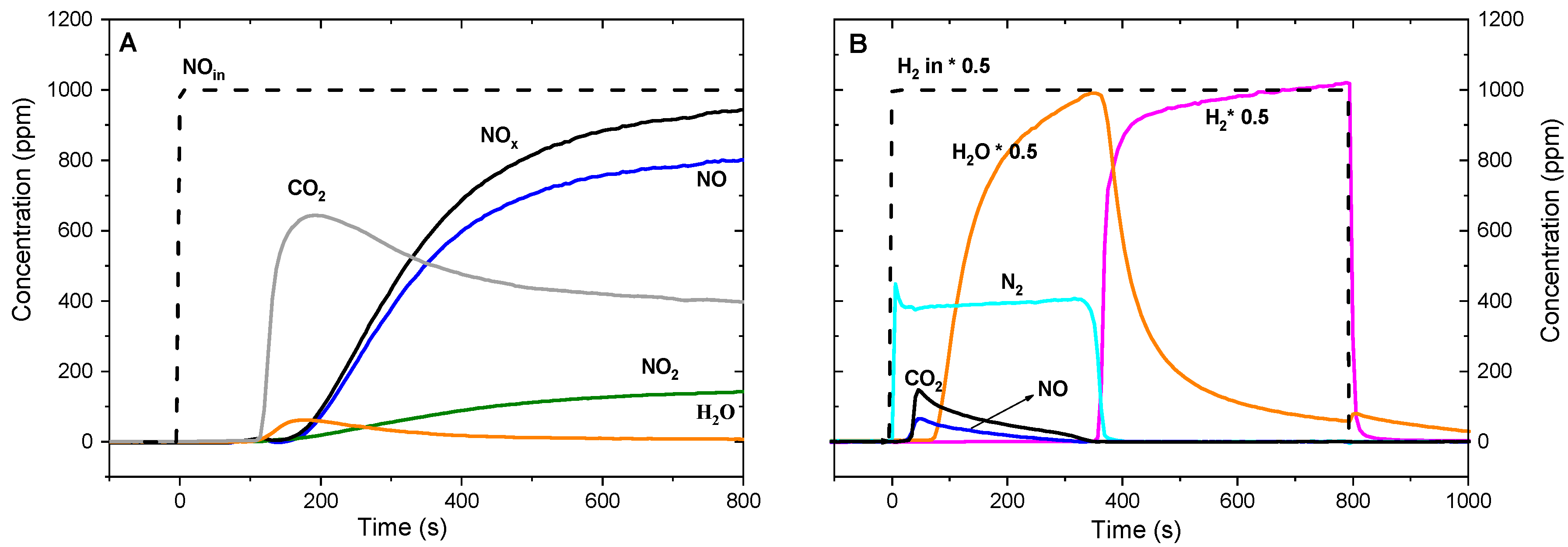
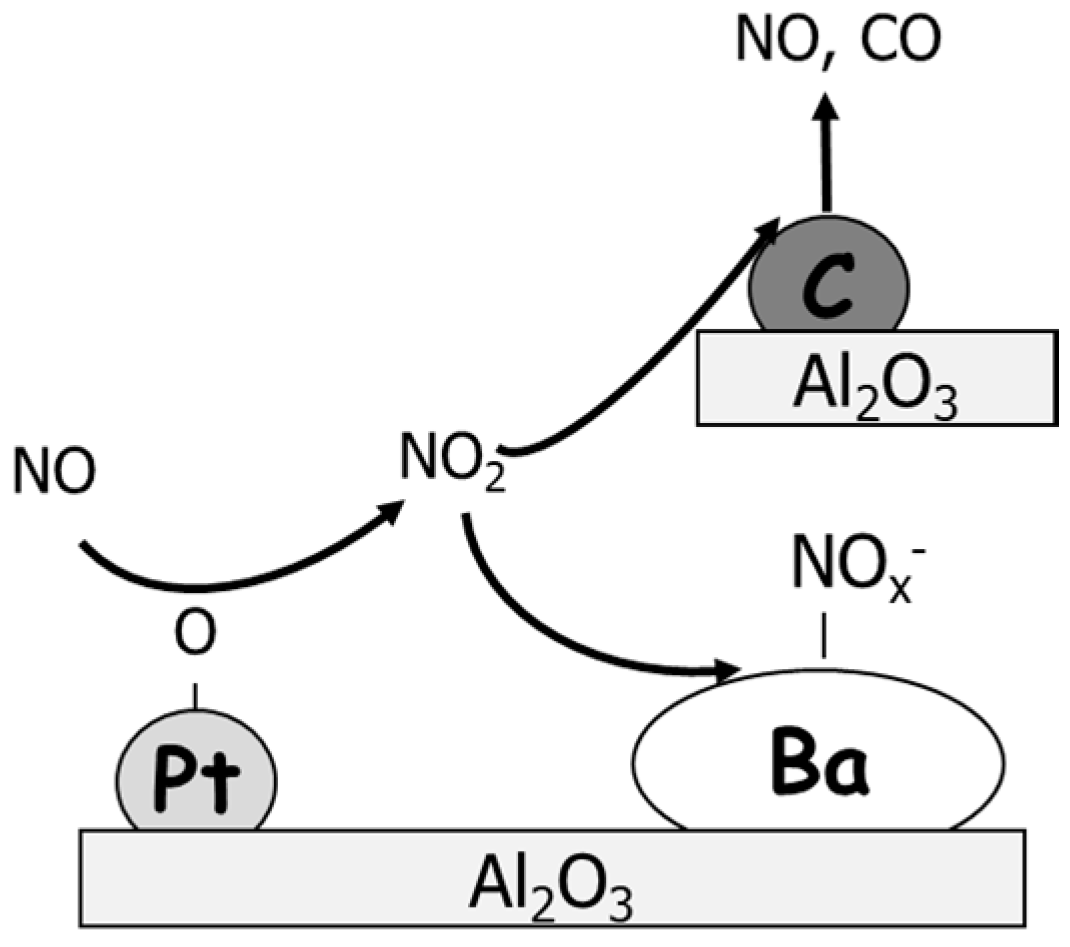
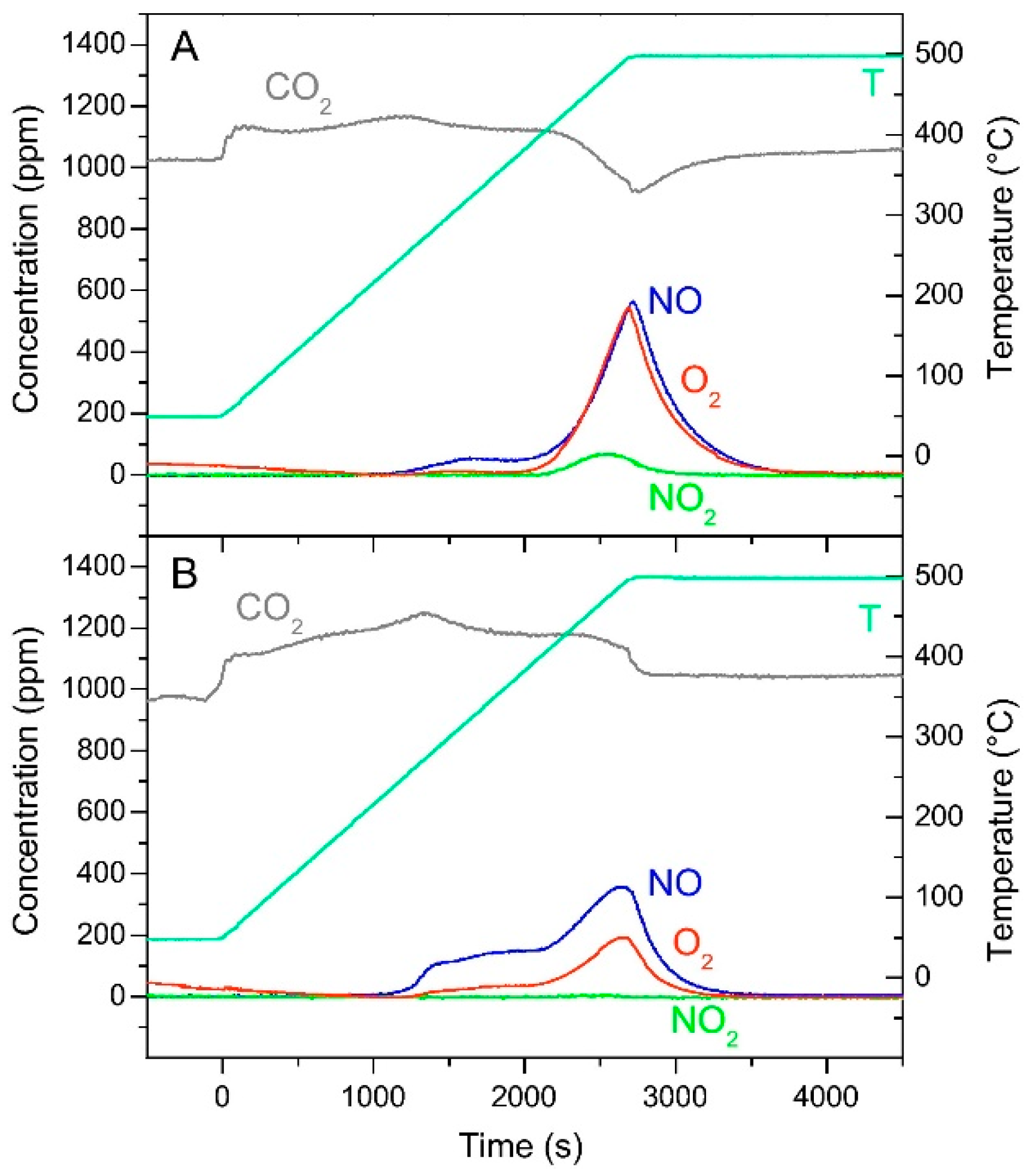
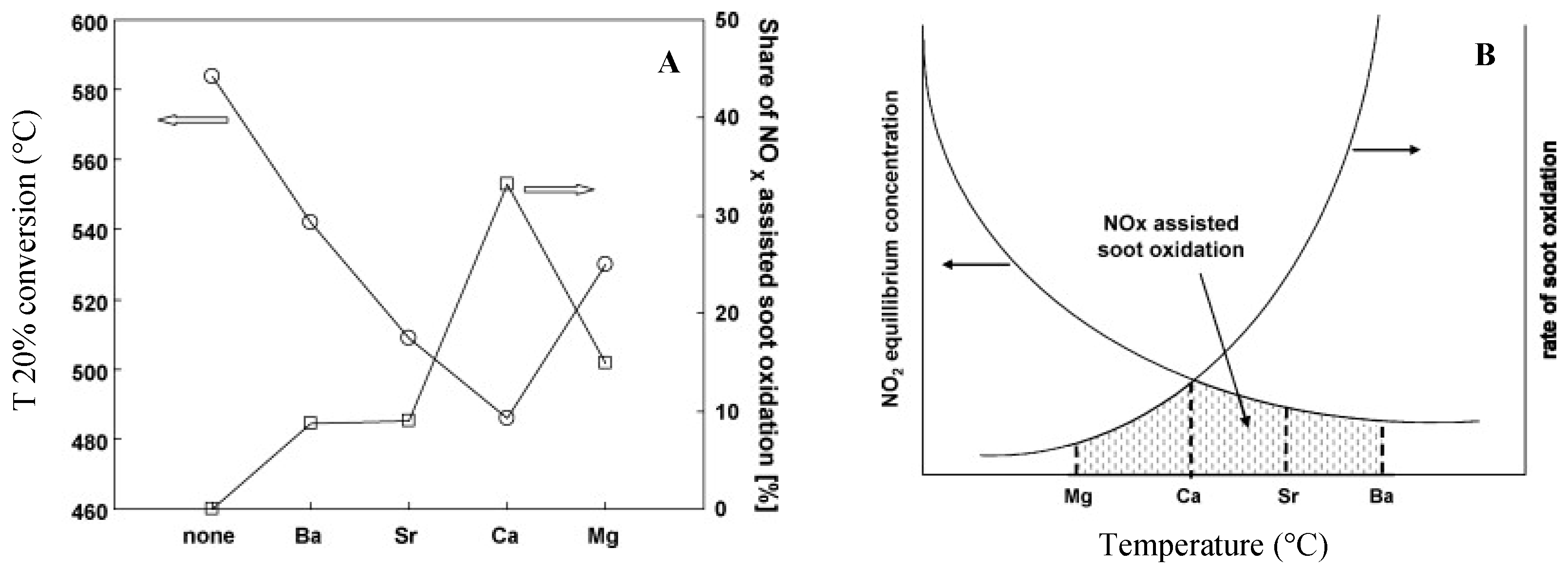

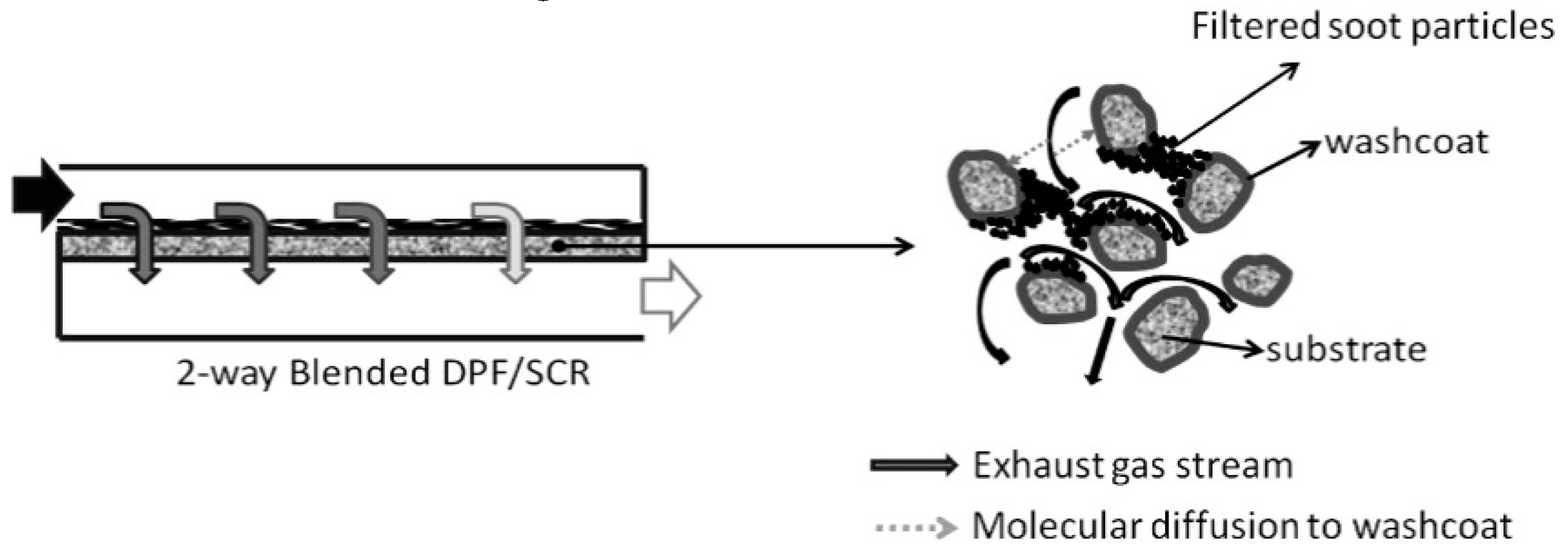
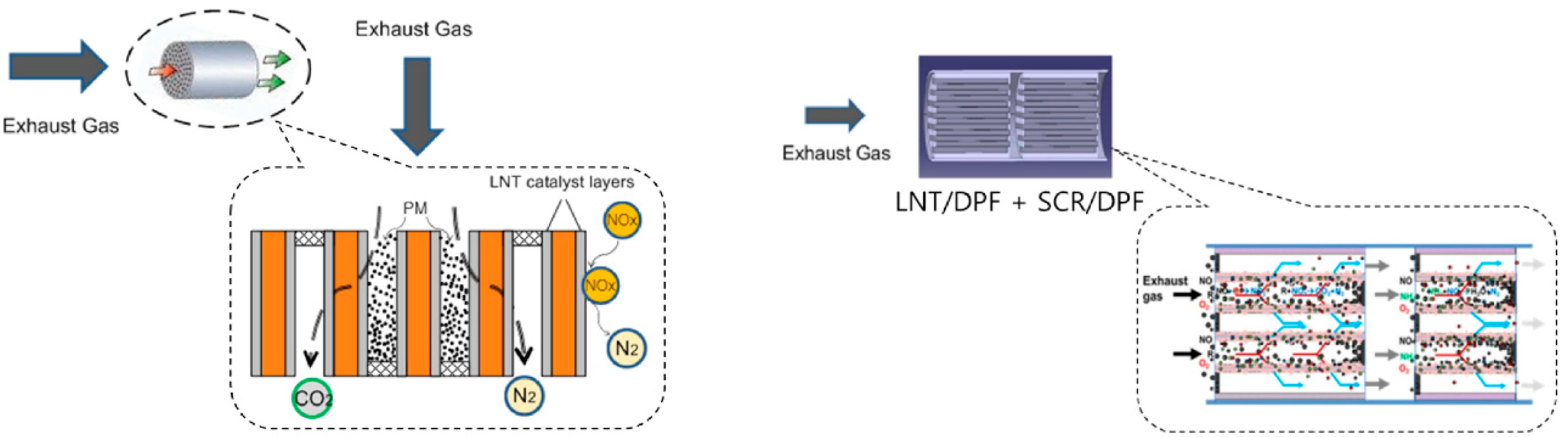
| Hydrotalcites Families | Catalysts | Preparation Method | Reaction Conditions | Ref. |
|---|---|---|---|---|
| Binary MgAlOx LDH | A series of K-supported MgAl with different amount of K doping (2, 5, 8 and 15 wt.% of K) | Co-precipitation; K addition by impregnation; calc. 600 °C | Cat/soot = 9/1; 9.97% O2 + He or 1050 ppm NO + He; total flow 100 mL/min; heating rate 5 or 10 °C/min | [22,23,24] |
| CuAlOx catalyst | Co-precipitation; calc. 800 °C | Cat/soot = 9/1; 0.25% NO + 5.0% O2 + He; total flow 20 cm3/min; heating rate 1.6 °C/min | [25] | |
| CoAlOx catalyst | Co-precipitation; calc. 500 °C e 800 °C | Cat/soot = 20/1; 0.25 vol.% NO + 5 vol.% O2 + He, total flow 80 cm3/min, heating rate 1.4 °C/min | [26] | |
| Ternary MgAlOx LDH | A series of Mn-doped MgAl with different amount of Mn doping (from 0.5 to 3) | Co-precipitation; calc. 800 °C | Cat/soot = 20/1; 750 ppm NO + 10 vol.% O2 + N2, total flow 240 cm3/min, heating rate 10 °C/min | [27] |
| A series of CuxMg3-xAl with different Cu contents (0.5, 1.0, 1.5, 2.0, 2.5, 3.0) | Co-precipitation; calc. 600 °C–700 °C–800 °C | Cat/soot = 20/1; 0.25 vol.% NO + 5 vol.% O2 + He; total flow 20 cm3/min, heating rate of 1.6 °C/min. | [28] | |
| Co2.5Mg0.5Al | Co-precipitation; calc. 500 °C, 600 °C, 700 °C or 800 °C | Cat/soot = 20/1; 400 ppm NO + 10 vol.% O2 + N2; total flow 400 mL/min; heating rate of 10 °C/min. | [12,13] | |
| Quaternary MgAlOx LDH | A series of K-doped Co2.5MgAl with different amount of K doping (1.5, 4.5, 7.5, 10) | Co-precipitation; K addition by impregnation; calc. 600 °C | Cat/soot = 20/1; 400 ppm NO + 10% O2 + N2; total flow 20 mL/min | [13] |
| A series of K-doped Mn1.5MgAl with different amount of K doping (x = 1.5, 4.5, 7.5, 10, 15, 20) | Co-precipitation; K addition by impregnation; calc. 800 °C | Cat/soot = 20/1; 750 ppm NO + 10 vol.% O2 + N2, total flow 240 cm3/min, heating rate 10 °C/min | [29] |
| Sample | NOx Uptake (µmol/gcat) | NOx Reduction Percentage (%) |
|---|---|---|
| Mn-free | 373 (100–272 °C) | 7.2 (278–700 °C) |
| Mn0.5 | 657 (100–404 °C) | 20.4 (327–614 °C) |
| Mn1.0 | 502 (100–386 °C) | 24.0 (295–554 °C) |
| Mn1.5 | 271 (100–336 °C) | 12.6 (308–700 °C) |
| Mn2.0 | 233 (100–355 °C) | 6.9 (253–460 °C) |
| Mn2.5 | 85 (100–202 °C) | 6.5 (263–618 °C) |
| Mn3.0 | 108 (100–213 °C) | 10.4 (212–648 °C) |
| Sample | NOx Uptake (mg/gcat) |
|---|---|
| Co-MgAlO | 24.10 |
| 1.5% K/Co-MgAlO | 31.75 |
| 4.5% K/Co-MgAlO | 51.88 |
| 7.5% K/Co-MgAlO | 56.03 |
| 10% K/Co-MgAlO | 61.24 |
| Perovskites Families | Chemical Composition | Catalysts | Preparation Method | Reaction Conditions | Ref. |
|---|---|---|---|---|---|
| Manganites | A1–x RxMnO3 | La1−xKxMnO3 (x = 0.1, 0.2, 0.25, 0.3) | Co-precipitation; calc. 850–950 °C | Cat/soot = 20/1; NO (0.5%) + O2 (5%) + He; total flow 20 cm3/min; heating rate 1 °C/min | [38] |
| La1−xKxMnO3 | Citrate-combustion route; calc. 750 °C | Cat/soot = 10/1; 10% O2 + 0.5% NO + He; total flow 100 mL/min; heating rate 1 °C/min | [39] | ||
| La1−xKxMnO3 | Complex combustion method; calc. 800 °C | Cat/soot = 10/1; NO 1000 ppm + O2 0.5 vol% + He. total flow 70 mL/min; heating rate 5 °C/min. | [40] | ||
| La0.7Ag0.3MnO3 | Solid-state method; calc. 600 °C | Cat/soot = 20/1; 10% NO-Ar mixture 30 mL/min, oxygen 10 mL/min, air 60 mL/min; heating rate 10 °C/min | [41] | ||
| Cobaltites | A1−xRxCoO3 | La1−xKxCoO3 | Citric acid-ligated method; calc. 800 °C | Cat/soot = 5/1; 5% O2 + 2000 ppm NO + He; total flow 50 cm3/min; heating rate 2 °C/min | [42] |
| La1−xKxCoO3 | Complex combustion method; calc. 800 °C | Cat/soot = 10/1; NO 1000 ppm + O2 0.5 vol% + He. total flow 70 mL/min; heating rate 5 °C/min. | [40] | ||
| Ferrites | A1−xRxFeO3 | La1−xKxFeO3 | complex combustion method; calc. 800 °C | Cat/soot = 10/1; NO 1000 ppm + O2 0.5 vol% + He. total flow 70 mL/min; heating rate 5 °C/min. | [40] |
| Chromites | A1−xRxCrO3 | La1−xKxCrO3 | citrate-combustion route; calc. 700 °C | Cat/soot = 10/1; 10% O2 + 0.5% NO + He; total flow 100 mL/min; heating rate 1 °C/min | [39] |
| Titanites | A1−xRxTiO3 | Sr0.8K0.2TiO3 | sol–gel method; calc. 850 °C | Cat/soot = 4/1; 500 ppm NOx + O2 5% + N2; heating rate 10 °C/min. | [43] |
| Catalyst | Ti | NOx Conversion |
|---|---|---|
| LaCoO3 | 300 °C | 45% @400 °C |
| La1−xKxCoO3 | 200 °C | 50% @305 °C |
| La1−xKxMnO3 | 150 °C | 53% @300 °C |
| La1−xKxFeO3 | 250 °C | 48% @320 °C |
| Soot Oxidation | NO Reduction | |||
|---|---|---|---|---|
| Catalyst | Ti (°C) | Tm (°C) | Tmax-NO (°C) | Conversion (%) |
| LaMnO3 | 205 | 380 | 386 | 45.3 |
| La0.65Ag0.35MnO3 | 152 | 300 | 301 | 53.2 |
| La0.65Ag0.1K0.25MnO3 | 135 | 355 | 368 | 64.4 |
| Description | Reaction | Reaction Rate |
|---|---|---|
| NH3 adsorption | NH3 + S → NH3-S | |
| NH3 desorption | NH3-S → NH3 + S | |
| NH3 oxidation | 2 NH3-S +3/2 O2 → N2 + 3 H2O + 2 S | |
| NH3 oxidation | NO + 1/2 O2 → NO2 | where |
| Standard NH3 SCR | 4 NH3-S + 4 NO + O2 → 4 N2 + 6 H2O + 4 S | |
| Rapid NH3 SCR | 2 NH3-S + NO + NO2 → 2 N2 + 3 H2O + 2 S | |
| NH3 SCR with NO2 | 4 NH3-S + 3 NO2 → 3.5 N2 + 6 H2O + 4 S | |
| N2O formation by SCR | 2 NH3-S + 2 NO2 → N2O + N2 + 3 H2O + 2 S |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castoldi, L. An Overview on the Catalytic Materials Proposed for the Simultaneous Removal of NOx and Soot. Materials 2020, 13, 3551. https://doi.org/10.3390/ma13163551
Castoldi L. An Overview on the Catalytic Materials Proposed for the Simultaneous Removal of NOx and Soot. Materials. 2020; 13(16):3551. https://doi.org/10.3390/ma13163551
Chicago/Turabian StyleCastoldi, Lidia. 2020. "An Overview on the Catalytic Materials Proposed for the Simultaneous Removal of NOx and Soot" Materials 13, no. 16: 3551. https://doi.org/10.3390/ma13163551
APA StyleCastoldi, L. (2020). An Overview on the Catalytic Materials Proposed for the Simultaneous Removal of NOx and Soot. Materials, 13(16), 3551. https://doi.org/10.3390/ma13163551





