Magnetic Calcium Phosphate Cement for Hyperthermia Treatment of Bone Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Magnetic CPC Preparation
2.2. Self-Setting Properties and Physical Characterization
2.3. Magnetic Characterization
2.4. Bioactivity
2.5. Cytocompatibility
2.6. Statistical Analysis
3. Results and Discussion
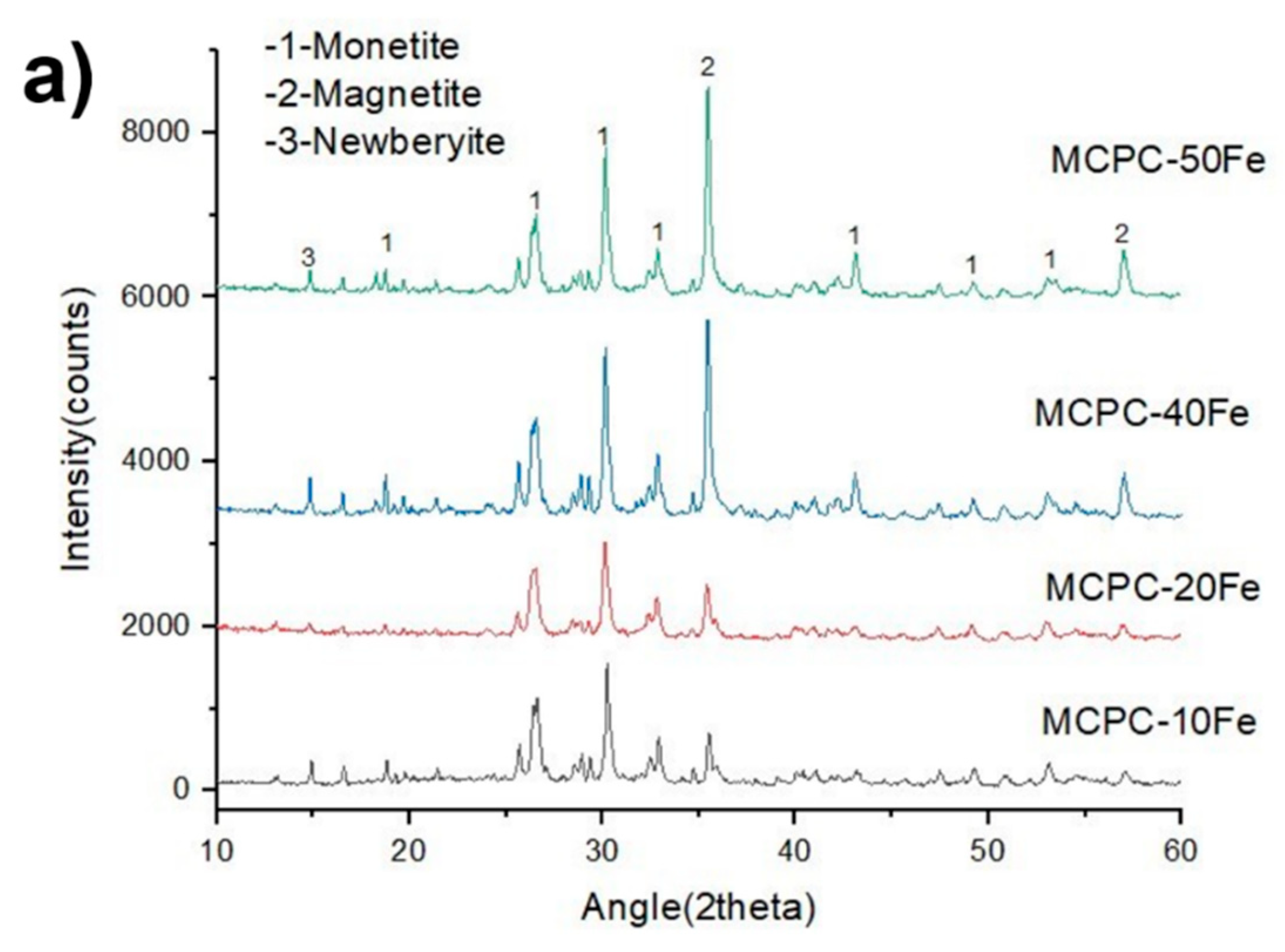
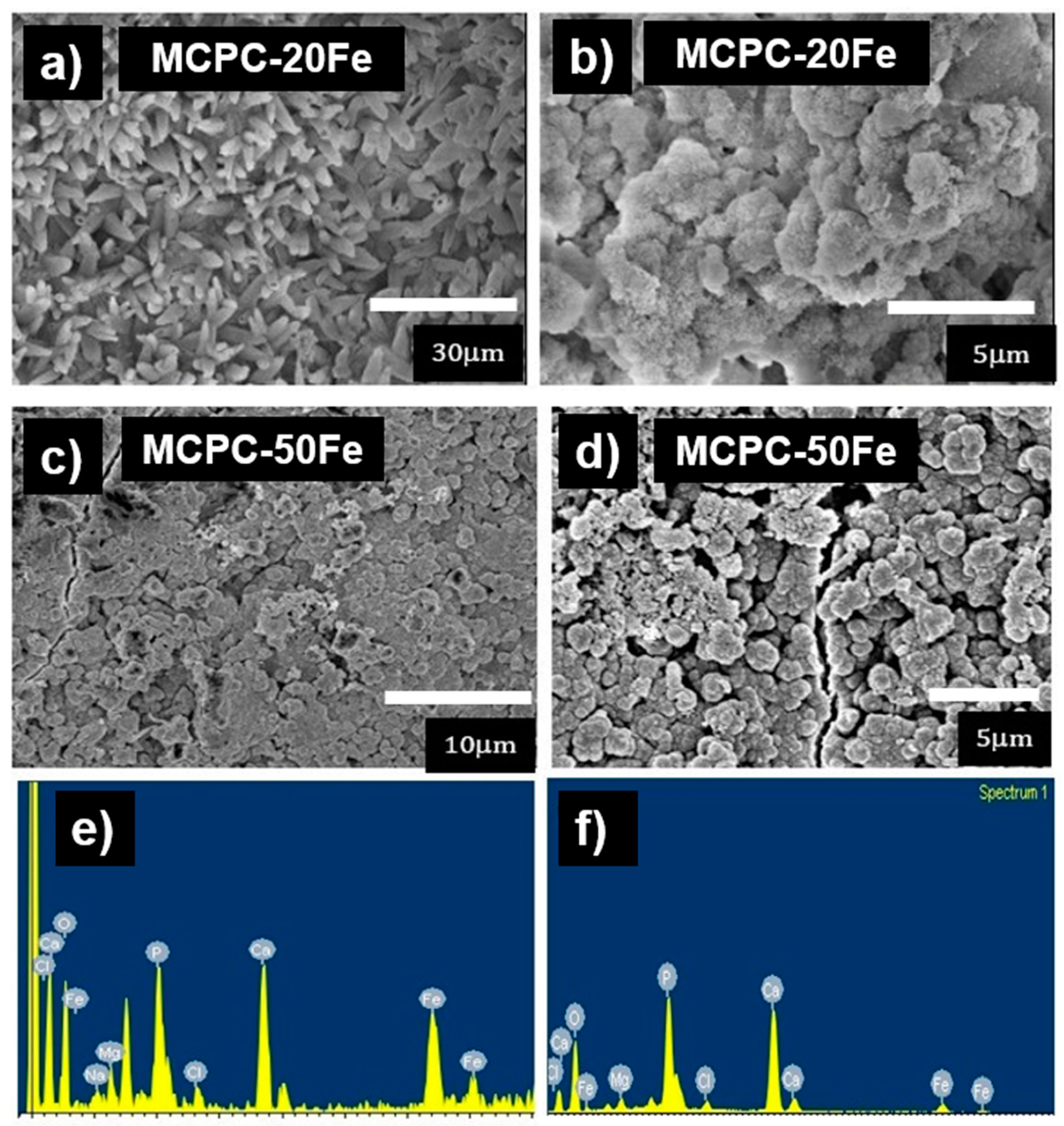
3.1. Self-Setting Properties and Material Characterization
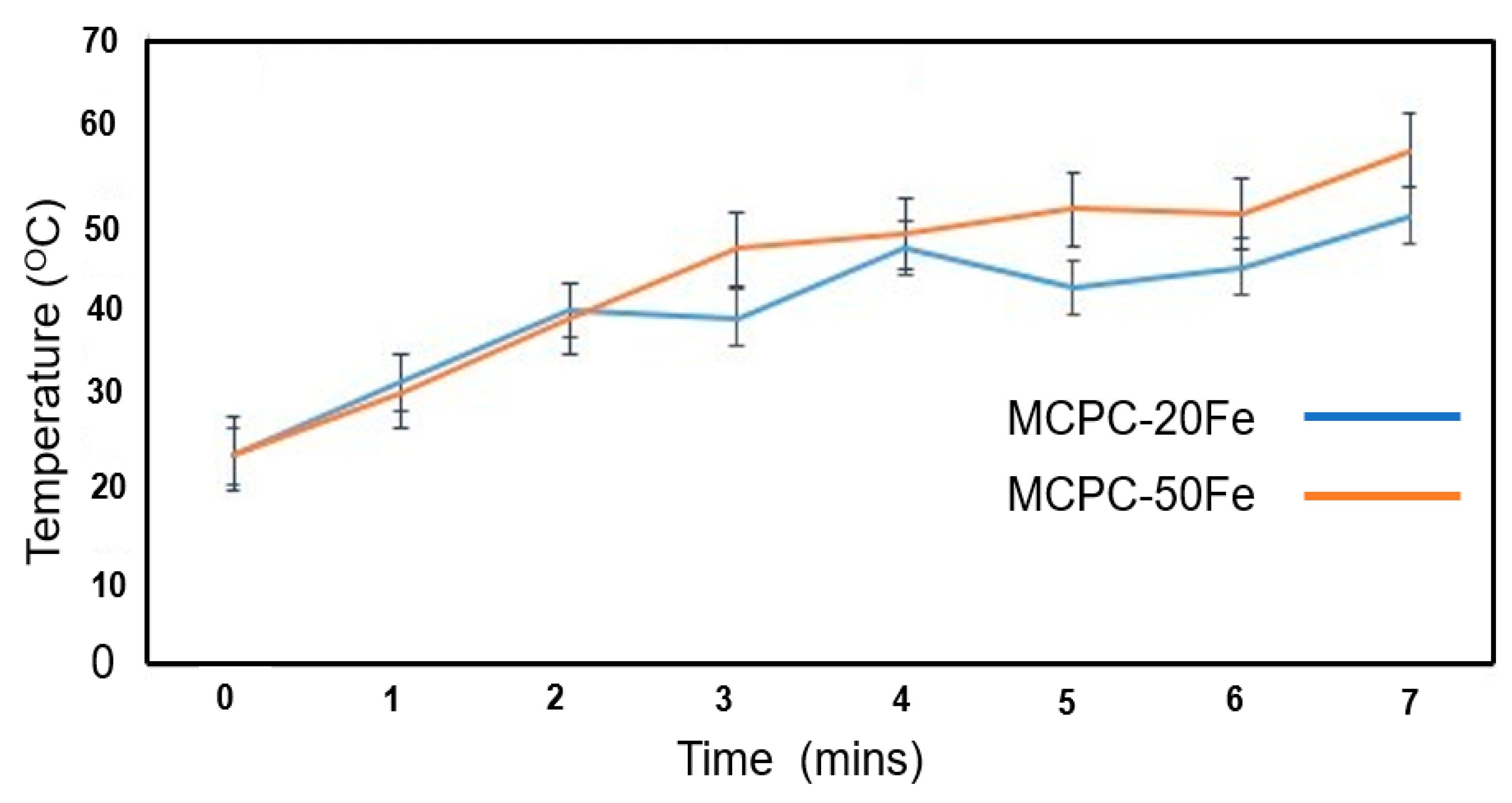
3.2. Heat Generation by MCPCs
3.3. Bioactivity
3.4. In Vitro Cytocompatibility
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Coleman, R. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Evola, F.R.; Costarella, L.; Pavone, V.; Caff, G.; Cannavò, L.; Sessa, A.; Avondo, S.; Sessa, G. Biomarkers of Osteosarcoma, Chondrosarcoma, and Ewing Sarcoma. Front. Pharmacol. 2017, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, S.; Aleya, L.; Kim, B.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. 2019, 27, 1–12. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the In Vitro and In Vivo Toxicity of Superparamagnetic Iron Oxide Nanoparticles. Chem. Rev. 2011, 112, 2323–2338. [Google Scholar] [CrossRef]
- Takegami, K.; Sano, T.; Wakabayashi, H.; Sonoda, J.; Yamazaki, T.; Morita, S.; Shibuya, T.; Uchida, A. New ferromagnetic bone cement for local hyperthermia. J. Biomed. Mater. Res. 1998, 43, 210–214. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, Y.; Gao, W.; Xu, J.; Zuo, G.; Chen, Y.; Zhao, M.; Li, J.; Song, J.; Zhang, N. Magnetic hyperthermia ablation of tumors using injectable Fe3O4/calcium phosphate cement. ACS Appl. Mater. Interfaces 2015, 7, 13866–13875. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Z.; Zhang, T.; Zhang, Q.; Chen, Y.; Xie, Y.; Lei, J.; Cai, L. Biphasic Injectable Bone Cement with Fe3O4/GO Nanocomposites for the Minimally Invasive Treatment of Tumor-Induced Bone Destruction. ACS Biomater. Sci. Eng. 2019, 5, 5833–5843. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhao, Y.; Zhang, F.; Li, X.; Wang, L.; Weir, M.D.; Ma, J.; Reynolds, M.A.; Gu, N.; et al. Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Mater. Sci. Eng. C 2018, 98, 30–41. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, Y.; Yang, Z.; Chen, H.; Ren, K.; Weir, M.D.; Chow, L.C.; Reynolds, M.A.; Zhang, F.; Gu, N.; et al. Iron oxide nanoparticle-calcium phosphate cement enhanced the osteogenic activities of stem cells through WNT/β-catenin signaling. Mater. Sci. Eng. C 2019, 104, 109955. [Google Scholar] [CrossRef]
- Boroujeni, N.M.; Zhou, H.; Luchini, T.J.; Bhaduri, S.B. Development of multi-walled carbon nanotubes reinforced monetite bionanocomposite cements for orthopedic applications. Mater. Sci. Eng. C 2013, 33, 4323–4330. [Google Scholar] [CrossRef] [PubMed]
- Koju, N.; Sikder, P.; Gaihre, B.; Bhaduri, S.B. Smart Injectable Self-Setting Monetite Based Bioceramics for Orthopedic Applications. Materials 2018, 11, 1258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Luchini, T.J.F.; Agarwal, A.K.; Goel, V.K.; Bhaduri, S.B. Development of monetite-nanosilica bone cement: A preliminary study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Luchini, T.J.; Boroujeni, N.M.; Agarwal, A.K.; Goel, V.; Bhaduri, S.B. Development of nanosilica bonded monetite cement from egg shells. Mater. Sci. Eng. C 2015, 50, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sikder, P.; Coomar, P.; Mewborn, J.; Bhaduri, S. Antibacterial calcium phosphate composite cements reinforced with silver-doped magnesium phosphate (newberyite) micro-platelets. J. Mech. Behav. Biomed. Mater. 2020. [Google Scholar] [CrossRef]
- Sikder, P.; Ren, Y.; Bhaduri, S.B. Microwave processing of calcium phosphate and magnesium phosphate based orthopedic bioceramics: A state-of-the-art review. Acta Biomater. 2020, 111, 29–53. [Google Scholar] [CrossRef]
- Sikder, P.; Grice, C.R.; Lin, B.; Goel, V.K.; Bhaduri, S.B. Single-Phase, Antibacterial Trimagnesium Phosphate Hydrate Coatings on Polyetheretherketone (PEEK) Implants by Rapid Microwave Irradiation Technique. ACS Biomater. Sci. Eng. 2018, 4, 2767–2783. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Self-Setting Calcium Orthophosphate Formulations. J. Funct. Biomater. 2013, 4, 209–311. [Google Scholar] [CrossRef]
- Sugimoto, T.; Matijević, E. Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. J. Colloid Interface Sci. 1980, 74, 227–243. [Google Scholar] [CrossRef]
- Vereda, F.; Rodríguez-González, B.; De Vicente, J.; Hidalgo-Alvarez, R.; Moratilla, F.V. Evidence of direct crystal growth and presence of hollow microspheres in magnetite particles prepared by oxidation of Fe(OH)2. J. Colloid Interface Sci. 2008, 318, 520–524. [Google Scholar] [CrossRef]
- Butterworth, M.; Bell, S.; Armes, S.; Simpson, A. Synthesis and Characterization of Polypyrrole–Magnetite–Silica Particles. J. Colloid Interface Sci. 1996, 183, 91–99. [Google Scholar] [CrossRef]
- Muzquiz-Ramos, E.; Cortes-Hernández, D.A.; Escobedo-Bocardo, J.C.; Zugasti-Cruz, A. In vitro bonelike apatite formation on magnetite nanoparticles after a calcium silicate treatment: Preparation, characterization and hemolysis studies. Ceram. Int. 2012, 38, 6849–6856. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H.; Liu, J.Q.; Yu, T.; Shen, Z.H.; Ye, J. Good hydration and cell-biological performances of superparamagnetic calcium phosphate cement with concentration-dependent osteogenesis and angiogenesis induced by ferric iron. J. Mater. Chem. B 2015, 3, 8782–8795. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Zhang, Y.; Yang, X.; Nutt, S.; Moradian-Oldak, J. An amelogenin-chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013, 9, 7289–7297. [Google Scholar] [CrossRef] [PubMed]
- Sikder, P.; Bhaduri, S.B. Microwave assisted synthesis and characterization of single-phase tabular hexagonal newberyite, an important bioceramic. J. Am. Ceram. Soc. 2018, 101, 2537–2544. [Google Scholar] [CrossRef]
- Iwasaki, T.; Nakatsuka, R.; Murase, K.; Takata, H.; Nakamura, H.; Watano, S. Simple and Rapid Synthesis of Magnetite/Hydroxyapatite Composites for Hyperthermia Treatments via a Mechanochemical Route. Int. J. Mol. Sci. 2013, 14, 9365–9378. [Google Scholar] [CrossRef]
- Sikder, P.; Koju, N.; Lin, B.; Bhaduri, S.B. Conventionally Sintered Hydroxyapatite–Barium Titanate Piezo-Biocomposites. Trans. Indian Inst. Met. 2019, 72, 2011–2018. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Takagi, S.; Quinn, J.B.; Chow, L.C. Fast-setting calcium phosphate scaffolds with tailored macropore formation rates for bone regeneration. J. Biomed. Mater. Res. 2004, 68, 725–734. [Google Scholar] [CrossRef]


| Specimen Name | CPC/ Monetite (g) | Magnetite (g) | MgO (g) | Na2H2PO4 (g) | Colloidal Silica Blend (mL) |
|---|---|---|---|---|---|
| MCPC-10Fe | 5 | 0.5 | 0.08 | 0.04 | 1.79 |
| MCPC-20Fe | 5 | 1 | 0.08 | 0.04 | 1.89 |
| MCPC-40Fe | 5 | 2.0 | 0.08 | 0.06 | 2.24 |
| MCPC-50Fe | 5 | 2.5 | 0.08 | 0.06 | 2.40 |
| Cement Compositions | Initial Setting Time (min) | Final Setting Time (min) | Handling |
|---|---|---|---|
| MCPC-10Fe | 5.23 ± 1.3 | 14.5 ± 4.5 | A sticky consistency that stuck to gloves during cement preparation. This allowed for a good injectability. |
| MCPC-20Fe | 5.72 ± 1.4 | 14.5 ± 4.5 | Had a stickier consistency as the iron content in the cement increased. Cement was difficult to wash off. |
| MCPC-40Fe | 4.40 ± 1.4 | 6.04 ± 0.15 * | Hardened at a faster rate compared to the other MCPC. Was difficult to work with. |
| MCPC-50Fe | 7.75 ± 0.43 * | 14.4 ± 1.8 | Cement had a good workability and handling. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruskin, E.I.; Coomar, P.P.; Sikder, P.; Bhaduri, S.B. Magnetic Calcium Phosphate Cement for Hyperthermia Treatment of Bone Tumors. Materials 2020, 13, 3501. https://doi.org/10.3390/ma13163501
Ruskin EI, Coomar PP, Sikder P, Bhaduri SB. Magnetic Calcium Phosphate Cement for Hyperthermia Treatment of Bone Tumors. Materials. 2020; 13(16):3501. https://doi.org/10.3390/ma13163501
Chicago/Turabian StyleRuskin, Ethel Ibinabo, Paritosh Perry Coomar, Prabaha Sikder, and Sarit B. Bhaduri. 2020. "Magnetic Calcium Phosphate Cement for Hyperthermia Treatment of Bone Tumors" Materials 13, no. 16: 3501. https://doi.org/10.3390/ma13163501
APA StyleRuskin, E. I., Coomar, P. P., Sikder, P., & Bhaduri, S. B. (2020). Magnetic Calcium Phosphate Cement for Hyperthermia Treatment of Bone Tumors. Materials, 13(16), 3501. https://doi.org/10.3390/ma13163501




