Experimental Investigation of NaOH and KOH Mixture in SCBA-Based Geopolymer Cement Composite
Abstract
1. Introduction
2. Experimental Investigation
2.1. Materials
2.1.1. Cement
2.1.2. SCBA
2.1.3. Mixing Water
2.1.4. Coarse and Fine Aggregate
2.1.5. NaOH and KOH Activator
2.2. Testing Program
2.2.1. Specimen Designation
2.2.2. Testing of SCBA (Phase 1)
2.2.3. Testing on Geopolymer Cement Paste (Phase 2)
2.2.4. Testing on Geopolymer Concrete (Phase 3)
3. Test Results and Discussion
3.1. Phase 1 Test Results
3.1.1. Chapelle’s Test
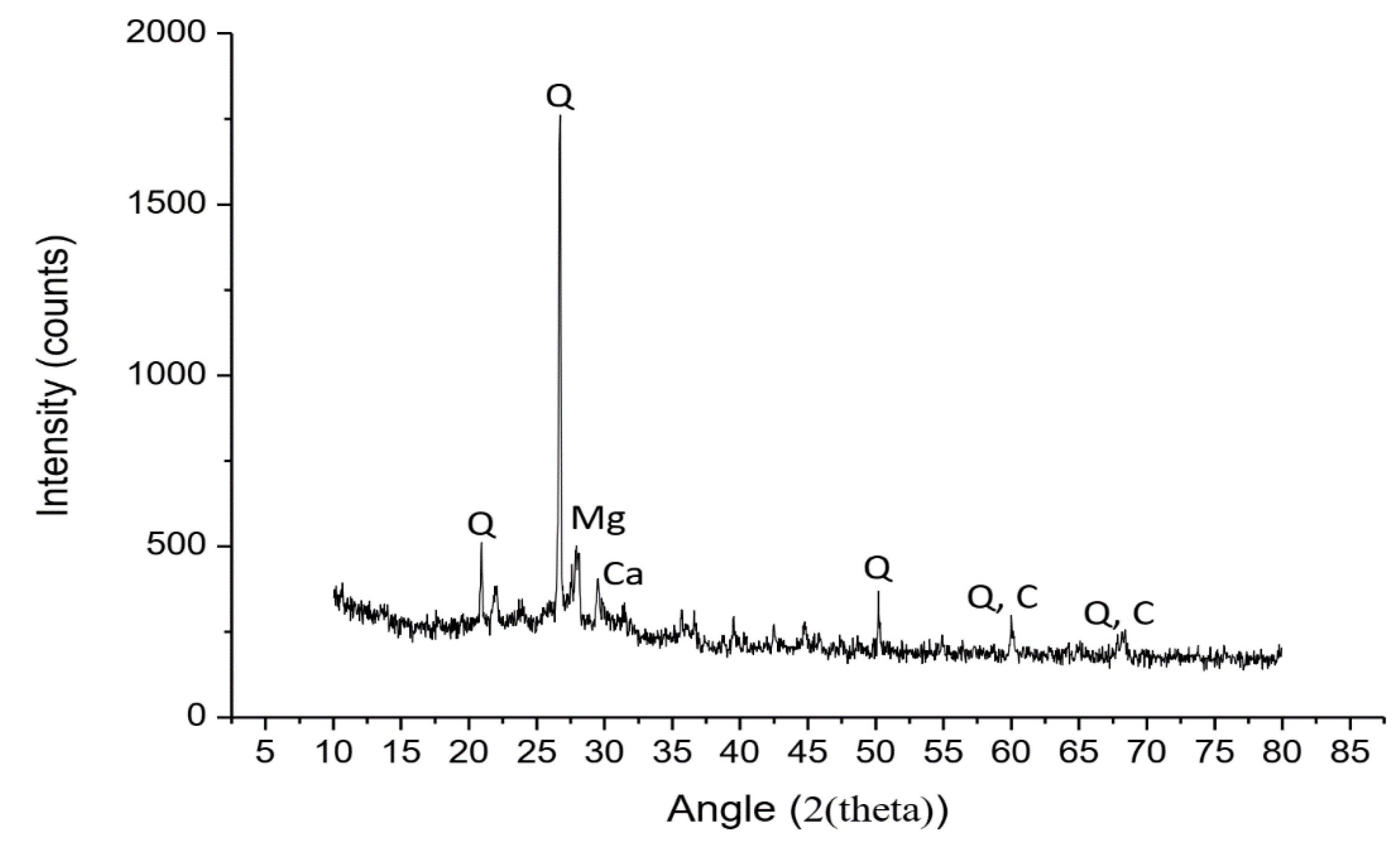
3.1.2. XRD and XRF Analysis
3.1.3. SEM Analysis
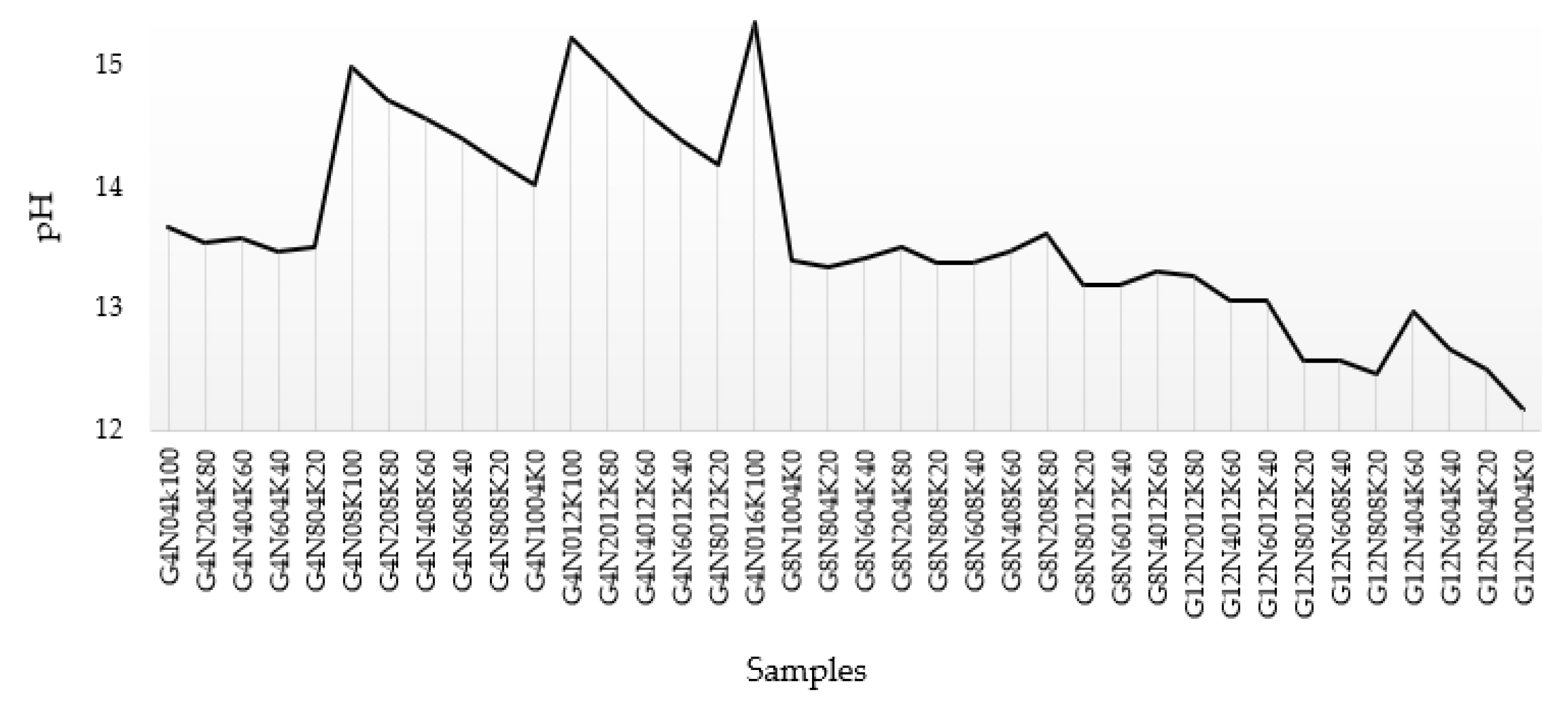
3.1.4. pH Test Results
3.2. Phase 2 Test Results
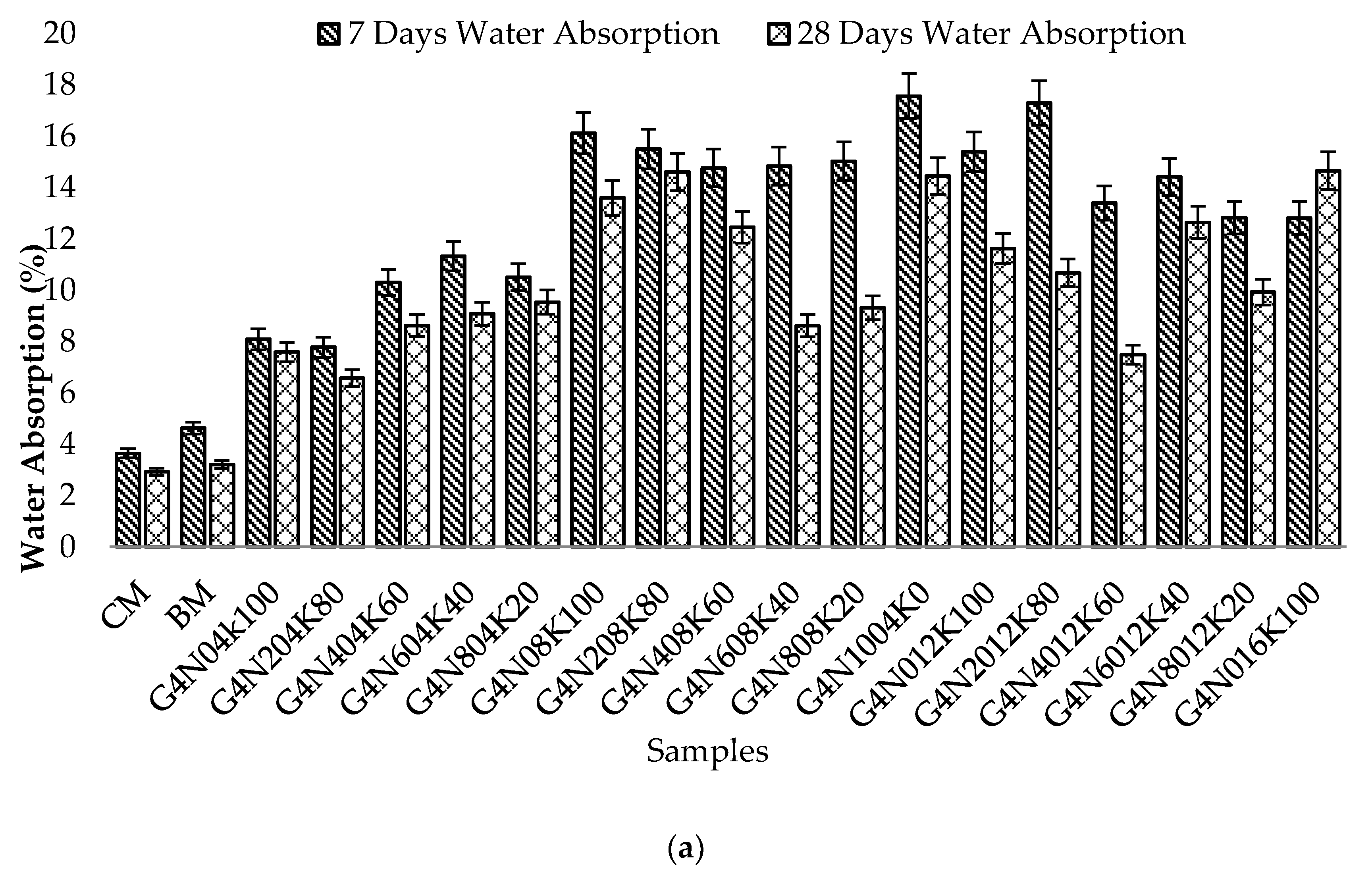
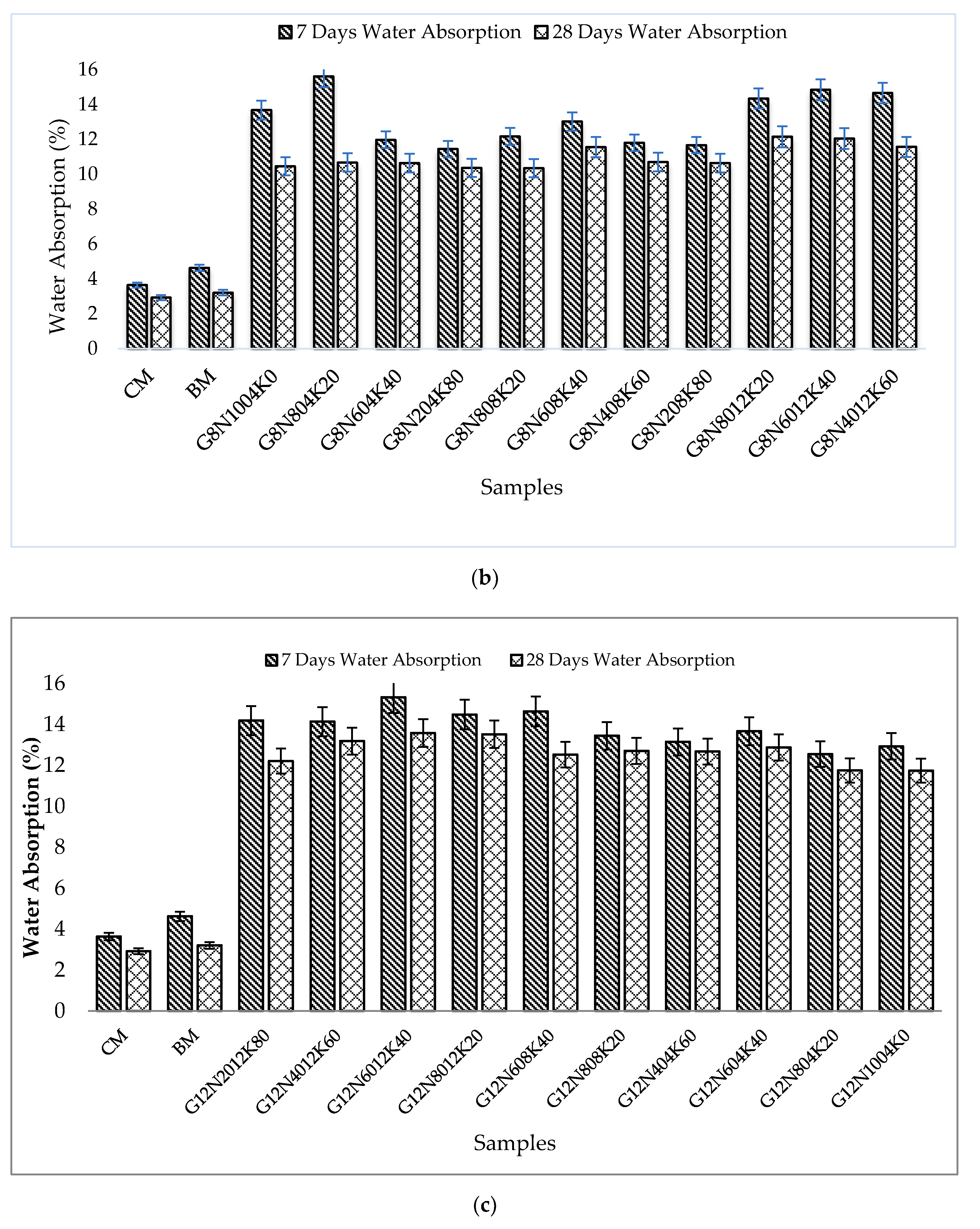
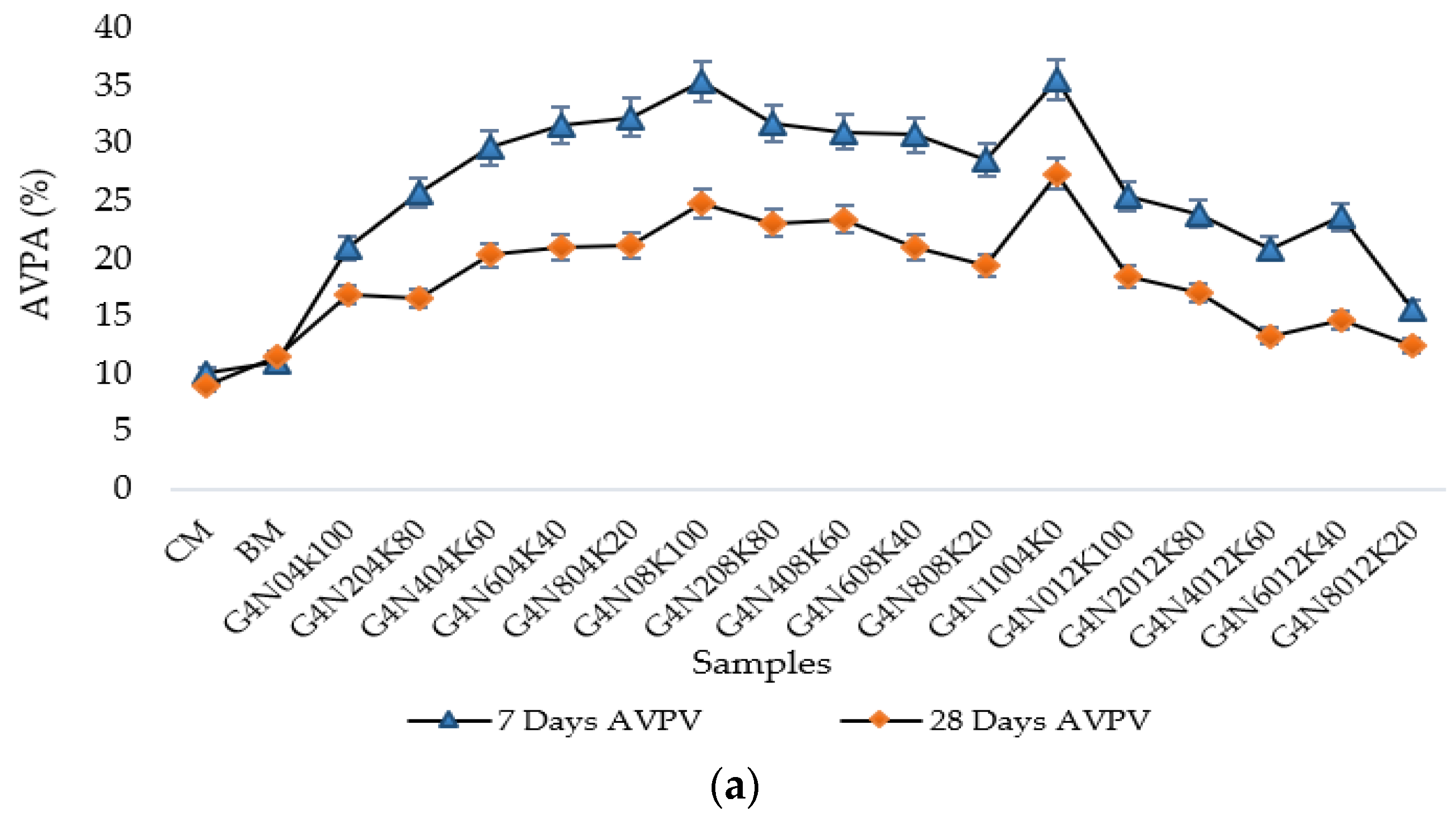
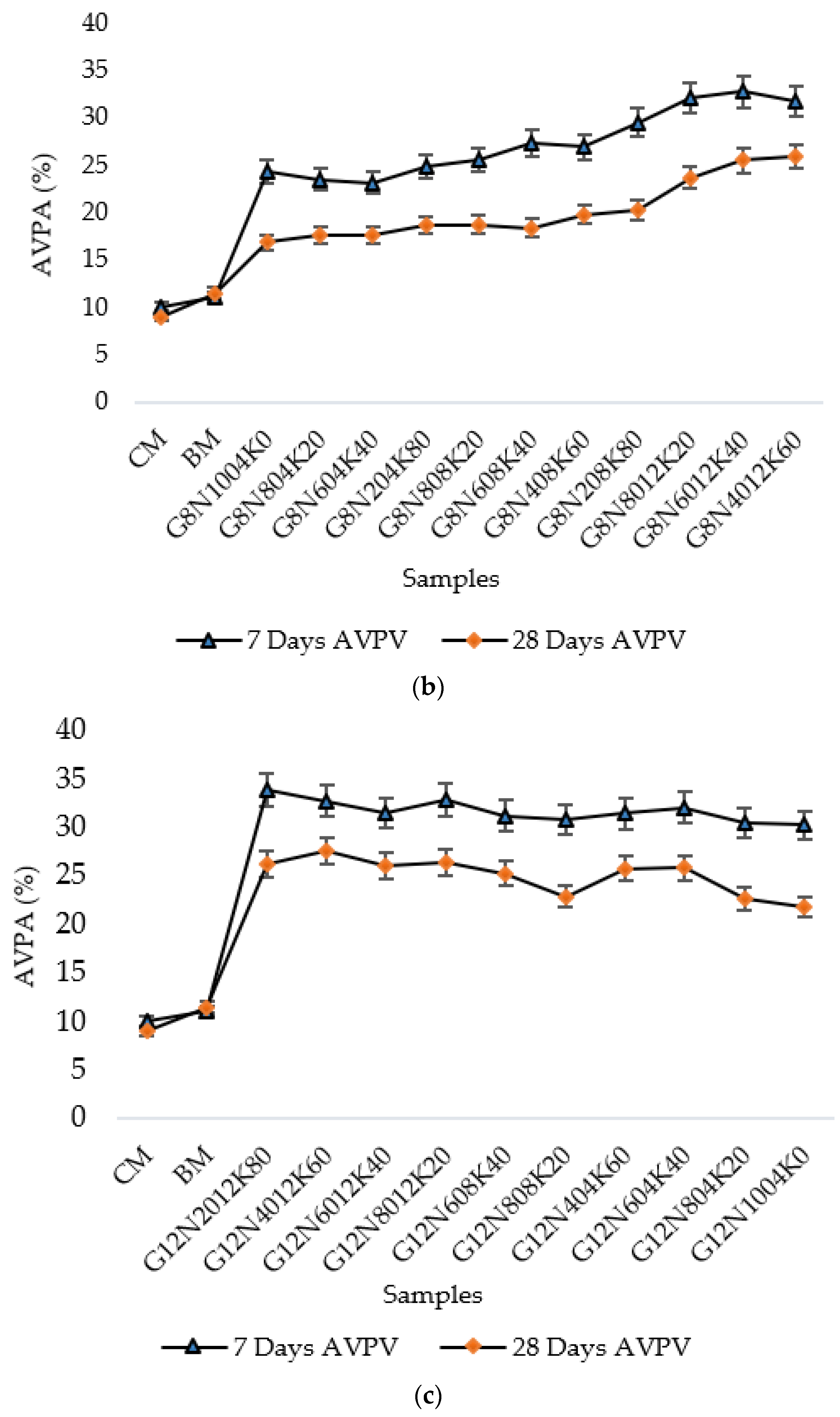
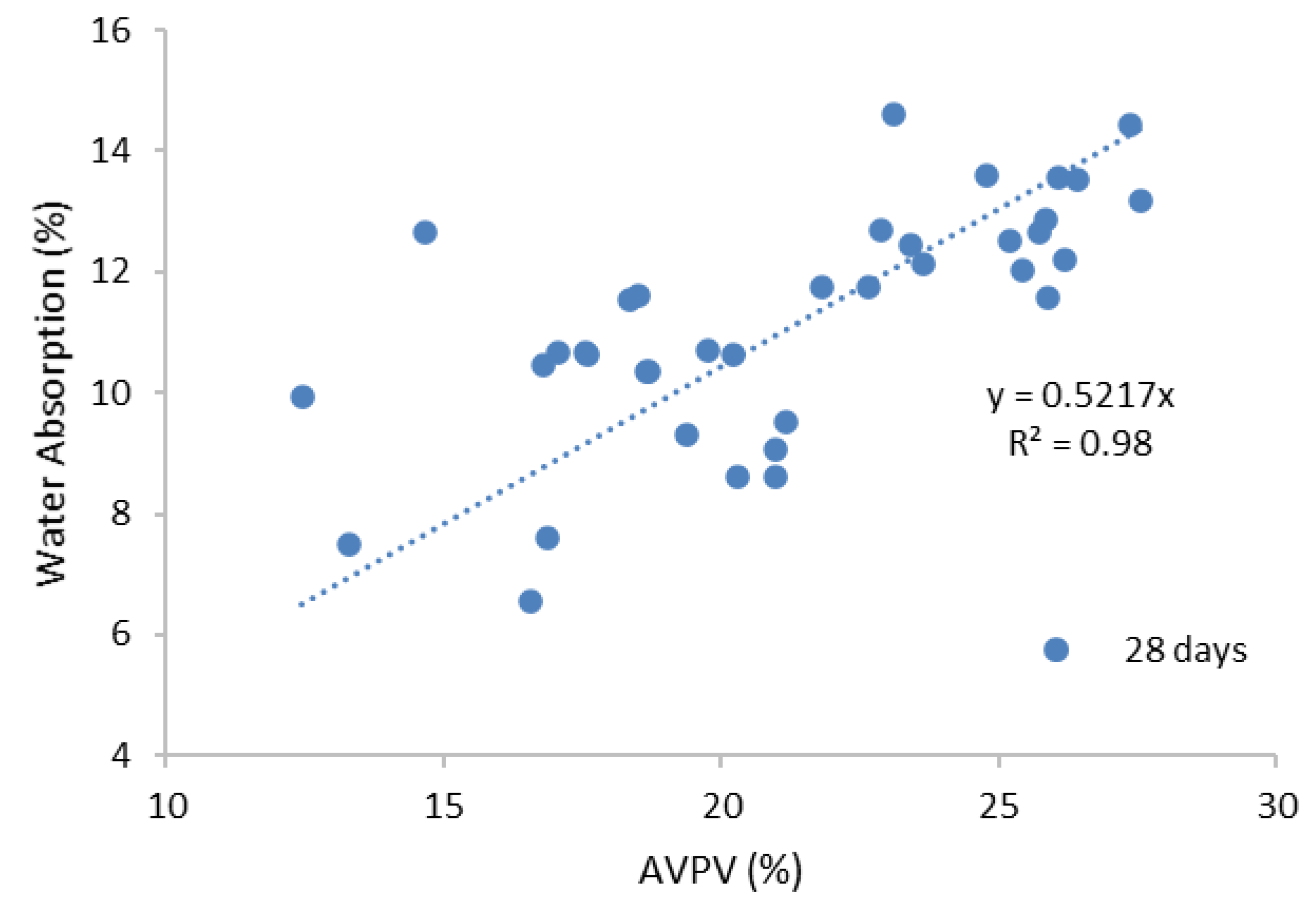
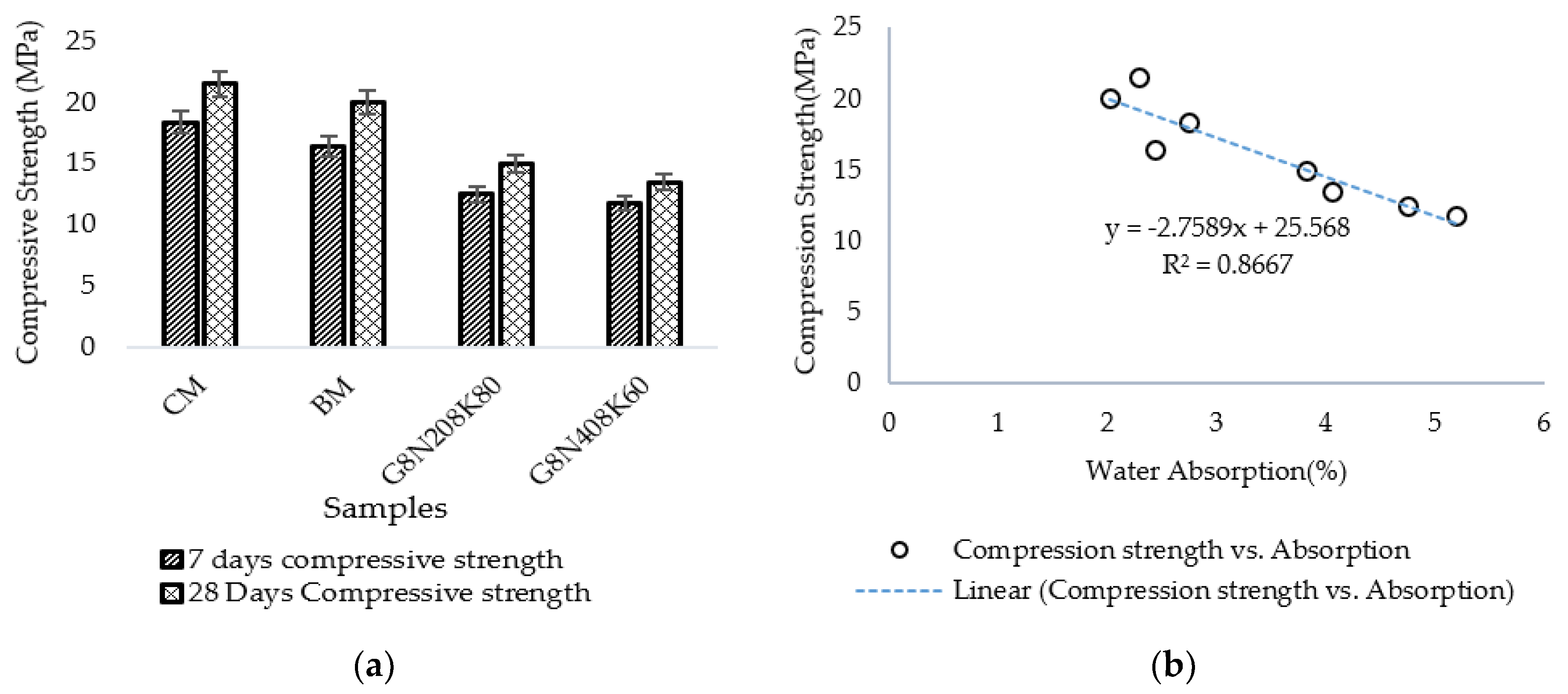
3.2.1. Water Absorption Test
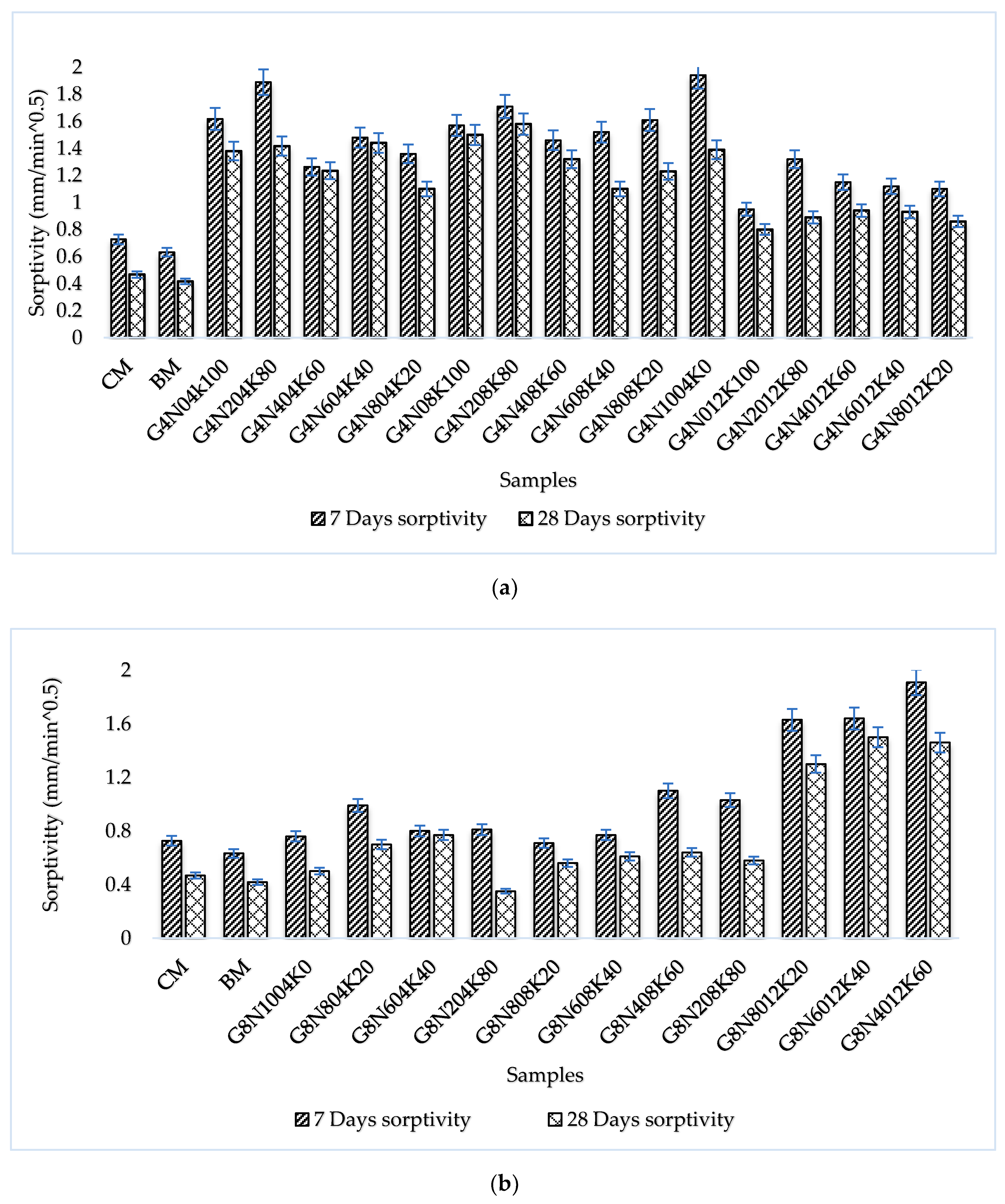
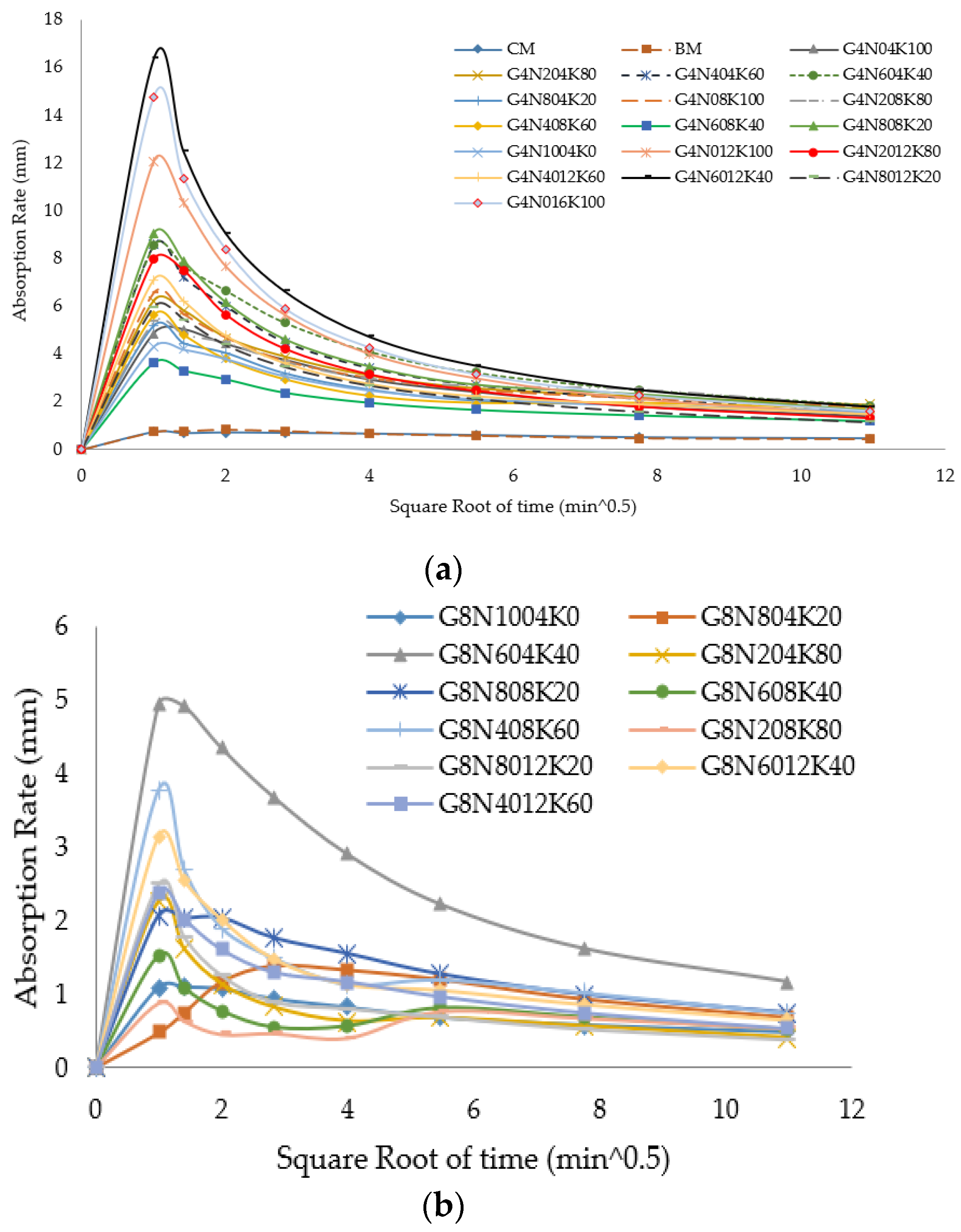
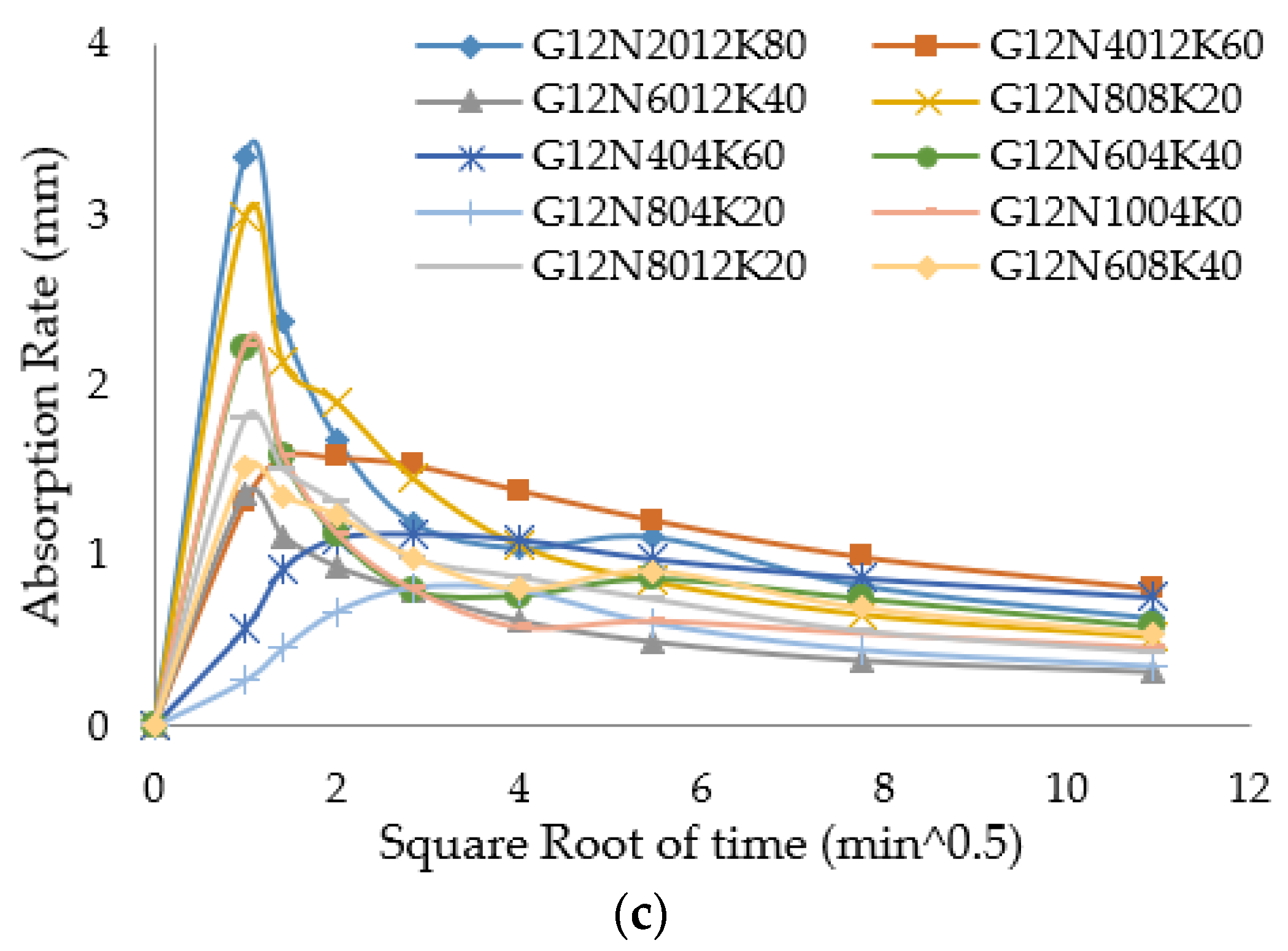
3.2.2. Sorptivity Test
3.2.3. Permeable Porosity Test
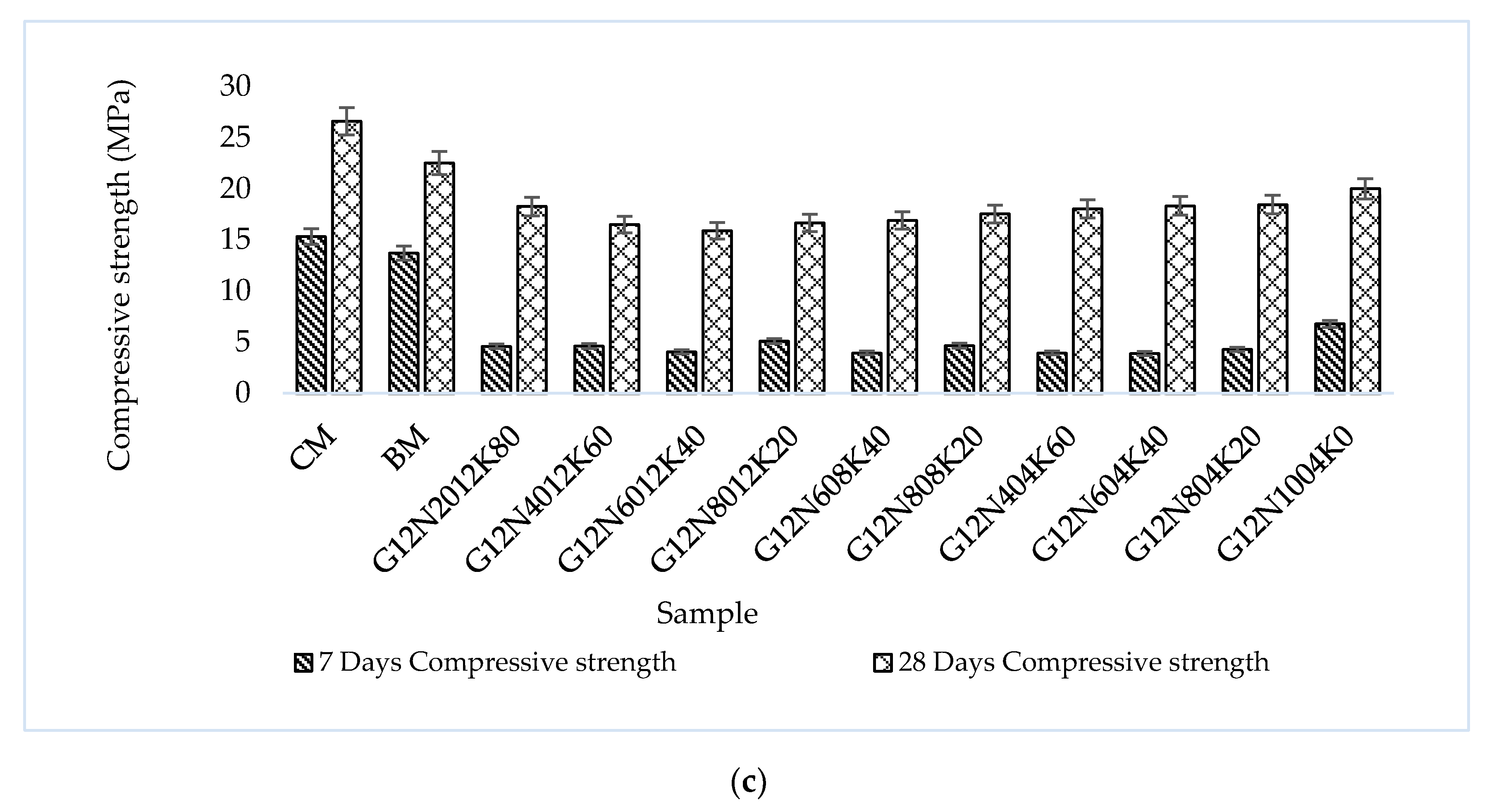
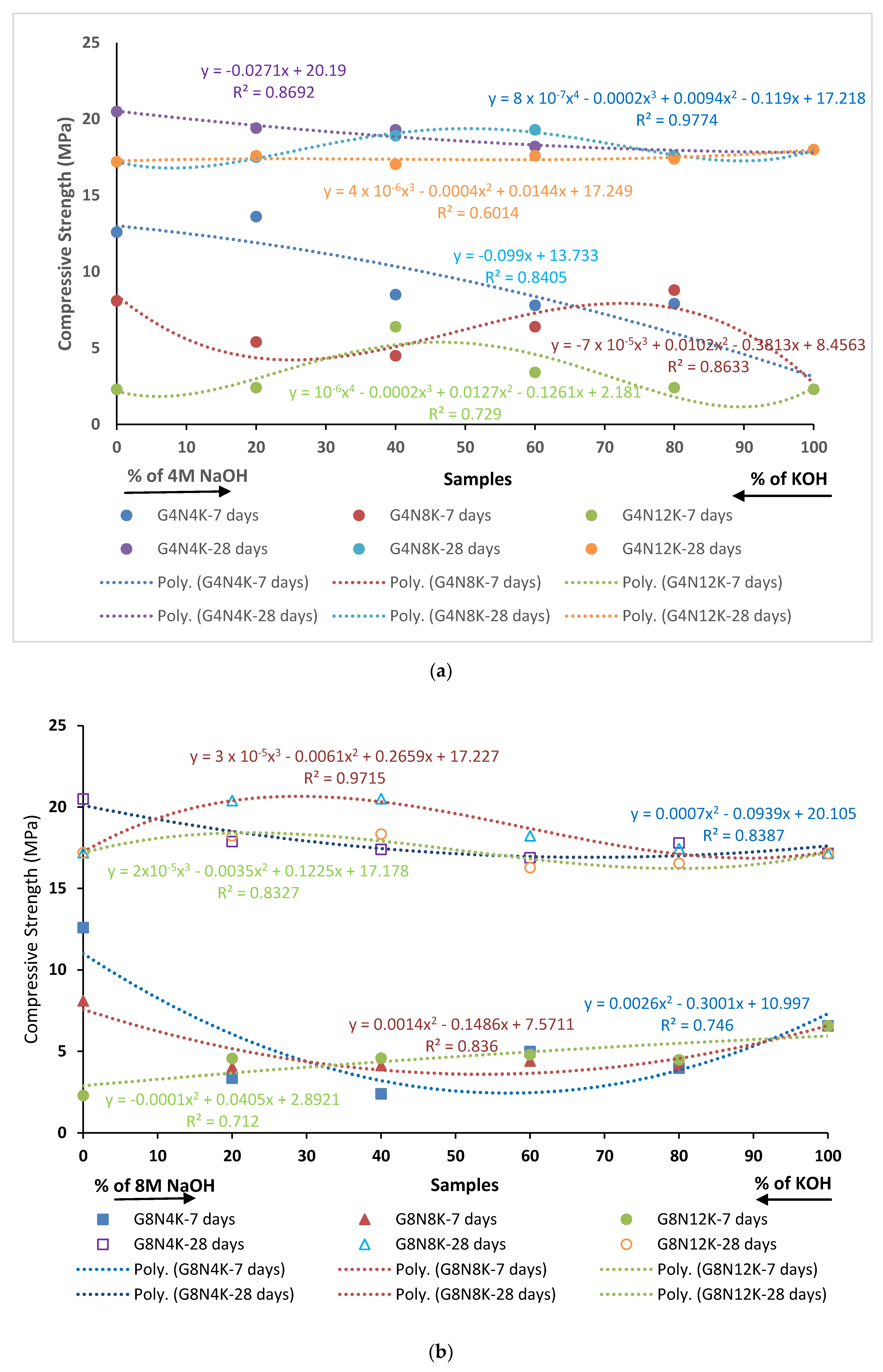
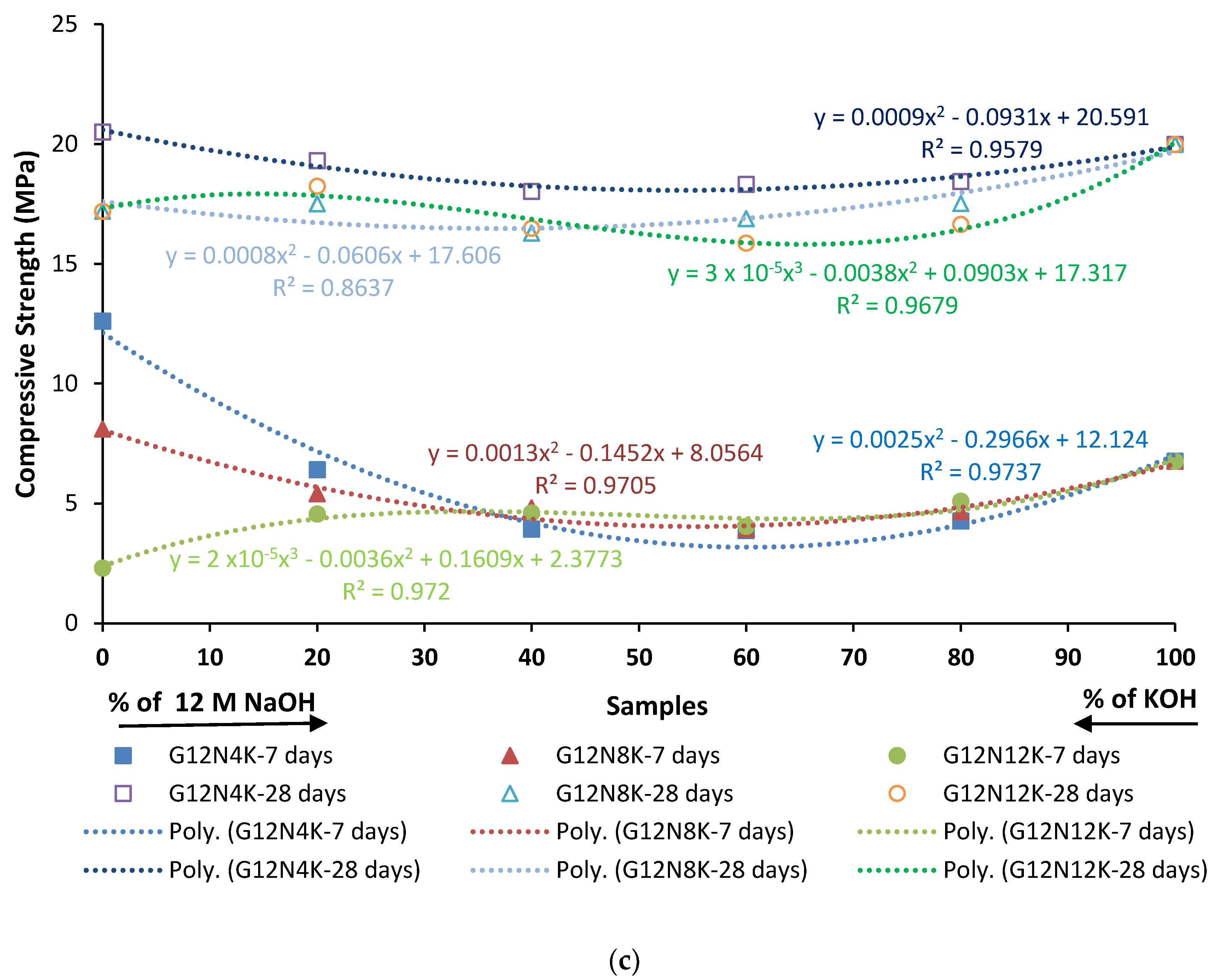
3.2.4. Compression Test
3.3. Phase 3 Test Results
3.3.1. Selection of Optimum Samples
3.3.2. Slump Test
3.3.3. Water Absorption Test
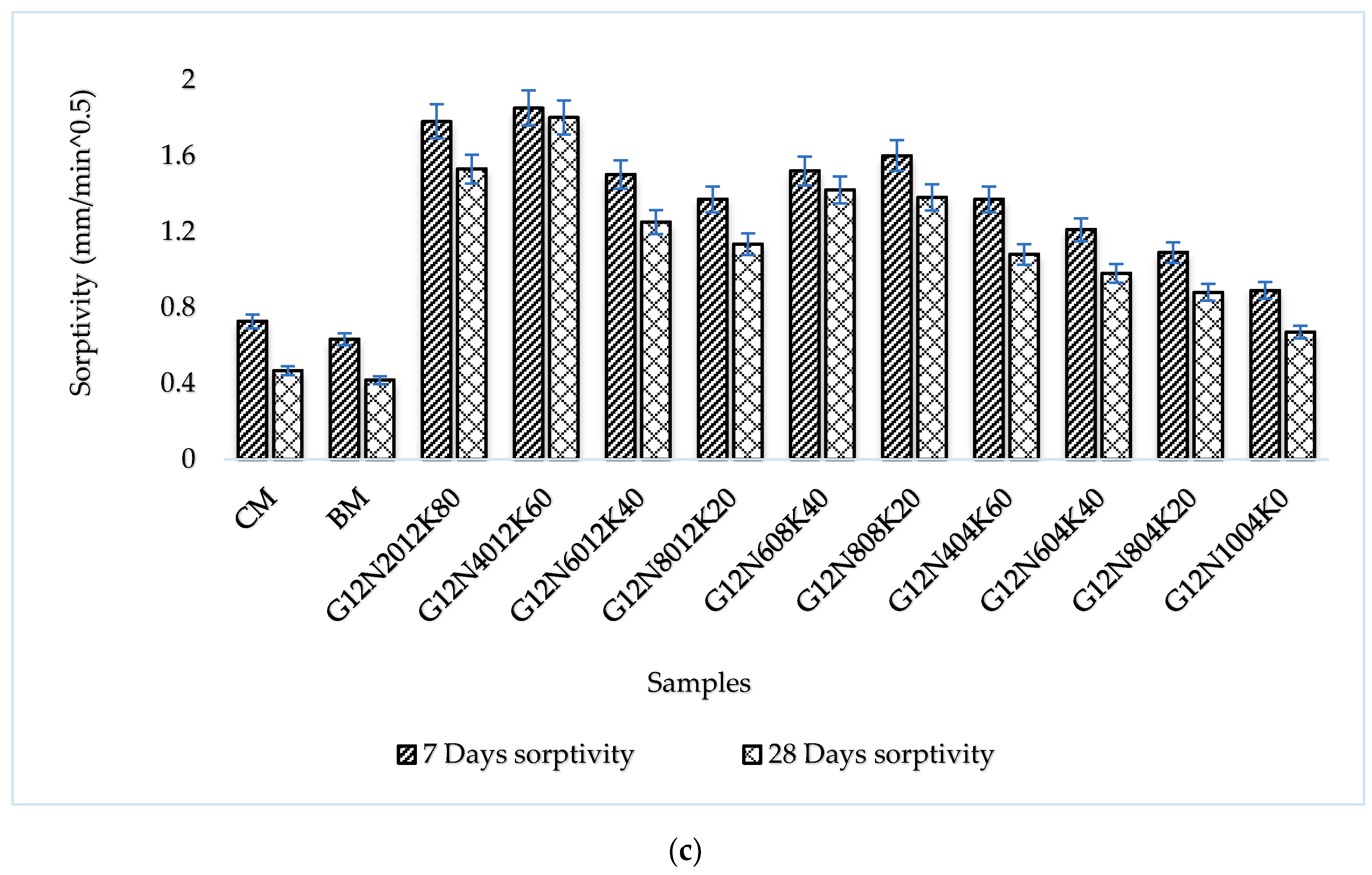
3.3.4. Sorptivity Test
3.3.5. Permeable Porosity Test
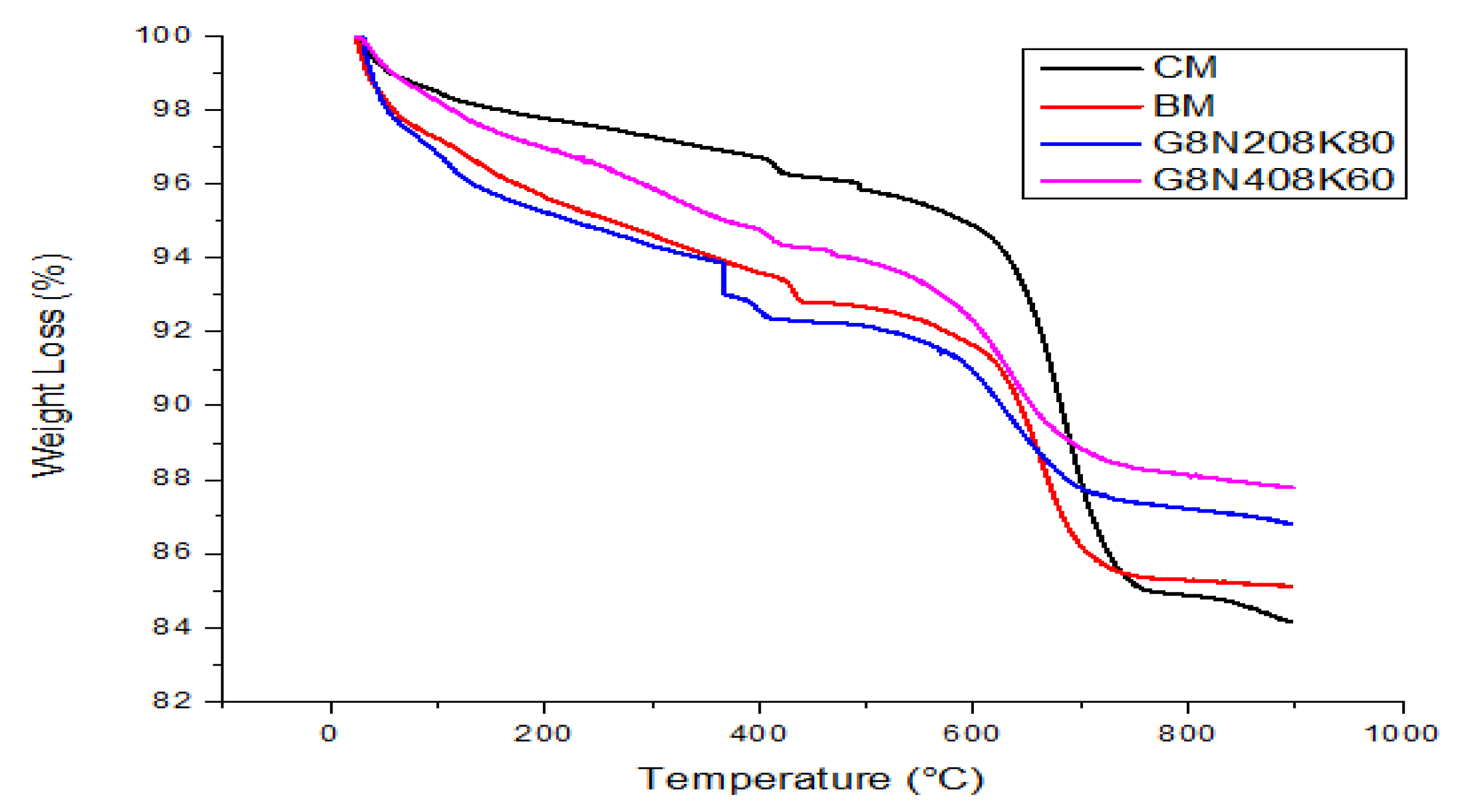
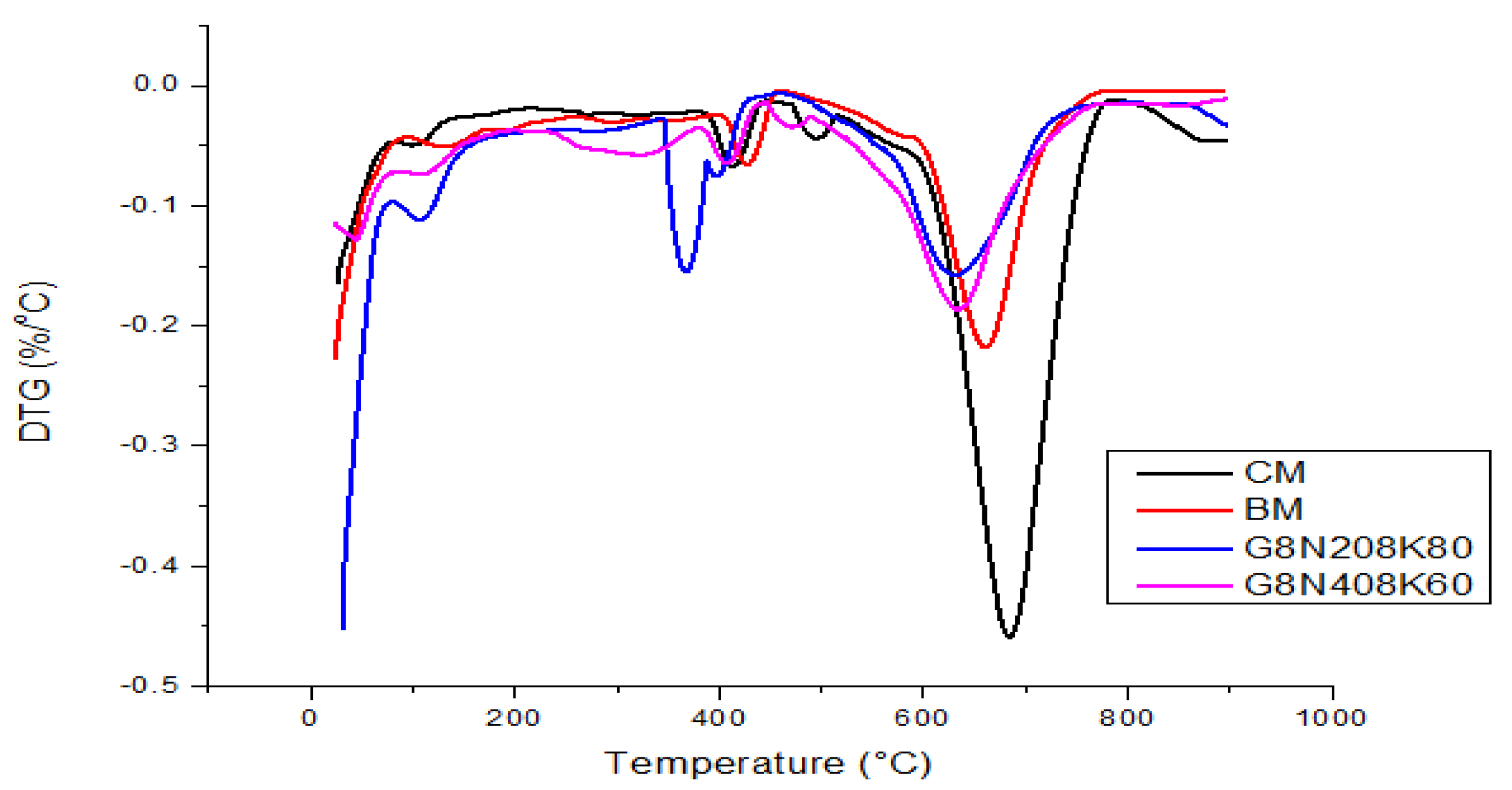
3.3.6. TGA
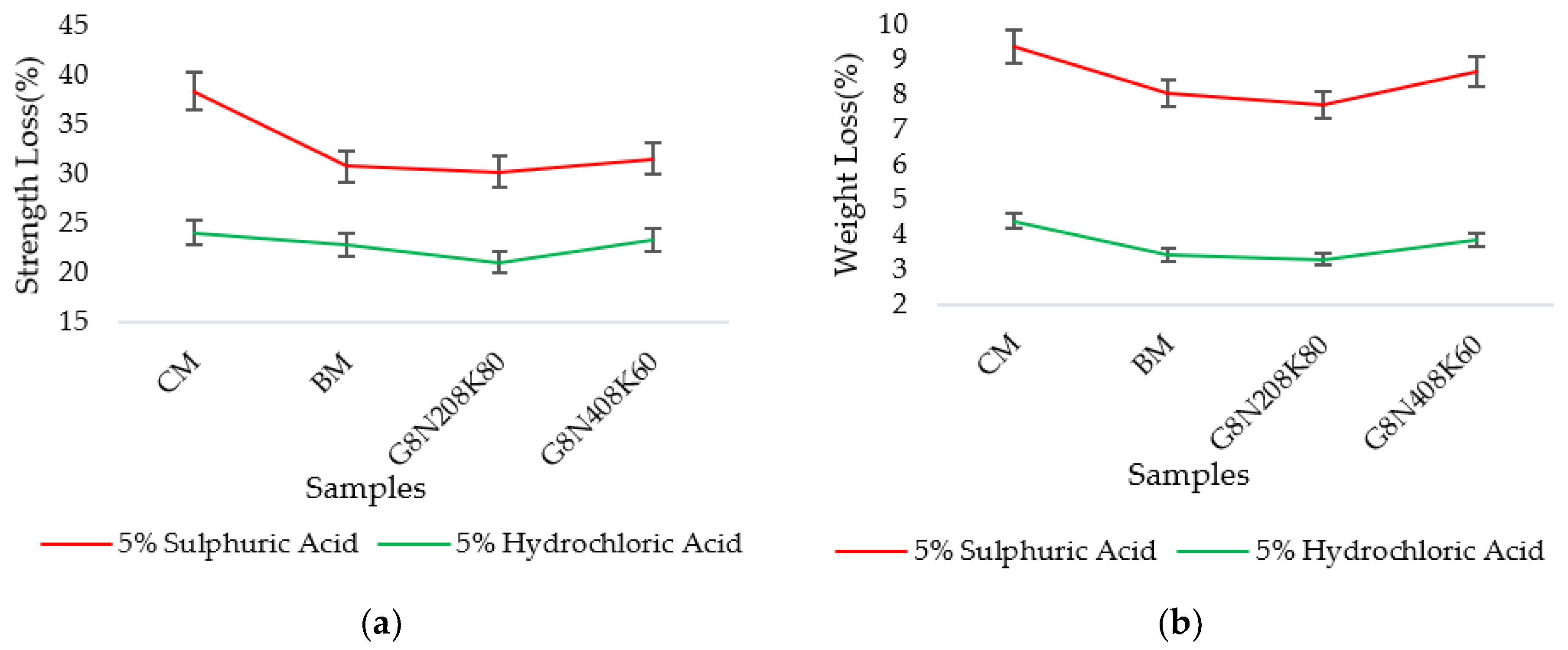
3.3.7. Acid Attack Test
3.3.8. Impact of GPC on Emission Reduction and Global Warming Potential (GWP)
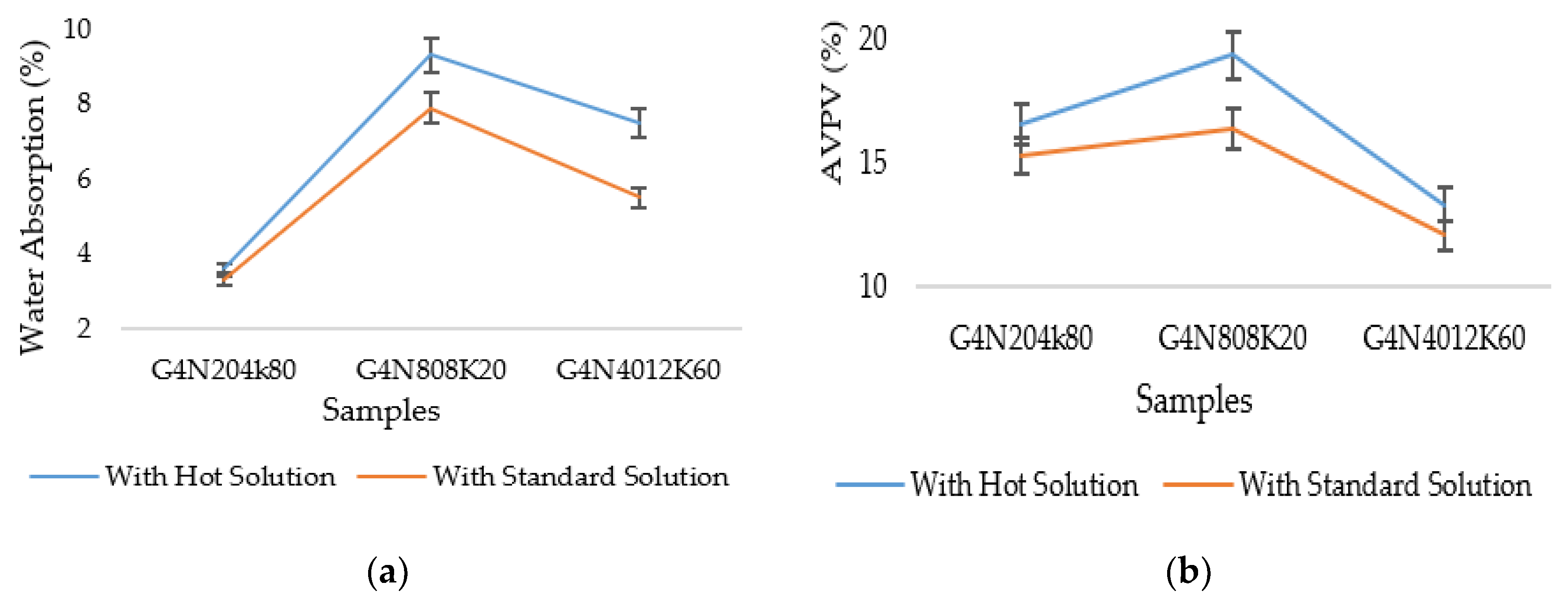
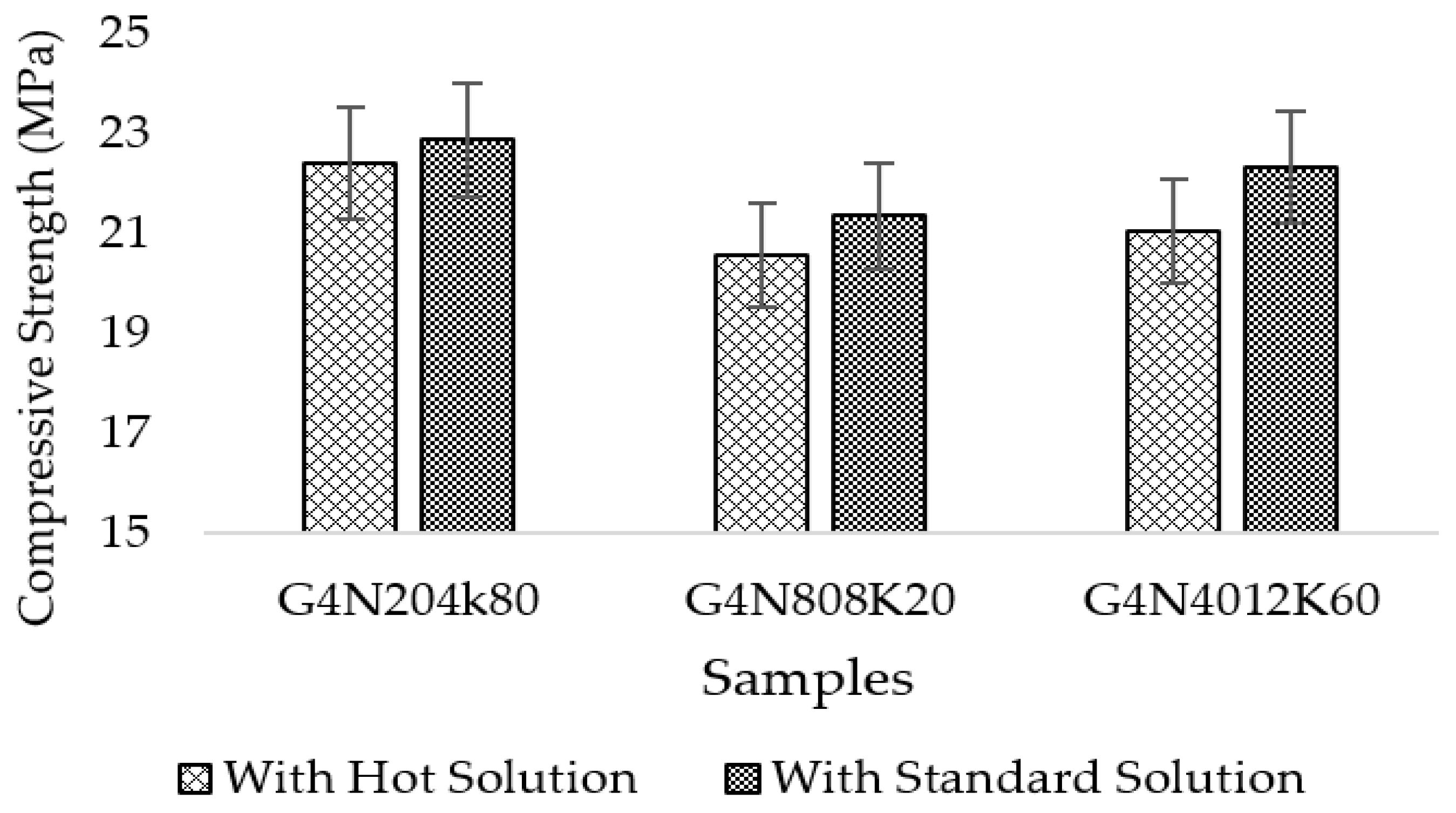
3.4. Temperature Characteristics on Molar Solutions
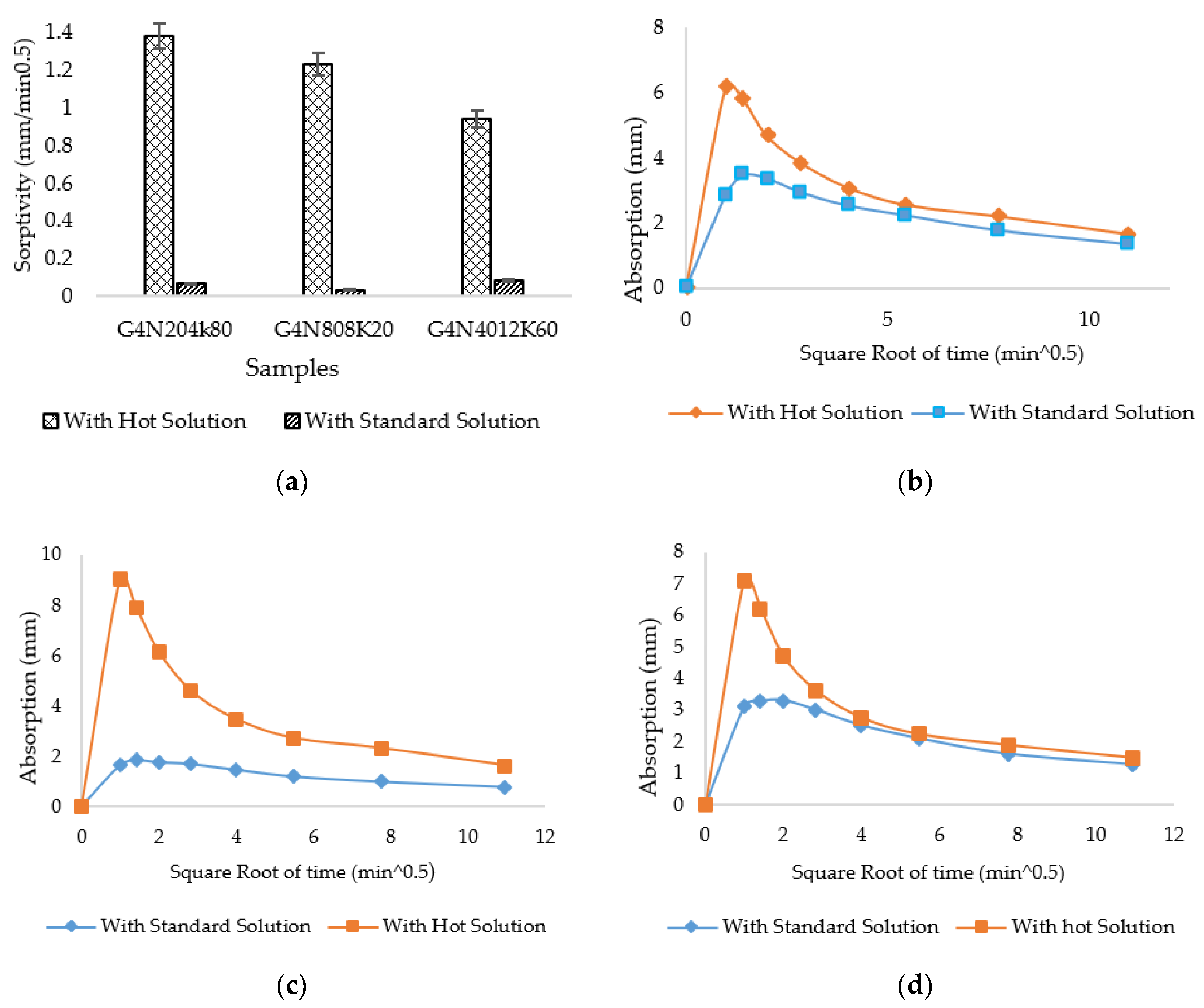
3.4.1. Sorptivity, Water Absorption, and Permeable Porosity Test Results
3.4.2. Compressive Strength Results
4. Conclusions and Recommendation
- An SCBA mixture can be used as both a pozzolan and an aluminosilicate source. From sieving and grinding, the SCBA exhibited better pozzolanic activity, as shown by its Chapelle activity. Likewise, SCBA can be used as an aluminosilicate source containing rich silica, as determined by XRD and XRF.
- The SCBA-based geopolymer cement with a higher molarity ratio had less compressive strength and higher absorption due to the presence of voids and pores caused by a vigorous exothermic reaction during the polymerization process. Furthermore, the SCBA-based geopolymer cement mixtures exhibited greater absorption and sorptivity values than the control mixtures due to the effect of temperature curing. However, the optimum geopolymer cement paste was selected from the 8 M combination solution mixture, at which better mechanical and durable properties were achieved. Thus, the two G8N208K80 and G8N408K60 samples were selected as the optimum samples, and a comparison between them was carried out.
- The SCBA-based geopolymer concrete had a higher workability than the control mixtures due to the effect of the concentration of NaOH and KOH activators. In addition, the water absorption and sorptivity of the SCBA-based geopolymer concrete were higher than the concrete and bagasse-based concrete mixtures due to the presence of voids and pores, as well as the alkali-aggregate effect. However, the durability performance of the geopolymer concrete was significantly better than the control cement and bagasse mixture. Furthermore, compared to the control mixture, the SCBA-based geopolymer concrete achieved a 21% reduction in global warming potential.
Author Contributions
Funding
Conflicts of Interest
References
- Abdullahi, M. Effect of aggregate type on compressive strength of concrete. Int. J. Civ. Struct. Eng. 2012, 2, 782. [Google Scholar] [CrossRef]
- Aprianti, E.; Shafigh, P.; Bahri, S.; Farahani, J.N. Supplementary cementitious materials origin from agricultural wastes—A review. Constr. Build. Mater. 2015, 74, 176–187. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Afghan, S. Sugarcane cultivation in Pakistan; Pakistan Society of Sugar Technologist: Karachi, Pakistan, 2005. [Google Scholar]
- Habert, G.; de Lacaillerie, J.D.E.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Rukzon, S.; Chindaprasirt, P. Utilization of bagasse ash in high-strength concrete. Mater. Des. 2012, 34, 45–50. [Google Scholar] [CrossRef]
- Srivastava, E.S.; Shukla, P.K.; Kumar, K.; Kumar, P. Studies on Partial Replacement of Cement by Bagasse Ash in Concrete. Int. J. 2015, 2, 43–45. [Google Scholar]
- Ganesan, K.; Rajagopal, K.; Thangavel, K. Evaluation of bagasse ash as supplementary cementitious material. Cem. Concr. Compos. 2007, 29, 515–524. [Google Scholar]
- Albitar, M.; Ali, M.; Visintin, P.; Drechsler, M.K. Durability evaluation of geopolymer and conventional concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Elmoaty, A.E.M.A.; Emam, M.A. Factors affecting the mechanical properties of alkali activated ground granulated blast furnace slag concrete. Constr. Build. Mater. 2019, 197, 339–355. [Google Scholar] [CrossRef]
- Vignesh, P.; Vivek, K. An experimental investigation on strength parameters of flyash based geopolymer concrete with GGBS. Int. Res. J. Eng. Technol. 2015, 2, 135–142. [Google Scholar]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Ma, C.K.; Awang, A.Z.; Omar, W. Structural and material performance of geopolymer concrete: A review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Castaldelli, V.N.; Tashima, M.M.; Melges, J.L.P.; Akasaki, J.L.U.I.S.; Monzo, J.; Borrachero, M.V.; Soriano, L.; Payá, J. Preliminary studies on the use of sugar cane bagasse ash (SCBA) in the manufacture of alkali activated binders. Key Eng. Mater. 2014, 600, 689–698. [Google Scholar]
- Bahurudeen, A.; Santhanam, M. Influence of different processing methods on the pozzolanic performance of sugarcane bagasse ash. Cem. Concr. Compos. 2015, 56, 32–45. [Google Scholar] [CrossRef]
- Cordeiro, G.; Filho, R.T.; Fairbairn, E. Effect of calcination temperature on the pozzolanic activity of sugar cane bagasse ash. Constr. Build. Mater. 2009, 23, 3301–3303. [Google Scholar] [CrossRef]
- ASTM-C.136; Standard Test Method for Sieve Analysis of Fine and Coarse Aggregates; American Society for Testing and Materials: West Conshohocken, PA, USA, 2006.
- NF P18 513 Standard Test Method to Find Pozzolanic Activity for Concrete; French Standard Institute: Paris, France, 2012.
- Chandrasekhar, S.; Pramada, P.; Majeed, J. Effect of calcination temperature and heating rate on the optical properties and reactivity of rice husk ash. J. Mater. Sci. 2006, 41, 7926–7933. [Google Scholar] [CrossRef]
- ASTM. “ASTM C39/C39M-18” ‘Standard Test Method to Find out Compressive Strength of Material’; American Society for Testing and Materials: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM C642-97; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete; American Society for Testing and Materials: West Conshohocken, PA, USA, 1997.
- ASTM. ASTM (2013). “C1585-13.” Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes; American Society for Testing and Materials: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM C642-06; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete; American Society for Testing and Materials: West Conshohocken, PA, USA, 2006.
- ASTM-C; ASTM, C. (2005). “ASTM E1131-03.” Standard Test Method for Compositional Analysis by Thermogravimetry; American Society for Testing and Materials: West Conshohocken, PA, USA, 2005.
- ASTM. ASTM (2001). “ASTM C267.” Standard Test Methods for Chemical Resistance of Mortars, Grouts, and Monolithic Surfacings and Polymer Concretes; American Society for Testing and Materials: West Conshohocken, PA, USA, 2001. [Google Scholar]
- Zghair, L.; Frayyeh, Q.; Salman, M. Performance of self-compacting concrete containing pozzolanic materials in aggressive environment. Eng. Technol. J. 2017, 35, 439–444. [Google Scholar]
- Braz, I.G.; Shinzato, M.C.; Montanheiro, T.J.; de Almeida, T.M.; Carvalho, F.M.S. Effect of the addition of aluminum recycling waste on the pozzolanic activity of sugarcane bagasse ash and zeolite. Waste Biomass Valorization 2018, 10, 1–21. [Google Scholar] [CrossRef]
- Govindarajan, D.; Jayalakshmi, G. XRD, FTIR and microstructure studies of calcined Sugarcane Bagasse Ash. Appl. Sci. Res. 2011, 2, 544–549. [Google Scholar]
- Jagadesh, P.; Ramachandramurthy, A.; Murugesan, R.; Sarayu, K. Micro-Analytical studies on sugar cane bagasse ash. Sadhana 2015, 40, 1629–1638. [Google Scholar] [CrossRef]
- Lee, N.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Sant, G.; Kumar, A.; Patapy, C.; le Saout, G.; Scrivener, K. The influence of sodium and potassium hydroxide on volume changes in cementitious materials. Cem. Concr. Res. 2012, 42, 1447–1455. [Google Scholar] [CrossRef]
- Jawed, I.; Skalny, J. Alkalies in cement: A review: II. Effects of alkalies on hydration and performance of Portland cement. Cem. Concr. Res. 1978, 8, 37–51. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Jennings, H.M. Effects of high alkalinity on cement pastes. Mater. J. 2001, 98, 251–255. [Google Scholar]
- Beltzung, F.; Wittmann, F.H. Role of disjoining pressure in cement based materials. Cem. Concr. Res. 2005, 35, 2364–2370. [Google Scholar] [CrossRef]
- Dias, W. Reduction of concrete sorptivity with age through carbonation. C Cem. Concr. Res. 2000, 30, 1255–1261. [Google Scholar] [CrossRef]
- Thokchom, S.; Ghosh, P.; Ghosh, S. Effect of water absorption, porosity and sorptivity on durability of geopolymer mortars. ARPN J. Eng. Appl. Sci. 2009, 4, 28–32. [Google Scholar]
- Matalkah, F.; Salem, T.; Shaafaey, M.; Soroushian, P. Drying shrinkage of alkali activated binders cured at room temperature. Constr. Build. Mater. 2019, 201, 563–570. [Google Scholar] [CrossRef]
- Vijai, K.; Kumutha, R.; Vishnuram, B. Effect of types of curing on strength of geopolymer concrete. Int. J. Phys. Sci. 2010, 5, 1419–1423. [Google Scholar]
- Vora, P.R.; Dave, U.V. Parametric studies on compressive strength of geopolymer concrete. Procedia Eng. 2013, 51, 210–219. [Google Scholar] [CrossRef]
- Sokołowska, J.J. Long-term compressive strength of polymer concrete-like composites with various fillers. Materials 2020, 13, 1207. [Google Scholar] [CrossRef]
- Memon, S.A.; Khan, M.K. Ash blended cement composites: Eco-friendly and sustainable option for utilization of corncob ash. J. Clean. Prod. 2018, 175, 442–455. [Google Scholar] [CrossRef]
- Aldred, J.; Day, J. Is geopolymer concrete a suitable alternative to traditional concrete. In Proceedings of the 37th Conference on Our World in Concrete & Structures, Singapore, 29–31 August 2012. [Google Scholar]
- AS-1530; AS-1530. AS 1530: Methods for fire tests on building materials, componentes and structures. In Australian Standards; Australian Standards: Sydney, Australia, 2005.
- ASTM C-1202; Standard test method for electrical indication of concrete’s ability to resist chloride ion penetration. In American Society of Testing Materials; ASTM: West Conshohocken, PA, USA, 2004.
- Hassan, A.; Arif, M.; Shariq, M. Use of geopolymer concrete for a cleaner and sustainable environment—A review of mechanical properties and microstructure. J. Clean. Prod. 2019, 223, 704–728. [Google Scholar] [CrossRef]
- AS-3600; Concrete structures. In Australian Standard; Australian Standard: Sydney, Australia, 2001.
- Nath, P.; Sarker, P.K. Use of OPC to improve setting and early strength properties of low calcium fly ash geopolymer concrete cured at room temperature. Cem. Concr. Compos. 2015, 55, 205–214. [Google Scholar] [CrossRef]
- Rangan, B.V. Studies on fly ash-based geopolymer concrete. In Proceedings of the World Congress Geopolymer, Saint Quentin, France, 29 June–1 July 2005. [Google Scholar]
- Assi, L.N.; Deaver, E.E.; Ziehl, P. Effect of source and particle size distribution on the mechanical and microstructural properties of fly Ash-Based geopolymer concrete. Constr. Build. Mater. 2018, 167, 372–380. [Google Scholar] [CrossRef]
- Bilal, H.; Yaqub, M.; Rehman, S.K.U.; Abid, M.; Alyousef, R.; Alabduljabbar, H.; Aslam, F. Performance of Foundry Sand Concrete under Ambient and Elevated Temperatures. Materials 2019, 12, 2645. [Google Scholar] [CrossRef]
- Sersale, R.; Sabatelli, V.; Valenti, G. Influence of some retarders on the hydration, at early ages of tricalciumaluminate. Proceeding 7th Intern. Congr. Chem. Cem. 1980, 4, 547–551. [Google Scholar]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Singh, L.; Agarwal, S.K.; Bhattacharyya, S.K.; Sharma, U.; Ahalawat, S. Preparation of silica nanoparticles and its beneficial role in cementitious materials. Nanomater. Nanotechnol. 2011, 1, 9. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Shadnia, R.; Zhang, L.; Li, P. Experimental study of geopolymer mortar with incorporated PCM. Constr. Build. Mater. 2015, 84, 95–102. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Lee, M.; Chen-Tan, N.; van Riessen, A. Characterisation of class F fly ash geopolymer pastes immersed in acid and alkaline solutions. Cem. Concr. Compos. 2011, 33, 1086–1091. [Google Scholar] [CrossRef]
- Rawal, P.; Nimityongskul, P. Evaluation of water permeability and acid attack of concrete incorporating high volume replacement of cement by fly ash and rice husk ash. 2004; Typescript. [Google Scholar]
- International Organization for Standardization. In ISO 14040: Environmental Management-Life Cycle Assessment-Principles and Framework; International Organization for Standardization: Geneva, Switzerland, 1997.
- Abbas, R.; Khereby, M.A.; Ghorab, H.Y.; Elkhoshkhany, N. Preparation of geopolymer concrete using Egyptian kaolin clay and the study of its environmental effects and economic cost. Clean Technol. Environ. Policy 2020, 22, 1–19. [Google Scholar] [CrossRef]




| Component | Content (%) |
|---|---|
| CaO | 63.34 |
| SiO2 | 20.82 |
| Al2O3 | 5.32 |
| Fe2O3 | 3.27 |
| MgO | 2.56 |
| SO3 | 2.31 |
| K2O | 0.98 |
| Na2O | 0.18 |
| Loss of Ignition (LOI) | 1.22 |
| Property | Fine Aggregate | Coarse Aggregate |
|---|---|---|
| Unit Weight (kg/m3) | 1730 | 1610 |
| Specific Gravity | 2.62 | 2.65 |
| Water Absorption (%) | 0.66 | 0.74 |
| Fineness Modulus | 2.5 | - |
| Mix | Activator to Binder Ratio (A/B) | Binder Content (g/cm3) | Cement (g/cm3) | Bagasse Ash (g/cm3) | Activator Content (g/m3) | NaOH (g/cm3) | KOH (g/cm3) |
|---|---|---|---|---|---|---|---|
| GxN0xk100 | 0.5 | 910 | 728 | 182 | 455 | 0 | 455 |
| GxN20xk80 | 0.5 | 910 | 728 | 182 | 455 | 91 | 364 |
| GxN40xk60 | 0.5 | 910 | 728 | 182 | 455 | 182 | 273 |
| GxN60xk40 | 0.5 | 910 | 728 | 182 | 455 | 273 | 182 |
| GxN80xk20 | 0.5 | 910 | 728 | 182 | 455 | 364 | 91 |
| GxN100xk0 | 0.5 | 910 | 728 | 182 | 455 | 455 | 0 |
| Mix | Activator to Binder Ratio (A/B) | Binder Content (kg/m3) | Cement (kg/m3) | Bagasse Ash (kg/m3) | Coarse Aggregate (kg/m3) | Fine Aggregate (kg/m3) | Activator Content (kg/m3) | NaOH (kg/m3) | KOH (kg/m3) |
|---|---|---|---|---|---|---|---|---|---|
| GxN0xk100 | 0.5 | 410 | 328 | 82 | 990 | 735 | 222 | 0 | 222 |
| GxN20xk80 | 0.5 | 410 | 328 | 82 | 990 | 735 | 222 | 44.4 | 177.6 |
| GxN40xk60 | 0.5 | 410 | 328 | 82 | 990 | 735 | 222 | 88.8 | 133.2 |
| GxN60xk40 | 0.5 | 410 | 328 | 82 | 990 | 735 | 222 | 133.2 | 88.8 |
| GxN80xk20 | 0.5 | 410 | 328 | 82 | 990 | 735 | 222 | 177.6 | 44.4 |
| GxN100xk0 | 0.5 | 410 | 328 | 82 | 990 | 735 | 222 | 222 | 0 |
| Titration Volume of HCl for Control Mixture (mL) (V1) | Titration Volume of HCL for Ash Sample (mL) (V2) | Chapelle Activity = CaO (mg/gm) = [(V1 − V2)/V1] * 2642.86 | Percent Increase w.r.t Standard (%) |
|---|---|---|---|
| 9.75 | 4.75 | 1355.31 | 310.7 |
| Composition | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | Na2O | P2O5 | MnO |
|---|---|---|---|---|---|---|---|---|---|
| Percentage (%) | 71.3 | 14.22 | 0.35 | 3.92 | 3.8 | 2.88 | 2.31 | 1.08 | 0.02 |
| Sample Name | W/C Ratio | Slump (mm) |
|---|---|---|
| CM | 0.5 | 91.44 |
| BM | 0.5 | 97.27 |
| G8N208K80 | 0.5 | 104.20 |
| G8N408K60 | 0.5 | 101.60 |
| Mixtures | Sulphuric Acid (H2SO4) | Hydrochloric Acid (HCl) | ||||
|---|---|---|---|---|---|---|
| Strength (P1) Before Test (MPa) | Strength (P2) After Test (MPa) | Strength Loss (%) | Strength (P1) Before Test (MPa) | Strength (P2) After Test (MPa) | Strength Loss (%) | |
| CM | 21.5 | 13.23 | 38.46 | 21.5 | 16.31 | 24.14 |
| BM | 20.3 | 13.85 | 30.85 | 20.3 | 15.45 | 22.87 |
| G8N208K80 | 15.01 | 10.46 | 30.31 | 15.01 | 11.84 | 21.11 |
| G8N408K60 | 13.5 | 9.23 | 31.63 | 13.5 | 10.34 | 23.4 |
| Mixtures | Sulphuric Acid(H2SO4) | Hydrochloric Acid(HCl) | ||||
|---|---|---|---|---|---|---|
| Weight (W1) Before Test (gm) | Weight (W2) After Test (gm) | Weight Loss (%) | Weight (W1) Before Test (gm) | Weight (W2) After Test (gm) | Weight Loss (%) | |
| CM | 8340 | 7560 | 9.35 | 8240 | 7880 | 4.37 |
| BM | 8340 | 7670 | 8.03 | 8500 | 8210 | 3.41 |
| G8N208K80 | 8320 | 7680 | 7.69 | 8160 | 7893 | 3.27 |
| G8N408K60 | 7980 | 7290 | 8.64 | 8100 | 7790 | 3.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, S.K.U.; Imtiaz, L.; Aslam, F.; Khan, M.K.; Haseeb, M.; Javed, M.F.; Alyousef, R.; Alabduljabbar, H. Experimental Investigation of NaOH and KOH Mixture in SCBA-Based Geopolymer Cement Composite. Materials 2020, 13, 3437. https://doi.org/10.3390/ma13153437
Rehman SKU, Imtiaz L, Aslam F, Khan MK, Haseeb M, Javed MF, Alyousef R, Alabduljabbar H. Experimental Investigation of NaOH and KOH Mixture in SCBA-Based Geopolymer Cement Composite. Materials. 2020; 13(15):3437. https://doi.org/10.3390/ma13153437
Chicago/Turabian StyleRehman, Sardar Kashif Ur, Lahiba Imtiaz, Fahid Aslam, Muhammad Khizar Khan, Muhammad Haseeb, Muhammad Faisal Javed, Rayed Alyousef, and Hisham Alabduljabbar. 2020. "Experimental Investigation of NaOH and KOH Mixture in SCBA-Based Geopolymer Cement Composite" Materials 13, no. 15: 3437. https://doi.org/10.3390/ma13153437
APA StyleRehman, S. K. U., Imtiaz, L., Aslam, F., Khan, M. K., Haseeb, M., Javed, M. F., Alyousef, R., & Alabduljabbar, H. (2020). Experimental Investigation of NaOH and KOH Mixture in SCBA-Based Geopolymer Cement Composite. Materials, 13(15), 3437. https://doi.org/10.3390/ma13153437