A Systematic Study on Polymer-Modified Alkali-Activated Slag–Part II: From Hydration to Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Polymer Addition Method
2.2.2. Sample Preparation
2.3. Methods
2.3.1. Calorimetry
2.3.2. X-ray Diffraction Experiment (XRD)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Mercury Intrusion Porosimetry (MIP)
2.3.5. Scanning Electron Microscope (SEM)
2.3.6. Mechanical Measurement
3. Results
3.1. Effects of Polymer Addition Methods
3.2. Hydration and Hydration Products
3.3. Microstructure Observation
3.4. Mechanical Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, C. Alkali-Activated Cements and Concretes: Theory and Application; Taylor & Francis: London, UK; New York, NY, USA, 2006. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. Alkali Activated Materials, State-of-the-Art Report, RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2014. [Google Scholar]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Alkali Activated Materials: State-of-the-Art Report; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Zhang, Y.R.; Kong, X.M.; Lu, Z.B.; Lu, Z.C.; Hou, S.S. Effect of the charge characteristic of the backbone of polycarboxylate superplasticizer on the adsorption and the retardation in cement pastes. Cem. Concr. Res. 2015, 67, 184–196. [Google Scholar] [CrossRef]
- Yoshioka, K.; Tazawa, E.; Kawai, K.; Enohata, T. Adsorption characteristics of superplasticizers on cement component minerals. Cem. Concr. Res. 2002, 32, 1507–1513. [Google Scholar] [CrossRef]
- Land, G.; Stephen, D. The influence of nano-silica on the hydration of ordinary Portland cement. J. Mater. Sci. 2012, 47, 1011–1017. [Google Scholar] [CrossRef]
- Alizadeh, R.; Raki, L.; Makar, J.; Beaudoin, J.J.; Moudrakovski, L. Hydration of tricalcium silicate in the presence of synthetic calcium–silicate–hydrate. J. Mater. Chem. 2009, 42, 7937–7946. [Google Scholar] [CrossRef] [Green Version]
- Collepardi, M.; Borsoi, A.; Collepardi, S.; Jacob, J.; Olagot, O.; Troli, R. Effects of shrinkage reducing admixture in shrinkage compensating concrete under non-wet curing conditions. Cem. Concr. Compos. 2005, 27, 704–708. [Google Scholar] [CrossRef]
- Palacios, M.; Houst, Y.F.; Bowen, P.; Puertas, F. Adsorption of superplasticizer admixtures on alkali-activated slag pastes. Cem. Concr. Res. 2009, 39, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Palacios, M.; Puertas, F. Effect of superplasticizer and shrinkage-reducing admixtures on alkali-activated slag pastes and mortars. Cem. Concr. Res. 2005, 35, 1358–1367. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J. Effect of different superplasticizers and activator combinations on workability and strength of fly ash based geopolymer. Mater. Des. 2014, 57, 667–672. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of shrinkage-reducing admixtures on the properties of alkali-activated slag mortars and pastes. Cem. Concr. Res. 2007, 37, 691–702. [Google Scholar] [CrossRef]
- Rattanasak, U.; Pankhet, K.; Chindaprasirt, P. Effect of chemical admixtures on properties of high-calcium fly ash geopolymer. Int. J. Miner. Metall. Mater. 2011, 18, 364–369. [Google Scholar] [CrossRef]
- Bilim, C.; Karahan, O.; Atisß, C.D.; Ilkentapar, S. Influence of admixtures on the properties of alkali-activated slag mortars subjected to different curing conditions. Mater. Des. 2013, 44, 540–547. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymer and other alkali activated materials: Why, how and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Pang, X.Y.; Jimenez, W.C.; Iverson, B.J. Hydration kinetics modelling of the effect of curing temperature and pressure on the heat evolution of oil well cement. Cem. Concr. Res. 2013, 54, 69–76. [Google Scholar] [CrossRef]
- Ray, I.; Gupta, A.P.; Biswas, M. Effect of latex and superplasticiser on Portland cement mortar in the hardened state. Cem. Concr. Compos. 1995, 17, 9–21. [Google Scholar] [CrossRef]
- Saija, L.M. Waterproofing of Portland cement mortars with a specially designed polyacrylic latex. Cem. Concr. Res. 1995, 25, 503–509. [Google Scholar] [CrossRef]
- Ribeiro, D.B.; Pinto, E.N.M.G.; Melo, D.M.A.; Martinelli, A.E.; Araújo, R.G.S. A study of compressive strength of the geopolymerics pastes additivated with nonionic latex. In Proceedings of the 11th International Conference on Advanced Materials, Rio da Janeiro, Brazil, 20–25 September 2009. [Google Scholar]
- Lee, N.K.; Kim, E.M.; Lee, H.K. Mechanical properties and setting characteristics of geopolymer mortar using styrene-butadiene (SB) latex. Constr. Build. Mater. 2016, 113, 264–272. [Google Scholar] [CrossRef]
- Kusbiantoro, A.; Rahman, N.; Chin, S.C.; Ridho, B.A. Effect of Poly(Ethylene-co-Vinyl Acetate) as a Self-Healing Agent in Geopolymer Exposed to Various Curing Temperatures. Mater. Sci. Forum 2016, 841, 16–20. [Google Scholar] [CrossRef]
- El-Yamany, H.E.; El-Salamawy, M.A.; El-Assal, N.T. Microstructure and mechanical properties of alkali-activated slag mortar modified with latex. Constr. Build. Mater. 2018, 191, 32–38. [Google Scholar] [CrossRef]
- Lu, Z.C.; Merkl, J.P.; Schmidtke, C.; Pulkin, M.; Deschner, F.; Wache, S.; Jin, Y.; Stephan, D. A systematic study on polymer modified alkali-activated slag—Part I: Stability analysis of colloidal polymer dispersion in sodium water glass. Constr. Build. Mater. 2019, 221, 40–49. [Google Scholar] [CrossRef]
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Ye, H.L.; Radlinska, A. Shrinkage mechanism of alkali-activated slag. Cem. Concr. Res. 2016, 88, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Salmas, C.; Androutsopoulos, G.; Porosimetry, M. Contact angle hysteresis of materials with controlled pore structure. J. Colloid. Interface Sci. 2001, 239, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Aligizaki, K. Pore Structure of Cement-Based Materials: Testing, Interpretation and Requirements; Taylor & Francis: Abingdon, UK; New York, NY, USA, 2006. [Google Scholar]
- Juenger, M.C.G.; Jennings, H.M. The use of nitrogen adsorption to assess of the microstructure of cement paste. Cem. Concr. Res. 2001, 31, 883–892. [Google Scholar] [CrossRef]
- Choi, Y.C.; Kim, J.; Choi, S. Mercury intrusion porosimetry characterization of micropore structures of high-strength cement pastes incorporating high volume ground granulated blast-furnace slag. Constr. Build. Mater. 2017, 137, 96–103. [Google Scholar] [CrossRef]
- Diamond, S. Mercury porosimetry—An inappropriate method for the measurement of pore size distributions in cement-based materials. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Diamond, S. A discussion of the paper ‘‘Effect of drying on cement-based materials pore structure as identified by mercury porosimetry—A comparative study between oven, vacuum, and freeze-drying’’ by C. Gallé. Cem. Concr. Res. 2003, 33, 169–170. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Yang, R.; Zhang, Y.; Jiang, L.; Wang, Z.M.; Wang, Q.C.; Liu, W.; Zeng, M.; Zhou, S.M.; et al. Oil Swellable Polymer Modified Cement Paste: Expansion and Crack Healing Upon Oil Absorption. Constr. Build. Mater. 2016, 114, 98–108. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, X.; Lu, Z.; Jansen, D.; Pakusch, J.; Wang, S. Pore structure of hardened cement paste containing colloidal polymers with varied glass transition temperature and surface charges. Cem. Concr. Compos. 2019, 95, 154–168. [Google Scholar] [CrossRef]
- Kaufmann, J.; Loser, R.; Leemann, A. Analysis of cement-bonded materials by multi-cycle mercury intrusion and nitrogen sorption. J. Colloid. Interface Sci. 2009, 336, 730–737. [Google Scholar] [CrossRef]
- Kong, X.; Emmerling, S.; Pakusch, J.; Rueckel, M.; Nieberle, J. Retardation effect of styrene-acrylate copolymer latexes on cement hydration. Cem. Concr. Res. 2015, 75, 23–41. [Google Scholar] [CrossRef]
- Krizan, D.; Zivanovic, B. Effects of dosage and modulus of water glass on early hydration of alkali–slag cements. Cem. Concr. Res. 2002, 32, 1181–1188. [Google Scholar] [CrossRef]
- Ben Haha, M.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part II: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Fernilndez-Jimhez, A.; Puertas, F. Alkali-activated slag cements: Kinetic studies. Cem. Concr. Res. 1997, 27, 359–368. [Google Scholar] [CrossRef]
- Fernilndez-Jimhez, A.; Puertas, F.; Arteaga, A. Determination of Kinetic Equations of Alkaline Activation of Blast Furnace Slag by Means of Calorimetric Data. J. Therm. Anal. Calorim. 1998, 52, 945–955. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.J.; Peethamparan, S. Temperature and activator effect on early-age reaction kinetics of alkali-activated slag binders. Constr. Build. Mater. 2016, 113, 783–793. [Google Scholar] [CrossRef] [Green Version]
- Zivica, V. Effects of type and dosage of alkaline activator and temperature on the properties of alkali-activated slag mixtures. Constr. Build. Mater. 2007, 21, 1463–1469. [Google Scholar] [CrossRef]
- Haha, M.B.; Saout, G.L.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Knapen, E.; Cizer, O.; Balen, K.V.; Gemert, D.V. Effect of free water removal from early-age hydrated cement pastes on thermal analysis. Constr. Build. Mater. 2009, 23, 3431–3438. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Gruskovnjak, A.; Lothenbach, B.; Winnefeld, F.; Münch, B.; Figi, R.; Ko, S.; Adler, M.; Mäder, U. Quantification of hydration phases in supersulphated cements: Review and new approaches. Adv. Cem. Res. 2011, 23, 265–275. [Google Scholar] [CrossRef]
- Walkley, B.; Nicolas, R.S.; Sani, M.; Rees, G.J.; Hanna, J.V.; van Deventer, J.S.J.; Provis, J.L. Phase evolution of C-(N)-A-S-H/N-A-S-H gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 2016, 89, 120–135. [Google Scholar] [CrossRef] [Green Version]
- Cruskovnjak, A.; Lothenbach, B.; Holzer, L.; Figi, R.; Winnefeld, F. Hydration of alkali-activated slag: Comparison with ordinary Portland cement. Adv. Cem. Res. 2006, 18, 119–128. [Google Scholar] [CrossRef]
- Li, L.; Wang, R.; Lu, Q. Influence of polymer latex on the setting time, mechanical properties and durability of calcium sulfoaluminate cement mortar. Constr. Build. Mater. 2018, 169, 911–922. [Google Scholar] [CrossRef]
- Puertas, F.; Palomo, A.; Fernández-Jiménez, A.; Izquierdo, J.D.; Granizo, M.L. Effect of superplasticisers on the behaviour and properties of alkaline cements. Adv. Cem. Res. 2003, 15, 23–28. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Tzevelekou, T.; Lampropoulou, P.; Giannakopoulou, P.P.; Rogkala, A.; Koutsovitis, P.; Koukouzas, N.; Petrounias, P. Valorization of Slags Produced by Smelting of Metallurgical Dusts and Lateritic Ore Fines in Manufacturing of Slag Cements. Appl. Sci. 2020, 10, 4670. [Google Scholar] [CrossRef]
- Jin, Y.; Stephan, D. The unusual solidification process of alkali activated slag and its relationship with the glass structure of the slag. Cem. Concr. Res. 2019, 121, 1–10. [Google Scholar] [CrossRef]
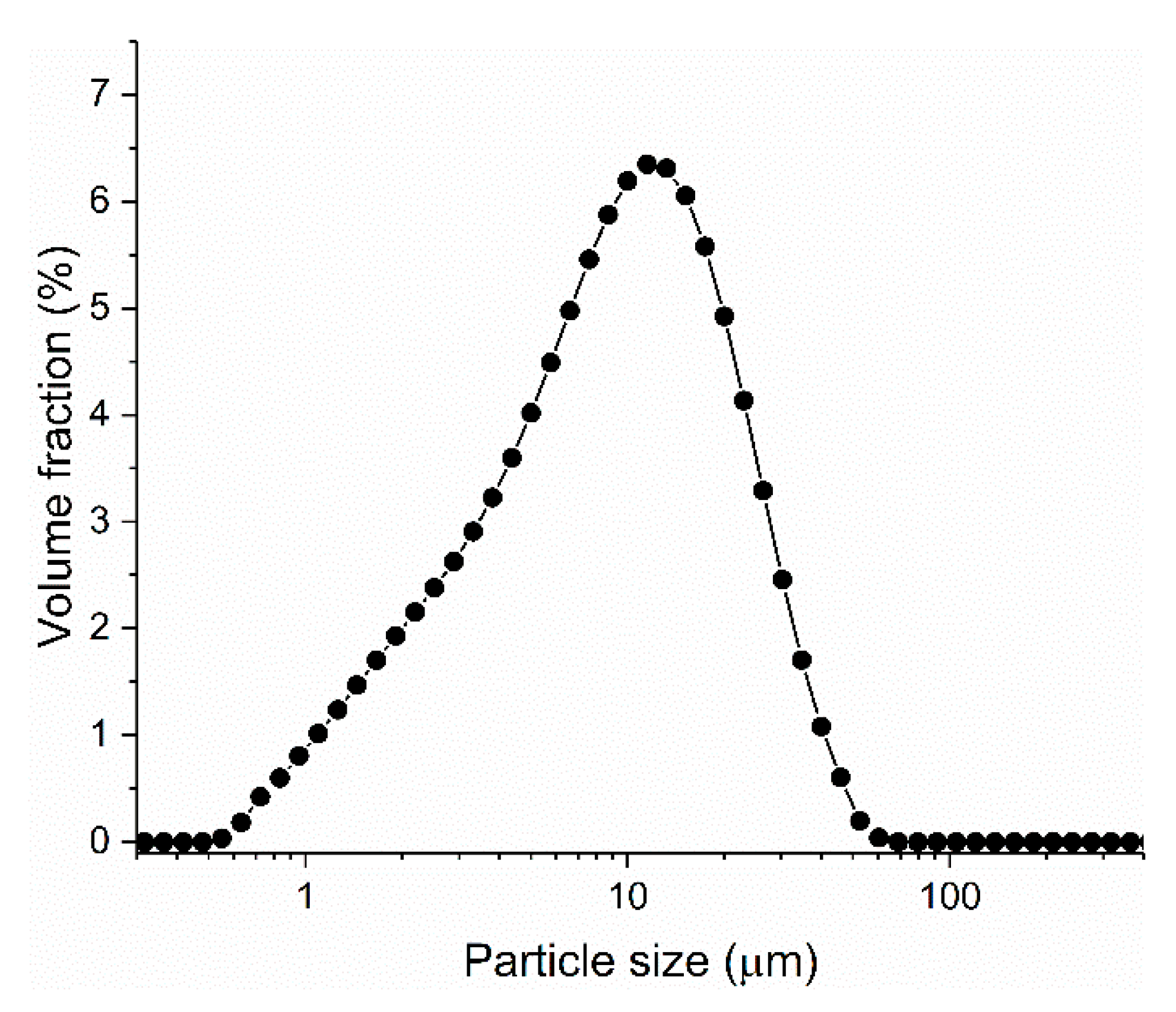
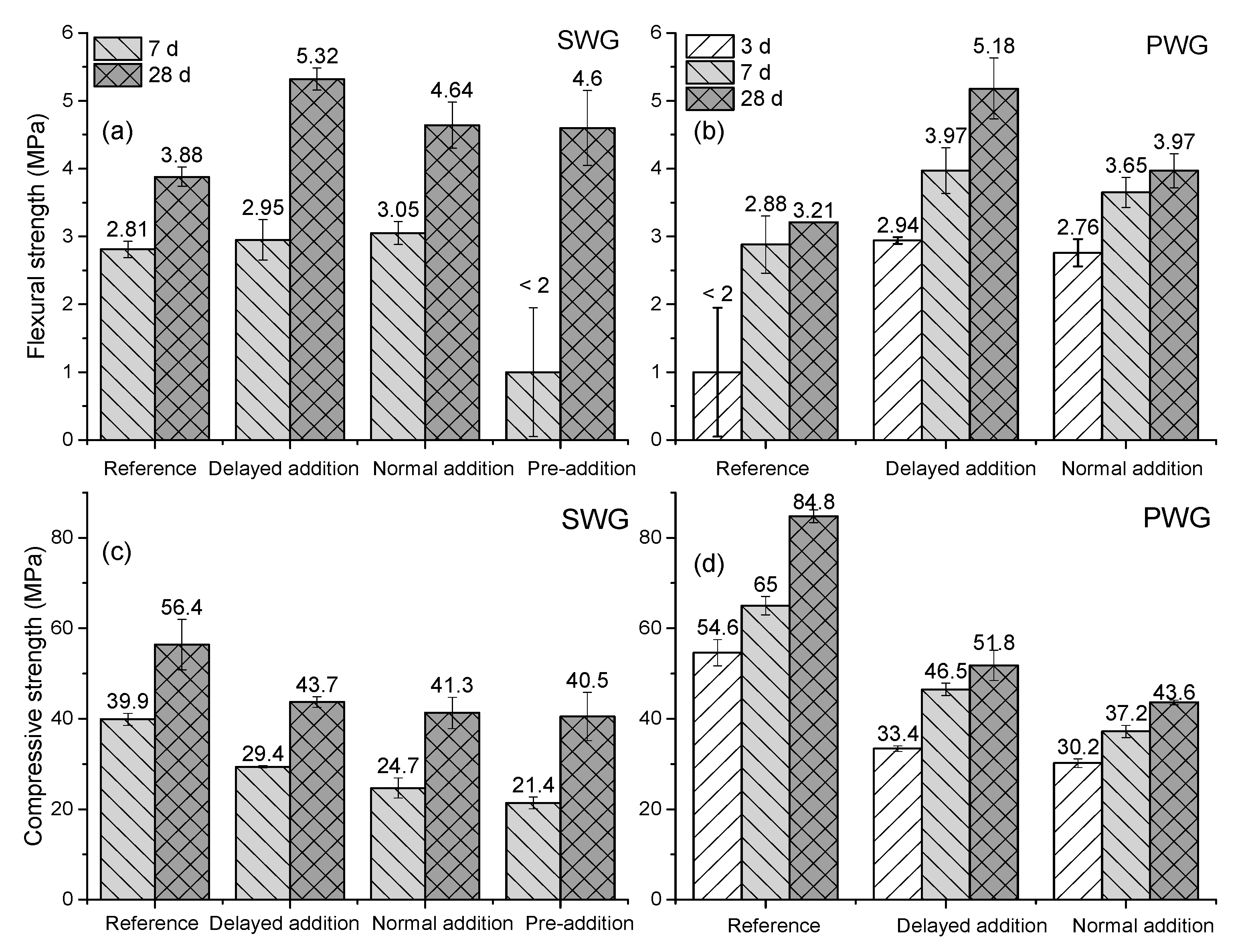

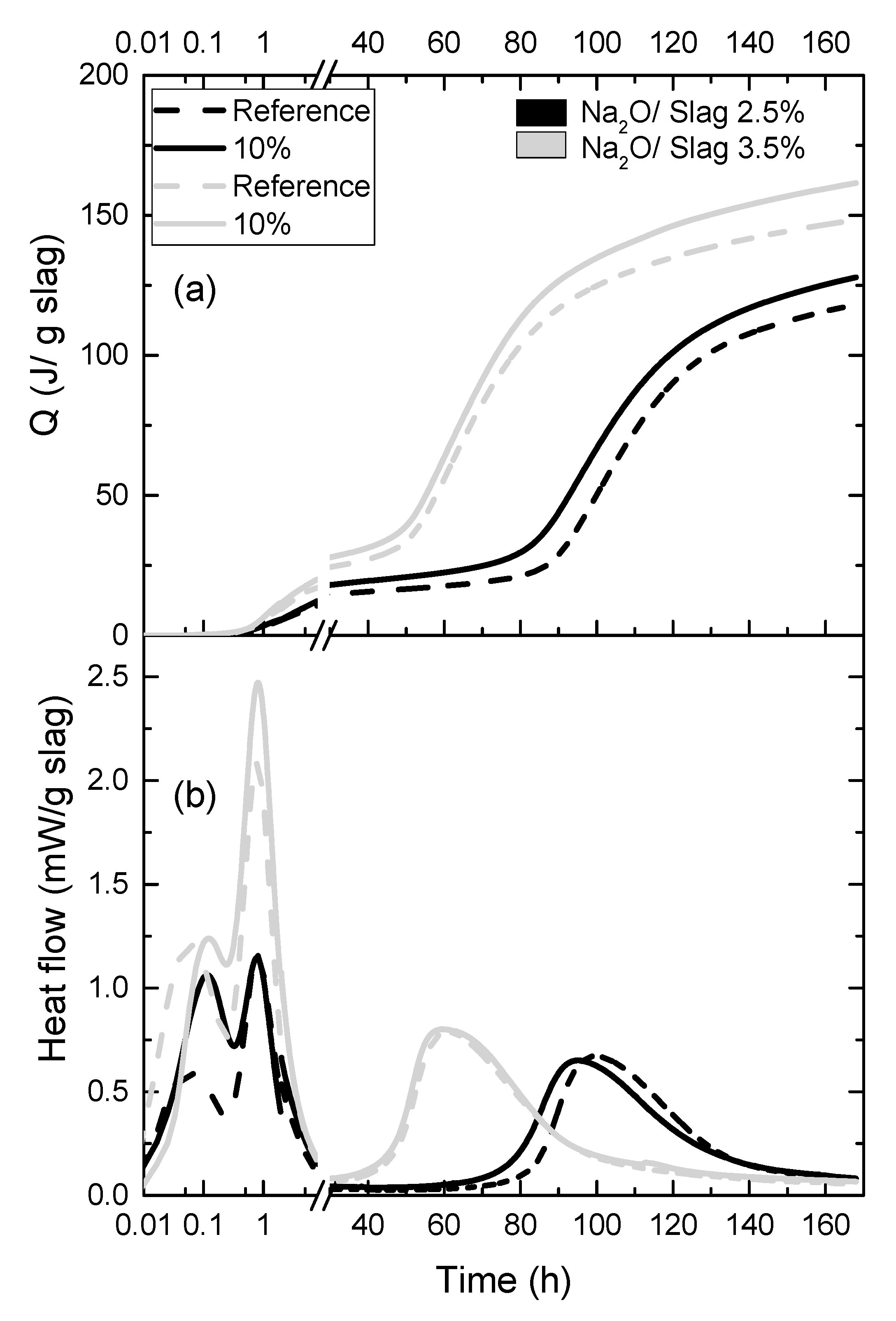

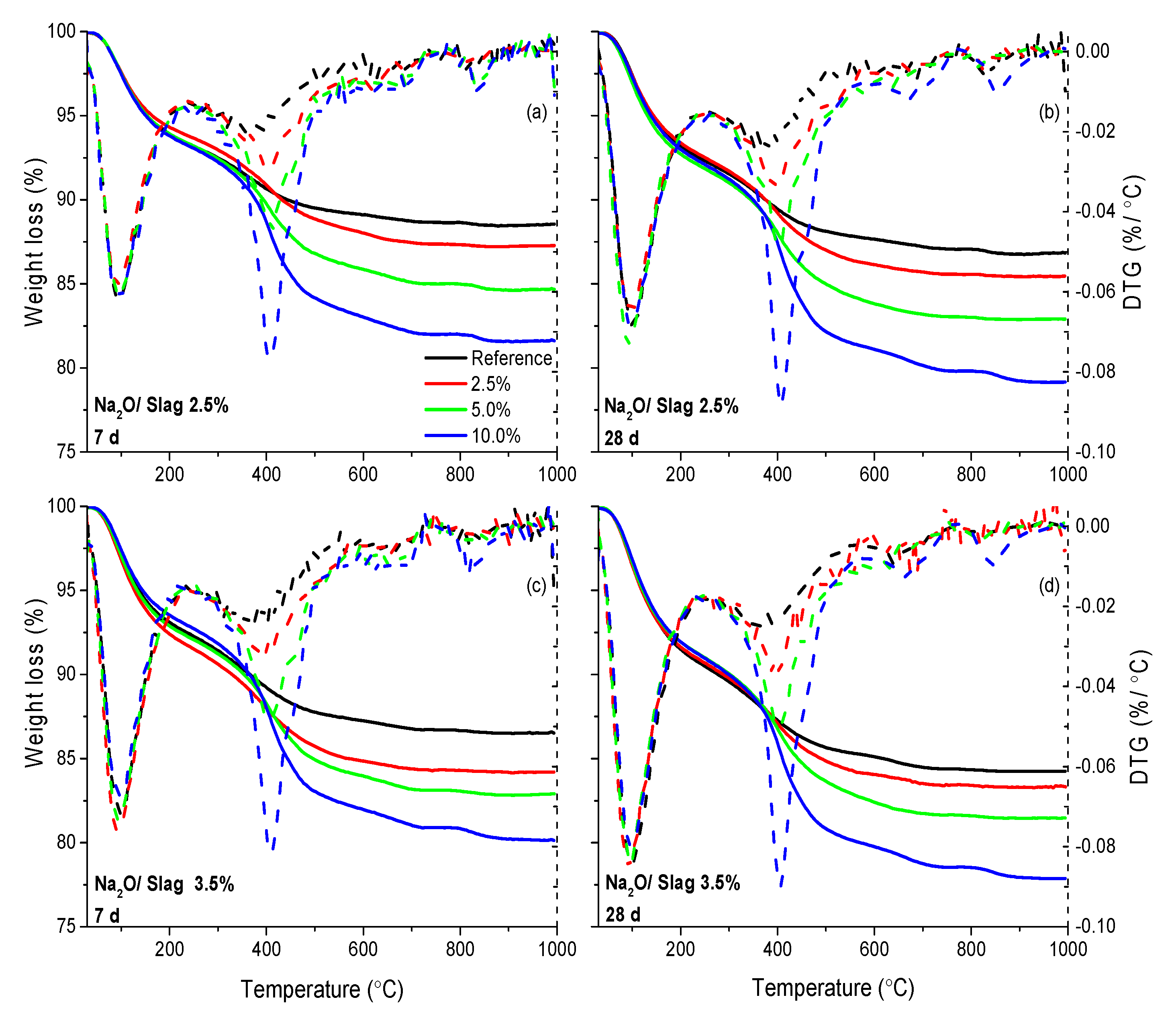
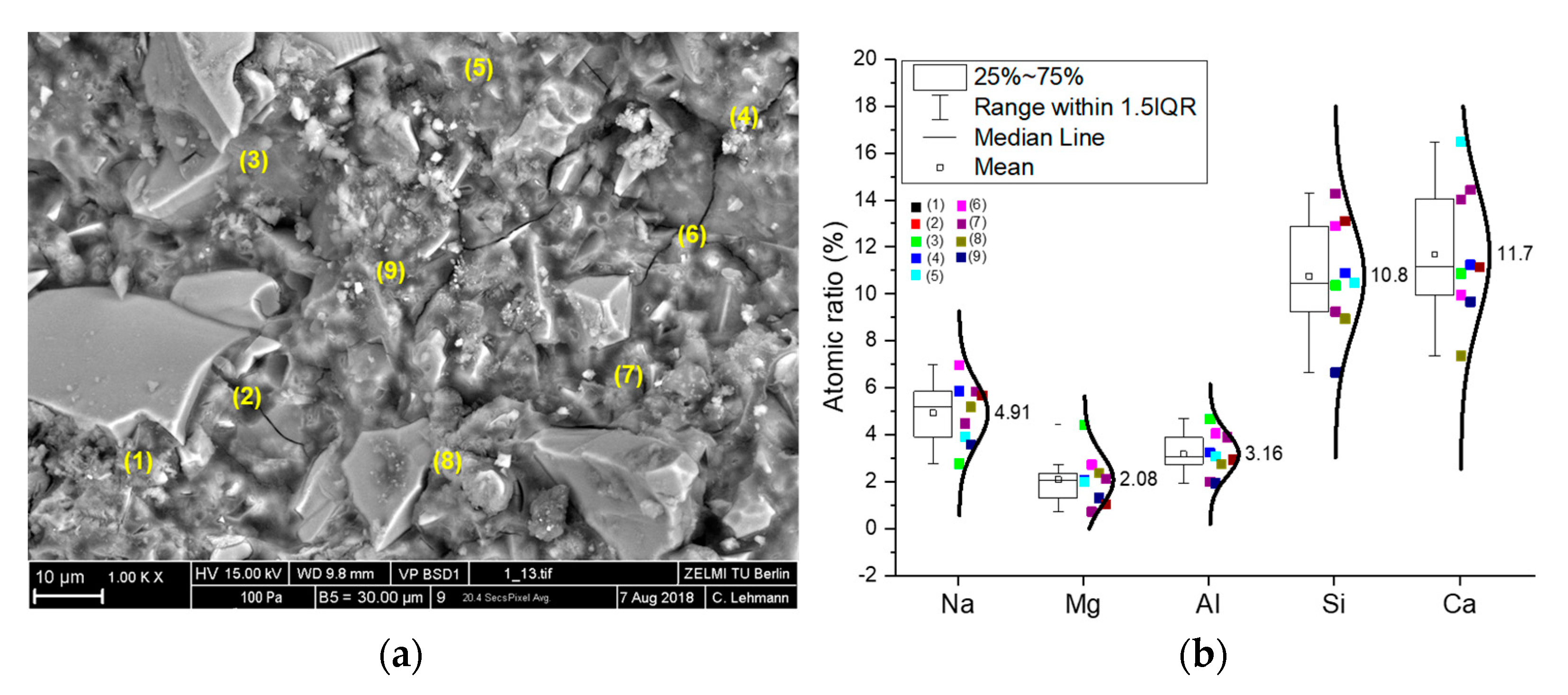
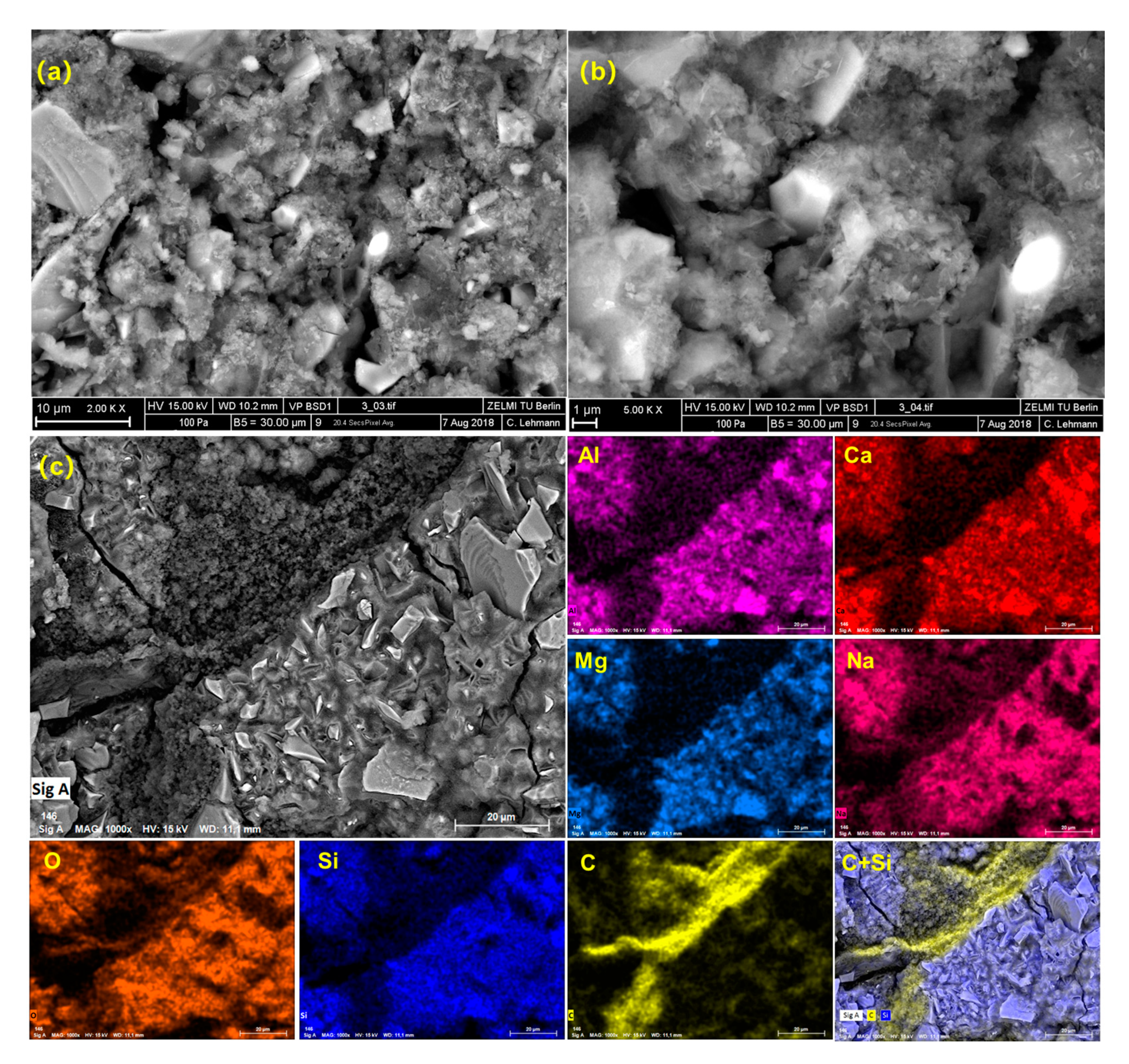
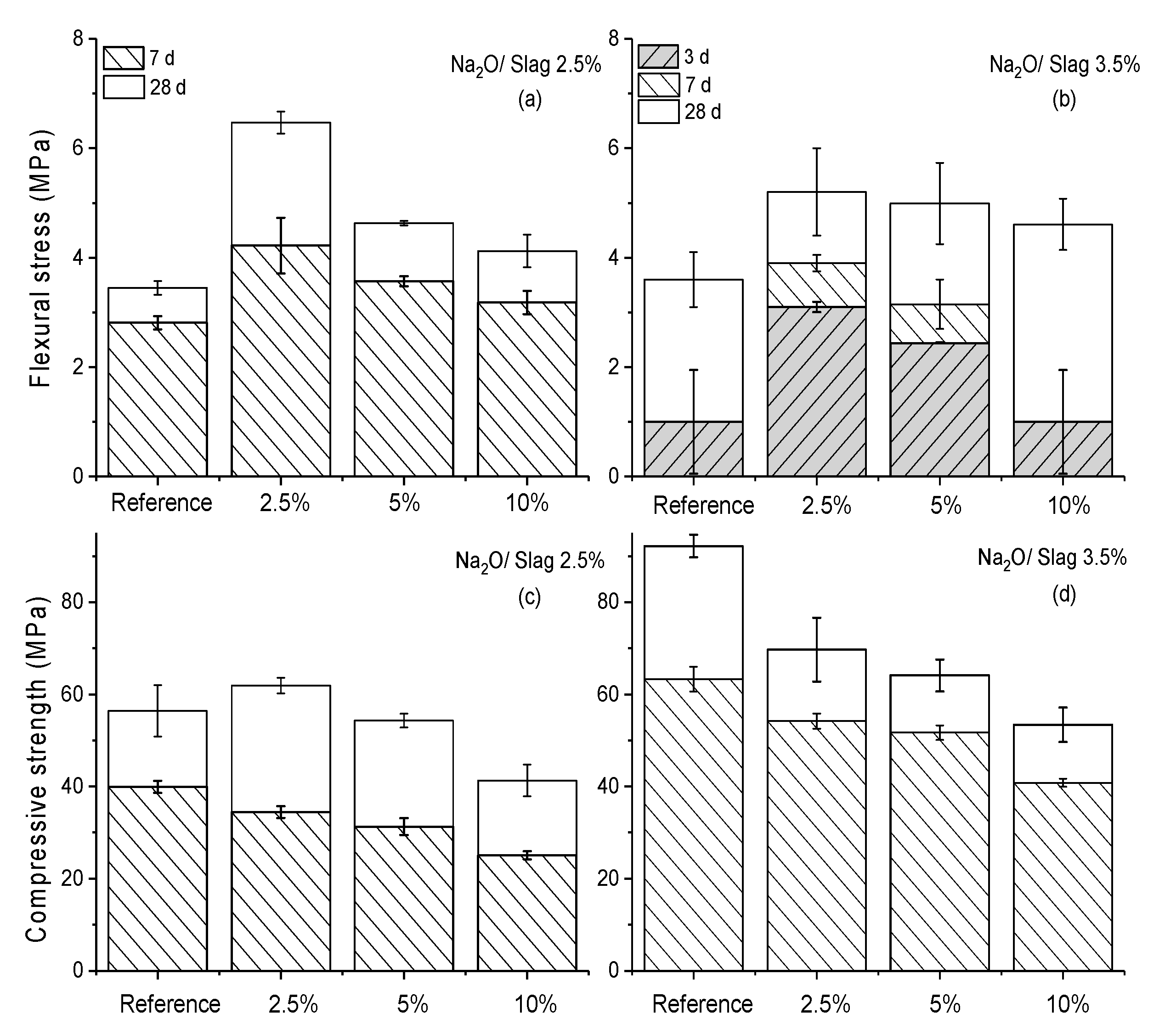
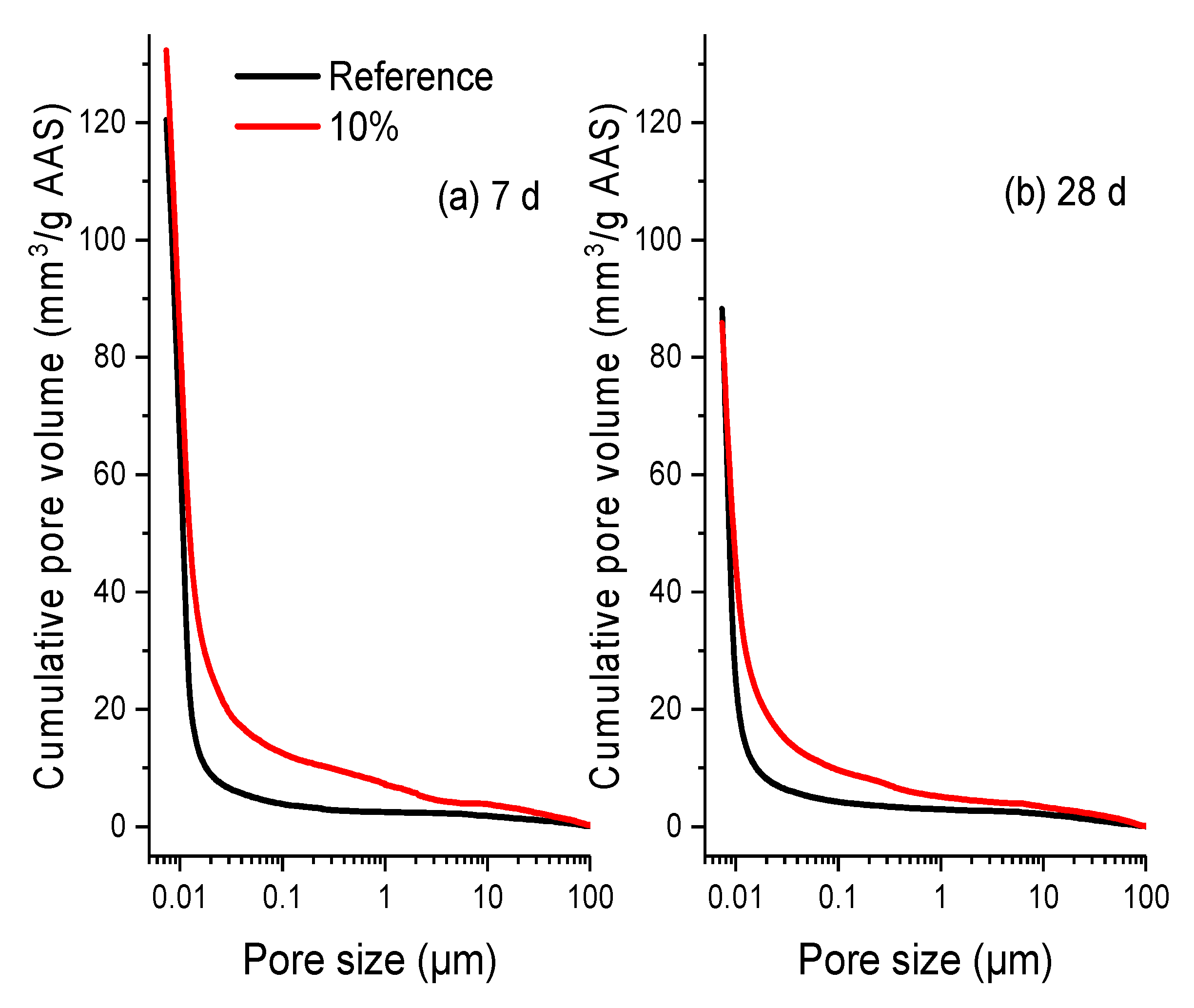
| Material | SiO2 | Al2O3 | CaO | MgO | Na2O | K2O | Fe2O3 | SO3 |
|---|---|---|---|---|---|---|---|---|
| Slag | 35.4 | 13.1 | 39.8 | 8.16 | 0.35 | 0.62 | <0.5 | 1.17 |
| Samples | Concentration (wt %) | SiO2 (wt %) | Modulus | pH |
|---|---|---|---|---|
| SWG | 44.5 | 28.3 | 1.81 | 13.5 |
| PWG | 40.0 | 15.8 | 1.02 | 14.3 |
| Dispersion | Solid Contents (wt %) | pH | Zeta Potential (mV) | Particle Size d50 (nm) | Tg | Stabilizer |
|---|---|---|---|---|---|---|
| SA | 53.5 | 6.05 | −22.2 | 185 | −15 | PEG-containing Surfactant |
| Water Glasses | Silica Modulus | Mass Ratio (wt %) | Conducted Experiments | ||||
|---|---|---|---|---|---|---|---|
| Water/Slag | M2O */Slag | WG/Slag | Defoamer/Slag | SA/Slag | |||
| SWG | 1.81 | 50 | 2.5 | 6.9 | 0.08 | 0.0 | Strength, hydration, XRD, TGA, SEM |
| 2.5 | Strength, TGA | ||||||
| 5.0 | Strength, TGA | ||||||
| 10.0 | Strength, hydration, XRD, TGA, SEM | ||||||
| 3.5 | 9.6 | 0.08 | 0 | Strength, hydration, XRD, TGA, MIP | |||
| 2.5 | Strength, TGA, | ||||||
| 5.0 | Strength, TGA, | ||||||
| 10.0 | Strength, hydration, XRD, TGA, MIP | ||||||
| PWG | 1.02 | 50 | 2.5 | 6.2 | 0.08 | 0 | Strength |
| 10.0 | Strength | ||||||
| Polymer Dosage | Na2O/Slag | Weight Loss (%) | |||||
|---|---|---|---|---|---|---|---|
| 7 d | 28 d | ||||||
| 50–200 °C | 200–500 °C (With Polymer) | 200–500 °C (Without Polymer) | 50–200 °C | 200–500 °C (With Polymer) | 200–500 °C (Without Polymer) | ||
| Reference | 2.5% | 6.2 | 4.3 | 4.3 | 6.9 | 5.0 | 5.0 |
| 3.5% | 6.9 | 5.3 | 5.3 | 8.6 | 5.8 | 5.8 | |
| 2.5% | 2.5% | 5.7 | 6.5 | 4.0 | 6.6 | 6.4 | 3.9 |
| 3.5% | 7.6 | 6.7 | 4.2 | 8.4 | 6.8 | 4.3 | |
| 5.0% | 2.5% | 6.1 | 7.1 | 2.7 | 7.3 | 7.7 | 3.3 |
| 3.5% | 7.2 | 7.9 | 3.5 | 8.1 | 8.3 | 3.9 | |
| 10.0% | 2.5% | 6.2 | 9.7 | 1.3 | 7.1 | 10.8 | 2.5 |
| 3.5% | 6.5 | 10.5 | 2.1 | 8.1 | 11.1 | 2.7 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Merkl, J.-P.; Pulkin, M.; Firdous, R.; Wache, S.; Stephan, D. A Systematic Study on Polymer-Modified Alkali-Activated Slag–Part II: From Hydration to Mechanical Properties. Materials 2020, 13, 3418. https://doi.org/10.3390/ma13153418
Lu Z, Merkl J-P, Pulkin M, Firdous R, Wache S, Stephan D. A Systematic Study on Polymer-Modified Alkali-Activated Slag–Part II: From Hydration to Mechanical Properties. Materials. 2020; 13(15):3418. https://doi.org/10.3390/ma13153418
Chicago/Turabian StyleLu, Zichen, Jan-Philip Merkl, Maxim Pulkin, Rafia Firdous, Steffen Wache, and Dietmar Stephan. 2020. "A Systematic Study on Polymer-Modified Alkali-Activated Slag–Part II: From Hydration to Mechanical Properties" Materials 13, no. 15: 3418. https://doi.org/10.3390/ma13153418
APA StyleLu, Z., Merkl, J.-P., Pulkin, M., Firdous, R., Wache, S., & Stephan, D. (2020). A Systematic Study on Polymer-Modified Alkali-Activated Slag–Part II: From Hydration to Mechanical Properties. Materials, 13(15), 3418. https://doi.org/10.3390/ma13153418





