Slag Blended Cement Paste Carbonation under Different CO2 Concentrations: Controls on Mineralogy and Morphology of Products
Abstract
1. Introduction
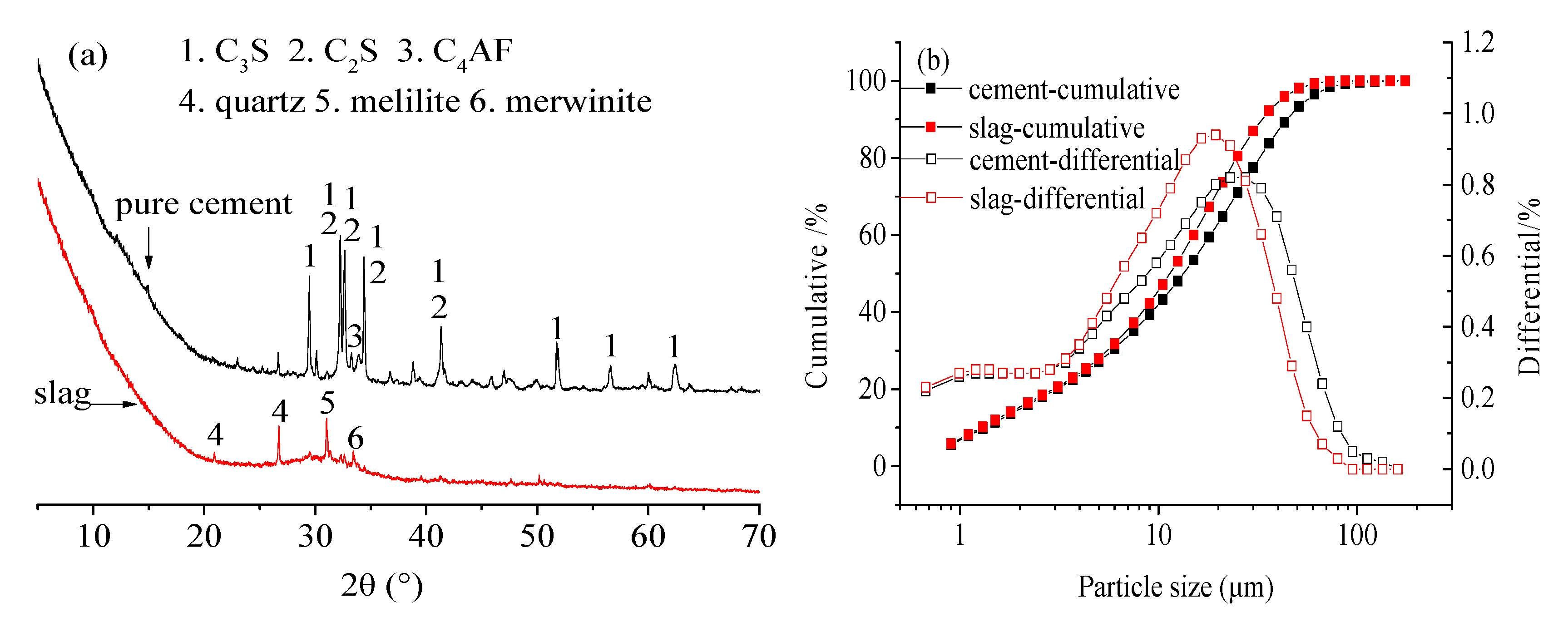
2. Materials and Methods
3. Analysis Tests
3.1. X-ray Diffraction (XRD) Analysis
3.2. Si Magic Angle Spinning Nuclear Magnetic Resonance (29Si MAS NMR) Analysis
3.3. Scanning Electron Microscope (SEM) Observations
4. Results and Discussion
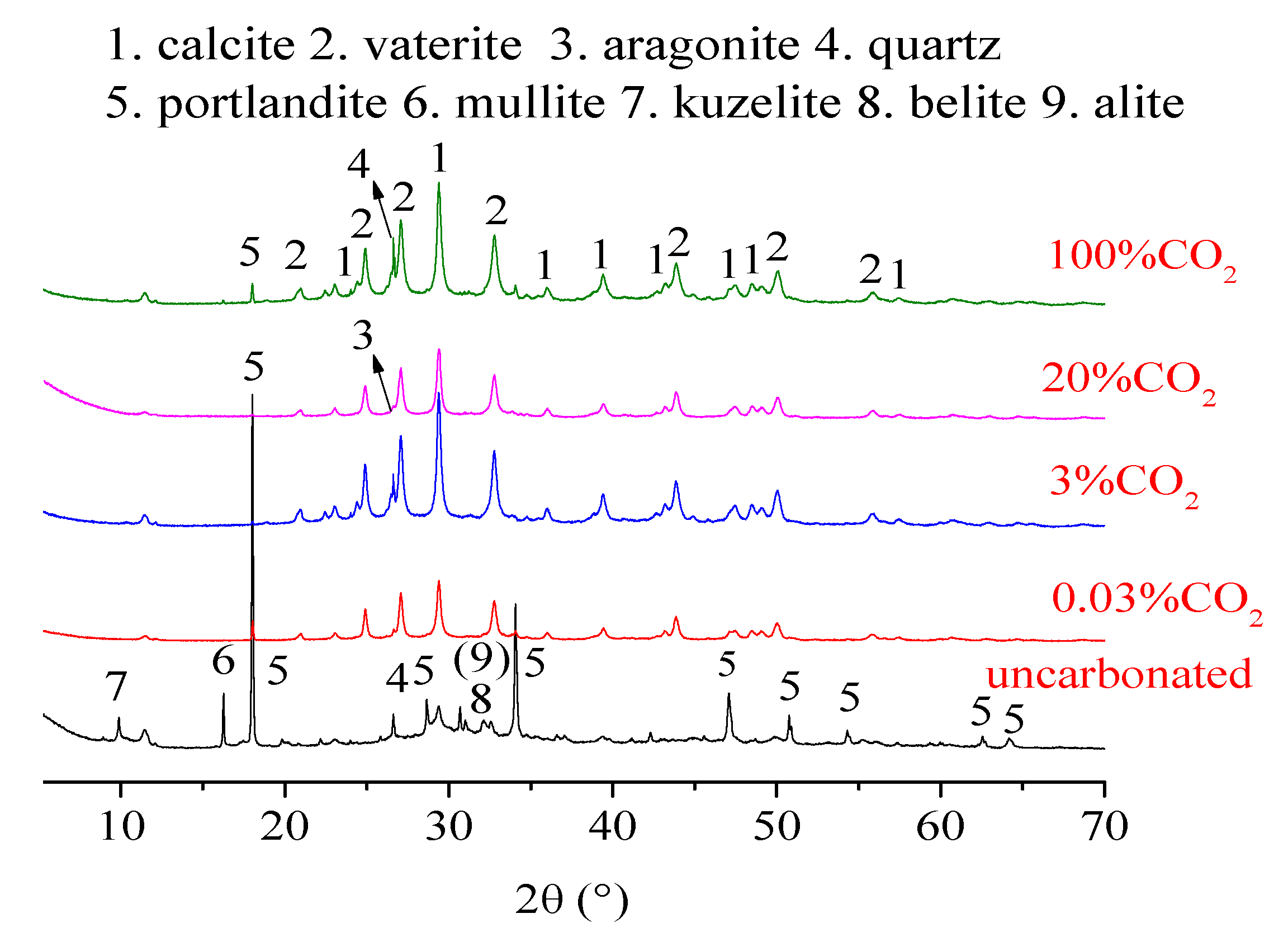
4.1. XRD Results
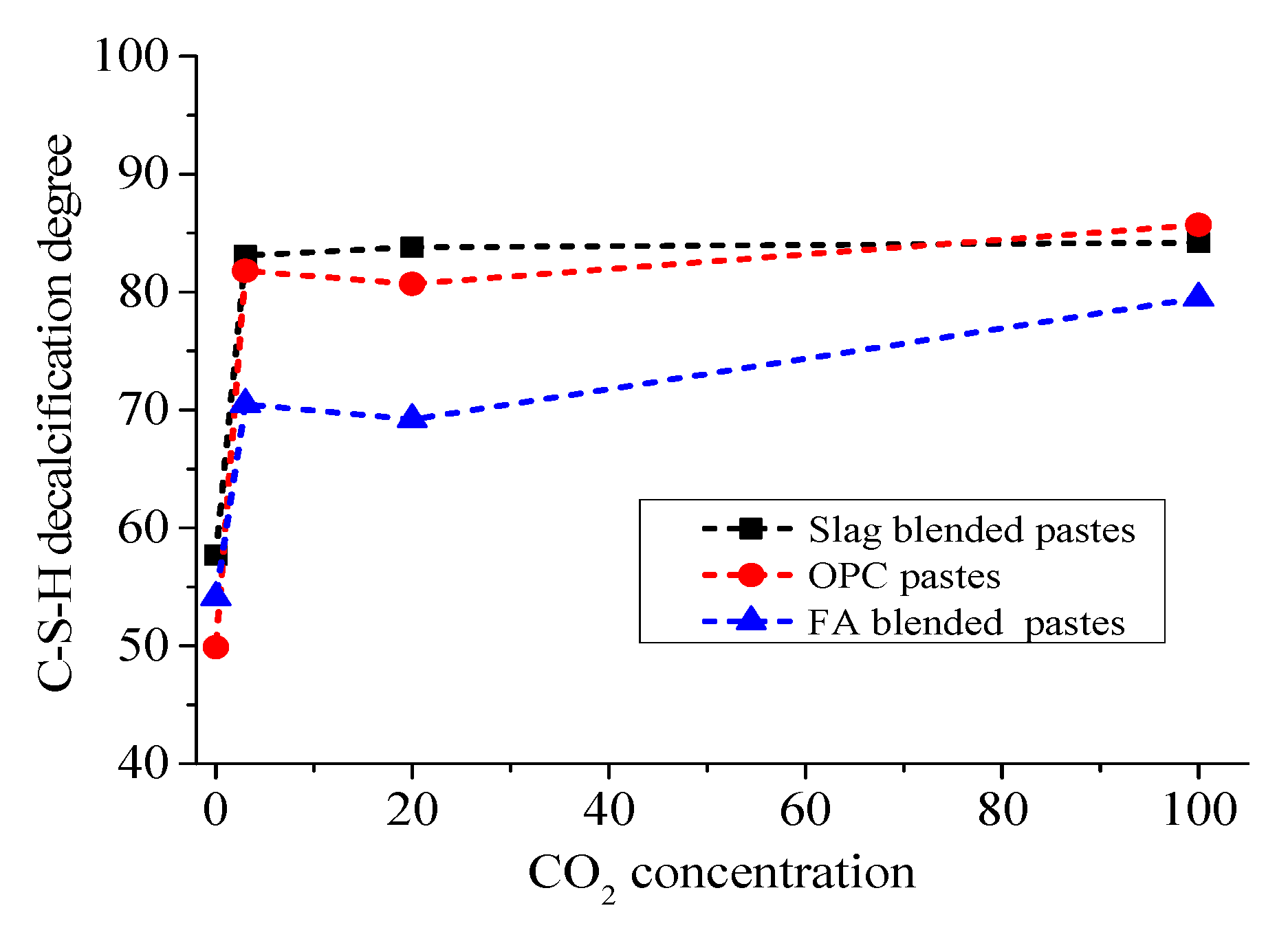
4.2. NMR Spectra
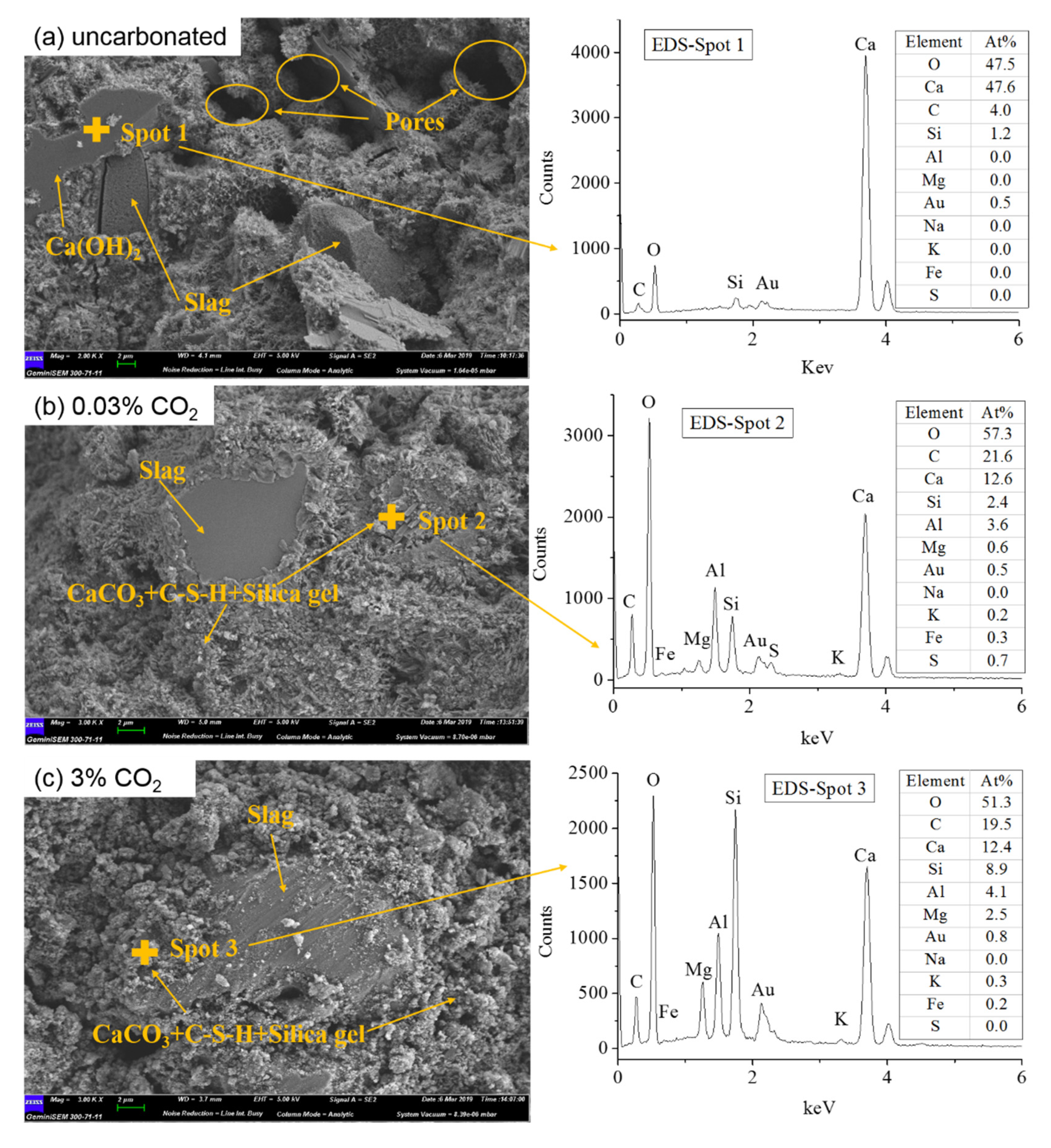
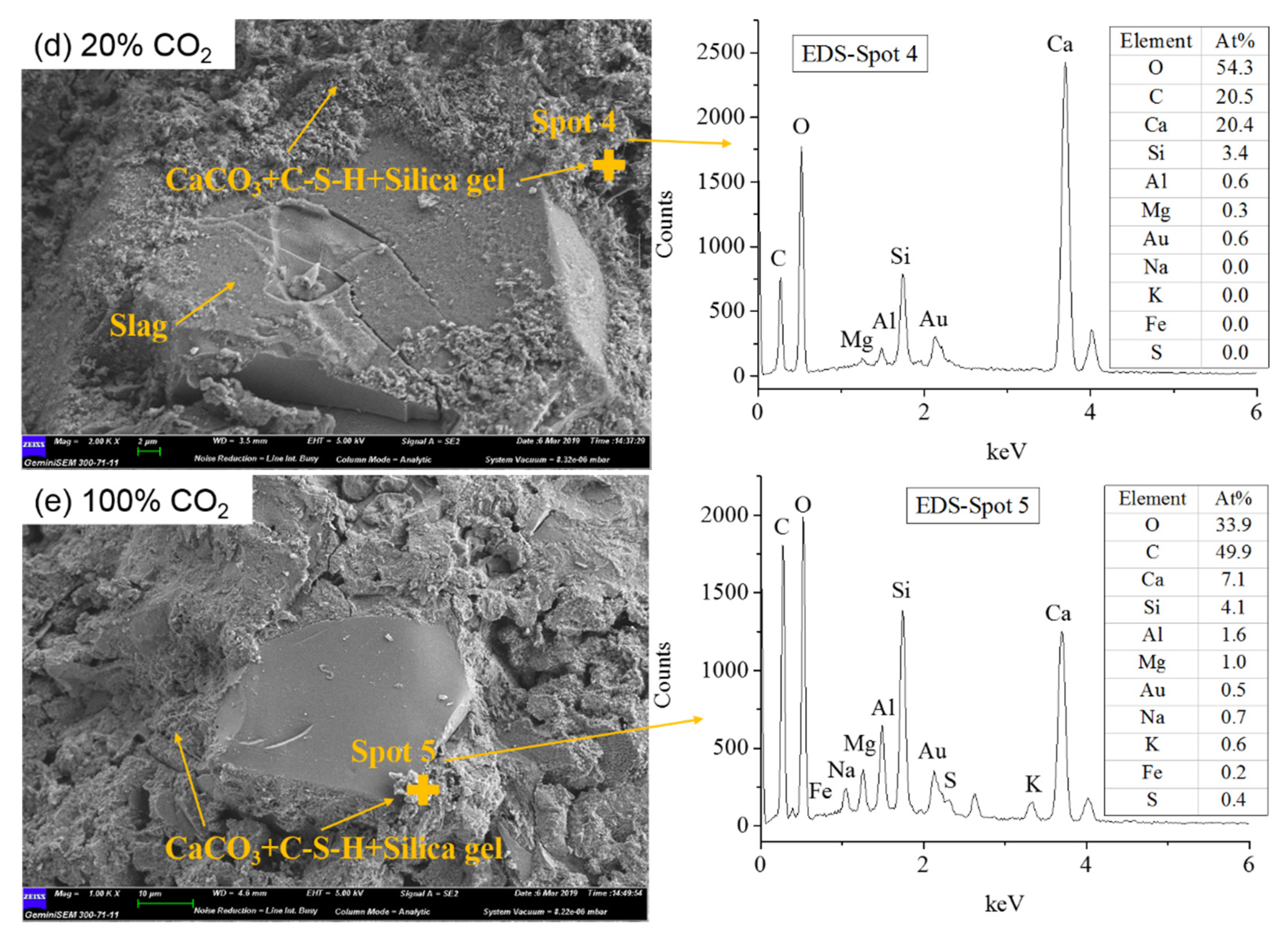
4.3. SEM-EDS Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fernandez Bertos, M.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar] [CrossRef]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction-a review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of supplementary cementing materials on concrete resistance against carbonation and chloride ingress. Cem. Concr. Res. 2000, 30, 291–299. [Google Scholar] [CrossRef]
- Thiery, M.; Villain, G.; Dangla, P.; Platret, G. Investigation of the carbonation front shape on cementitious materials: Effects of the chemical kinetics. Cem. Concr. Res. 2007, 37, 1047–1058. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, M.; Ding, B.; Liu, F.; Gao, F. The experimental investigation of width of semi-carbonation zone in carbonated concrete. Constr. Build. Mater. 2014, 65, 67–75. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Fundamental modeling and experimental investigation of concrete carbonation. ACI Mater. J. 1991, 88, 363–373. [Google Scholar]
- Dutzer, V.; Dridi, W.; Poyet, S.; Le Bescop, P.; Bourbon, X. The link between gas diffusion and carbonation in hardened cement pastes. Cem. Concr. Res. 2019, 123, 105795. [Google Scholar] [CrossRef]
- Jin, M.; Gao, S.; Jiang, L.; Chu, H.; Lu, M.; Zhi, F.F. Degradation of concrete with addition of mineral admixture due to free chloride ion penetration under the effect of carbonation. Corros. Sci. 2018, 138, 42–53. [Google Scholar] [CrossRef]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Walkley, B.; San Nicolas, R.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Shah, V.; Scrivener, K.; Bhattacharjee, B.; Bishnoi, S. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Wu, B.; Ye, G. Development of porosity of cement paste blended with supplementary cementitious materials after carbonation. Constr. Build. Mater. 2017, 145, 52–61. [Google Scholar] [CrossRef]
- Qiu, Q.; Gu, Z.; Xiang, J.; Huang, C.; Hong, S.; Xing, F.; Dong, B. Influence of slag incorporation on electrochemical behavior of carbonated cement. Constr. Build. Mater. 2017, 147, 661–668. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Gil-Maroto, A.; Vázquez, T. Alkali-aggregate behaviour of alkali-activated slag mortars: Effect of aggregate type. Cem. Concr. Compos. 2009, 31, 277–284. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Chen, W.; Brouwers, H.J.H. The hydration of slag, part 2: Reaction models for blended cement. J. Mater. Sci. 2007, 42, 444–464. [Google Scholar] [CrossRef]
- Escalante-García, J.I.; Sharp, J.H. The microstructure and mechanical properties of blended cements hydrated at various temperatures. Cem. Concr. Res. 2001, 31, 695–702. [Google Scholar] [CrossRef]
- Karellas, S.; Leontaritis, A.D.; Panousis, G.; Bellos, E.; Kakaras, E. Energetic and exergetic analysis of waste heat recovery systems in the cement industry. Energy 2013, 58, 147–156. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Andrade, C.; Cheyrezy, M. Concrete carbonation tests in natural and accelerated conditions. Adv. Cem. Res. 2003, 15, 171–180. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Tang, L.; Dong, Z. XRD and 29Si MAS NMR study on carbonated cement paste under accelerated carbonation using different concentration of CO2. Mater. Today Commun. 2019, 19, 464–470. [Google Scholar] [CrossRef]
- Richardson, I.G.; Groves, G.W. Microstructure and microanalysis of hardened cement pastes involving ground granulated blast-furnace slag. J. Mater. Sci. 1992, 27, 6204–6212. [Google Scholar] [CrossRef]
- Gruyaert, E.; Van den Heede, P.; De Belie, N. Carbonation of slag concrete: Effect of the cement replacement level and curing on the carbonation coefficient-Effect of carbonation on the pore structure. Cem. Concr. Compos. 2013, 35, 39–48. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Costa, J.O.; Milestone, N.B.; Lynsdale, C.J.; Streatfield, R.E. Carbonation of CH and C-S-H in composite cement pastes containing high amounts of BFS. Cem. Concr. Res. 2010, 40, 284–292. [Google Scholar] [CrossRef]
- He, Z.; Li, L.; Du, S. Mechanical properties, drying shrinkage, and creep of concrete containing lithium slag. Constr. Build. Mater. 2017, 147, 296–304. [Google Scholar] [CrossRef]
- Luke, K.; Lachowski, E. Internal Composition of 20-Year-Old Fly Ash and Slag-Blended Ordinary Portland Cement Pastes. J. Am. Ceram. Soc. 2008, 91, 4084–4092. [Google Scholar] [CrossRef]
- Šavija, B.; Luković, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef]
- Anstice, D.J.; Page, C.L.; Page, M.M. The pore solution phase of carbonated cement pastes. Cem. Concr. Res. 2005, 35, 377–383. [Google Scholar] [CrossRef]
- Auroy, M.; Poyet, S.; Le Bescop, P.; Torrenti, J.M.; Charpentier, T.; Moskura, M.; Bourbon, X. Comparison between natural and accelerated carbonation (3% CO2): Impact on mineralogy, microstructure, water retention and cracking. Cem. Concr. Res. 2018, 109, 64–80. [Google Scholar] [CrossRef]
- Šauman, Z. Carbonization of porous concrete and its main binding components. Cem. Concr. Res. 1971, 1, 645–662. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M.; Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 2007, 37, 1182–1192. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Lin, S.; Tang, L.; Dong, Z.; Xing, F.; Dong, B. Changes in chemical phases and microscopic characteristics of fly ash blended cement pastes in different CO2 concentrations. Constr. Build. Mater. 2020, 257, 119598. [Google Scholar] [CrossRef]
- Zajac, M.; Skibsted, J.; Durdzinski, P.; Bullerjahn, F.; Skocek, J.; Ben Haha, M. Kinetics of enforced carbonation of cement paste. Cem. Concr. Res. 2020, 131, 106013. [Google Scholar] [CrossRef]
- Girão, A.V.; Richardson, I.G.; Taylor, R.; Brydsonb, R.M.D. Composition, morphology and nanostructure of C–S–H in 70% white Portland cement–30% fly ash blends hydrated at 55 °C. Cem. Concr. Res. 2010, 40, 1350–1359. [Google Scholar] [CrossRef]
- Castellote, M.; Fernandez, L.; Andrade, C.; Alonso, C. Chemical changes and phase analysis of OPC pastes carbonated at different CO2 concentrations. Mater. Struct. 2008, 42, 515–525. [Google Scholar] [CrossRef]
- Sevelsted, T.F.; Skibsted, J. Carbonation of C–S–H and C–A–S–H samples studied by 13C, 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2015, 71, 56–65. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Xing, F.; Wang, S.; Tang, L.; Lin, S.; Dong, Z. Carbonation of the synthetic calcium silicate hydrate (C-S-H) under different concentrations of CO2: Chemical phases analysis and kinetics. J. CO2 Util. 2020, 35, 303–313. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Xu, D.; Du, P.; Zhou, Z.; Yuan, L.; Cheng, X. Accelerated carbonation of hardened cement pastes: Influence of porosity. Constr. Build. Mater. 2019, 225, 159–169. [Google Scholar] [CrossRef]


| Component | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | Loss |
|---|---|---|---|---|---|---|---|---|---|
| Cement | 20.89 | 5.1 | 2.98 | 64.94 | 1.76 | 3.36 | 0.16 | 0.81 | 1.22 |
| Slag | 31.54 | 13.6 | 0.7 | 41.22 | 9.11 | 2.84 | 0.47 | 0.52 | - |
| Sample | OPC + Slag | OPC [20] | OPC + FA [31] | |||
|---|---|---|---|---|---|---|
| c | a | v | c/(a + v) | c/(a + v) | c/(a + v) | |
| 0.03% CO2 | 33.4 | 2.2 | 64.4 | 0.5 | 0.77 | 0.51 |
| 3% CO2 | 33.1 | 0.2 | 66.7 | 0.5 | 0.30 | 0.64 |
| 20% CO2 | 32.2 | 1.8 | 66 | 0.48 | 0.28 | 0.58 |
| 100% CO2 | 32.4 | 3.2 | 64.4 | 0.48 | 0.51 | 0.79 |
| Deconvolved Peak | Peak Location (ppm) | Uncarbonated | 0.03% CO2 | 3% CO2 | 20% CO2 | 100% CO2 |
|---|---|---|---|---|---|---|
| Q0 | −66.8 to −75.5 | 22.4 | 18.6 | 8.7 | 8.9 | 6.9 |
| Q1 | −77.8 to −79.6 | 33.7 | 8.2 | 1.1 | 1.3 | 4.6 |
| Q2(Al) | −81.1 to −82.6 | 1.9 | 1.8 | 3.8 | 3.2 | 1.3 |
| Q2 | −84.8 to −86.1 | 40.2 | 22.1 | 7.9 | 7.8 | 6.1 |
| Q3(Al) | −91.2 to −92.9 | 1.8 | 7.3 | 9.7 | 12.4 | 15.0 |
| Q3 | −97.6 to −99.9 | 0.0 | 26.1 | 18.9 | 25.7 | 18.1 |
| Q4 | −106.1 to −108.5 | 0.0 | 15.9 | 49.9 | 40.7 | 48.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Lin, S.; Li, Y.; Long, W.; Dong, Z.; Tang, L. Slag Blended Cement Paste Carbonation under Different CO2 Concentrations: Controls on Mineralogy and Morphology of Products. Materials 2020, 13, 3404. https://doi.org/10.3390/ma13153404
Liu W, Lin S, Li Y, Long W, Dong Z, Tang L. Slag Blended Cement Paste Carbonation under Different CO2 Concentrations: Controls on Mineralogy and Morphology of Products. Materials. 2020; 13(15):3404. https://doi.org/10.3390/ma13153404
Chicago/Turabian StyleLiu, Wei, Shifa Lin, Yongqiang Li, Wujian Long, Zhijun Dong, and Luping Tang. 2020. "Slag Blended Cement Paste Carbonation under Different CO2 Concentrations: Controls on Mineralogy and Morphology of Products" Materials 13, no. 15: 3404. https://doi.org/10.3390/ma13153404
APA StyleLiu, W., Lin, S., Li, Y., Long, W., Dong, Z., & Tang, L. (2020). Slag Blended Cement Paste Carbonation under Different CO2 Concentrations: Controls on Mineralogy and Morphology of Products. Materials, 13(15), 3404. https://doi.org/10.3390/ma13153404





