Functional Coatings for Orthodontic Archwires—A Review
Abstract
1. Introduction
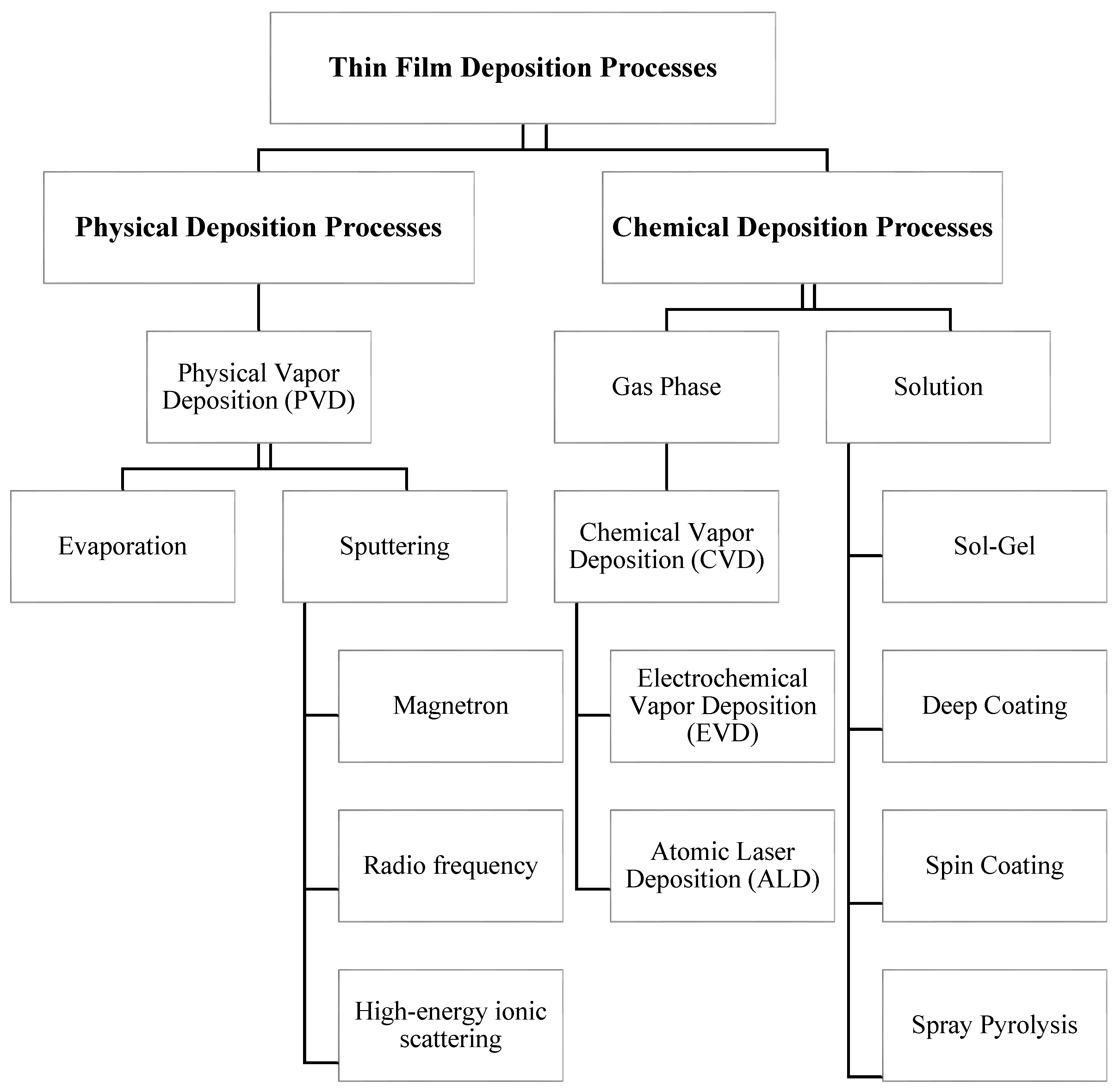
1.1. Physical Deposition Processes
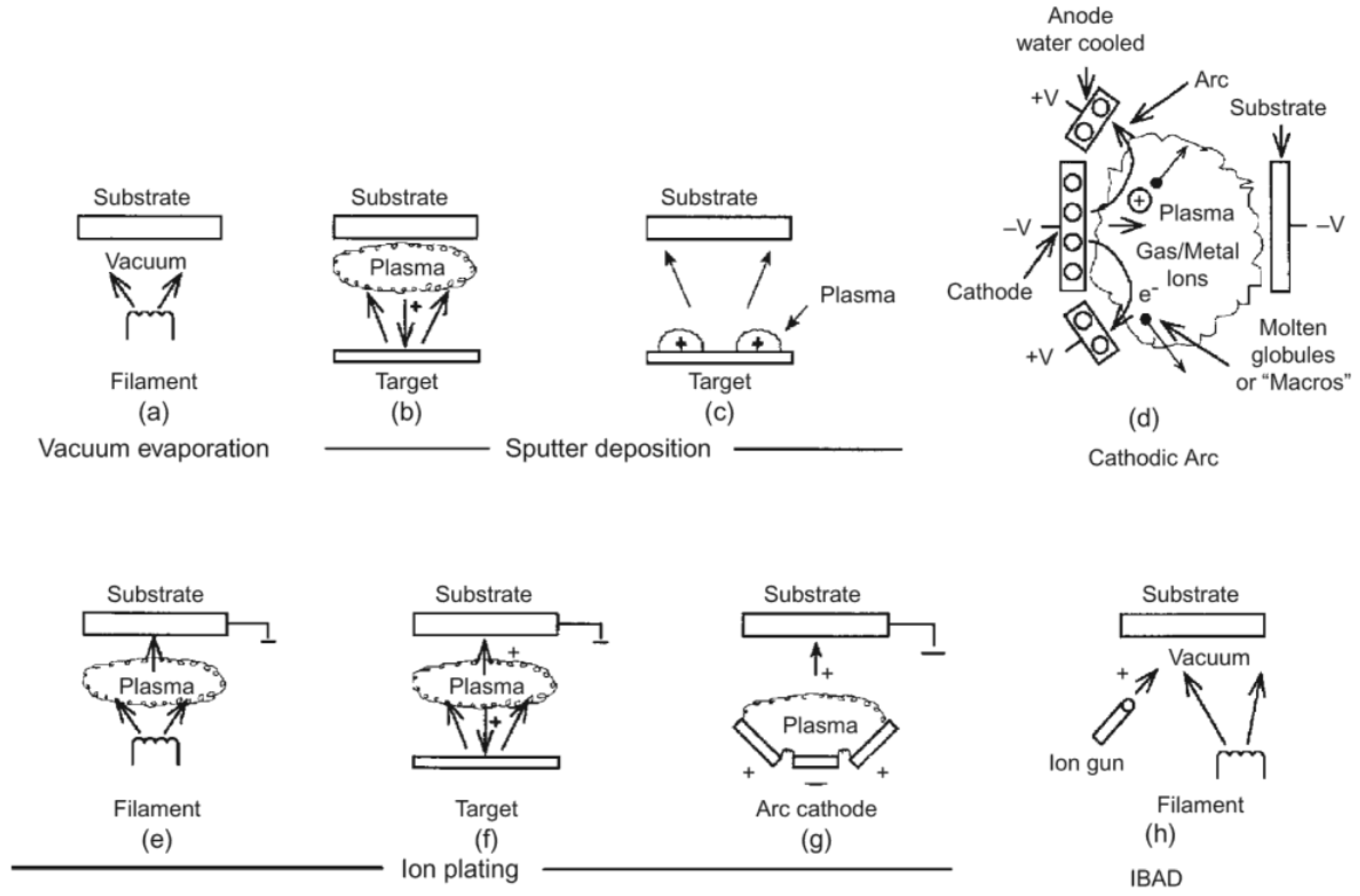
1.1.1. Physical Vapor Deposition (PVD)
1.1.2. Evaporation
1.1.3. Physical Sputtering
1.2. Chemical Deposition Processes
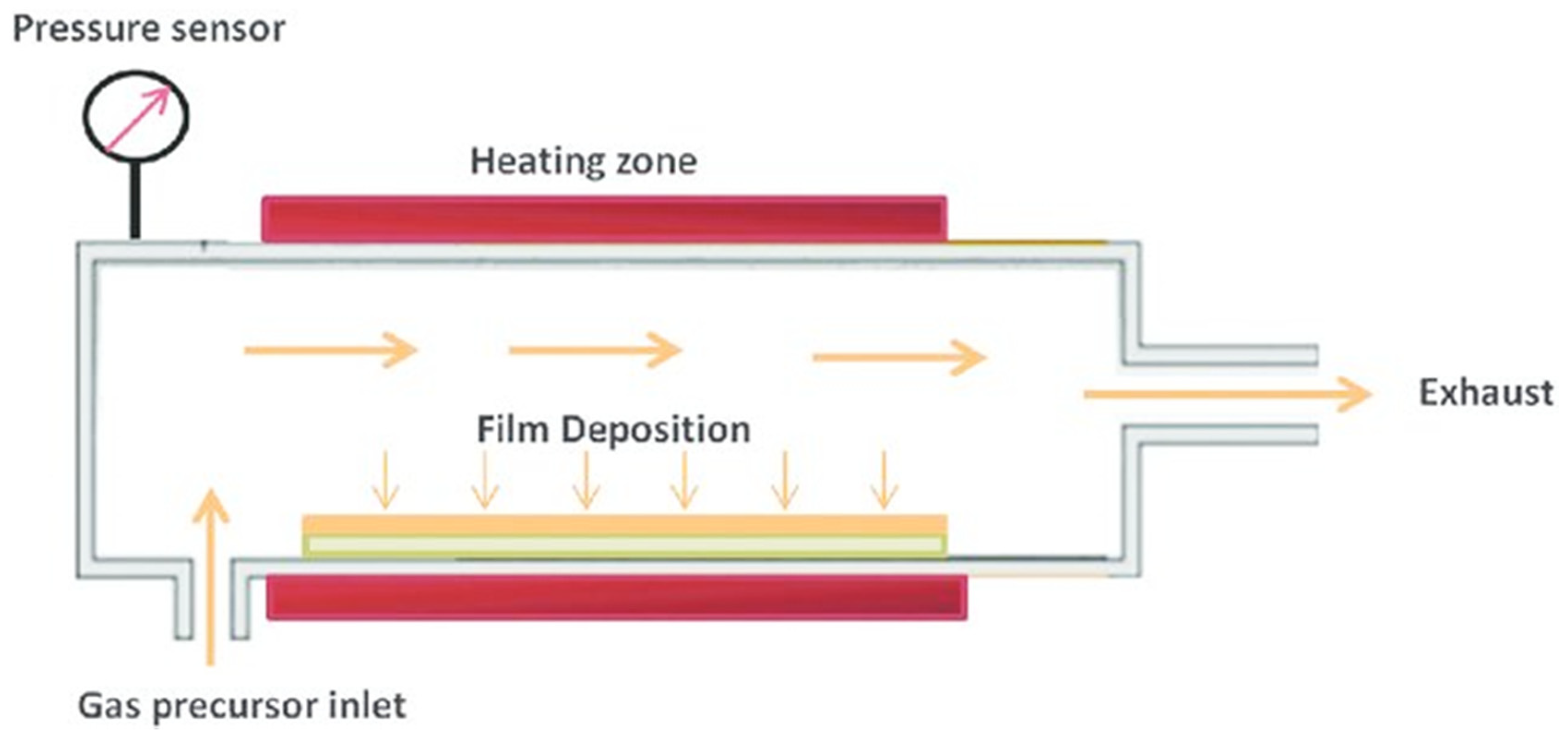
1.2.1. Chemical Vapor Deposition (CVD)
1.2.2. Electrodeposition
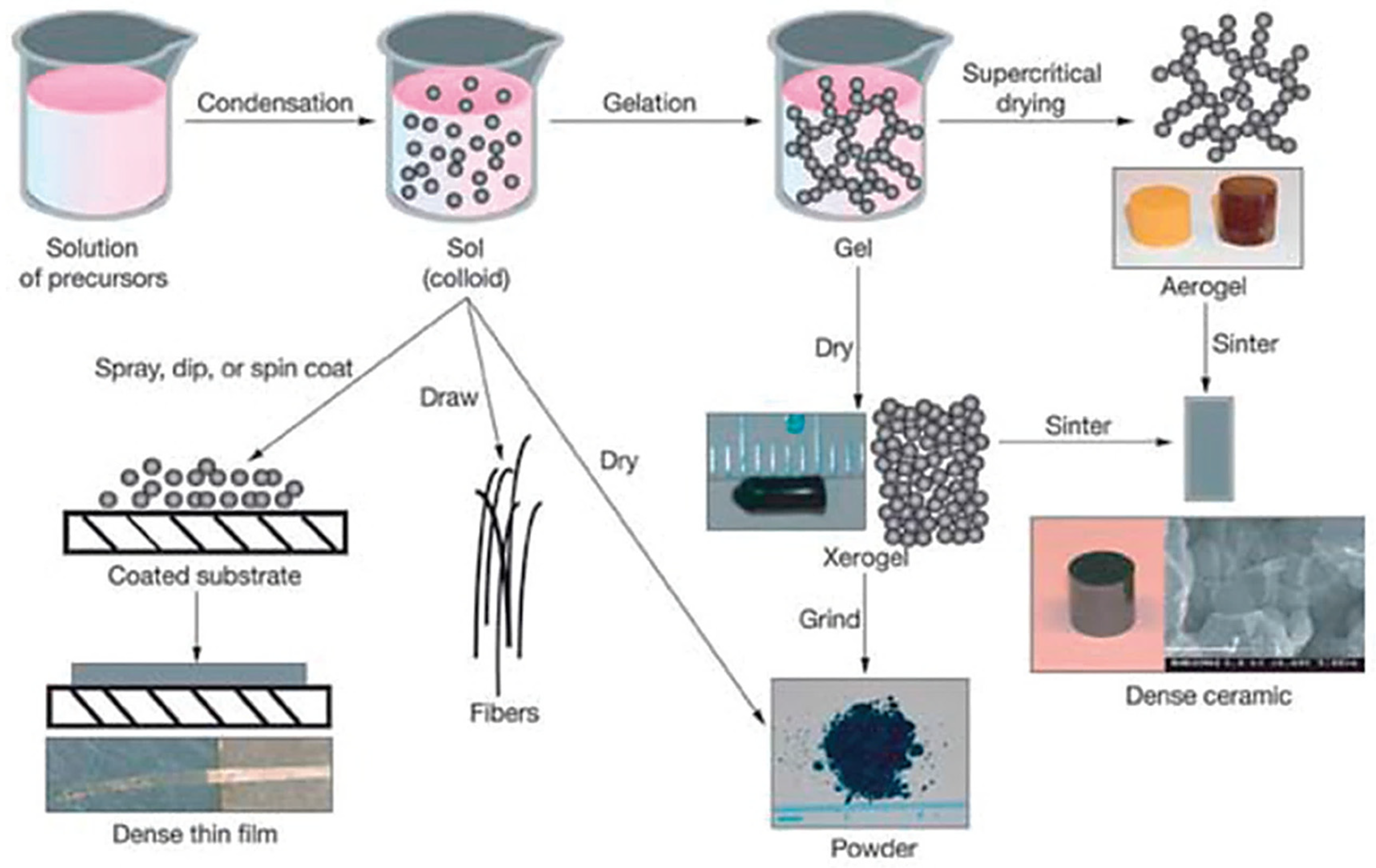
1.2.3. Sol-Gel
- Better homogeneity of structure;
- High purity of starting materials;
- Sol-gels are obtained at low temperature, which results in trapping particles with low chemical durability;
- Possibility to control the porosity and structure of a fixed, three-dimensional network of gels;
- Possibility to control the conductivity of the resulting material.
2. Materials and Method
- Both in-vivo and in-vitro studies were included;
- Studies exclusively on functional coatings for orthodontic archwires and brackets;
- Studies on different types of archwires and brackets;
- Studies in English language only;
- Any type of studies, including review articles, descriptive studies and case control trials, were considered.
3. Results
3.1. Antimicrobial Properties of Coating for Orthodontic Elements
3.1.1. Common oral Microbes
3.1.2. Reduction in the Initial Adhesion of Microorganisms on Orthodontic Appliances
3.1.3. Nanoparticles in Orthodontic Elements
3.1.4. Coating Orthodontic Archwires and Brackets with a Thin Film of Nitrogen-Doped TiO2 NPs
- Reduction under 20% results in no bactericidal activity;
- Reduction of 20–50% suggests low antibacterial activity;
- Reduction of 50–70% indicates strong bactericidal effect;
- Reduction above 70% is regarded as strong antibacterial activity.
3.2. Mechanical Properties of Coating for Orthodontics Elements
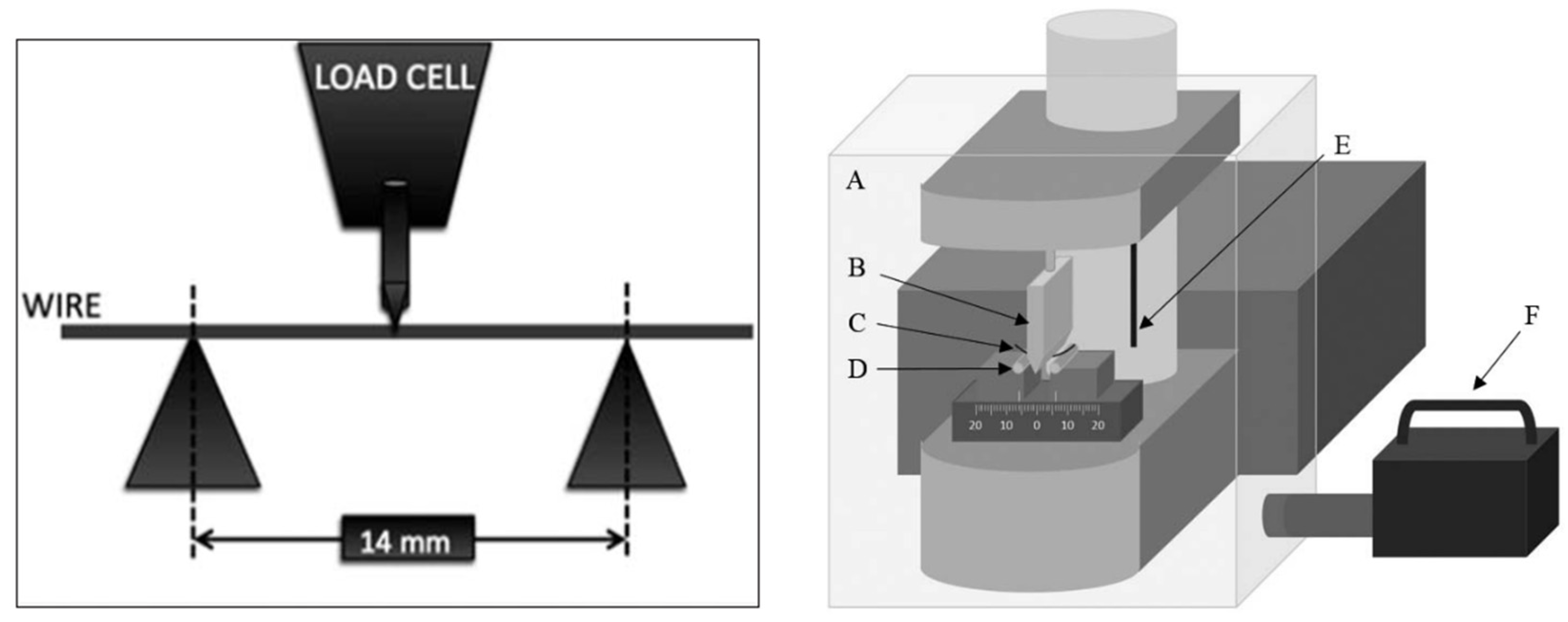
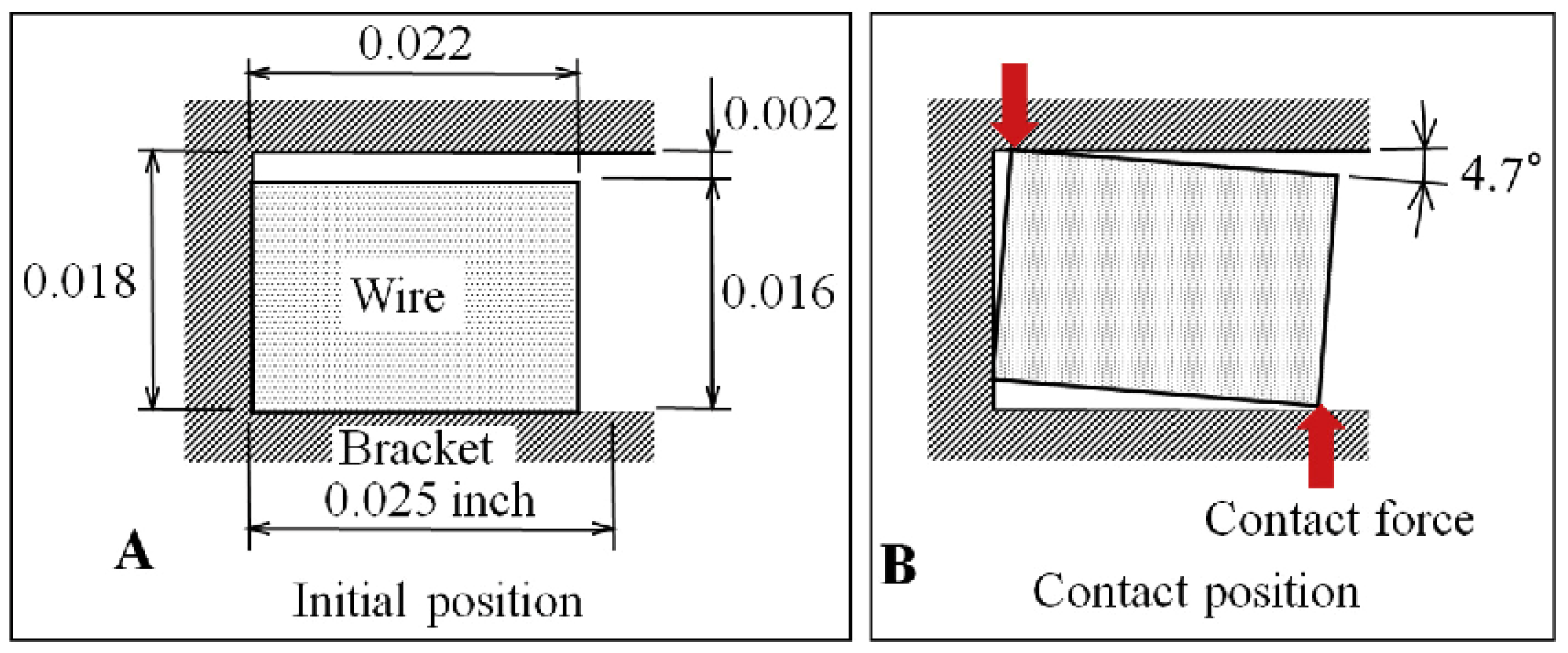
3.2.1. Mechanical Properties of Load and Deflection (the Three-Point Bending Test)
3.2.2. Properties of the Surface Roughness and the Mechanical Sliding Mechanism
3.2.3. Corrosion Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nguee, A.A.M.; Ongkosuwito, E.M.; Jaddoe, V.W.V.; Wolvius, E.B.; Kragt, L. Impact of orthodontic treatment need and deviant occlusal traits on oral health–related quality of life in children: A cross-sectional study in the Generation R cohort. Am. J. Orthod. Dentofac. Orthop. 2020, 157, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, F.; Papoutsi, A.; Dianiskova, S.; Dalessandri, D.; Bonetti, S.; Tsoi, J.K.H.; Matinlinna, J.P.; Paganelli, C. Resistance to sliding in orthodontics: Misconception or method error? A systematic review and a proposal of a test protocol. Korean J. Orthod. 2018, 48, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cameán, A.; Jos, Á.; Mellado-García, P.; Iglesias-Linares, A.; Solano, E.; Cameán, A.M. In vitro and in vivo evidence of the cytotoxic and genotoxic effects of metal ions released by orthodontic appliances: A review. Environ. Toxicol. Pharmacol. 2015, 40, 86–113. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.M.; Ponces, M.J.; Lopes, J.D.; Vasconcelos, M.; Pollmann, M.C.F. Orthodontic wires and its corrosion—The specific case of stainless steel and beta-titanium. J. Dent. Sci. 2015, 10, 1–7. [Google Scholar] [CrossRef]
- Bourauel, C.; Fries, T.; Drescher, D.; Plietsch, R. Surface roughness of orthodontic wires via atomic force microscopy, laser specular reflectance, and profilometry. Eur. J. Orthod. 1998, 20, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, W.; Gizani, S.; Nassika, M.; Kontou, E.; Nakou, M. Adhesion of Streptococcus mutans to Different Types of Brackets. Angle Orthod. 2007, 77, 1090–1095. [Google Scholar] [CrossRef]
- Ahn, S.J.; Lee, S.J.; Kook, J.K.; Lim, B.S. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent. Mater. 2009, 25, 206–213. [Google Scholar] [CrossRef]
- Borzabadi-Farahani, A.; Borzabadi, E.; Lynch, E. Nanoparticles in orthodontics, a review of antimicrobial and anti-caries application. Acta Odontol. Scand. 2013, 72, 413–417. [Google Scholar] [CrossRef]
- Derks, A.; Katsaros, C.; Frencken, J.E.; Van’t Hof, M.A.; Kuijpers-Jagtman, A.M. Caries-inhibiting effect of preventive measures during orthodontic treatment with fixed appliances. A systematic review. Caries Res. 2004, 38, 413–420. [Google Scholar] [CrossRef]
- Ghasemi, T.; Arash, V.; Rabiee, S.M.; Rajabnia, R.; Pourzare, A.; Rakhshan, V. Antimicrobial effect, frictional resistance, and surface roughness of stainless steel orthodontic brackets coated with nanofilms of silver and titanium oxide: A preliminary study. Microsc. Res. Tech. 2017, 80, 599–607. [Google Scholar] [CrossRef]
- Chun, M.J.; Shim, E.; Kho, E.H.; Park, K.J.; Jung, J.; Kim, J.M.; Kim, B.; Lee, K.H.; Cho, D.L.; Bai, D.H.; et al. Surface modification of orthodontic wires with photocatalytic titanium oxide for its antiadherent and antibacterial properties. Angle Orthod. 2007, 77, 483–488. [Google Scholar] [CrossRef]
- Mikulewicz, M.; Gronostajski, Z.; Wielgus, A.; Chojnacka, K. Transparent orthodontic archwires: A systematic literature review. Arch. Civ. Mech. Eng. 2017, 17, 651–657. [Google Scholar] [CrossRef]
- Affi, J.; Ihsan, F.; Fajri, H.; Gunawarman, G. Corosion behavior of new type titanium alloy as candidate for dental wires in artificial saliva on fluctuating temperatures. IOP Conf. Ser. Mater. Sci. Eng. 2018, 547, 012022. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, X.; Hou, X.; Li, H.; Sun, D. The corrosion resistance of composite arch wire laser-welded ny NiTi shape memory alloy and stainless steel wires with Cu interlayer in artificial saliva with protein. Int. J. Med. Sci. 2013, 10, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lou, Y.; Zhang, C.; Yin, S.; Li, H.; Sun, D.; Sun, X. Improved corrosion resistance and antibacterial properties of composite arch-wires by N-doped TiO2 coating. RSC Adv. 2017, 7, 43938–43949. [Google Scholar] [CrossRef]
- Akaike, S.; Hayakawa, T.; Kobayashi, D.; Aono, Y.; Hirata, A.; Hiratsuka, M.; Nakamura, Y. Reduction in static friction by deposition of a homogeneous diamond-like carbon (DLC) coating on orthodontic brackets. Dent. Mater. J. 2015, 34, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Akaike, S.; Kobayashi, D.; Aono, Y.; Hiratsuka, M.; Hirata, A.; Hayakawa, T.; Nakamura, Y. Relationship between static friction and surface wettability of orthodontic brackets coated with diamond-like carbon (DLC), fluorine-or silicone-doped DLC coatings. Diam. Relat. Mater. 2016, 61, 109–114. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ohgoe, Y.; Ozeki, K.; Sato, K.; Sumiya, T.; Hirakuri, K.K.; Aoki, H. Diamond-like carbon coatings on orthodontic archwires. Diam. Relat. Mater. 2005, 14, 1094–1097. [Google Scholar] [CrossRef]
- Yu, J.; Huang, H. Surface roughness and topography of four commonly used types of orthodontic archwire. J. Med. Biol. Eng. 2011, 31, 367–370. [Google Scholar] [CrossRef]
- Sheibaninia, A.; Salehi, A.; Asatourian, A. Comparison of Spring Characteristics of Titanium-Molybdenum Alloy and Stainless Steel. J. Clin. Exp. Dent. 2018, 9, 84–90. [Google Scholar] [CrossRef]
- Syed, S.S.; Kulkarni, D.; Todkar, R.; Bagul, R.S.; Parekh, K.; Bhujbal, N. A novel method of coating orthodontic archwires with nanoparticles. J. Int. Oral Heal. 2015, 7, 30–33. [Google Scholar]
- Iijima, M.; Muguruma, T.; Brantley, W.A.; Choe, H.C.; Nakagaki, S.; Alapati, S.B.; Mizoguchi, I. Effect of coating on properties of esthetic orthodontic nickel-titanium wires. Angle Orthod. 2012, 82, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Park, H.; Kyung, Y.; Kim, K.; Kwon, T. Comparative short-term in vitro analysis of mutans streptococci adhesion on esthetic, nickel-titanium, and stainless-steel arch wires. Angle Orthod. 2014, 84, 680–686. [Google Scholar] [CrossRef]
- Ryu, S.H.; Lim, B.S.; Kwak, E.J.; Lee, G.J.; Choi, S.; Park, K.H. Surface ultrastructure and mechanical properties of three different white-coated NiTi archwires. Scanning 2015, 37, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.L.; Mattos, C.T.; Sant Anna, E.F.; Ruellas, A.C.; Elias, C.N. Cross-section dimensions and mechanical properties of esthetic orthodontic coated archwires. Am. J. Orthod. Dentofac. Orthop. 2013, 143, S85–S91. [Google Scholar] [CrossRef]
- Bradley, T.G.; Berzins, D.W.; Valeri, N.; Pruszynski, J.; Eliades, T.; Katsaros, C. An investigation into the mechanical and aesthetic properties of new generation coated nickel-titanium wires in the as-received state and after clinical use. Eur. J. Orthod. 2014, 36, 290–296. [Google Scholar] [CrossRef]
- Katić, V.; Ćurković, H.O.; Semenski, D.; Baršić, G.; Marušić, K.; Špalj, S. Influence of surface layer on mechanical and corrosion properties of nickel-titanium orthodontic wires. Angle Orthod. 2014, 84, 1041–1048. [Google Scholar] [CrossRef]
- Washington, B.; Evans, C.A.; Viana, G.; Bedran-Russo, A.; Megremis, S. Contemporary esthetic nickel-titanium wires: Do they deliver the same forces? Angle Orthod. 2015, 85, 95–101. [Google Scholar] [CrossRef]
- Da Silva, D.L.; Santos, E.; de Souza Camargo, S.; de Oliveira Ruellas, A.C. Infrared spectroscopy, nano-mechanical properties, and scratch resistance of esthetic orthodontic coated archwires. Angle Orthod. 2015, 85, 777–783. [Google Scholar] [CrossRef]
- Rudge, P.; Sherriff, M.; Bister, D. A comparison of roughness parameters and friction coefficients of aesthetic archwires. Eur. J. Orthod. 2015, 37, 49–55. [Google Scholar] [CrossRef]
- Choi, S.; Park, D.J.; Kim, K.A.; Park, K.H.; Park, H.K.; Park, Y.G. In vitro sliding-driven morphological changes in representative esthetic NiTi archwire surfaces. Microsc. Res. Tech. 2015, 78, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Mhaske, A.R.; Shetty, P.C.; Bhat, N.S.; Ramachandra, C.S.; Laxmikanth, S.M.; Nagarahalli, K.; Tekale, P.D. Antiadherent and antibacterial properties of stainless steel and NiTi orthodontic wires coated with silver against Lactobacillus acidophilus—An in vitro study. Prog. Orthod. 2015, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Elayyan, F.; Silikas, N.; Bearn, D. Ex vivo surface and mechanical properties of coated orthodontic archwires. Eur. J. Orthod. 2008, 30, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Arango, S.; Peláez-Vargas, A.; García, C. Coating and surface treatments on orthodontic metallic materials. Coatings 2013, 3, 1–15. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials. Synthesis, Properties and Applications, 2nd ed.; World Scientific Series in Nanoscience and Nanotechnology: Singapore, 2011. [Google Scholar]
- Tripi, T.R.; Bonaccorso, A.; Condorelli, G.G. Fabrication of hard coatings on NiTi instruments. J. Endod. 2003, 29, 132–134. [Google Scholar] [CrossRef]
- Zuo, J.; Keil, P.; Grundmeier, G. Synthesis and characterization of photochromic Ag-embedded TiO2 nanocomposite thin films by non-reactive RF-magnetron sputter deposition. Appl. Surf. Sci. 2012, 258, 7231–7237. [Google Scholar] [CrossRef]
- Kuo, C.G.; Hsu, C.Y.; Wang, S.S.; Wen, D.C. Photocatalytic characteristics of TiO2 films deposited by magnetron sputtering on polycarbonate at room temperature. Appl. Surf. Sci. 2012, 258, 6952–6957. [Google Scholar] [CrossRef]
- Shah, A.G.; Shetty, P.C.; Ramachandra, C.S.; Bhat, N.S.; Laxmikanth, S.M. In vitro assessment of photocatalytic titanium oxide surface modified stainless steel orthodontic brackets for antiadherent and antibacterial properties against Lactobacillus acidophilus. Angle Orthod. 2011, 81, 1028–1035. [Google Scholar] [CrossRef]
- Zuo, J. Deposition of Ag Nanostructures on TiO2. Thin Films by RF Magnetron Sputtering. Appl. Surf. Sci. 2010, 256, 7096–7101. [Google Scholar] [CrossRef]
- Dimca, V.; Suchea, M.; di Pietrantonio, F.; Cannatà, D.; Benett, M. Chapter 8: Biosensor Technologies Based on Nanomaterials. In Functional Nanostructured Interfaces for Environmental and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Makhlouf, A.S.H. Current and Advanced Coating Technologies for Industrial Applications, Nanocoatings and Ultra-Thin Films Technologies and Applications. In Nanocoatings and Ultra-Thin Films; Woodhead Publishing: Sawston, UK, 2011; pp. 3–23. [Google Scholar] [CrossRef]
- Zhang, Q.; Sando, D.; Nagarajan, V. Chemical route derived bismuth ferrite thin films and nanomaterials. J. Mater. Chem. C 2016, 4, 4092–4124. [Google Scholar] [CrossRef]
- Grainger, S.; Blunt, J. Engineering Coatings: Design and Application, 2nd ed.; Woodhead Publishing: Sawston, UK, 1998. [Google Scholar]
- Redlich, M.; Gorodnev, A.; Feldman, Y.; Kaplan-Ashiri, I.; Tenne, R.; Fleischer, N.; Genut, M.; Feuerstein, N. Friction reduction and wear resistance of electro-co-deposited inorganic fullerene-like WS2 coating for improved stainless steel orthodontic wires. J. Mater. Res. 2008, 23, 2909–2915. [Google Scholar] [CrossRef]
- Zein El Abedin, S.; Welz-Biermann, U.; Endres, F. A study on the electrodeposition of tantalum on NiTi alloy in an ionic liquid and corrosion behaviour of the coated alloy. Electrochem. Commun. 2005, 7, 941–946. [Google Scholar] [CrossRef]
- Qiu, D.; Wang, A.; Yin, Y. Characterization and corrosion behavior of hydroxyapatite/zirconia composite coating on NiTi fabricated by electrochemical deposition. Appl. Surf. Sci. 2010, 257, 1774–1778. [Google Scholar] [CrossRef]
- Patil, K.R.; Hwang, Y.K.; Kim, M.J.; Chang, J.S.; Park, S.E. Preparation of thin films comprising palladium nanoparticles by a solid-liquid interface reaction technique. J. Colloid Interface Sci. 2004, 276, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.D.; Bescher, E.P. Physical properties of sol-gel coatings. J. Sol Gel Sci. Technol. 2000, 19, 23–29. [Google Scholar] [CrossRef]
- Collins, A.M. Practical, Reliable and Jargon-Free Experimental Procedures. In Nanotechnology Cookbook; Elsevier Science: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bach, H.; Krause, D. Thin Films on Glass; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Alagiri, M.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and characterization of NiO nanoparticles by sol-gel method. J. Mater. Sci. Mater. Electron. 2012, 23, 728–732. [Google Scholar] [CrossRef]
- Sajjadi, S.P. Sol-gel process and its application in Nanotechnology. J. Polym. Eng. Technol. 2005, 13, 38–41. [Google Scholar]
- Lee, B.-S.; Lin, H.-P.; Chan, J.C.C.; Wang, W.-C.; Hung, P.-H.; Tsai, Y.-H.; Lee, Y.-L. A novel sol-gel-derived calcium silicate cement with short setting time for application in endodontic repair of perforations. Int. J. Nanomed. 2018, 13, 261–271. [Google Scholar] [CrossRef]
- Guadalupe, A. Introductory Chapter: A Brief Semblance of the Sol-Gel Method in Research. In Sol-Gel Method—Design and Synthesis of New Materials with Interesting Physical, Chemical and Biological Properties; Department of Nanotechonology and Functional Materials, CICATA Unidad Legaria, Instituto Politécnico: Nacional, Mexico, 2018; pp. 1–6. [Google Scholar]
- Gill, J.K.; Orsat, V.; Kermasha, S. Screening trials for the encapsulation of laccase enzymatic extract in silica sol-ge. J. Sol Gel Sci. Technol. 2018, 85, 657–662. [Google Scholar] [CrossRef]
- Strawbridge, I.; James, P.F. Thin silica films prepared by dip coating. J. Non Cryst. Solids 1986, 82, 366–372. [Google Scholar] [CrossRef]
- Rao, A. Modeling bending response of shape memory alloy wires/beams under superelastic conditions -A two species thermodynamic Preisach approach. Int. J. Struct. Chang. Solids 1992, 5, 1–26. [Google Scholar]
- Donesz-Sikorska, A.; Marycz, K.; Śmieszek, A.; Grzesiak, J.; Kaliński, K.; Krzak-Roś, J. Biologicznie aktywne powłoki otrzymywane metodą zol – żel na metalicznych materiałach implantacyjnych. Przem. Chem. 2013, 92, 1110–1116. [Google Scholar]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Sanches-Giraud, C.; Silva, C.; Guimaraes, W.; Gomez, D. The battle against microbial pathogens: Basic science, technological advances and educational programs. Formatex Res. Cent. 2015, 1, 717–732. [Google Scholar]
- Tejero-Martin, D.; Rezvani Rad, M.; McDonald, A.; Hussain, T. Beyond Traditional Coatings: A Review on Thermal-Sprayed Functional and Smart Coatings; Springer: Berlin/Heidelberg, Germany, 2019; Volume 28. [Google Scholar]
- Chhattani, S.; Shetty, P.C. In vitro assessment of photocatalytic titanium oxide surface-modified stainless steel and nickel titanium orthodontic wires for its antiadherent and antibacterial properties against Streptococcus mutans. J. Indian Orthod. Soc. 2014, 48, 82–87. [Google Scholar] [CrossRef]
- Mirhashemi, A.; Bahador, A.; Kassaee, M.; Daryakenari, G.; Ahmad-Akhoundi, M.; Sodagar, A. Antimicrobial Effect of Nano-Zinc Oxide and Nano-Chitosan Particles in Dental Composite Used in Orthodontics. J. Med. Bacteriol. 2015, 2, 1–10. [Google Scholar]
- Metin-Gürsoy, G.; Taner, L.; Akca, G. Nanosilver coated orthodontic brackets: In vivo antibacterial properties and ion release. Eur. J. Orthod. 2017, 39, 9–16. [Google Scholar] [CrossRef]
- Ryu, H.S.; Bae, I.H.; Lee, K.G.; Hwang, H.S.; Lee, K.H.; Koh, J.T.; Cho, J.H. Antibacterial effect of silver-platinum coating for orthodontic appliances. Angle Orthod. 2012, 82, 151–157. [Google Scholar] [CrossRef]
- Supriadi, S.; Suharno, B.; Nugraha, N.K.; Yasinta, A.O.; Annur, D. Adhesiveness of TiO2 PVD coating on electropolished stainless steel 17-4 PH orthodontic bracket. Mater. Res. Express 2019, 6, 094003. [Google Scholar] [CrossRef]
- Cao, S.; Wang, Y.; Cao, L.; Wang, Y.; Lin, B.; Lan, W.; Cao, B. Preparation and antimicrobial assay of ceramic brackets coated with TiO2 thin films. Korean J. Orthod. 2016, 46, 146–154. [Google Scholar] [CrossRef]
- Abraham, K.S.; Jagdish, N.; Kailasam, V.; Padmanabhan, S. Streptococcus mutans adhesion on nickel titanium (NiTi) and copper-NiTi archwires: A comparative prospective clinical study. Angle Orthod. 2017, 87, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; El-Fallal, A.; Degla, H. In vitro and in vivo biofilm adhesion to esthetic coated arch wires and its correlation with surface roughness. Angle Orthod. 2016, 86, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Li, N.; Liu, B.; Zhang, Y. Preparation of an orthodontic bracket coated with an nitrogen-doped TiO2—xNy thin film and examination of its antimicrobial performance. Dent. Mater. J. 2013, 32, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Judi, C.; Oh, K.; Choi, Y.; Kim, K. Photocatalytic Antibacterial Effect of TiO2 Film of TiAg on Streptococcus mutans. Angle Orthod. 2009, 79, 528–532. [Google Scholar] [CrossRef]
- Uysal, T.; Yagci, A.; Uysal, B.; Akdogan, G. Are nano-composites and nano-ionomers suitable for orthodontic bracket bonding? Eur. J. Orthod. 2010, 32, 78–82. [Google Scholar] [CrossRef]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The role of oral cavity biofilm on metallic biomaterial surface destruction–corrosion and friction aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef]
- Dodds, M.; Roland, S.; Edgar, M.; Thornhill, M. Saliva A review of its role in maintaining oral health and preventing dental disease. BDJ Team 2015, 2, 15123. [Google Scholar] [CrossRef]
- Pasich, E.; Walczewska, M.; Pasich, A.; Marcinkiewicz, J. Mechanizm i czynniki ryzyka powstawania biofilmu bakteryjnego jamy ustnej, Mechanism and risk factors of oral biofilm formation. Post. Hig. Med. 2013, 67, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.; Lim, B.; Park, J.; Hyun, J.; Ahn, S. Effect of orthodontic bonding steps on the initial adhesion of mutans streptococci in the presence of saliva. Angle Orthod. 2011, 81, 326–333. [Google Scholar] [CrossRef]
- Maruthamuthu, S.; Rajasekar, A.; Sadagopan, S.; Nagu, M.; Palaniswamy, N. Electrochemical behaviour of microbes on orthodontic wires. Curr. Sci. 2005, 89, 988–996. [Google Scholar]
- Chung, S.H.; Cho, S.; Kim, K.; Lim, B.S.; Ahn, S.J. Antimicrobial and physical characteristics of orthodontic primers containing antimicrobial agents. Angle Orthod. 2017, 87, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.; Morais, W.A.; Passos, V.F.; Lima, J.; Rodrigues, L.K. Fluoride releasing and enamel demineralization around orthodontic brackets by fluoride-releasing composites containing nanoparticles. J. Dent. 2014, 18, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garrido, N.; Lozano, C.; Giacaman, R.A. Frequency of sucrose exposure on the cariogenicity of a biofilm-caries model. Eur. J. Dent. 2016, 10, 345–350. [Google Scholar] [CrossRef]
- Inagaki, L.T.; Dainezi, V.B.; Alonso, R.C.B.; de Bolzan, P.A.; Garcia-Godoy, F.; Puppin-Rontani, R.M.; Pascon, F.M. Evaluation of sorption/solubility, flexural strength and elastic modulus of experimental resin blends with chlorhexidine. J. Dent. 2016, 49, 40–45. [Google Scholar] [CrossRef]
- Mei, L.; Busscher, H.J.; van der Mei, H.C.; Ren, Y. Influence of surface roughness on streptococcal adhesion forces to composite resins. Dent. Mater. 2011, 27, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Altmann, A.; Collares, F.; Ogliari, F.; Samuel, S. Effect of methacrylated-based antibacterial monomer on orthodontic adhesive system properties. Am. J. Orthod. Dentofac. Orthop. 2015, 147, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Chang, L.; Liu, X.; Lin, J.; Liu, H.; Han, B.; Wang, S. Antibacterial property of a polyethylene glycol-grafted dental material. ACS Appl. Mater. Interfaces 2017, 9, 17688–17692. [Google Scholar] [CrossRef]
- Yu, F.; Dong, Y.; Yu, H.; Lin, P.; Zhang, L.; Sun, X.; Liu, Y.; Xia, Y.; Huang, L.; Chen, J. Antibacterial Activity and Bonding Ability of an Orthodontic Adhesive Containing the Antibacterial Monomer 2-Methacryloxylethyl Hexadecyl Methyl Ammonium Bromide. Sci. Rep. 2017, 7, 41787. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Altmann, A.S.P.; Ferreira, C.J.; Arthur, R.A.; Leitune, V.C.B.; Samuel, S.M.W.; Collares, F.M. Evaluation of an antibacterial orthodontic adhesive incorporated with niobium-based bioglass: An in situ study. Braz. Oral Res. 2019, 33, e010. [Google Scholar] [CrossRef]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Torii, M.; Russell, R.R.B.; McCabe, J.F. Incorporation of antibacterial monomer MDPB into dentin primer. J. Dent. Res. 1997, 76, 768–772. [Google Scholar] [CrossRef]
- Cao, L.; Wu, J.; Zhang, Q.; Baras, B.; Bhadila, G.; Li, Y.; Melo, M.A.S.; Weir, M.D.; Bai, Y.; Zhang, N.; et al. Novel protein-repellent and antibacterial resins and cements to inhibit lesions and protect teeth. Int. J. Polym. Sci. 2019, 2019, 5602904. [Google Scholar] [CrossRef]
- Mani, S.; Manickam, S.; Muthusamy, V.; Thangaraj, R. Antimicrobial activity and photocatalytic degradation properties of zinc sulfide nanoparticles synthesized by using plant extracts. J. Nanostruct. 2018, 8, 107–118. [Google Scholar] [CrossRef]
- Pansambal, S.; Deshmukh, K.; Savale, A.; Ghotekar, S.; Pardeshi, O.; Jain, G.; Aher, Y.; Pore, D. Phytosynthesis and biological activities of fluorescent CuO nanoparticles using Acanthospermum hispidum L. extract. J. Nanostruct. 2017, 7, 165–174. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 72, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P. The use of nanoparticles to control oral biofilm formation. J. Dent. Res. 2010, 89, 1175–1186. [Google Scholar] [CrossRef]
- Sodagar, A.; Bahador, A.; Pourhajibagher, M.; Ahmadi, B.; Baghaeian, P. Effect of Addition of Curcumin Nanoparticles on Antimicrobial Property and Shear Bond Strength of Orthodontic Composite to Bovine Enamel. J. Dent. 2016, 13, 373–382. [Google Scholar]
- Tahmasbi, S.; Mohamadian, F.; Hosseini, S.; Eftekhar, L. A review on the applications of nanotechnology in orthodontics. Nanomed. J. 2019, 6, 11–18. [Google Scholar] [CrossRef]
- Stobie, N.; Duffy, B.; McCormack, D.E.; Colreavy, J.; Hidalgo, M.; McHale, P.; Hinder, S.J. Prevention of Staphylococcus epidermidis biofilm formation using a low-temperature processed silver-doped phenyltriethoxysilane sol-gel coating. Biomaterials 2008, 29, 963–969. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; Ruvollo–Filho, A.C.; de Camargo, E.R.; Barbosa, D.B. The growing importance of materials that prevent microbial adhesion: Antimicrobial effect of medical devices containing silver. Int. J. Antimicrob. Agents 2009, 34, 103–110. [Google Scholar] [CrossRef]
- Hernández-Sierra, J.F.; Ruiz, F.; Pena, D.C.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; de Guillén, A.J.; Tapia-Peréz, H.; Castañón, G.M. The antimicrobial sensi- tivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 2008, 4, 237–240. [Google Scholar] [CrossRef]
- Luo, M.L.; Zhao, J.Q.; Tang, W.; Pu, C.S. Hydrophilic modification of poly(ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Cao, S.; Liu, B.; Fan, L.; Yue, Z.; Liu, B.; Cao, B. Highly antibacterial activity of N-doped TiO2 thin films coated on stainless steel brackets under visible light irradiation. Appl. Surf. Sci. 2014, 309, 119–127. [Google Scholar] [CrossRef]
- Armi, V.D.; Donati, V.; Rizza, F.; Giaquinto, S. Aesthetic orthodontic archwires: The state of art. Oral Implantol. 2014, 29, 115–122. [Google Scholar]
- Raked, T. Fixed Appliances and Orthodontic Instruments. In Orthodontics for Dental Hygienists and Dental Therapists; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 97–170. [Google Scholar]
- Walker, M.; White, R.J.; Kula, K.S. Effect of fluoride prophylactic agents on the mechanical properties of nickle-titanium-based orthodontic wires. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 662–669. [Google Scholar] [CrossRef]
- Brantley, W.A. Orthodontic Wires. In Orthodontic Materials: Scientific and Clinical Aspects; Brantley, W.A., Eliades, T., Eds.; Thieme: New York, NY, USA, 2001. [Google Scholar]
- Aksakalli, S.; Malkoc, S. Esthetic orthodontic archwires: Literature review. Mater. Sci. 2013, 1, 5–7. [Google Scholar] [CrossRef]
- Muguruma, T.; Iijima, M.; Yuasa, T.; Kawaguchi, K.; Mizoguchi, I. Characterization of the coatings covering esthetic orthodontic archwires and their influence on the bending and frictional properties. Angle Orthod. 2017, 87, 610–617. [Google Scholar] [CrossRef]
- Matias, M.; de Freitas, M.R.; de Freitas, K.M.S.; Janson, G.; Higa, R.H.; Francisconi, M.F. Comparison of deflection forces of esthetic archwires combined with ceramic brackets. J. Appl. Oral Sci. 2018, 26, 1–9. [Google Scholar] [CrossRef]
- Doshi, U.H.; Bhad-Patil, W.A. Static frictional force and surface roughness of various bracket and wire combinations. Am. J. Orthod. Dentofac. Orthop. 2011, 139, 74–79. [Google Scholar] [CrossRef]
- Souza, J.C.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.P.; Rocha, L.A. Do oral biofilms influence the wear and corrosion behavior of titanium? Biofouling 2010, 26, 471–478. [Google Scholar] [CrossRef]
- Chae, J.M.; Park, J.H.; Kojima, Y.; Tai, K.; Kook, Y.A.; Kyung, H.M. Biomechanical analysis for total distalization of the mandibular dentition: A finite element study. Am. J. Orthod. Dentofac. Orthop. 2019, 155, 388–397. [Google Scholar] [CrossRef]
- Farronato, G.; Maijer, R.; Carìa, M.P.; Esposito, L.; Alberzoni, D.; Cacciatore, G. The effect of Teflon coating on the resistance to sliding of orthodontic archwires. Eur. J. Orthod. 2012, 34, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, S.; Wang, D.; Zhou, T.; Wang, L.; Ma, J. Effects of nanostructured, diamondlike, carbon coating and nitrocarburizing on the frictional properties and biocompatibility of orthodontic stainless steel wires. Angle Orthod. 2016, 86, 782–788. [Google Scholar] [CrossRef]
- Redlich, M.; Katz, A.; Rapoport, L.; Wagnert, H.; Feldman, Y.; Tenne, R. Improved orthodontic stainless steel wires coated with inorganic fullerene-like nano- particles of WS(2) impregnated in electroless nickel-phosphorus film. Dent. Mater. 2008, 24, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Bartzela, T.N.; Senn, C.; Wichelhaus, A. Load-deflection characteristics of superelastic nickel-titanium wires. Angle Orthod. 2007, 77, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, R.; Soni, S.; Garg, V.; Kaur, M. Esthetic orthodontic appliances—A review. Ann. Geriatrric Educ. Med. Sci. 2018, 5, 10–14. [Google Scholar] [CrossRef]
- Mirhashemi, A.H.; Jahangiri, S.; Kharrazifard, M.J. Release of nickel and chromium ions from orthodontic wires following the use of teeth whitening mouthwashes. Prog. Orthod. 2018, 19, 2–6. [Google Scholar] [CrossRef]
- Krishnan, M.; Seema, S.; Kumar, A.V.; Varthini, N.P.; Sukumaran, K.; Pawar, V.R.; Arora, V. Corrosion resistance of surface modified nickel titanium archwires. Angle Orthod. 2014, 84, 358–367. [Google Scholar] [CrossRef]
| Material /Substrate | Coating Composition | Coating Technique | Bacteria/Microbial Strains | Methods | Results | Ref. |
|---|---|---|---|---|---|---|
| stainless steel (SS) and NiTi archwires | pure silver (99.9%) | Thermal evaporation method | Lactobacillus acidophilus (LA) | Antiadherent was evaluated by the weight change in the wires; Antibacterial by the dilution agar plate method. | The silver coating prevented the adhesion of L. acidophilus and demonstrated antibacterial effect against bacteria. | Mhaske et al. [32] |
| SS brackets | photocatalytic TiO2 | Radiofrequency magnetron sputtering | Lactobacillus acidophilus | Antiadherent was evaluated by the weight change in the brackets and antibacterial the dilution agar plate method. | The photocatalytic TiO2 coating prevented the adhesion of L. acidophilus and demonstrated antibacterial effect against bacteria. | Shah et al. [40] |
| SS and NiTi archwires | photocatalytic TiO2 | Sol-gel thin film dip-coating | Streptococcus mutans (SM) | Antiadherent was evaluated by the weight change in the wires; Antibacterial by the dilution agar plate method. | The photocatalytic TiO2 coating prevented the adhesion of S. mutans and demonstrated antibacterial effect against bacteria. | Chhattani et al. [64] |
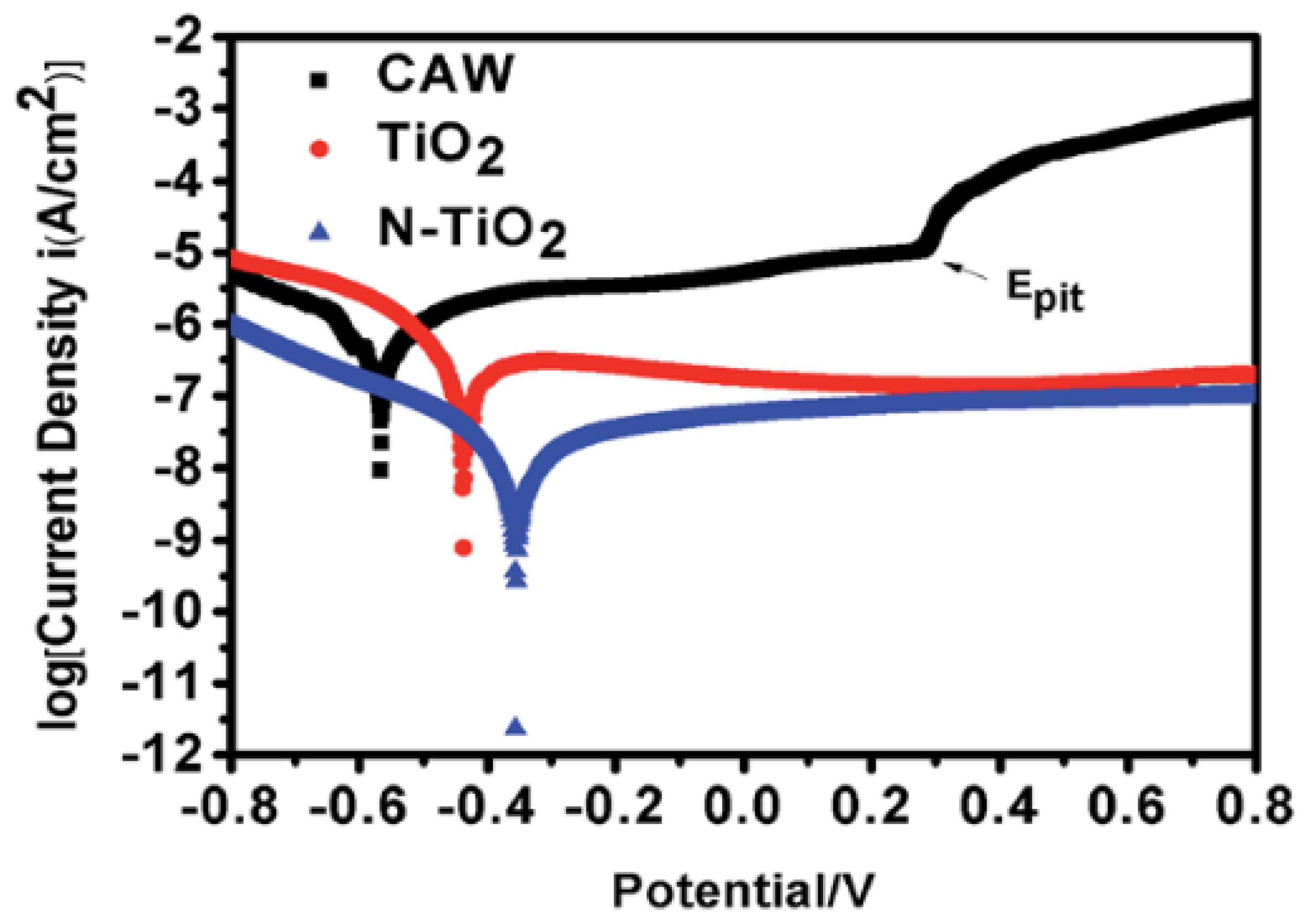
| composite archwires (CAWs): NiTi and SS wires | 99.99% ceramic TiO2 and N-doped TiO2 | Radiofrequency magnetron sputtering | Streptococcus mutans | Antiadherent was evaluated by the weight change in the wires; Antibacterial by the dilution agar plate method. | Wires coated with N-doped TiO2 thin film showed the most effective antimicrobial effects. | Liu et al. [15] |
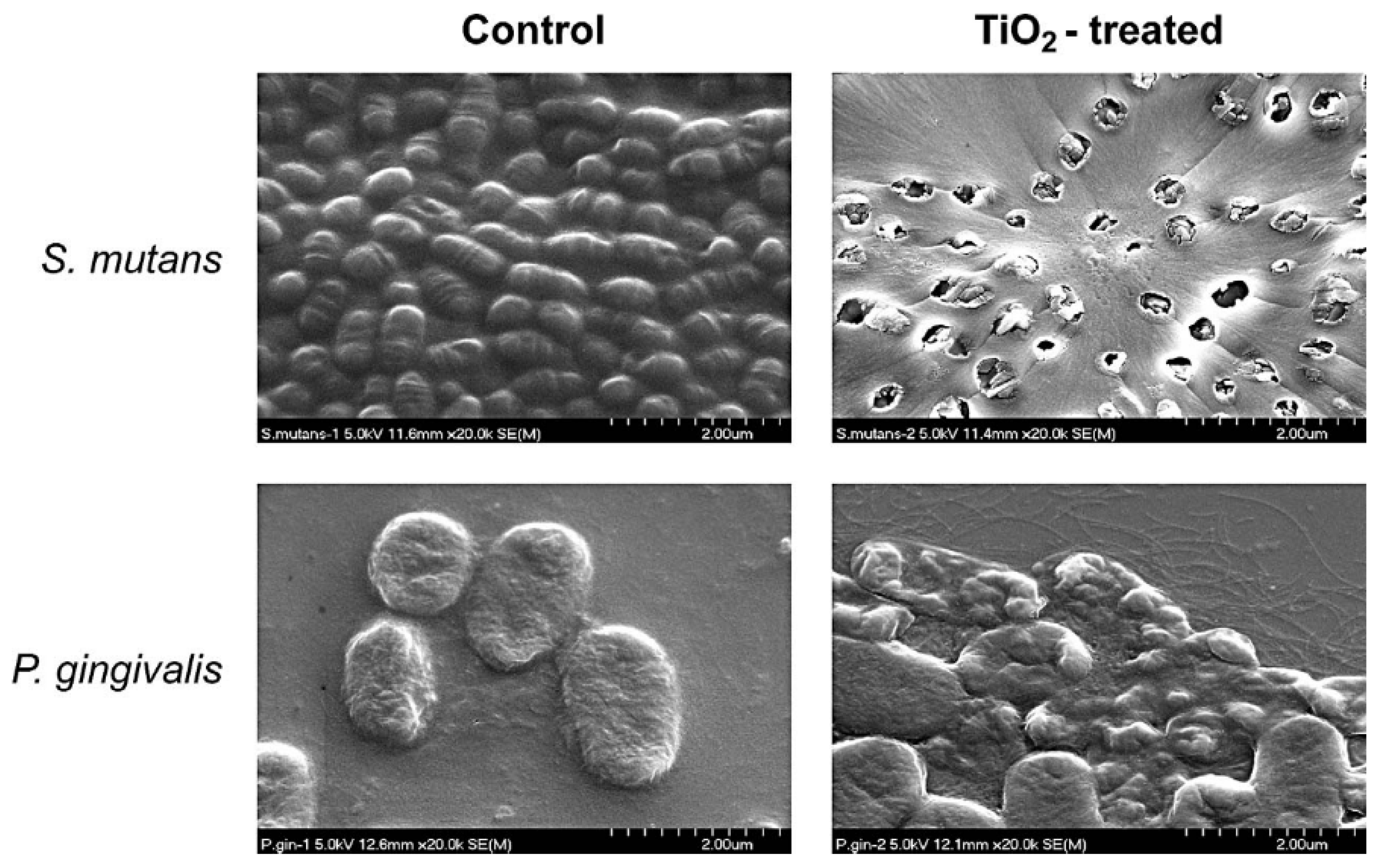
| SS orthodontic wires | photocatalytic TiO2 | Sol-gel thin film dip-coating | Streptococcus mutans and Porphyromonas gingivalis | Antiadherent was evaluated by the weight change in the wires; Antibacterial by the dilution agar plate method for Streptococcus mutans and by spectrophotometry for Porphyromonas gingivalis. | The TiO2-coated orthodontic wires showed an antiadherent effect against S. mutans and bactericidal effect on S. mutans and P. gingivalis. | Chun et al. [11] |
| orthodontic composite | Chitosan (CS) and zinc oxide (ZnO)nanoparticles (NPs) | Streptococcusmutans, S. anguis and Lactobacillus acidophilus | Antibacterial by the dilution agar plate method. | Antibacterial by the dilution agar plate method. | Mirhashemi et al. [65] | |
| orthodontic brackets | Nanosilver | beam evaporation method | Streptococcus mutans | Antibacterial was evaluated by the dilution agar plate method. | Nanosilver coated orthodontic bracket favoured the inhibition of S. mutans on day 30 and reduction of caries on the smooth surfaces. | Metin-Gürsoy et al. [66] |
| SS surfaces | Ag and Ag-Pt alloys | physical vapor deposition | Streptococcus mutans and Aggregatibacter actinomycetemo-mitans | Antibacterial was evaluated by the dilution agar plate method. | The coatings released sufficient Ag ions when immersed in phosphate-buffered saline and showed antimicrobial effect on S. mutans and Aggregatibacter actinomycetemcomitans strains. | Ryu et al. [67] |
| SS brackets | TiO2 | physical vapor deposition | correlation of coating adhesion and surface roughness | The adhesiveness of TiO2 coating was assessed quantitatively and qualitatively by measuring hardness by micro Vickers. | Surface roughness correlates with coating adhesion, if the surface roughness increased then the adhesion of coating would be decreasing. | Supriadi et al. [68] |
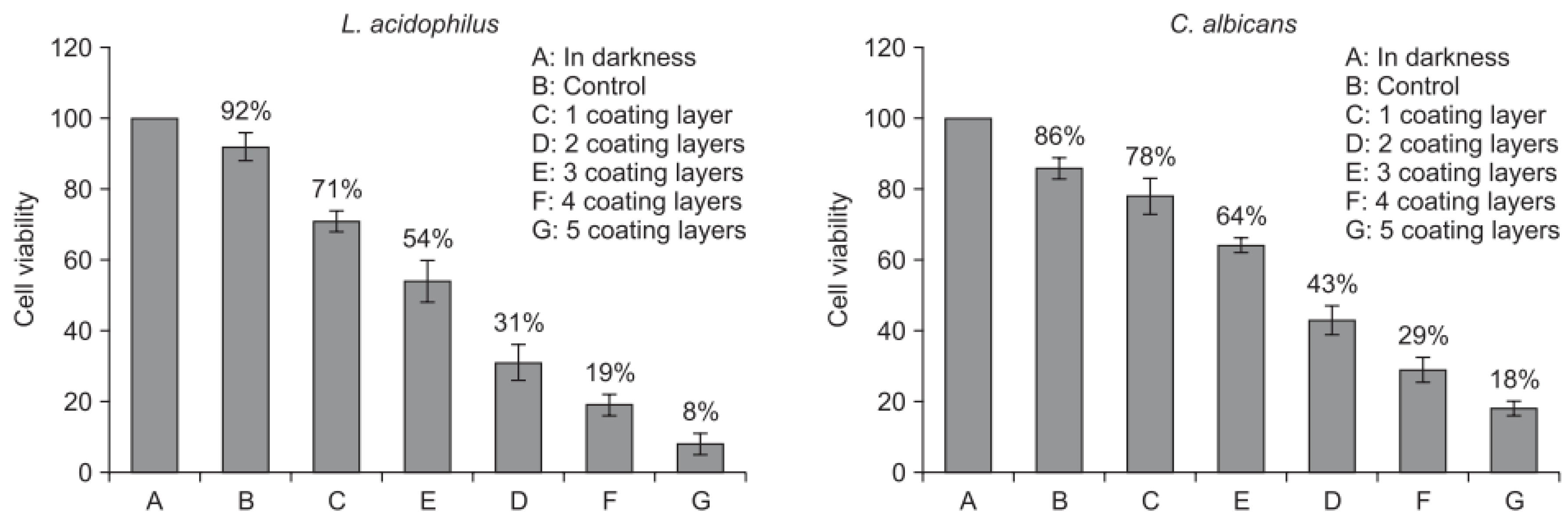
| ceramic brackets | photocatalytic TiO2 | sol-gel dip coating | Lactobacillus acidophilus and Candida albicans | The antibacterial effect was measured by the numbers of colonies these bacteria strains on a plate and by the photocatalytic antibacterial test under UV-A light irradiation. | The films with 5 coating layers and annealed at 700 °C exhibited the greatest antibacterial activity against L. acidophilus and C. albicans under UV-A light irradiation. | Cao et al. [69] |
| NiTi and Cu-NiTi archwires | without coating | Streptococcus mutans | The S. mutans adhesion was evaluated by using real-time polymerase chain reaction. | S. mutans adhesion, surface roughness, and surface free energy were greater in Cu-NiTi than NiTi archwires. | Abraham et al. [70] | |
| NiTi archwires after 4 and 8 weeks of intraoral use | - Esthetic coated NiTi wires: - Ortho Organizers (Sao Marcos, Calif) - Forestadent (Pforzheim, Germany) - TP Orthodontics (Laporte, Ind) | Streptococcus mutans, Staphylococcus aureus and Candida albicans | The amount of bacterial adhesion was quantified using the colony-count method. | Biofilm adhesion increased after intraoral use at all time intervals. There was a positive correlation between surface roughness and biofilm adhesion in vivo only. | Taha et al. [71] | |
| SS brackets | nitrogen-doped (N-doped) | Radiofrequency magnetron sputtering | Streptococcus mutans, Lactobacillus acidophilus, Actinomyces viscous and Candida albicans | The antimicrobial activity was assessed on the basis of colony counts. | The bracket coated with thin film shows high antimicrobial activity against bacteria and strongly prevents the adherence of them. | Cao et al. [72] |
| Ti and TiAg metals | photocatalytic TiO2 | anodic oxidation (AO) and thermal oxidation (TO) | Streptococcus mutans | Photocatalytic antibacterial activity with ultraviolet A (UVA) illumination. | The antibacterial effect of a TiO2 film formed by AO superior to that formed by TO. The addition of Ag to Ti specimen indicated a synergistic effect on the photocatalytic antibacterial property against S. mutans. | Choi et al. [73] |
| SS, ceramic and plastic orthodontic brackets | without coating | Streptococcus mutans and Streptococcus sanguis | Adhesion was quantitated by a microbial culture technique by treating the brackets with adhering bacteria with trypsin and enumerating the total viable counts of bacteria recovered after cultivation. | There were no differences in the adherence to stainless steel, ceramic, or plastic brackets. The presence of an early salivary pellicle and S. sanguis reduced the number of adhering S. mutans to all three types of brackets. | Papaioannou et al. [6] | |
| 4 different kinds of esthetic wires Ultraesthetic, Dany Coated, Bioforce Sentalloy White, TruGold and 2 uncoated: SS and NiTi wires | - Epoxy-coated nickel-titanium alloy - Bio-polymer (outer) and silver (inner) - coated nickel-titanium alloy - Rhodium-coated nickel-titanium alloy - 24K gold-plated stainless-steel | Streptococcus mutans and Streptococcus sanguis | The amount of bacteria adhering to the wires was quantified using the colony-counting method and by observing by scanning electron microscopy. | Adhesion of S. mutans to wires was greater than that of S. sobrinus. Some esthetic coatings on NiTi alloy might reduce S. mutans SM adhesion in vitro in the short term. | Kim et al. [23] | |
| a conventional orthodontic composite material (Transbond XT) + experimental composite adhesive material (ECA) and two conventional adhesives (composite and resin-modified glass ionomer) | ECA was modified by the addition of silica nanofillers and silver nanoparticles | cariogenic Streptococci | Effect of surface characteristics, physical properties, and antibacterial activities of ECA against cariogenic Streptococci. | ECA had rougher surfaces than conventional adhesives due to addition of silver nanoparticles and bacterial adhesion to ECA was less than to conventional adhesives. No significant difference in shear bond strength and bond failure interface between ECA and conventional adhesives were noted. | Uysal et al. [74], Ahn et al. [7] | |
| Group | Weight | Significance | |||
|---|---|---|---|---|---|
| Initial | Final | Change | |||
| 1. Control group | Includes 10 uncoated stainless steel orthodontic wires | 0.240 ± 0.021 (0.200–0.250) | 0.325 ± 0.035 (0.250–0.350) | 0.085 ± 0.024 (35.4%) | p < 0.001 ** |
| 2. Experimental group | Includes 10 surface-modified stainless steel orthodontic wires coated with silver | 0.245 ± 0.028 (0.200–0.300) | 0.255 ± 0.037 (0.200–0.300) | 0.010 ± 0.020 (4.08%) | p = 0.168 |
| 3. Control group | Includes 10 uncoated nickel titanium wires | 0.220 ± 0.026 (0.200–0.250) | 0.265 ± 0.033 (0.250–0.350) | 0.045 ± 0.028 (20.5%) | p < 0.001 ** |
| 4. Experimental group | Includes 10 surface-modified nickel titanium orthodontic wires coated with silver | 0.225 ± 0.042 (0.150–0.300) | 0.235 ± 0.047 (0.150–0.300) | 0.010 ± 0.021 (4.4%) | p = 0.169 |
| Group | Colony Count Range | Mean ± SD | p Value | |
|---|---|---|---|---|
| 1. Control group | Includes 10 uncoated stainless steel orthodontic wires | 776–934 | 836.60 ± 48.97 | p < 0.001 ** |
| 2. Experimental group | Includes 10 surface-modified stainless steel orthodontic wires coated with silver | 176–262 | 220.90 ± 30.73 | - |
| 3. Control group | Includes 10uncoated nickel titanium wires | 710–810 | 748.90 ± 35.64 | p < 0.001 ** |
| 4. Experimental group | Includes 10 surface-modified nickel titanium orthodontic wires coated with silver | 117–264 | 203.20 ± 41.94 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bącela, J.; Łabowska, M.B.; Detyna, J.; Zięty, A.; Michalak, I. Functional Coatings for Orthodontic Archwires—A Review. Materials 2020, 13, 3257. https://doi.org/10.3390/ma13153257
Bącela J, Łabowska MB, Detyna J, Zięty A, Michalak I. Functional Coatings for Orthodontic Archwires—A Review. Materials. 2020; 13(15):3257. https://doi.org/10.3390/ma13153257
Chicago/Turabian StyleBącela, Justyna, Magdalena Beata Łabowska, Jerzy Detyna, Anna Zięty, and Izabela Michalak. 2020. "Functional Coatings for Orthodontic Archwires—A Review" Materials 13, no. 15: 3257. https://doi.org/10.3390/ma13153257
APA StyleBącela, J., Łabowska, M. B., Detyna, J., Zięty, A., & Michalak, I. (2020). Functional Coatings for Orthodontic Archwires—A Review. Materials, 13(15), 3257. https://doi.org/10.3390/ma13153257









