The Impact of Ag Nanoparticles and CdTe Quantum Dots on Expression and Function of Receptors Involved in Amyloid-β Uptake by BV-2 Microglial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Amyloid β
2.2. Cell Culture
2.3. Nanoparticle Preparation and Characterization
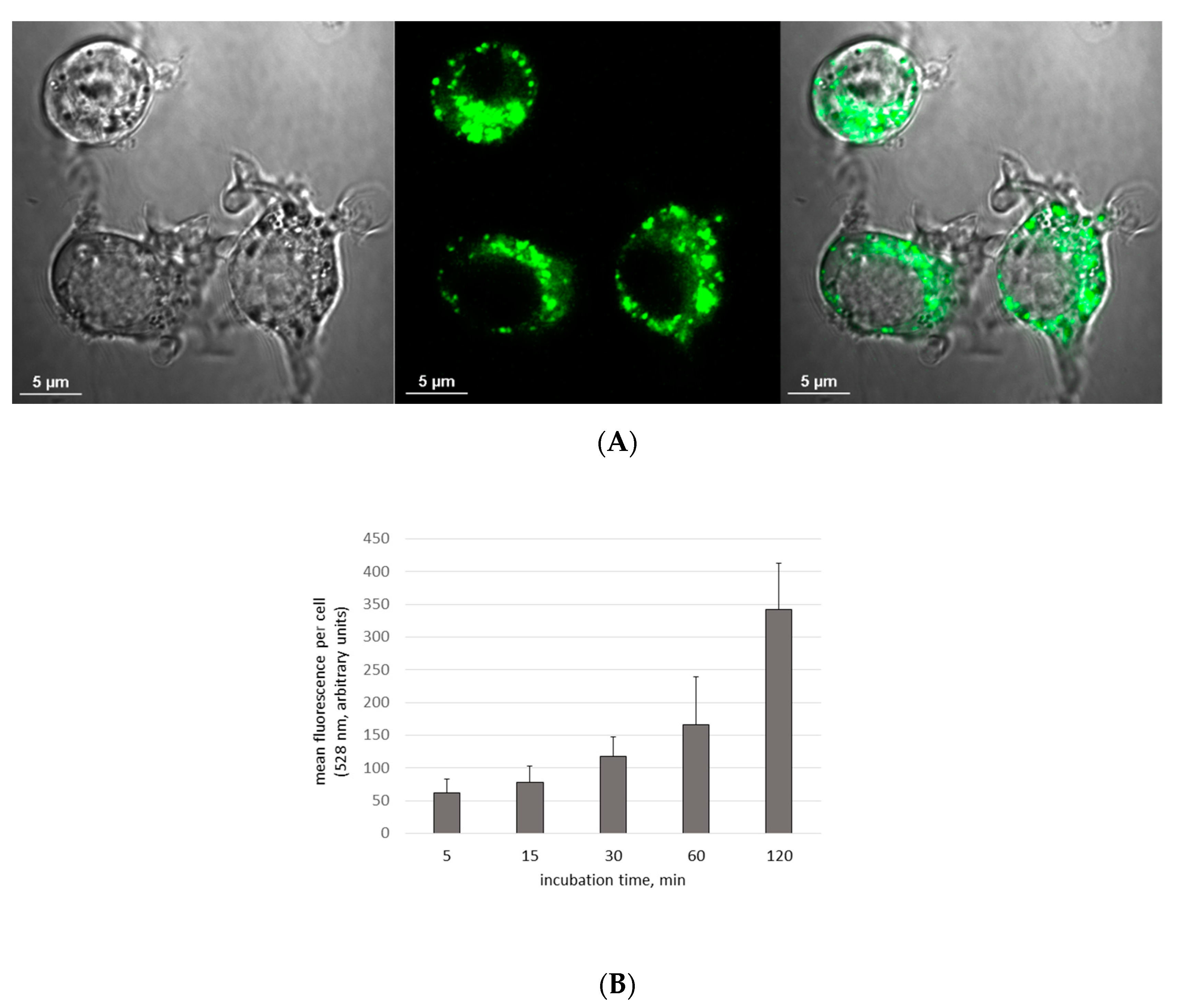
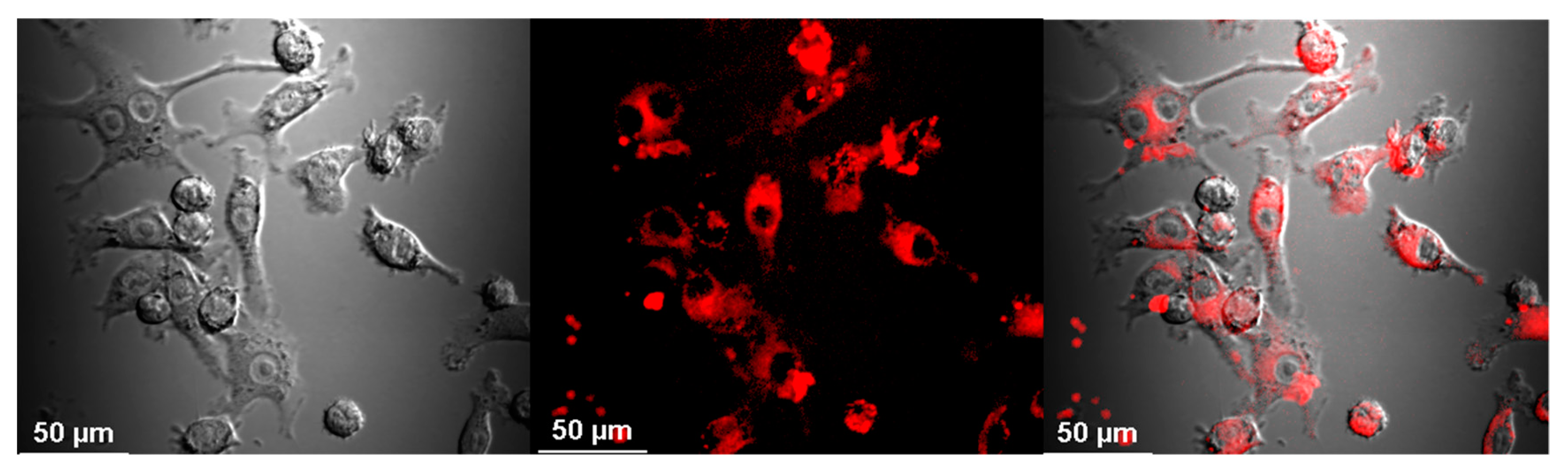
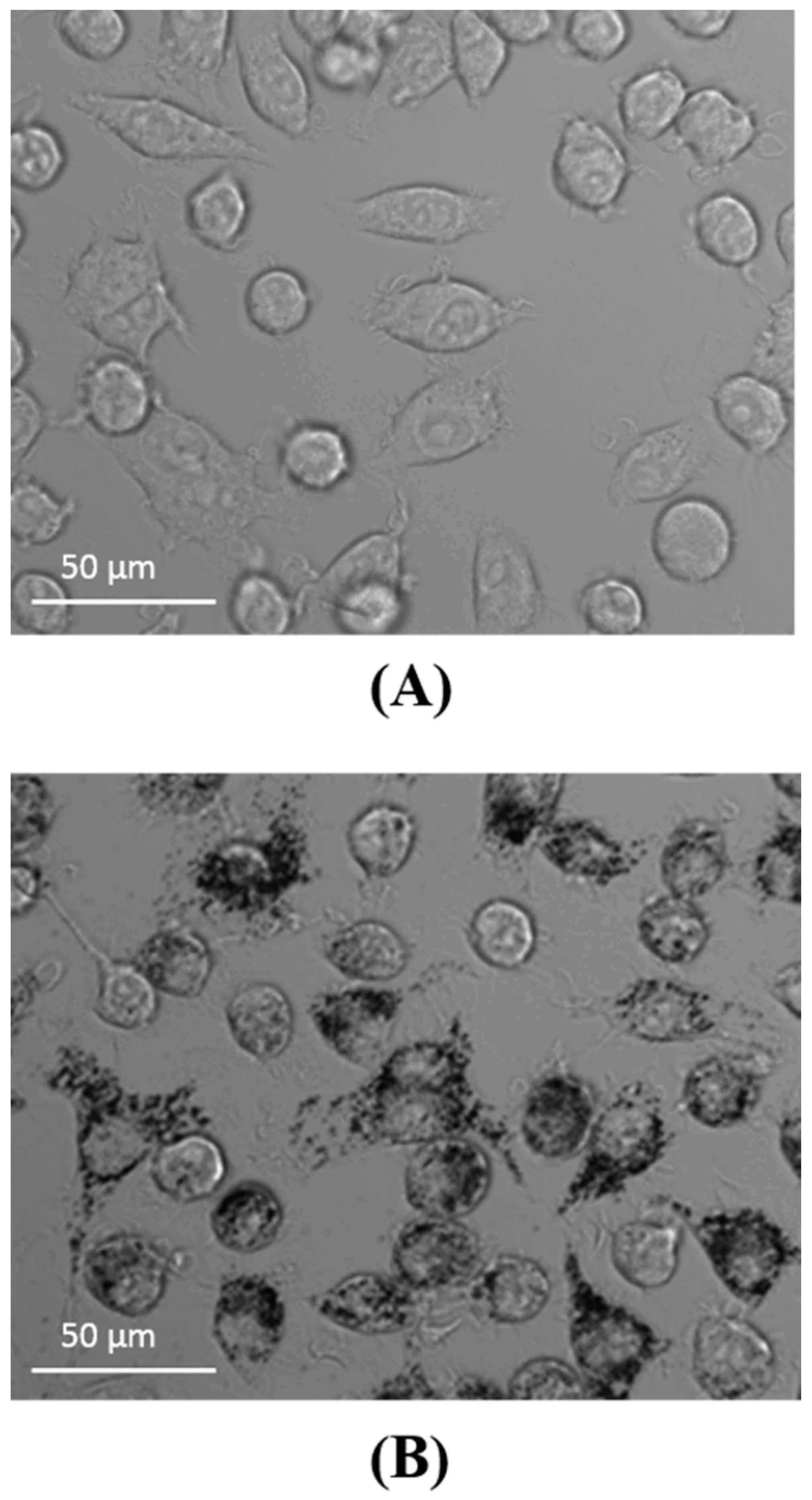
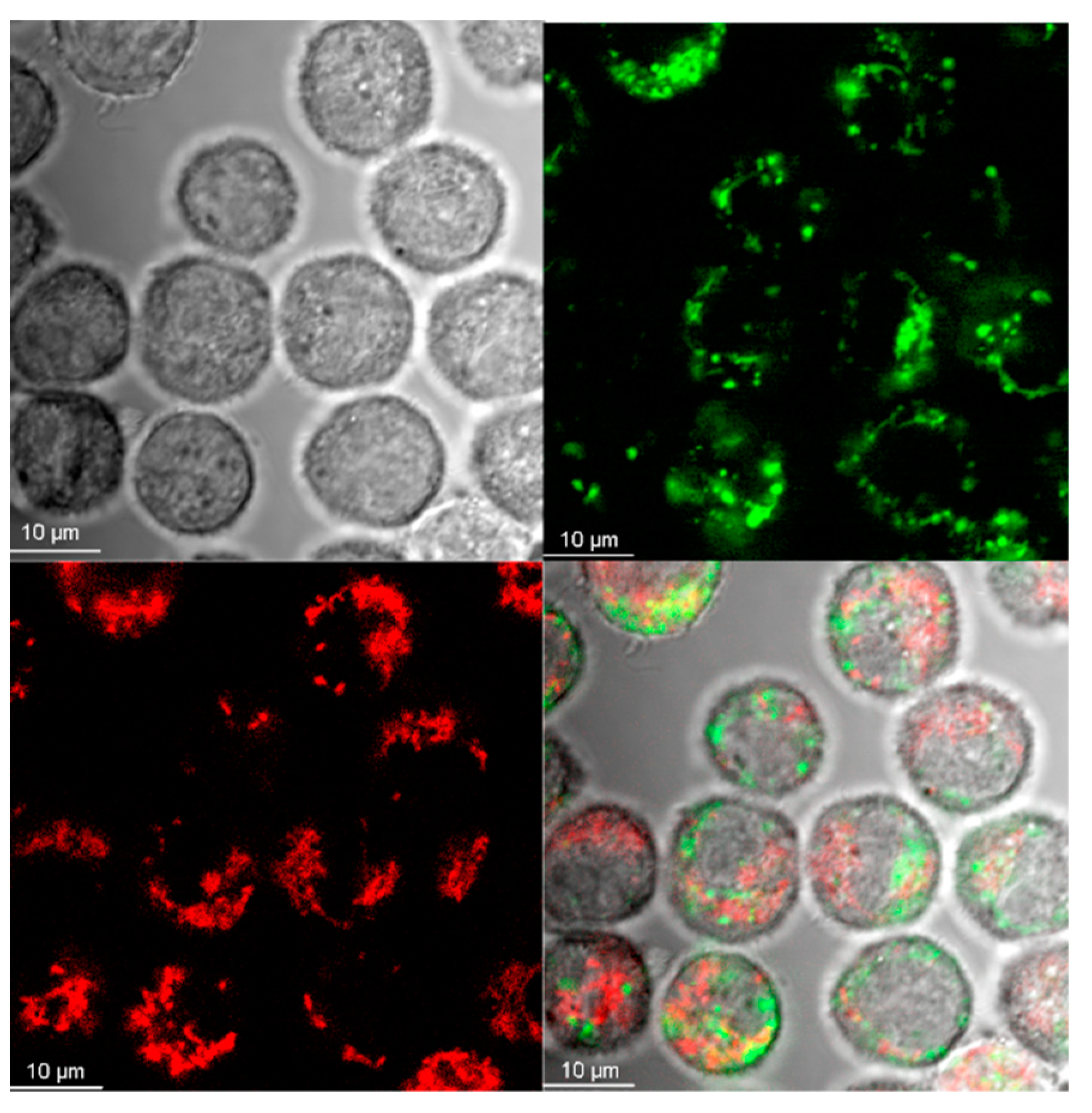
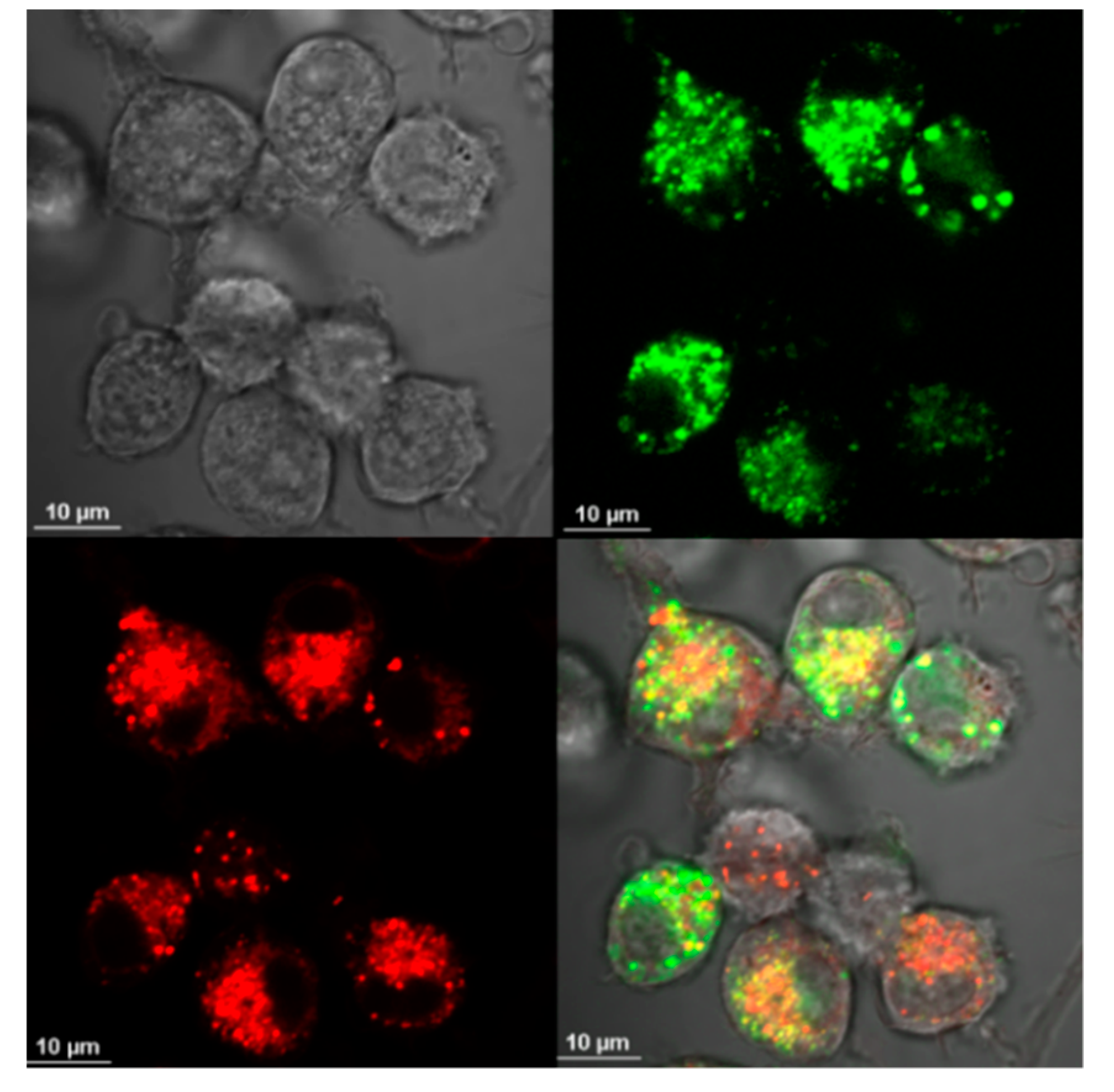
2.4. Visualization of Aβ and NP Uptake and Localization
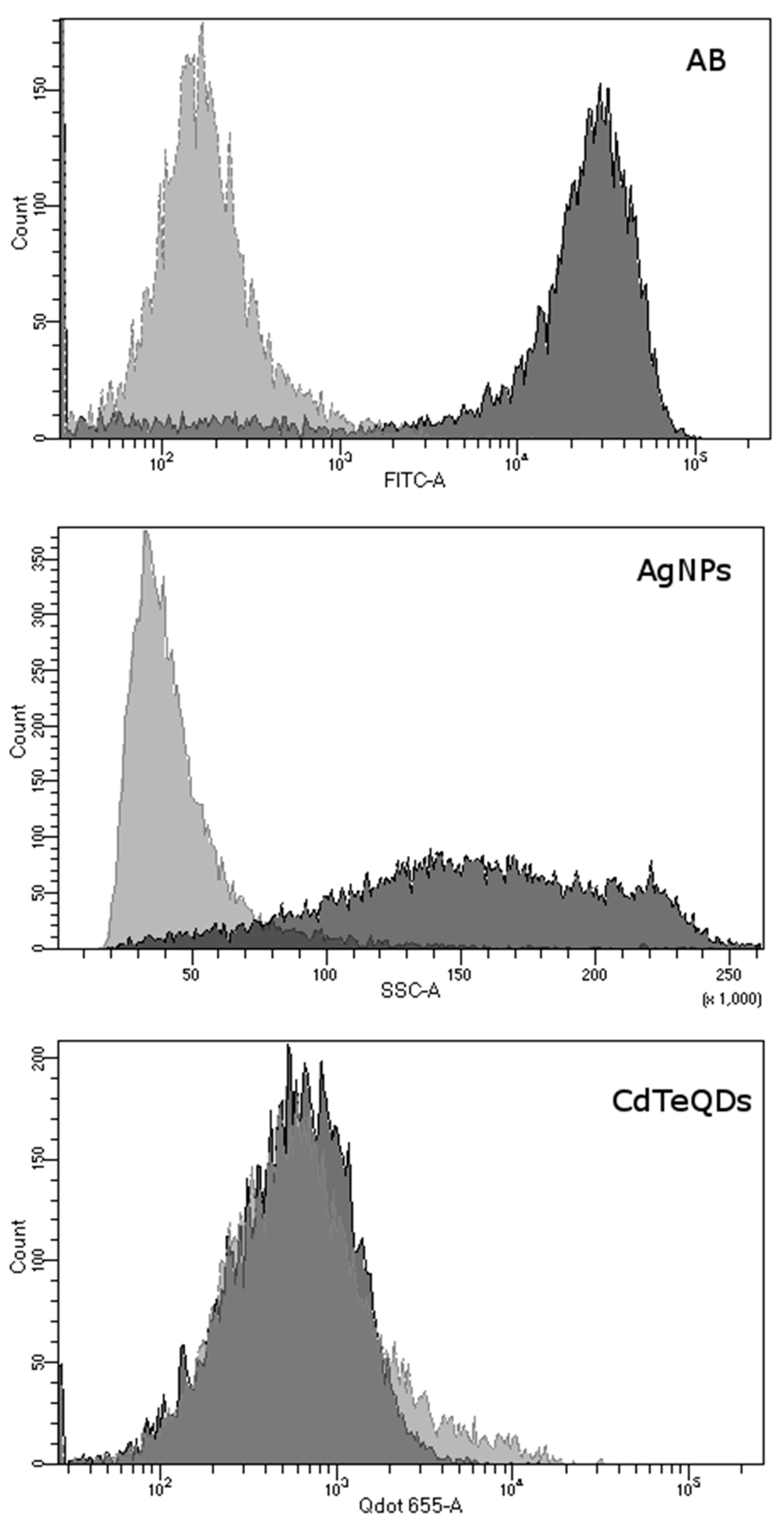
2.5. Measurement of Aβ and NP Uptake by Flow Cytometry
2.6. Assessment of the Role of Scavenger Receptors Class A in Aβ and NP Uptake
2.7. Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. The Uptake of Aβ and NP by Microglial Cells
3.2. Analysis of Gene Expression in BV-2 Cells Treated with Aβ or NP
3.3. Role of Scavenger Receptors Class A in Aβ and NP Uptake
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Crescenzi:, O.; Tomaselli, S.; Guerrini, R.; Salvadori, S.; D’Ursi, A.M.; Temussi, P.A.; Picone, D. Solution structure of the Alzheimer amyloid b-peptide (1–42) in an apolar microenvironment. Similarity with a virus fusion domain. Eur. J. Biochem. 2002, 269, 5642–5648. [Google Scholar] [CrossRef] [PubMed]
- Welikovitch, L.A.; Carmo, S.D.; Maglóczky, Z.; Malcolm, J.C.; Loke, J.; Klein, W.L.; Freund, T.; Cuello, A.C. Early intraneuronal amyloid triggers neuron-derived inflammatory signaling in APP transgenic rats and human brain. Pro. Natl. Acad. Sci. USA 2020, 24, 6844–6854. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, N. Cure for Alzheimer’s Disease. World J. Neurosci. 2015, 5, 328–330. [Google Scholar] [CrossRef][Green Version]
- Hadavi, D.; Poot, A.A. Biomaterials for the Treatment of Alzheimer’s Disease. Front. Bioeng. Biotechnol. 2016, 4, 49. [Google Scholar] [CrossRef]
- Nazem, N.; Mansoori, G.A. Nanotechnology Solutions for Alzheimer’s Disease: Advances in Research Tools. Diagnostic Methods and Therapeutic Agents. J. Alzheimer’s Dis. 2018, 13, 199–223. [Google Scholar] [CrossRef]
- Brambilla, D.; Le Droumaguet, B.; Nicolas, J.; Hashemi, S.H.; Wu, L.P.; Moghimi, S.M.; Couvreur, P.; Andrieux, K. Nanotechnologies for Alzheimer’s disease: Diagnosis, therapy and safety issues. Nanomedicine 2011, 7, 521–540. [Google Scholar] [CrossRef]
- Re, F.; Gregori, M.; Masserini, M. Nanotechnology for neurodegenerative disorders. Maturitas 2012, 73, 45–51. [Google Scholar] [CrossRef]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.L.; Wang, Y.X.J.; Chow, A.H.L.; Wu, E.X.; Baum, L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, D.Y.; Lee, T.; Kim, S.U.; Oh, B.K.; Choil, J.W. Signal enhancement of surface plasmon resonance based immunosensor using gold nanoparticle-antibody complex for beta-amyloid (1–40) detection. J. Nanosci. Nanotechnol. 2009, 9, 7155–7160. [Google Scholar] [CrossRef]
- Brambilla, D. Polymeric Nanoparticles as Original Theranostic Approach for Alzheimers Disease. Ph.D. Thesis, Paris-Saclay University, Paris, France, 2012. [Google Scholar]
- Chan, H.M.; Xiao, L.; Yeung, K.M.; Ho, S.L.; Zhao, D.; Chan, W.H.; Li, H.W. Effect of surface-functionalized nanoparticles on the elongation phase of beta-amyloid (1e40) fibrillogenesis. Biomaterials 2012, 33, 4443–4450. [Google Scholar] [CrossRef]
- Ge, L.; Li, Q.; Wang, M.; Ouyang, J.; Li, X.; Xing, M.M.Q. Nanosilver particles in medical applications: Synthesis, performance, and toxicity. Int. J. Nanomed. 2014, 9, 2399–2407. [Google Scholar]
- Hsiao, I.L.; Hsieh, Y.K.; Wang, C.F.; Chen, I.C.; Huang, Y.J. Trojan-Horse Mechanism in the Cellular Uptake of Silver Nanoparticles Verified by Direct Intra- and Extracellular Silver Speciation Analysis. Environ. Sci. Technol. 2015, 49, 3813–3821. [Google Scholar] [CrossRef] [PubMed]
- Derecki, N.C.; Katzmarski, N.; Kipnis, J.; Meyer-Luehmann, M. Microglia as a critical player in both developmental and late-life CNS pathologies. Acta Neuropathol. 2014, 128, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Choucair, N.; Laporte, V.; Levy, R.; Arnold, A.S.; Gies, J.P.; Poindron, P.; Lombard, Y. Phagocytic functions of microglial cells in the central nervous system and their importance in two neurodegenerative diseases: Multiple sclerosis and Alzheimer’s disease. Cent. Eur. J. Biol. 2006, 4, 463–493. [Google Scholar] [CrossRef]
- Crehan, H. Complement Receptor 1 in Microglia; Implications for Alzheimer’s Disease. Ph.D. Thesis, University College London, London, UK, 2014. [Google Scholar]
- Thériault, P.; ElAli, A.; Rivest, S. The dynamics of monocytes and microglia in Alzheimer’s disease. Alzheimer’s Res. Ther. 2015, 7, 1–10. [Google Scholar]
- Chung, H.; Brazil, M.L.; Soe, T.T.; Maxfield, F.R. Uptake, Degradation and Release of Fibrillar and Soluble Forms of Alzheimer’s Amyloid b-Peptide by Microglial Cells. J. Biol. Chem. 1999, 274, 32301–32308. [Google Scholar] [CrossRef]
- Koenigsknecht, J.; Landreth, G. Microglial Phagocytosis of Fibrillar β-Amyloid through a β1 Integrin-Dependent Mechanism. J. Neurosci. 2004, 24, 9838–9846. [Google Scholar] [CrossRef]
- Sokolowski, J.D.; Mandell, J.W. Phagocytic Clearance in Neurodegeneration. Am. J. Pathol. 2011, 178, 1416–1428. [Google Scholar] [CrossRef]
- Tahara, K.; Kim, H.D.; Jin, J.J.; Maxwell, J.A.; Li, L.; Fukuchi, K.I. Role of toll-like receptor signalling in Aβ uptake and clearance. Brain 2006, 129, 3006–3019. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.J.; Zhou, W.W.; Wang, S.W.; Xu, P.X.; Yu, X.L.; Liu, R.T. Activated Scavenger Receptor A Promotes Glial Internalization of Ab. PLoS ONE 2014, 9, 1–11. [Google Scholar]
- Sikorska, K.; Grądzka, I.; Sochanowicz, B.; Presz, A.; Męczyńska-Wielgosz, S.; Brzóska, K.; Kruszewski, M.K. Diminished amyloid-β uptake by mouse microglia upon treatment with quantum dots, silver or cerium oxide nanoparticles. Nanoparticles and amyloid-β uptake by microglia. Hum. Exp. Toxicol. 2020, 39, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tatsushi, T.; Ibuki, Y. Simple and Easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis. Environ. Sci. Technol. 2007, 41, 3018–3024. [Google Scholar] [CrossRef] [PubMed]
- Mukaka, M.M. Statistics Corner: A guide to appropriate use of Correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Frenkel, D.; Wilkinson, K.; Zhao, L.; Hickman, S.E.; Means, T.K.; Puckett, L.; Farfara, D.; Kingery, N.D.; Weiner, H.L.; El Khoury, J. Scara1 deficiency impairs clearance of soluble amyloid-β by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nat. Commun. 2013, 4, 2030. [Google Scholar] [CrossRef]
- Józefowski, S. Rola Receptorów Zmiataczy Klasy, A. SR-A i MARCO. W Układzie Odpornościowym. Część 1. Budowa Receptorów. Repertuar Wiązanych Ligandów i Zdolność Do Transdukcji Sygnału. Postepy Hig. Med. Dosw. 2012, 66, 104–119. [Google Scholar]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue Rachal Love, B.; Perry, S.; Paquette, N.; Deane, R.J.; Thiyagarajan, M.; et al. A multimodal RAGE-specific inhibitor reduces amyloid β–mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Invest. 2012, 122, 1377–1392. [Google Scholar] [CrossRef]
- Wilkinson, K.; El Khoury, J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int. J. Alzheimers Dis 2012, 2012, 456–489. [Google Scholar] [CrossRef]
- Coraci, I.S.; Husemann, J.; Berman, J.W.; Hulette, C.; Dufour, J.H.; Campanella, G.K.; Luster, A.D.; Silverstein, S.C.; El-Khoury, J.B. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am. J. Pathol. 2002, 160, 101–112. [Google Scholar] [CrossRef]
- El Khoury, J.B.; Moore, K.J.; Means, T.K.; Leung, J.; Terada, K.; Toft, M.; Freeman, M.W.; Luster, A.D. CD36 mediates the innate host response to beta-amyloid. J. Exp. Med. 2003, 197, 1657–1666. [Google Scholar] [CrossRef]
- Griciuc, A.; Serrano-Pozo, A.; Parrado, A.R.; Lesinski, A.N.; Asselin, C.N.; Mullin, K.; Hooli, B.; Choi, B.H.; Hyman, B.T.; Tanzi, R.E. Alzheimer’s Disease Risk Gene CD33 Inhibits Microglial Uptake of Amyloid Beta. Neuron 2013, 78, 631–643. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, J.T.; Hu, N.; Tan, M.S.; Zhu, X.C.; Tan, L. CD33 in Alzheimer’s Disease. Mol. Neurobiol. 2014, 49, 529–535. [Google Scholar] [CrossRef]
- Dong, L. The role of phospholipase a2 in amyloid β uptake by microglia. Master’s Thesis, University of Missouri-Columbia, Columbia, MO, USA, 2015. [Google Scholar]
- Domert, J.; Rao, S.B.; Agholme, L.; Brorsson, A.C.; Marcusson, J.; Hallbeck, M.; Nath, S. Spreading of amyloid-β peptides via neuritic cell-to-cell transfer is dependent on insufficient cellular clearance. Neurobiol. Dis. 2014, 65, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Bao, J.; Zhao, X.; Shen, H.; Lv, J.; Ma, S.; Zhang, X.; Li, Z.; Wang, S.; Wang, Q.; et al. Activated Cyclin-Dependent Kinase 5 Promotes Microglial Phagocytosis of Fibrillar β-Amyloid by Up-regulating Lipoprotein Lipase Expression. Mol. Cell. Proteom. 2013, 12, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, S.; Jiang, Q.; Lee, C.Y.D.; Koenigsknecht-Talboo, J.; Holtzman, D.M.; Landreth, G.E. Microglia Mediate the Clearance of Soluble Aβ through Fluid Phase Macropinocytosis. J. Neurosci. 2009, 29, 4252–4262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Shi, X.Y.; Bi, D.C.; Fang, W.S.; Wei, G.B.; Xu, X. Alginate-Derived Oligosaccharide Inhibits Neuroinflammation and Promotes Microglial Phagocytosis of β-Amyloid. Mar. Drugs. 2015, 13, 5828–5846. [Google Scholar] [CrossRef] [PubMed]
- Minami, S.S.; Sun, B.; Popat, K.; Kauppinen, T.; Pleiss, M.; Zhou, Y.; Ward, M.E.; Floreancig, P.; Mucke, L.; Desai, T.; et al. Selective targeting of microglia by quantum dots. J. Neuroinflammation 2012, 9, 22. [Google Scholar] [CrossRef]
- Gonzalez-Carter, D.A.; Leo, B.F.; Ruenraroengsak, P.; Chen, S.; Goode, A.E.; Theodorou, J.G.; Chung, K.F.; Carzaniga, R.; Shaffer, M.S.P.; Dexter, D.T.; et al. Silver nanoparticles reduce brain inflammation and related neurotoxicity through induction of H2S-synthesizing enzymes. Sci. Rep. 2017, 7, 42871. [Google Scholar] [CrossRef]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef]
- Aldossari, A.A.; Shannahan, J.H.; Podila, R.; Brown, J.M. Scavenger receptor B1 facilitates macrophage uptake of silver nanoparticles and cellular activation. J. Nanopart. Res. 2015, 17, 313. [Google Scholar] [CrossRef]
- Ezzat, K.; Aoki, Y.; Koo, T.; McClorey, G.; Benner, L.; Coenen-Stass, A.; O’Donovan, L.; Lehto, T.; Garcia-Guerra, A.; Nordin, J.; et al. Self-Assembly into Nanoparticles Is Essential for Receptor Mediated Uptake of Therapeutic Antisense Oligonucleotide. Nano Lett. 2015, 15, 4364–4373. [Google Scholar]
- Patel, P.C.; Giljohann, D.A.; Daniel, W.L.; Zheng, D.; Prigodich, A.E.; Mirkin, C.A. Scavenger Receptors Mediate Cellular Uptake of Polyvalent Oligonucleotide-Functionalized Gold Nanoparticles. Bioconjugate Chem. 2010, 15, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Ramaro, P. Cellular uptake, intracellular trafficking and cytotoxicity of silver nanoparticles. Toxicol. Lett. 2012, 213, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Poblete, H.; Agarwal, A.; Thomas, S.S.; Bohne, C.; Ravichandran, R.; Phopase, J.; Comer, J.; Alarcon, E.I. New Insights Into Peptide-Silver Nanoparticle Interaction: Deciphering the Role of Cysteine and Lysine in the Peptide Sequence. Langmuir 2016, 32, 265–273. [Google Scholar] [CrossRef]
- Yap, N.V.L.; Whelan, F.J.; Bowdish, D.M.E.; Golding, G.B. The evolution of the scavenger receptor cysteine-rich domain of the class A scavenger receptors. Front. Immunol. 2015, 6, 342. [Google Scholar] [CrossRef]
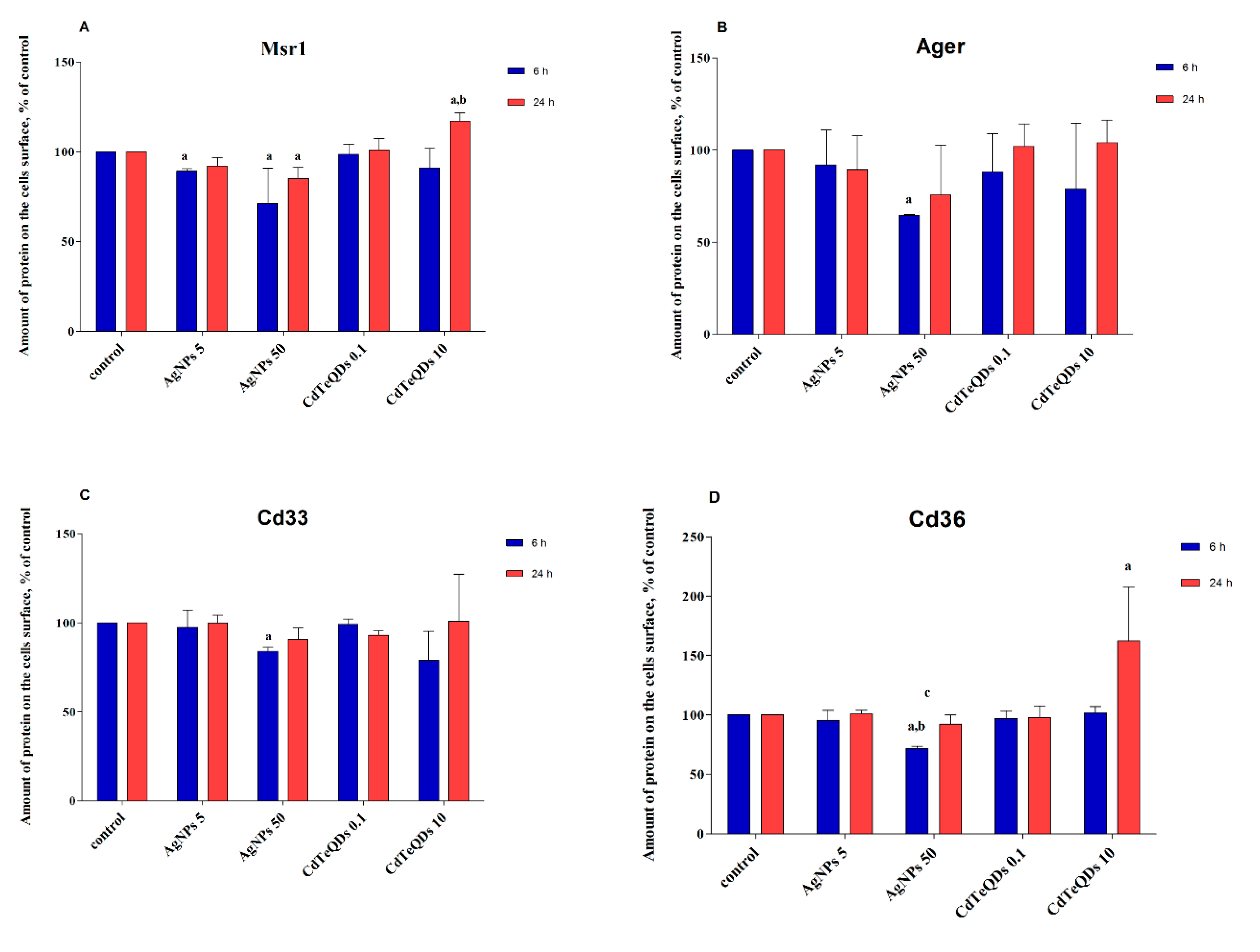
| Sample | Gene | Mean Rq (Relative Quantification) Value | Rq Min | Rq Max | p Value |
|---|---|---|---|---|---|
| Control | Ager | 1 | 0.913 | 1.096 | 1 |
| Cd33 | 1 | 0.974 | 1.026 | 1 | |
| Cd36 | 1 | 0.644 | 1.553 | 1 | |
| Msr1 | 1 | 0.525 | 1.906 | 1 | |
| AgNP 5 μg mL−1 | Ager | 0.913 | 0.809 | 1.031 | 0.363 |
| Cd33 | 0.839 | 0.756 | 0.932 | 0.093 | |
| Cd36 | 1.041 | 0.653 | 1.658 | 0.919 | |
| Msr1 | 0.993 | 0.514 | 1.92 | 0.99 | |
| AgNP 50 μg mL−1 | Ager | 0.959 | 0.791 | 1.163 | 0.757 |
| Cd33 | 0.903 | 0.826 | 0.987 | 0.177 | |
| Cd36 | 1.327 | 0.804 | 2.189 | 0.504 | |
| Msr1 | 0.988 | 0.504 | 1.937 | 0.983 | |
| CdTeQD 0.1 μg mL−1 | Ager | 0.897 | 0.577 | 1.393 | 0.713 |
| Cd33 | 0.800 | 0.732 | 0.875 | 0.040 | |
| Cd36 | 1.393 | 0.879 | 2.207 | 0.419 | |
| Msr1 | 0.997 | 0.478 | 2.079 | 0.996 | |
| CdTeQD 10 μg mL−1 | Ager | 0.849 | 0.686 | 1.05 | 0.315 |
| Cd33 | 1.168 | 1.107 | 1.232 | 0.022 | |
| Cd36 | 3.781 | 2.397 | 5.963 | 0.022 | |
| Msr1 | 1.664 | 0.891 | 3.105 | 0.382 |
| Treatment | Parameter Measured (Arbitrary Units) | ||
|---|---|---|---|
| SSC (Side Scatter Value) | Fluorescence | ||
| Aβ | −PA | Not applicable | 34,016 ± 5567 |
| +PA | Not applicable | 207 ± 35.4 a | |
| AgNP | −PA | 169 ± 17.6 | Not applicable |
| +PA | 46 ± 2.9 a | Not applicable | |
| CdTeQD | −PA | Not applicable | 864 ± 136 |
| +PA | Not applicable | 1337 ± 599 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikorska, K.; Grądzka, I.; Wasyk, I.; Brzóska, K.; Stępkowski, T.M.; Czerwińska, M.; Kruszewski, M.K. The Impact of Ag Nanoparticles and CdTe Quantum Dots on Expression and Function of Receptors Involved in Amyloid-β Uptake by BV-2 Microglial Cells. Materials 2020, 13, 3227. https://doi.org/10.3390/ma13143227
Sikorska K, Grądzka I, Wasyk I, Brzóska K, Stępkowski TM, Czerwińska M, Kruszewski MK. The Impact of Ag Nanoparticles and CdTe Quantum Dots on Expression and Function of Receptors Involved in Amyloid-β Uptake by BV-2 Microglial Cells. Materials. 2020; 13(14):3227. https://doi.org/10.3390/ma13143227
Chicago/Turabian StyleSikorska, Katarzyna, Iwona Grądzka, Iwona Wasyk, Kamil Brzóska, Tomasz M. Stępkowski, Malwina Czerwińska, and Marcin K. Kruszewski. 2020. "The Impact of Ag Nanoparticles and CdTe Quantum Dots on Expression and Function of Receptors Involved in Amyloid-β Uptake by BV-2 Microglial Cells" Materials 13, no. 14: 3227. https://doi.org/10.3390/ma13143227
APA StyleSikorska, K., Grądzka, I., Wasyk, I., Brzóska, K., Stępkowski, T. M., Czerwińska, M., & Kruszewski, M. K. (2020). The Impact of Ag Nanoparticles and CdTe Quantum Dots on Expression and Function of Receptors Involved in Amyloid-β Uptake by BV-2 Microglial Cells. Materials, 13(14), 3227. https://doi.org/10.3390/ma13143227






