Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Water Adsorption Calorimetry
3. Results and Discussion
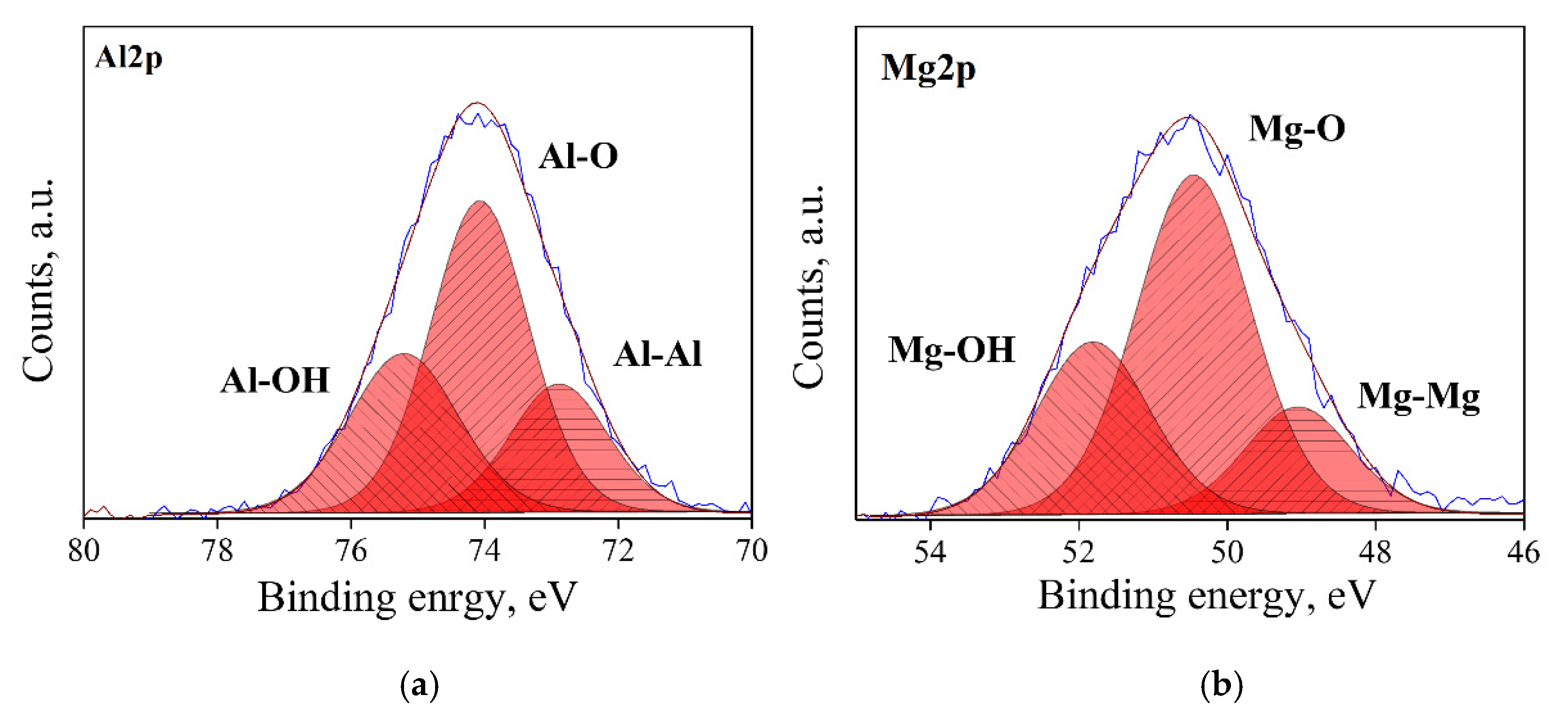
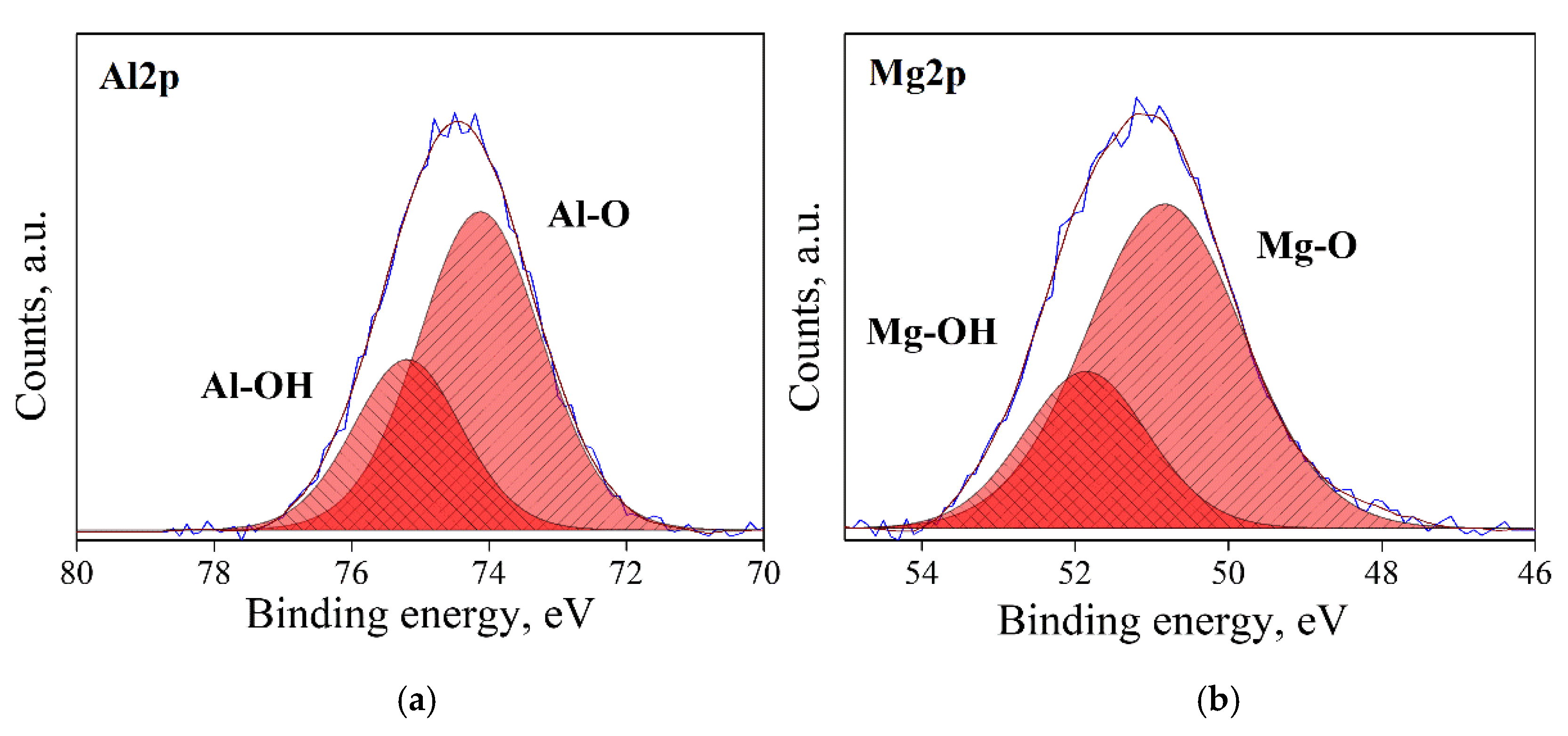
3.1. Surface State Analysis
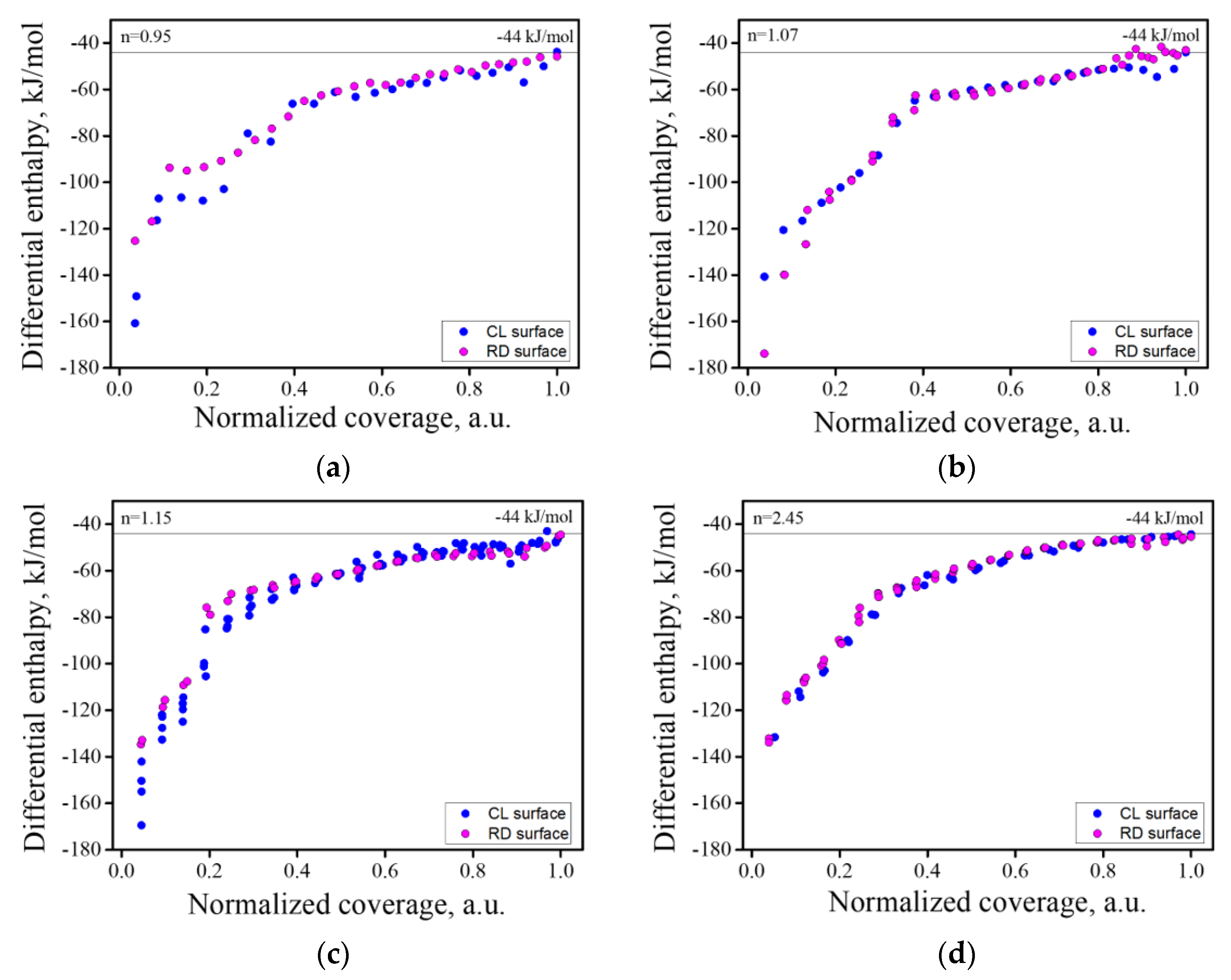
3.2. Water Adsorption Measurments
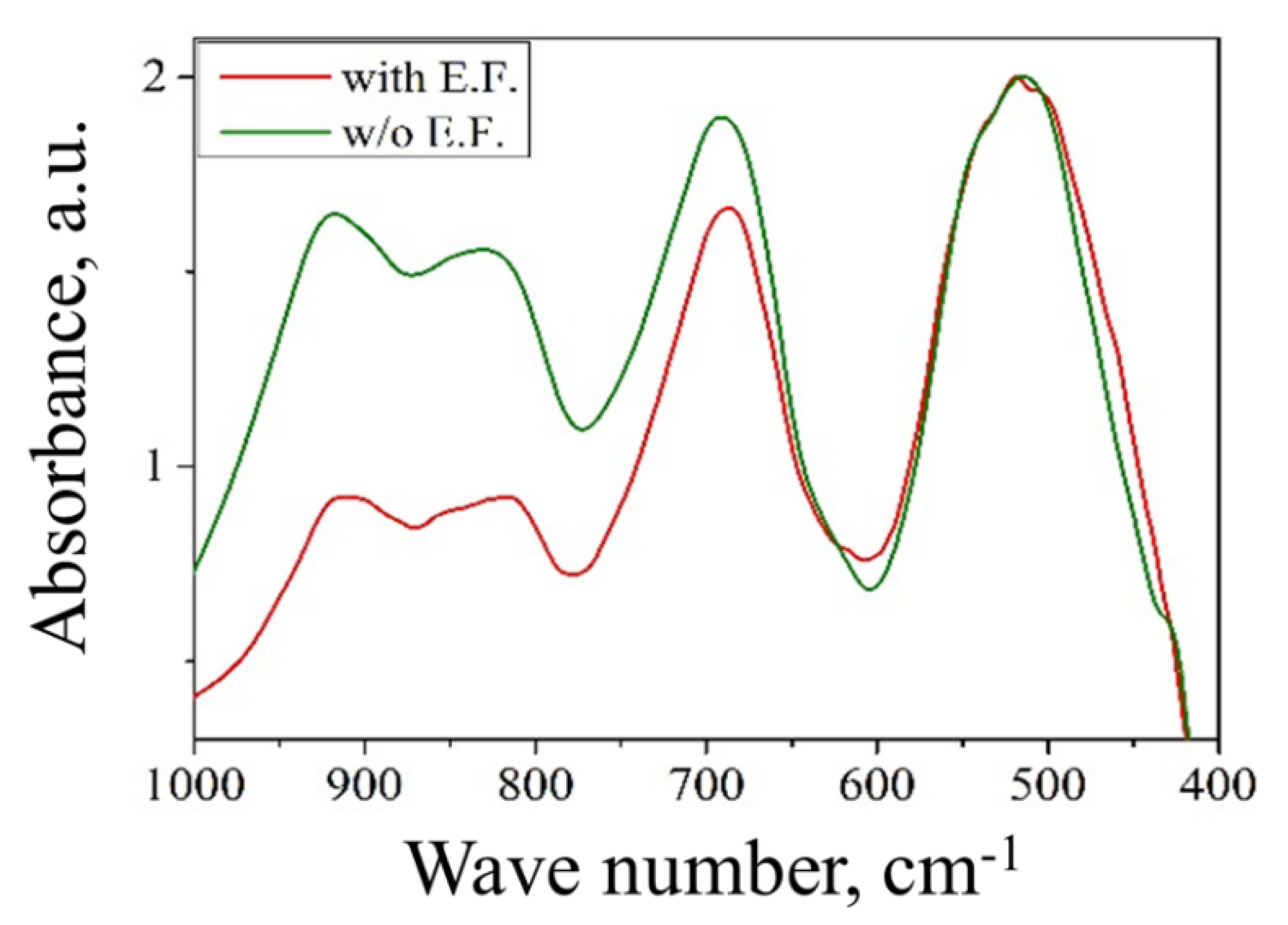
3.3. Effect of Anti-Site Defects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gusmano, G.; Montesperelli, G.; Traversa, E.; Mattogno, G. Microstructure and electrical properties of MgAl2O4 thin films for humidity sensing. J. Am. Ceram. Soc. 1993, 76, 743–750. [Google Scholar] [CrossRef]
- Govindaraj, A.; Flahaut, E.; Laurent, C.; Peigney, A.; Rousset, A.; Rao, C.N.R. An investigation of carbon nanotubes obtained from the decomposition of methane over reduced Mg1−xMxAl2O4 spinel catalysts. J. Mater. Res. 1999, 14, 2567–2576. [Google Scholar] [CrossRef]
- Mei, D.; Lebarbier Dagle, V.; Xing, R.; Albrecht, K.O.; Dagle, R.A. Steam reforming of ethylene glycol over MgAl2O4 supported Rh, Ni, and Co Catalysts. ACS Catal. 2016, 6, 315–325. [Google Scholar] [CrossRef]
- Villa, A.; Gaiassi, A.; Rossetti, I.; Bianchi, C.L.; Van Benthem, K.; Veith, G.M.; Prati, L. Au on MgAl2O4 spinels: The effect of support surface properties in glycerol oxidation. J. Catal. 2010, 275, 108–116. [Google Scholar] [CrossRef]
- Mei, D.; Glezakou, V.A.; Lebarbier, V.; Kovarik, L.; Wan, H.; Albrecht, K.O.; Gerber, M.; Rousseau, R.; Dagle, R.A. Highly active and stable MgAl2O4-supported Rh and Ir catalysts for methane steam reforming: A combined experimental and theoretical study. J. Catal. 2014, 316, 11–23. [Google Scholar] [CrossRef]
- Mei, D.; Lebarbier, V.M.; Rousseau, R.; Glezakou, V.A.; Albrecht, K.O.; Kovarik, L.; Flake, M.; Dagle, R.A. Comparative investigation of benzene steam reforming over spinel supported Rh and Ir catalysts. ACS Catal. 2013, 3, 1133–1143. [Google Scholar] [CrossRef]
- Li, W.Z.; Kovarik, L.; Mei, D.; Liu, J.; Wang, Y.; Peden, C.H.F. Stable platinum nanoparticles on specific MgAl2O4 spinel facets at high temperatures in oxidizing atmospheres. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.M.; de With, G. Computer simulation of dissociative adsorption of water on the surfaces of spinel MgAl2O4. J. Am. Ceram. Soc. 2001, 84, 1553–1558. [Google Scholar] [CrossRef]
- Hallstedt, B. Thermodynamic assessment of the system MgO-Al2O3. J. Am. Ceram. Soc. 1992, 75, 1497–1507. [Google Scholar] [CrossRef]
- Erukhimovitch, V.; Mordekoviz, Y.; Hayun, S. Spectroscopic study of ordering in non-stoichiometric magnesium aluminate spinel. Am. Mineral. 2015, 100, 1744–1751. [Google Scholar] [CrossRef]
- Mordekovitz, Y.; Hayun, S. On the effect of lithium on the energetics and thermal stability of nano-sized nonstoichiometric magnesium aluminate spinel. J. Am. Ceram. Soc. 2016, 99, 1–9. [Google Scholar] [CrossRef]
- Hilklin, T.R.; Laine, R.M. Synthesis of metastable phases in the magnesium spinel− alumina system. Chem. Mater. 2008, 20, 553–558. [Google Scholar] [CrossRef]
- Halabi, M.; Ezersky, V.; Kohn, A.; Hayun, S. Charge distribution in nano-scale grains of magnesium aluminate spinel. J. Am. Ceram. Soc. 2017, 100, 800–811. [Google Scholar] [CrossRef]
- Rubat du Merac, M.; Kleebe, H.-J.; Müller, M.M.; Reimanis, I.E. Fifty years of research and development coming to fruition; Unraveling the complex interactions during processing of transparent magnesium aluminate (MgAl2O4) spinel. J. Am. Ceram. Soc. 2013, 96, 3341–3365. [Google Scholar] [CrossRef]
- Reimanis, I.; Kleebe, H.-J. A review on the sintering and microstructure development of transparent spinel (MgAl2O4). J. Am. Ceram. Soc. 2009, 92, 1472–1480. [Google Scholar] [CrossRef]
- Ball, J.A.; Murphy, S.T.; Grimes, R.W.; Bacorisen, D.; Smith, R.; Uberuaga, B.P.; Sickafus, K.E. Defect processes in MgAl2O4 spinel. Solid State Sci. 2008, 10, 717–724. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Foster, A.S.; Hinnemann, B.; Canova, F.F.; Helveg, S.; Meinander, K.; Martin, N.M.; Knudsen, J.; Vlad, A.; Lundgren, E.; et al. Stable cation inversion at the MgAl2O4(100) surface. Phys. Rev. Lett. 2011, 107, 2–5. [Google Scholar] [CrossRef]
- Simeone, D.; Dondane-Thiriiet, C.; Gosset, D.; Daniel, P.; Beauvy, M. Comment—Disorder phase transition induced by swift ions in MgAl2O4 and ZnAl2O4 spinels. J. Nucl. Mater. 2002, 300, 151–160. [Google Scholar] [CrossRef]
- Méducin, F.; Redfern, S.A.T.; Le Godec, Y.; Stone, H.J.; Tucker, M.G.; Dove, M.T.; Marshall, W.G. Study of cation order-disorder in MgAl2O4 spinel by in situ neutron diffraction up to 1600 K and 3.2 GPa. Am. Mineral. 2004, 89, 981–986. [Google Scholar] [CrossRef]
- Wood, B.J.; Kirkpatrick, R.J.; Montez, B. Order-disorder phenomena in MgAl2O4 spinel. Am. Mineral. 1986, 71, 999–1006. [Google Scholar]
- O’Neill, H.S.C.; Navrotsky, A. Simple spinels: Crystallographic parameters, cation radii, lattice energies, and cation distribution. Am. Mineral. 1983, 68, 181–194. [Google Scholar]
- Sickafus, K.E.; Yu, N.; Nastasi, M. Radiation resistance of the oxide spinel: The role of stoichiometry on damage response. Nucl. Imstrum. Meth. B 1996, 116, 85–91. [Google Scholar] [CrossRef]
- Ting, C.J.; Lu, H.Y. Defect reactions and the controlling mechanism in the sintering of magnesium aluminate spinel. J. Am. Ceram. Soc. 1999, 82, 841–848. [Google Scholar] [CrossRef]
- Chiang, Y.-M. Grain Boundary Mobility and Segregation in Non-Stoichiometric Solid Solutions of Magnesium Aluminate Spinel; Massachusetts Institute of Technology: Cambridge, MA, USA, 1980. [Google Scholar]
- Chiang, Y.-M.; Kingery, W.D. Grain-boundary migration in nonstoichiometric solid solutions of magnesium aluminate spinel: I, grain growth studies. J. Am. Ceram. Soc. 1989, 72, 271–277. [Google Scholar] [CrossRef]
- Sutorik, A.C.; Gilde, G.; Swab, J.J.; Cooper, C.; Gamble, R.; Shanholtz, E. Transparent solid solution magnesium aluminate spinel polycrystalline ceramic with the alumina-rich composition MgO·1.2 Al2O3. J. Am. Ceram. Soc. 2012, 95, 636–643. [Google Scholar] [CrossRef]
- Barzilai, S.; Aizenshtein, M.; Mintz, M.H.; Hayun, S. Effect of adsorbed oxygen on the dissociation of water over gadolinium oxide surfaces: Density functional theory calculations and experimental results. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Ianoş, R.; Lazǎu, I.; Pǎcurariu, C.; Barvinschi, P. Solution combustion synthesis of MgAl2O4 using fuel mixtures. Mater. Res. Bull. 2008, 43, 3408–3415. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Navrotsky, A. Direct measurements of water adsorption enthalpy on hafnia and zirconia. Appl. Phys. Lett. 2005, 87, 1–3. [Google Scholar] [CrossRef]
- Jing, S.-Y.; Lin, L.-B.; Houng, N.-K.; Zhang, J.; Lu, Y. Investigation on lattice constants of Mg-Al spinels. J. Mater. Sci. Lett. 2000, 19, 225–227. [Google Scholar] [CrossRef]
- Navrotsky, A.; Wechsler, B.A.; Geisinger, K.; Seifert, F. Thermochemistry of MgAl2O4-Al8/3O4 defect spinels. J. Am. Cerum. Soc 1986, 69, 418–422. [Google Scholar] [CrossRef]
- Corsi, J.S.; Fu, J.; Wang, Z.; Lee, T.; Ng, A.K.; Detsi, E. Hierarchical bulk nanoporous aluminum for on-site generation of hydrogen by hydrolysis in pure water and combustion of solid fuels. ACS Sustain. Chem. Eng. 2019, 7, 11194–11204. [Google Scholar] [CrossRef]
- Hinnen, C.; Imbert, D.; Siffre, J.M.; Marcus, P. An in situ XPS study of sputter-deposited aluminium thin films on graphite. Appl. Surf. Sci. 1994, 78, 219–231. [Google Scholar] [CrossRef]
- He, H.; Alberti, K.; Barr, T.L.; Klinowski, J. ESCA studies of aluminophosphate molecular sieves. J. Phys. Chem. 1993, 97, 13703–13707. [Google Scholar] [CrossRef]
- Grigorova, E.; Khristov, M.; Peshev, P.; Nihtianova, D.; Velichkova, N.; Atanasova, G. Hydrogen sorption properties of a MgH2–V2O5 composite prepared by ball milling. Bulg. Chem. Commun. 2013, 45, 280–287. [Google Scholar]
- Shelly, L.; Schweke, D.; Zalkind, S.; Shamir, N.; Barzilai, S.; Gouder, T.; Hayun, S. Effect of U content on the activation of H2O on Ce1-xUxO2+δ surfaces. Chem. Mater. 2018, 30, 8650–8660. [Google Scholar] [CrossRef]
- Hayun, S.; Shvareva, T.Y.; Navrotsky, A. Nanoceria—Energetics of surfaces, interfaces and water adsorption. J. Am. Ceram. Soc. 2011, 94, 3992–3999. [Google Scholar] [CrossRef]
- Uner, D.; Uner, M. Adsorption calorimetry in supported catalyst characterization: Adsorption structure sensitivity on Pt/γ-Al2O3. Thermochim. Acta 2005, 434, 107–112. [Google Scholar] [CrossRef]
- Garcia-Cuello, V.; Moreno-Piraján, J.C.; Giraldo-Gutiérrez, L.; Sapag, K.; Zgrablich, G. Determination of differential enthalpy and isotherm by adsorption calorimetry. Res. Lett. Phys. Chem. 2008, 2008, 127328. [Google Scholar] [CrossRef][Green Version]
- Hayun, S.; Tran, T.; Ushakov, S.V.; Thron, A.M.; Van Benthem, K.; Navrotsky, A.; Castro, R.H.R. Experimental methodologies for assessing the surface energy of highly hygroscopic materials: The case of nanocrystalline magnesia. J. Phys. Chem. C 2011, 115, 23929–23935. [Google Scholar] [CrossRef]
- Jia, C.; Fan, W.; Yang, F.; Zhao, X.; Sun, H.; Li, P.; Liu, L. A theoretical study of water adsorption and decomposition on low-index spinel ZnGa2O4 surfaces: Correlation between surface structure and photocatalytic properties. Langmuir 2013, 29, 7025–7037. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, J.G.; Wang, Y.; Mei, D. First-principles hermodynamics study of spinel MgAl2O4 surface stability. J. Phys. Chem. C 2016, 120, 19087–19096. [Google Scholar] [CrossRef]






| n | (MgxAlyO4) | Lattice Parameter, Å | Crystallite Size, nm | Surface Area, m2/g | ||
|---|---|---|---|---|---|---|
| (x) Mg | (y) Al | XRD | BET | |||
| 0.95 | 1.04 | 1.97 | 8.089(2) | 14.0 ± 0.2 | 117.7 ± 1.6 | 32.1 ± 0.2 |
| 1.07 | 0.95 | 2.03 | 8.078(2) | 13.3 ± 0.2 | 123.9 ± 1.7 | 37.6 ± 0.2 |
| 1.15 | 0.72 | 2.18 | 8.065(6) | 10.2 ± 0.3 | 161.6 ± 4.9 | 56.4 ± 0.2 |
| 2.45 | 0.48 | 2.35 | 7.989(4) | 15.5 ± 0.8 | 106.3 ± 5.2 | 41.2 ± 0.2 |
| n | Heat of Adsorption, kJ/mol | Hydroxides, mol. % | H2O Coverage, Molecules/nm2 | |||
|---|---|---|---|---|---|---|
| Al–OH | Mg–OH | Total | ||||
| Clean | 0.95 | −75.1 ± 0.2 | 6.2 ± 0.3 | 13.7 ± 0.7 | 20.0 ± 1.0 | 12.2 ± 1.0 |
| 1.07 | −73.3 ± 0.4 | 34.0 ± 1.7 | 25.5 ± 1.3 | 59.4 ± 3.0 | 12.9 ± 0.1 | |
| 1.15 | −71.0 ± 0.7 | 35.4 ± 1.8 | 12.4 ± 0.6 | 47.0 ± 2.4 | 11.5 ± 0.1 | |
| 2.45 | −67.2 ± 0.2 | 32.1 ± 1.0 | 2.2 ± 0.1 | 34.3 ± 1.7 | 10.1 ± 0.1 | |
| Reduced | 0.95 | −71.0 ± 1.0 | 8.3 ± 0.4 | 16.9 ± 0.8 | 25.1 ± 1.2 | 15.3 ± 0.3 |
| 1.07 | −75.6 ± 0.9 | 30.3 ± 1.5 | 18.9 ± 0.9 | 48.2 ± 2.5 | 11.3 ± 0.2 | |
| 1.15 | −68.5 ± 0.4 | 37.5 ± 1.9 | 11.5 ± 0.6 | 49.0 ± 2.4 | 13.4 ± 0.1 | |
| 2.45 | −66.3 ± 0.3 | 41.3 ± 2.1 | 7.8 ± 0.4 | 49.0 ± 2.4 | 13.5 ± 0.2 | |
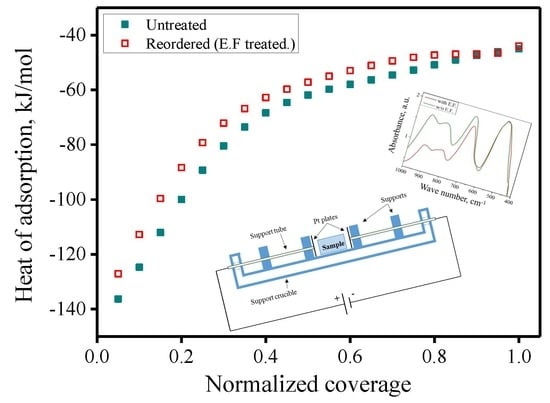
| n | Inversion (i) | Heat of Adsorption, kJ/mol | Extent of Hydroxides Formed, mol. % | H2O Coverage, Molecules/nm2 | |||
|---|---|---|---|---|---|---|---|
| Al–OH | Mg–OH | Total | |||||
| 2.45 | Untreated | 0.44 | −67.2 ± 0.3 | 32.1 ± 1.6 | 2.2 ± 0.1 | 34.3 ± 1.7 | 10.1 ± 0.1 |
| Treated/(EF) | 0.33 | −62.5 ± 0.5 | 25.0 ± 1.3 | 1.8 ± 0.1 | 26.8 ± 1.4 | 8.8 ± 0.1 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mordekovitz, Y.; Shoval, Y.; Froumin, N.; Hayun, S. Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption. Materials 2020, 13, 3195. https://doi.org/10.3390/ma13143195
Mordekovitz Y, Shoval Y, Froumin N, Hayun S. Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption. Materials. 2020; 13(14):3195. https://doi.org/10.3390/ma13143195
Chicago/Turabian StyleMordekovitz, Yuval, Yael Shoval, Natali Froumin, and Shmuel Hayun. 2020. "Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption" Materials 13, no. 14: 3195. https://doi.org/10.3390/ma13143195
APA StyleMordekovitz, Y., Shoval, Y., Froumin, N., & Hayun, S. (2020). Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption. Materials, 13(14), 3195. https://doi.org/10.3390/ma13143195