Synthesis of Ti4O7/Ti3O5 Dual-Phase Nanofibers with Coherent Interface for Oxygen Reduction Reaction Electrocatalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of H2Ti3O7 Precursor Nanofibers
2.2. Synthesis of Ti4O7/Ti3O5 Dual Phase Nanofibers
2.3. Structural Characterization of the Nanofibers
2.4. Electrochemical Performance Characterization
3. Results and Discussions
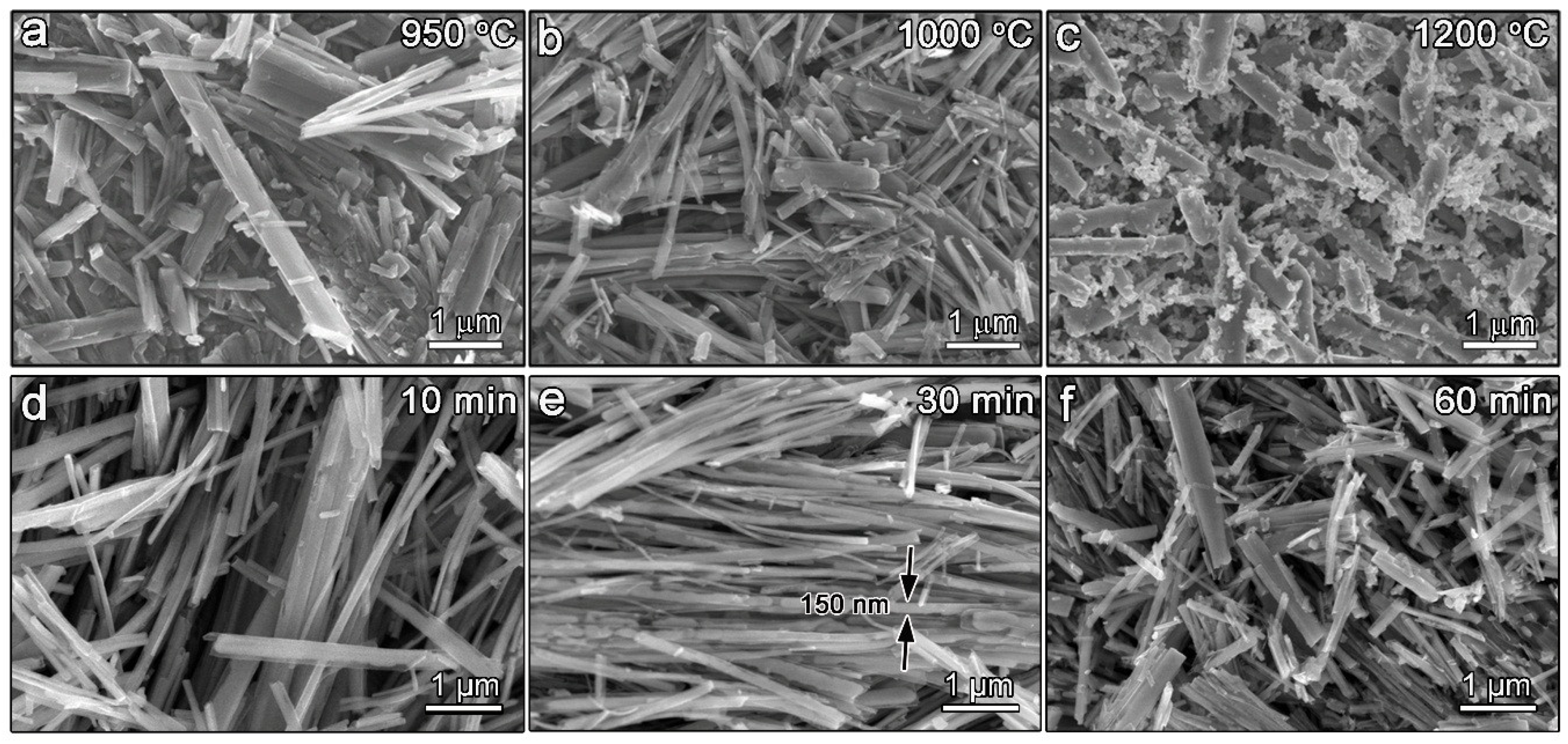
3.1. Morphology and Phase Evolution of TinO2n−1 Dual-Phase Nanofibers
3.2. TEM Analysis of T4/T3 Interface Structure
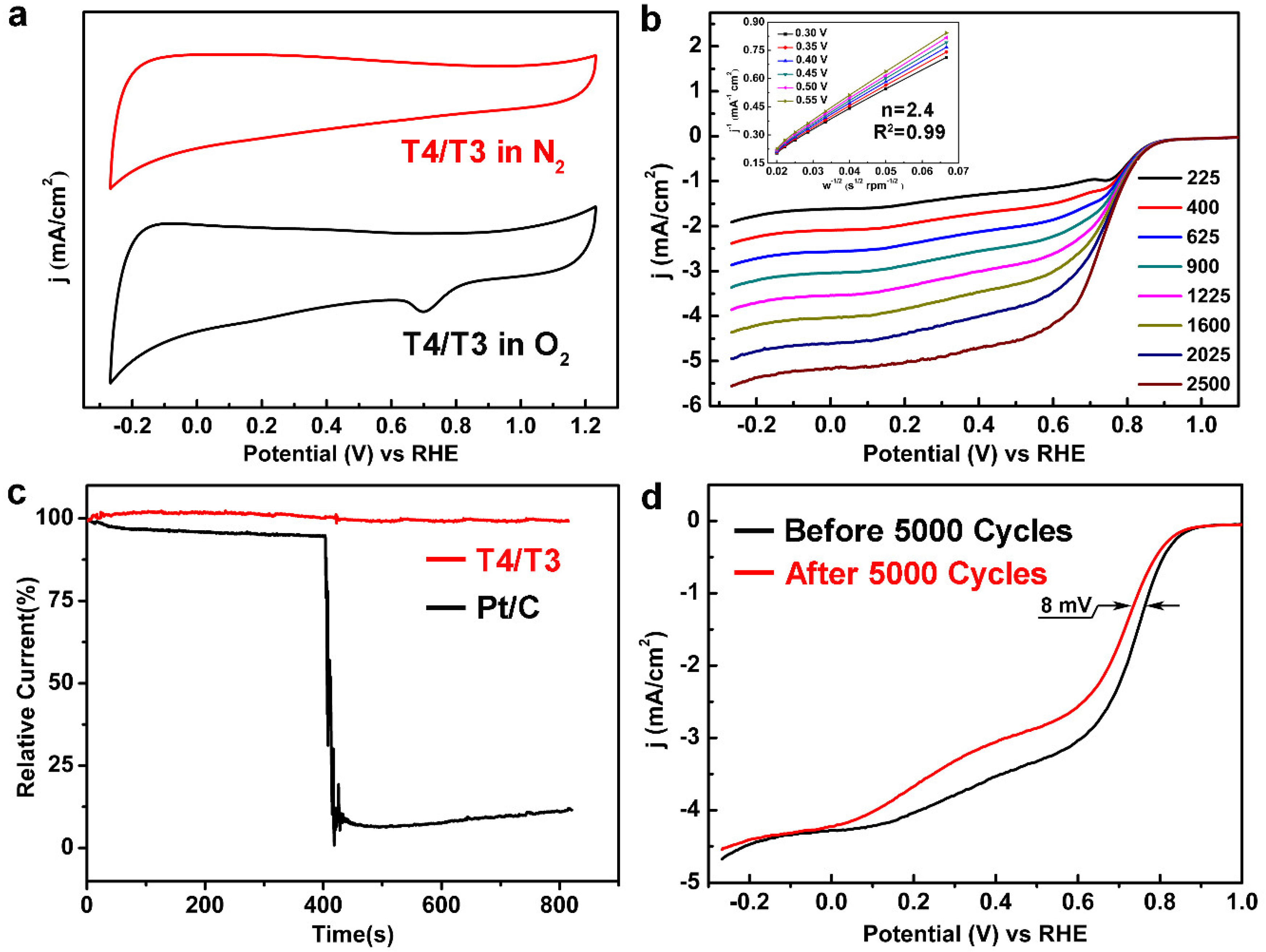
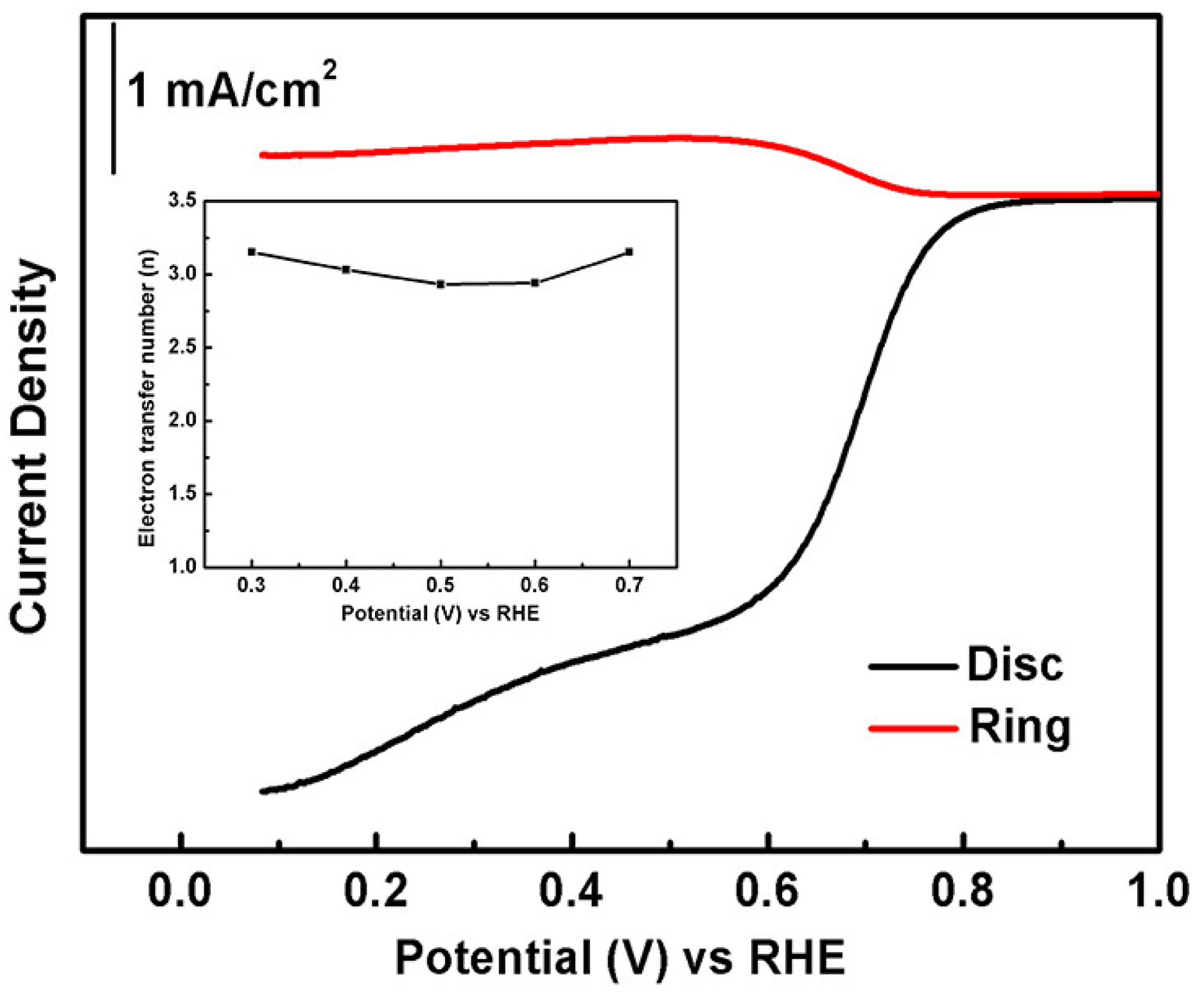
3.3. Electrochemical Performance of T4/T3 Dual-Phase Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jacobson, M.Z. Review of solutions to global warming, air pollution, and energy security. Energy Environ. Sci. 2009, 2, 148–173. [Google Scholar] [CrossRef]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F.; Brandon, N.P. Hydrogen and fuel cells: Towards a sustainable energy future. Energy Policy 2008, 36, 4356–4362. [Google Scholar] [CrossRef]
- Li, X.; Zhu, A.L.; Wei, Q.; Wang, H.; Hui, R.; Lei, Z.; Zhang, J. Magneli phase Ti4O7 electrode for oxygen reduction reaction and its implication for zinc-air rechargeable batteries. Electrochim. Acta 2010, 55, 5891–5898. [Google Scholar] [CrossRef]
- Wang, Y.J.; Long, W.; Wang, L.; Yuan, R.; Ignaszak, A.; Fang, B.; Wilkinson, D.P. Unlocking the door to highly active ORR catalysts for PEMFC applications: Polyhedron-engineered Pt-based nanocrystals. Energy Environ. Sci. 2018, 11, 258–275. [Google Scholar] [CrossRef]
- Xu, N.; Cai, Y.; Peng, L.; Qiao, J.; Wang, Y.D.; Chirdon, W.M.; Zhou, X.D. Superior stability of bifunctional oxygen electrode for primary, rechargeable and flexible Zn-air batteries. Nanoscale 2018, 10, 13626–13637. [Google Scholar] [CrossRef] [PubMed]
- Ioroi, T.; Yasuda, K.; Siroma, Z.; Fujiwara, N.; Miyazaki, Y. Thin film electrocatalyst layer for unitized regenerative polymer electrolyte fuel cells. J. Power Sources 2002, 112, 583–587. [Google Scholar] [CrossRef]
- Lee, D.U.; Kim, B.J.; Chen, Z. One-pot synthesis of a mesoporous NiCo2O4 nanoplatelet and graphene hybrid and its oxygen reduction and evolution activities as an efficient bi-functional electrocatalyst. J. Mater. Chem. A 2013, 1, 4754–4762. [Google Scholar] [CrossRef]
- Ovrum, E.; Bergh, T.F. Modelling lithium-ion battery hybrid ship crane operation. Appl. Energy 2015, 152, 162–172. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Marković, N.M. Chemistry. Just a dream—Or future reality? Science 2009, 324, 48–49. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Ben, F.; Bongjin Simon, M.; Guofeng, W.; Ross, P.N.; Lucas, C.A.; Markovi, N.M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef]
- Xiong, W.; Feng, D.U.; Liu, Y.; Albert, P.; Michael, S.; Ramakrishnan, T.S.; Dai, L.; Jiang, L.I. 3-D Carbon nanotube structures used as high performance catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2010, 132, 15839–15841. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Zhang, L.; Adzic, R.R. Niobium oxide-supported platinum ultra-low amount electrocatalysts for oxygen reduction. Phys. Chem. Chem. Phys. 2008, 10, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, J.; Wang, F.; Dai, L. Efficient oxygen reduction reaction (ORR) catalysts based on single iron atoms dispersed on a hierarchically structured porous carbon framework. Angew. Chem. 2018, 57, 9038–9043. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Chen, C.; You, W.; Chen, G.; Xue, S.; Zhang, J.; Liu, J.; Feng, Y.; Wang, P.; Wang, Y.; et al. Tuning strain effect and surface composition in PdAu hollow nanospheres as highly efficient ORR electrocatalysts and SERS substrates. Appl. Catal. B Environ. 2020, 262, 118298. [Google Scholar] [CrossRef]
- Lv, H.; Li, D.; Strmcnik, D.; Paulikas, A.P.; Markovic, N.M.; Stamenkovic, V.R. Recent advances in the design of tailored nanomaterials for efficient oxygen reduction reaction. Nano Energy 2016, 29, 149–165. [Google Scholar] [CrossRef]
- Stephens, I.E.L.; Bondarenko, A.S.; Grønbjerg, U.; Rossmeisl, J.; Chorkendorff, I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 2012, 5, 6744–6762. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 2013, 46, 1848. [Google Scholar] [CrossRef]
- Blizanac, B.B.; Ross, P.N.; Markovic, N.M. Oxygen electroreduction on Ag (1 1 1): The pH effect. Electrochim. Acta 2007, 52, 2264–2271. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Yu, S.K.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Lee, K.S.; Jeon, T.Y.; Yoo, S.J.; Park, I.S.; Cho, Y.H.; Kang, S.H.; Choi, K.H.; Sung, Y.E. Effect of PtRu alloying degree on electrocatalytic activities and stabilities. Appl. Catal. B Environ. 2011, 102, 334–342. [Google Scholar] [CrossRef]
- Ibrahim, K.B.; Su, W.-N.; Tsai, M.-C.; Chala, S.A.; Kahsay, A.W.; Yeh, M.-H.; Chen, H.-M.; Duma, A.D.; Dai, H.; Hwang, B.-J. Robust and conductive Magnéli Phase Ti4O7 decorated on 3D-nanoflower NiRu-LDH as high-performance oxygen reduction electrocatalyst. Nano Energy 2018, 47, 309–315. [Google Scholar] [CrossRef]
- Wang, W.; Long, K.; Cao, W.; Huttula, M.; Geng, B. Mass-production of Mesoporous MnCo2O4 Spinel with MnIV- and CoII-rich surface for superior bifunctional oxygen electrocatalysis. Angew. Chem. Int. Ed. 2017, 56, 14977–14981. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Li, M.; Zhang, Y.; Lei, Y.; Zhu, Y.; Jiang, R.; He, X.; Lei, Z.; Liu, Z.; Zhu, H.; et al. Design and synthesis of carbon nanofibers decorated by dual-phase TinO2n−1 nanoparticles with synergistic catalytic effect as high performance oxygen reduction reaction catalysts. Electrochim. Acta 2020, 344, 136120. [Google Scholar] [CrossRef]
- Wu, M.C.; Hiltunen, J.; Sápi, A.; Avila, A.; Larsson, W.; Liao, H.C.; Huuhtanen, M.; Tóth, G.; Shchukarev, A.; Laufer, N. Nitrogen-doped anatase nanofibers decorated with noble metal nanoparticles for photocatalytic production of hydrogen. ACS Nano 2015, 5, 5025–5030. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.C. A portion of the system silica-water. Econ. Geol. 1950, 45, 629–653. [Google Scholar] [CrossRef]
- Feng Shouhua, X.R. Advances in inorganic synthesis and preparative chemistry. Prog. Chem. 2000, 12, 445–457. [Google Scholar]
- Wang, F.; Ding, X.; Shi, R.; Li, M.; Lei, Y.; Lei, Z.; Jiang, G.; Xu, F.; Wang, H.; Jia, L.; et al. Facile Synthesis of Ti4O7 on hollow carbon spheres with enhanced polysulfide binding for high performance lithium-sulfur batteries. J. Mater. Chem. A 2019, 7, 10494–10504. [Google Scholar] [CrossRef]
- Wang, F.; Shi, R.; Lei, Y.; Lei, Z.; Jiang, R.; Wang, D.; Liu, Z.; Sun, J. Formation mechanisms of interfaces between different TinO2n-1 phases prepared by carbothermal reduction reaction. CrystEngComm 2019, 21, 524–534. [Google Scholar] [CrossRef]
- Lei, Y.; Li, J.; Wang, Z.; Sun, J.; Chen, F.; Liu, H.; Ma, X.; Liu, Z. Atomic-scale investigation of a new phase transformation process in TiO2 nanofibers. Nanoscale 2017, 9, 4601–4609. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J. SP2-a computer program for plotting stereographic projection and exploring crystallographic orientation relationships. J. Appl. Crystallogr. 2012, 45, 130–134. [Google Scholar] [CrossRef]
- Esfahani, R.A.M.; Vankova, S.K.; Videla, A.H.M.; Specchia, S. Innovative carbon-free low content Pt catalyst supported on Mo-doped titanium suboxide (Ti3O5-Mo) for stable and durable oxygen reduction reaction. Appl. Catal. B Environ. 2017, 201, 419–429. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, R.; Huang, Y.; Li, M.; Zhu, Y.; He, X.; Jiang, R.; Lei, Z.; Liu, Z.; Sun, J. Synthesis of Ti4O7/Ti3O5 Dual-Phase Nanofibers with Coherent Interface for Oxygen Reduction Reaction Electrocatalysts. Materials 2020, 13, 3142. https://doi.org/10.3390/ma13143142
Shi R, Huang Y, Li M, Zhu Y, He X, Jiang R, Lei Z, Liu Z, Sun J. Synthesis of Ti4O7/Ti3O5 Dual-Phase Nanofibers with Coherent Interface for Oxygen Reduction Reaction Electrocatalysts. Materials. 2020; 13(14):3142. https://doi.org/10.3390/ma13143142
Chicago/Turabian StyleShi, Ruyue, Ying Huang, Miaoran Li, Ying Zhu, Xuexia He, Ruibin Jiang, Zhibin Lei, Zonghuai Liu, and Jie Sun. 2020. "Synthesis of Ti4O7/Ti3O5 Dual-Phase Nanofibers with Coherent Interface for Oxygen Reduction Reaction Electrocatalysts" Materials 13, no. 14: 3142. https://doi.org/10.3390/ma13143142
APA StyleShi, R., Huang, Y., Li, M., Zhu, Y., He, X., Jiang, R., Lei, Z., Liu, Z., & Sun, J. (2020). Synthesis of Ti4O7/Ti3O5 Dual-Phase Nanofibers with Coherent Interface for Oxygen Reduction Reaction Electrocatalysts. Materials, 13(14), 3142. https://doi.org/10.3390/ma13143142




