An Experimental Study on the Thermal Stability of Mg2Si/Ni Interface under Thermal Cycling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Mg2Si/Ni co-sintering
2.2. Temperature Cycling and Characterization
3. Results and Discussion
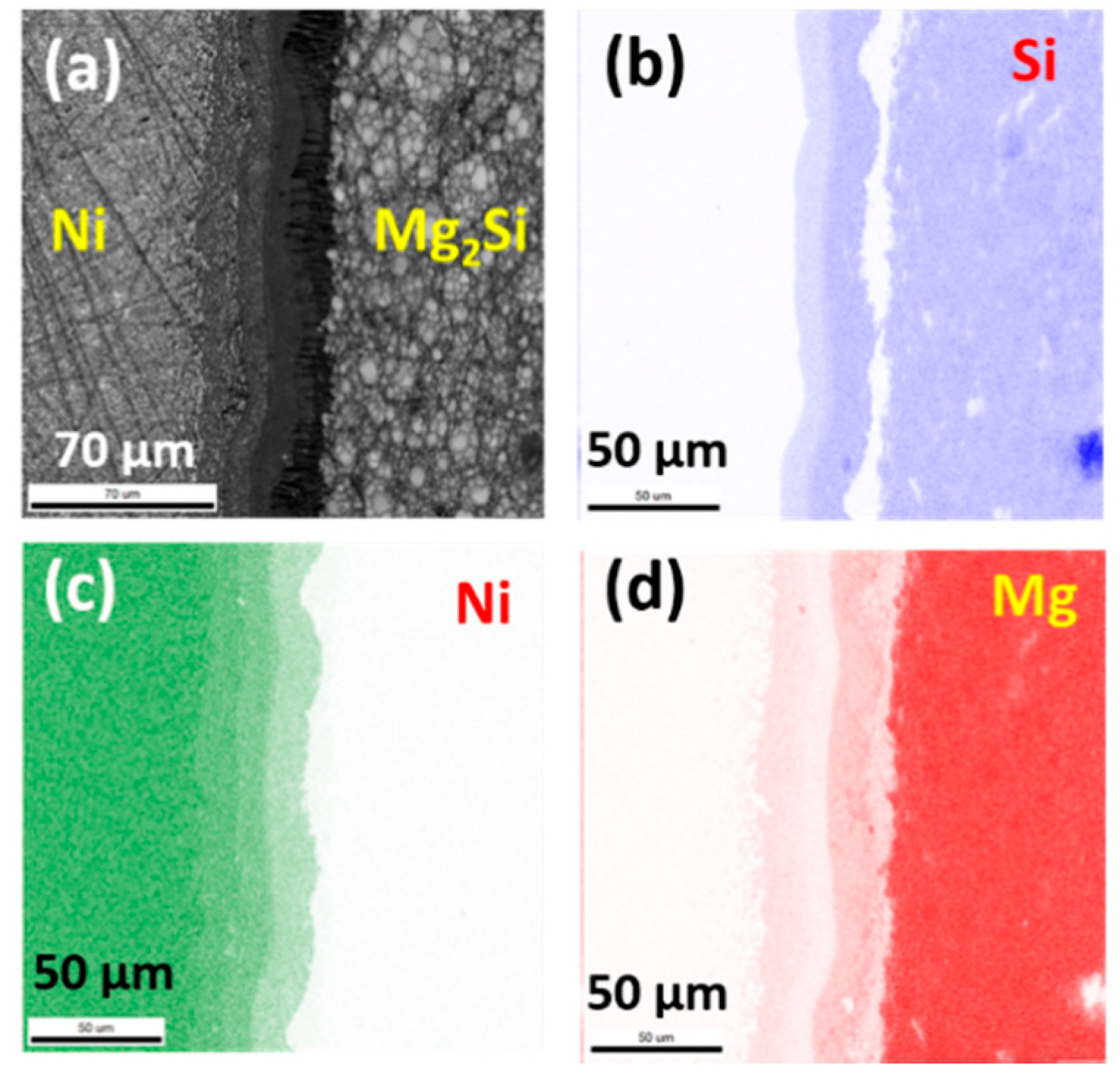
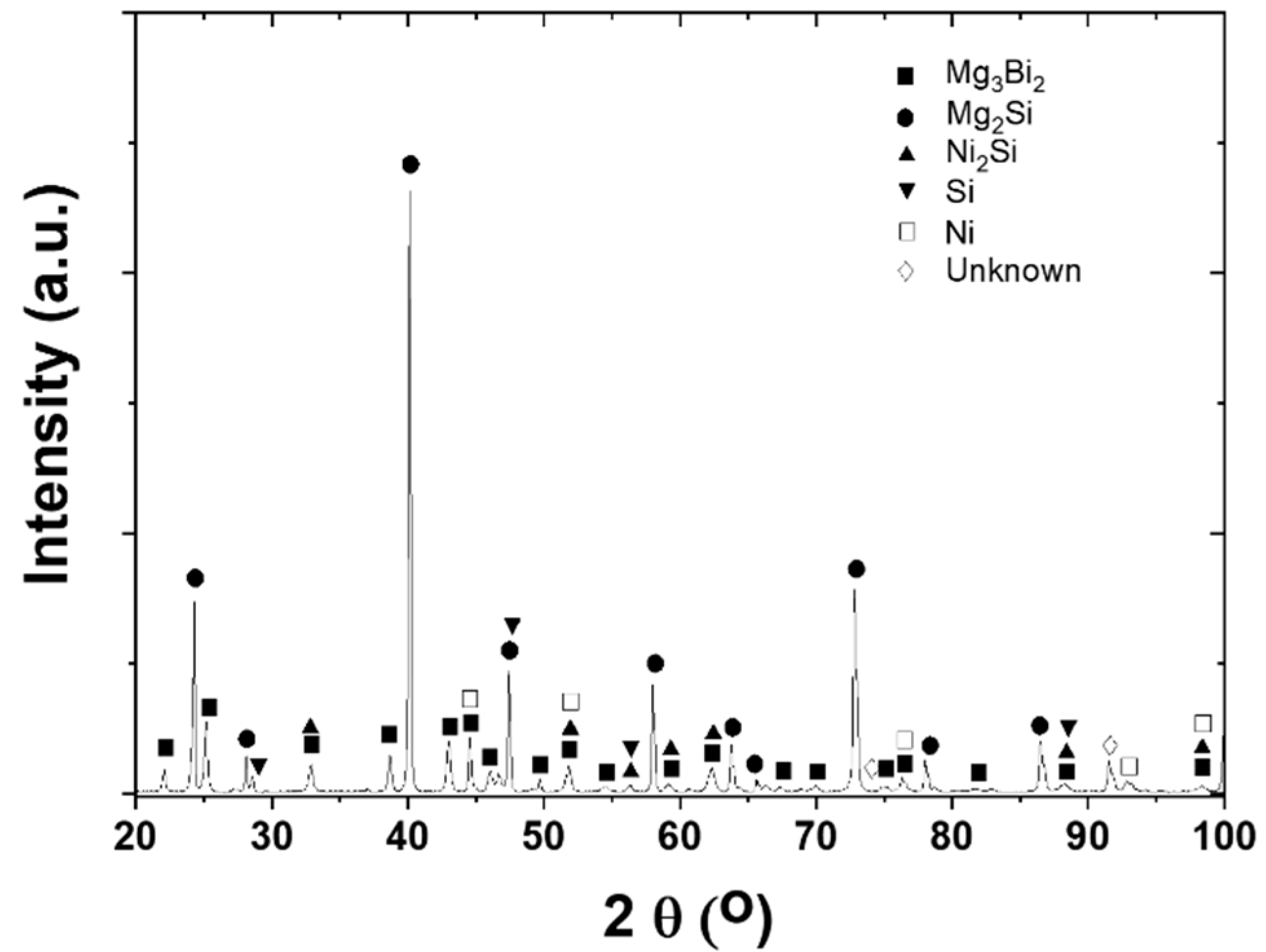
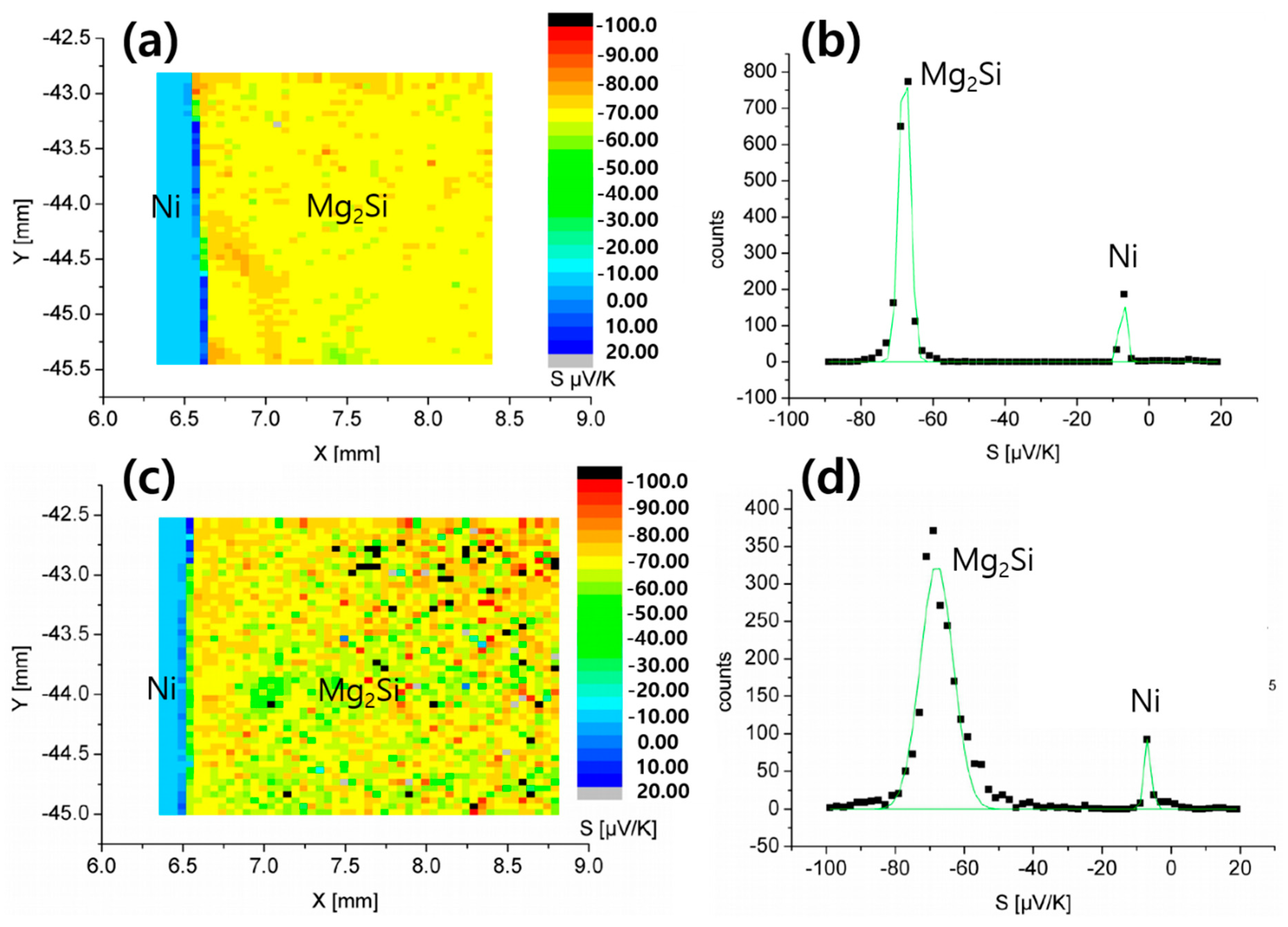
3.1. As-Sintered Mg2Si/Ni Interfaces
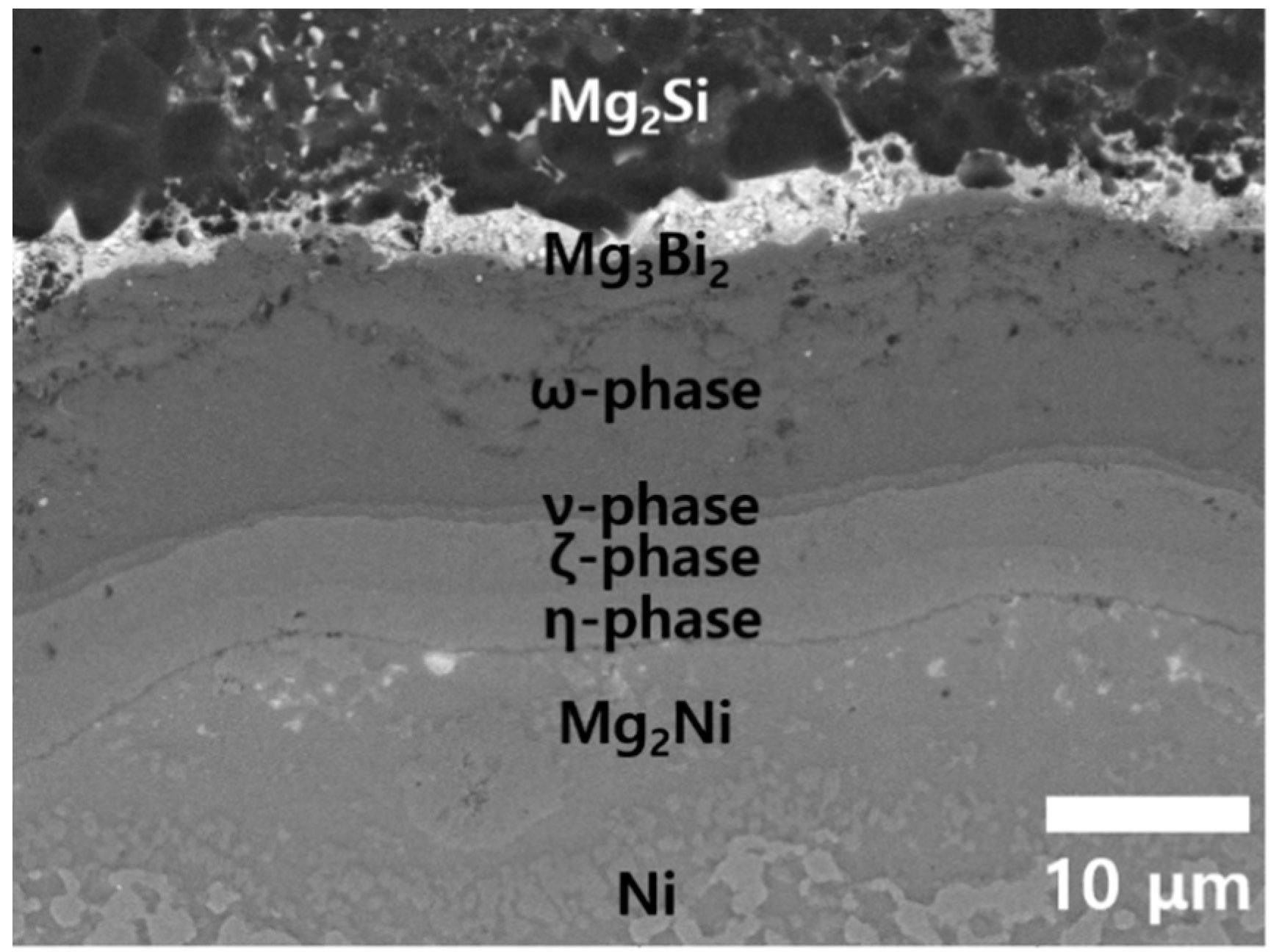
3.2. Mg2Si/Ni Interfaces after Temperature Cycling
3.3. PSM Analysis of Mg2Si/Ni Interfaces
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zaitsev, V.K.; Fedorov, M.I.; Gurieva, E.A.; Eremin, I.S.; Konstantinov, P.P.; Samunin, A.Y.; Vedernikov, M.V. Highly effective Mg2Si1−xSnx thermoelectrics. Phys. Rev. B 2006, 74, 045207. [Google Scholar] [CrossRef]
- Liu, W.; Tan, X.; Yin, K.; Liu, H.; Tang, X.; Shi, J.; Zhang, Q.; Uher, C. Convergence of conduction bands as a means of enhancing thermoelectric performance of n-type Mg2Si(1−x)Snx solid solutions. Phys. Rev. Lett. 2012, 108, 166601. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, T.; Wang, H.; Hu, L.; Xie, H.; Jiang, G.; Snyder, G.J.; Zhao, X. Low electron scattering potentials in high performance Mg2Si0.45Sn0.55 based thermoelectric solid solutions with band convergence. Adv. Energy Mater. 2013, 3, 1238–1244. [Google Scholar] [CrossRef]
- Imai, M.; Isoda, Y.; Udono, H. Thermal expansion of semiconducting silicides β-FeSi2 and Mg2Si. Intermetallics 2015, 67, 75–80. [Google Scholar] [CrossRef]
- de Boor, J.; Gloanec, C.; Kolb, H.; Sottong, R.; Ziolkowski, P.; Müller, E. Fabrication and characterization of nickel contacts for magnesium silicide based thermoelectric generators. J. Alloys Compd. 2015, 632, 348–353. [Google Scholar] [CrossRef]
- Yang, R.; Chen, S.; Fan, W.; Gao, X.; Long, Y.; Wang, W.; Munir, Z.A. Interfacial properties of Cu/Ni/Mg2Si joints prepared in one step by the spark plasma sintering method. J. Alloys Compd. 2017, 704, 545–551. [Google Scholar] [CrossRef]
- de Boor, J.; Droste, D.; Schneider, C.; Janek, J.; Müller, E. Thermal stability of magnesium silicide/nickel contacts. J. Electron. Mater. 2016, 45, 5313–5320. [Google Scholar] [CrossRef]
- Platzek, D.; Karpinski, G.; Stiewe, C.; Ziolkowski, Z.; Drasar, C.; Müller, E. Potential-Seebeck-Microprobe: Measuring the spatial resolution of the Seebeck coefficient and the electric potential. In Proceedings of the 24th International Conference on Thermoelectrics, Clemson, SC, USA, 19–23 June 2005; pp. 13–16. [Google Scholar]
- Oh, C.S.; Kang, S.Y.; Lee, D.N. Assessment of the Mg-Bi system. Calphad 1992, 16, 181–191. [Google Scholar]
- Song, Y.K.; Varin, R.A. Phase equilibria and intermetallic phases in the Ni-Si-Mg ternary system. Metall. Mater. Trans. A 2001, 32, 5–18. [Google Scholar] [CrossRef]
- Song, Y.K.; Varin, R.A. New intermetallic phases in the Ni-Si-Mg ternary system. Intermetallics 1998, 6, 43–59. [Google Scholar] [CrossRef]
- Barri, N.; Salasel, A.R.; Abbasi, A.; Mirzadeh, H.; Emamy, M.; Malekan, M. A new intermetallic phase formation in Mg-Si-Ni magnesium-based in-situ formed alloys. Vacuum 2019, 164, 349–354. [Google Scholar] [CrossRef]
- Iida, T.; Kogo, Y.; Yasumori, A.; Nishio, K.; Hirayama, N. Silicide Modules—Practical Issues in Developing Mg2Si with Good Stability for Generating Power from Waste Heat Sources. In Advanced Thermoelectrics—Materials, Contacts, Devices, and Systems; CRC Press: Boca Raton, FL, USA, 2018; pp. 697–717. [Google Scholar]
- Domae, S.; Eto, R.; Okuma, K. Stress-induced voiding in stacked tungsten via structure. Microelectron. Reliab. 1999, 39, 507–513. [Google Scholar] [CrossRef]
- Dalleau, D.; Weide-Zaage, K.; Danto, Y. Simulation of time depending void formation in copper, aluminum and tungsten plugged via structures. Microelectron. Reliab. 2003, 43, 1821–1826. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Salm, C.; Krabbenborg, B.H.; Bisschop, J.; Ton Mouthaan, A.J.; Kuper, F.G. Fast thermal cycling-enhanced electromigration in power metallization. IEEE Trans. Device Mater. Reliab. 2004, 4, 246–255. [Google Scholar] [CrossRef]
- Tio Castro, D.; Hoofman, R.J.O.M.; Michelon, J.; Gravesteijn, D.J.; Bruynseraede, C. Void growth modeling upon electromigration stressing in narrow copper lines. J. Appl. Phys. 2007, 102, 123515. [Google Scholar] [CrossRef]

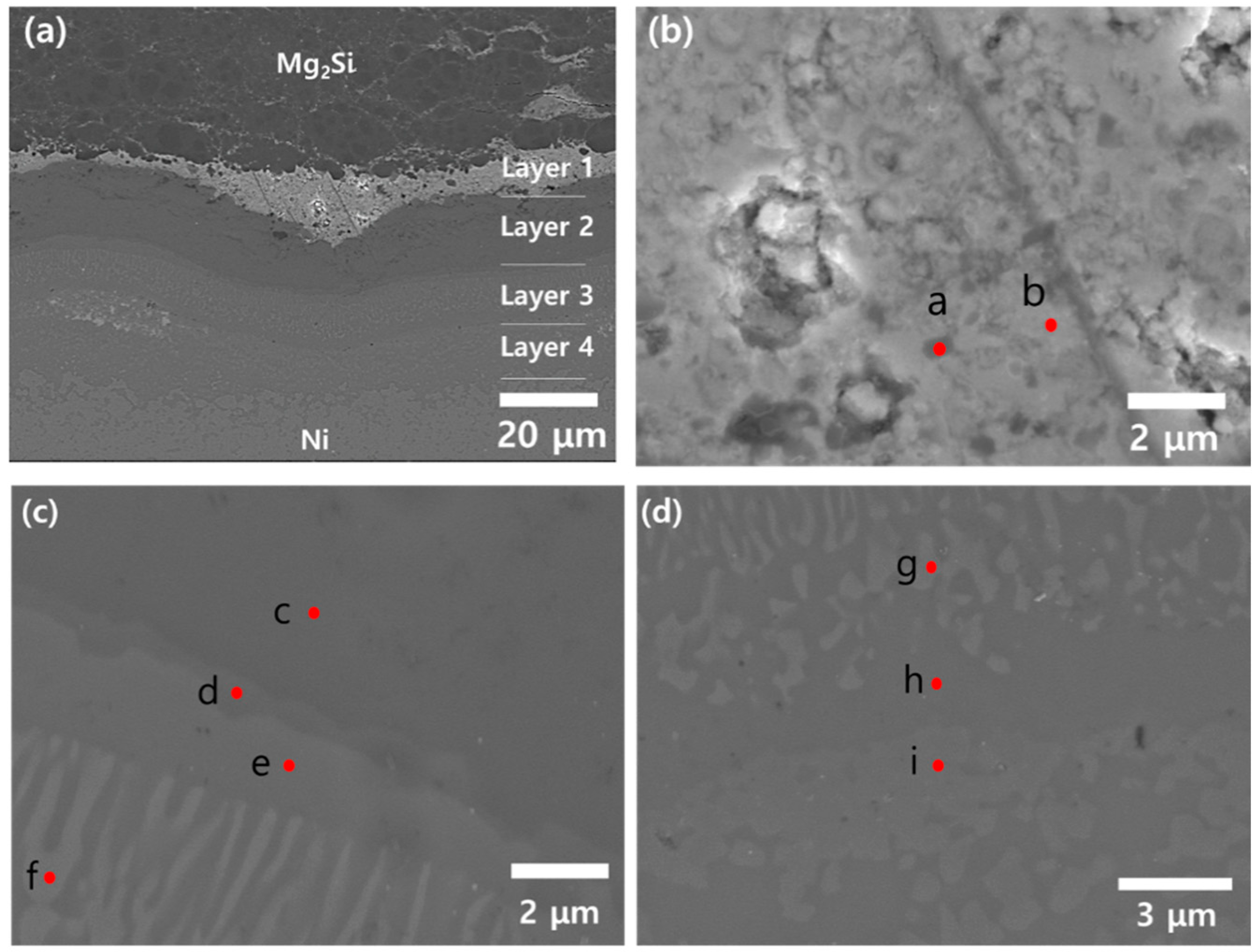


| Spot | Mg (at.%) | Si (at.%) | Ni (at.%) | Bi (at.%) | O (at.%) | C (at.%) | Candidate Phase |
|---|---|---|---|---|---|---|---|
| a | 41.12 | - | 1.81 | 16.57 | 33.26 | 7.24 | Mg3Bi2 + oxides |
| b | 30.95 | - | 2.21 | 18.63 | 36.06 | 12.15 | Mg3Bi2 + oxides |
| c | 20.75 | 22.67 | 18.04 | - | 3.27 | 35.26 | (Mg0.52Ni0.48)7Si4 (ω-phase) |
| d | 17.21 | 21.32 | 26.40 | - | 3.08 | 31.99 | Mg11Si10Ni12(ν-phase) |
| e | 12.84 | 22.57 | 30.41 | - | 3.71 | 30.47 | Mg3Si7Ni10 (ζ-phase) |
| f | 9.97 | 18.23 | 36.93 | - | 2.98 | 31.89 | |
| g | 2.56 | 20.28 | 39.46 | - | 2.97 | 34.74 | Ni2Si |
| h | 14.20 | 15.93 | 35.49 | - | 2.66 | 31.72 | Mg20Si24Ni56(η-phase) |
| i | 16.85 | - | 45.20 | - | 2.89 | 35.07 | MgNi2, Ni |
| As-Sintered Sample | Temperature Cycled Sample (Th = 400 °C, 500 Cycles) |
|---|---|
| 43.4 ± 24.5 μΩ·cm2 | 11.4 ± 5.0 μΩ·cm2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, S.-J.; Lee, J.E.; Kim, B.-S.; Min, B.-K. An Experimental Study on the Thermal Stability of Mg2Si/Ni Interface under Thermal Cycling. Materials 2020, 13, 3117. https://doi.org/10.3390/ma13143117
Joo S-J, Lee JE, Kim B-S, Min B-K. An Experimental Study on the Thermal Stability of Mg2Si/Ni Interface under Thermal Cycling. Materials. 2020; 13(14):3117. https://doi.org/10.3390/ma13143117
Chicago/Turabian StyleJoo, Sung-Jae, Ji Eun Lee, Bong-Seo Kim, and Bok-Ki Min. 2020. "An Experimental Study on the Thermal Stability of Mg2Si/Ni Interface under Thermal Cycling" Materials 13, no. 14: 3117. https://doi.org/10.3390/ma13143117
APA StyleJoo, S.-J., Lee, J. E., Kim, B.-S., & Min, B.-K. (2020). An Experimental Study on the Thermal Stability of Mg2Si/Ni Interface under Thermal Cycling. Materials, 13(14), 3117. https://doi.org/10.3390/ma13143117






