Insights into Characterization Methods and Biomedical Applications of Nanoparticle–Protein Corona
Abstract
1. Introduction
2. Characterization Approaches
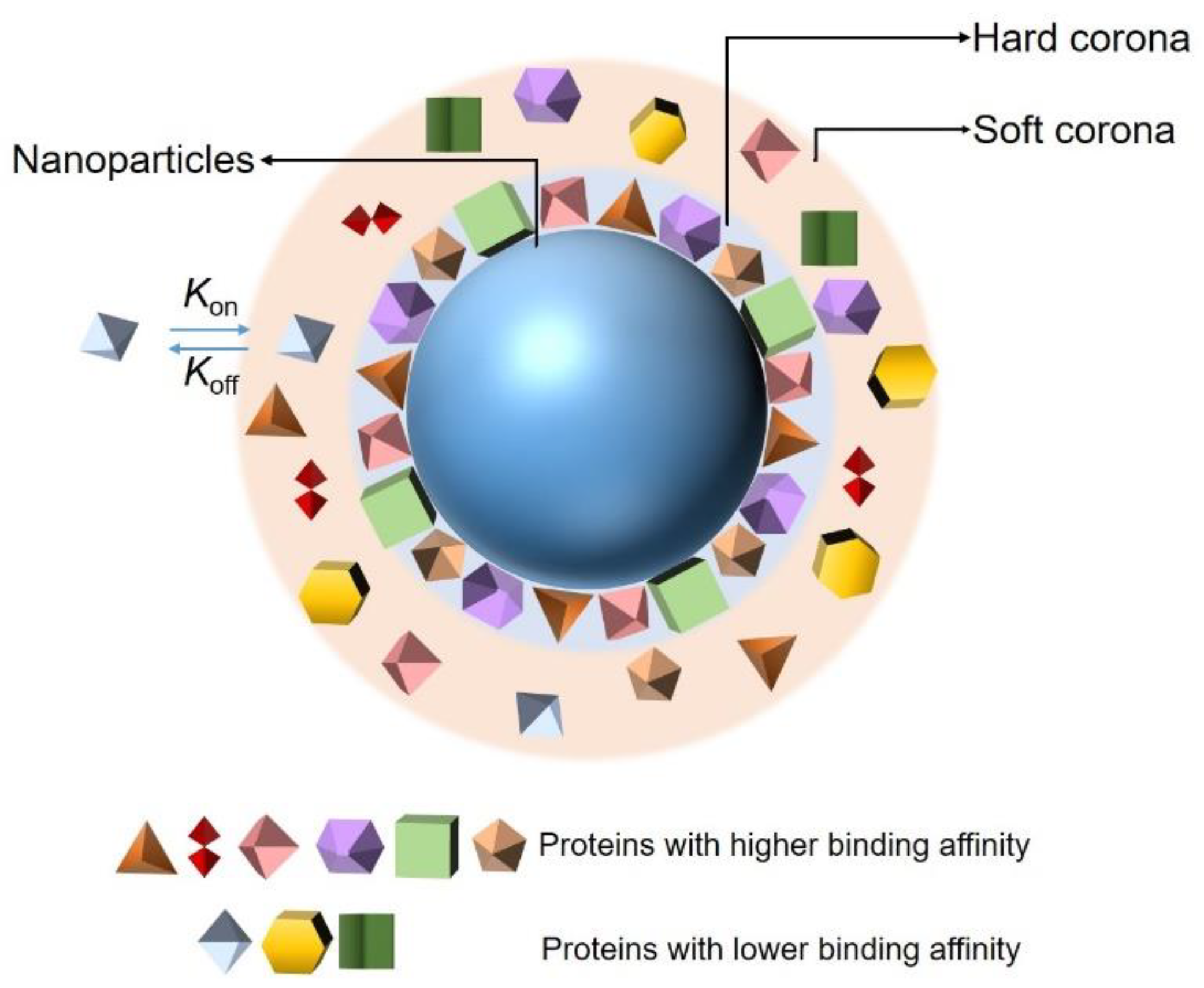
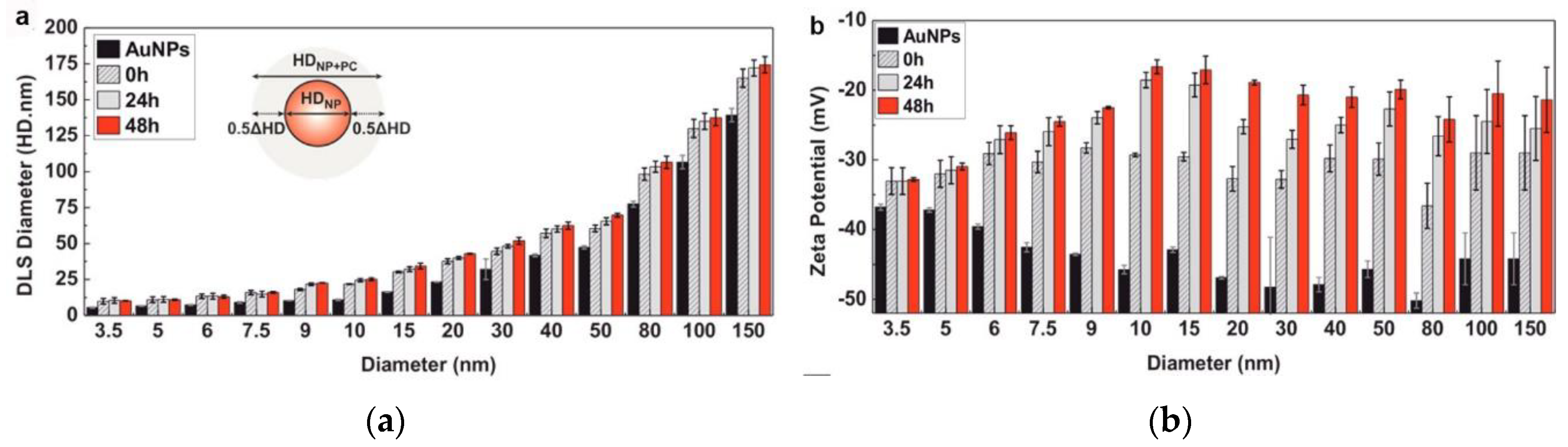
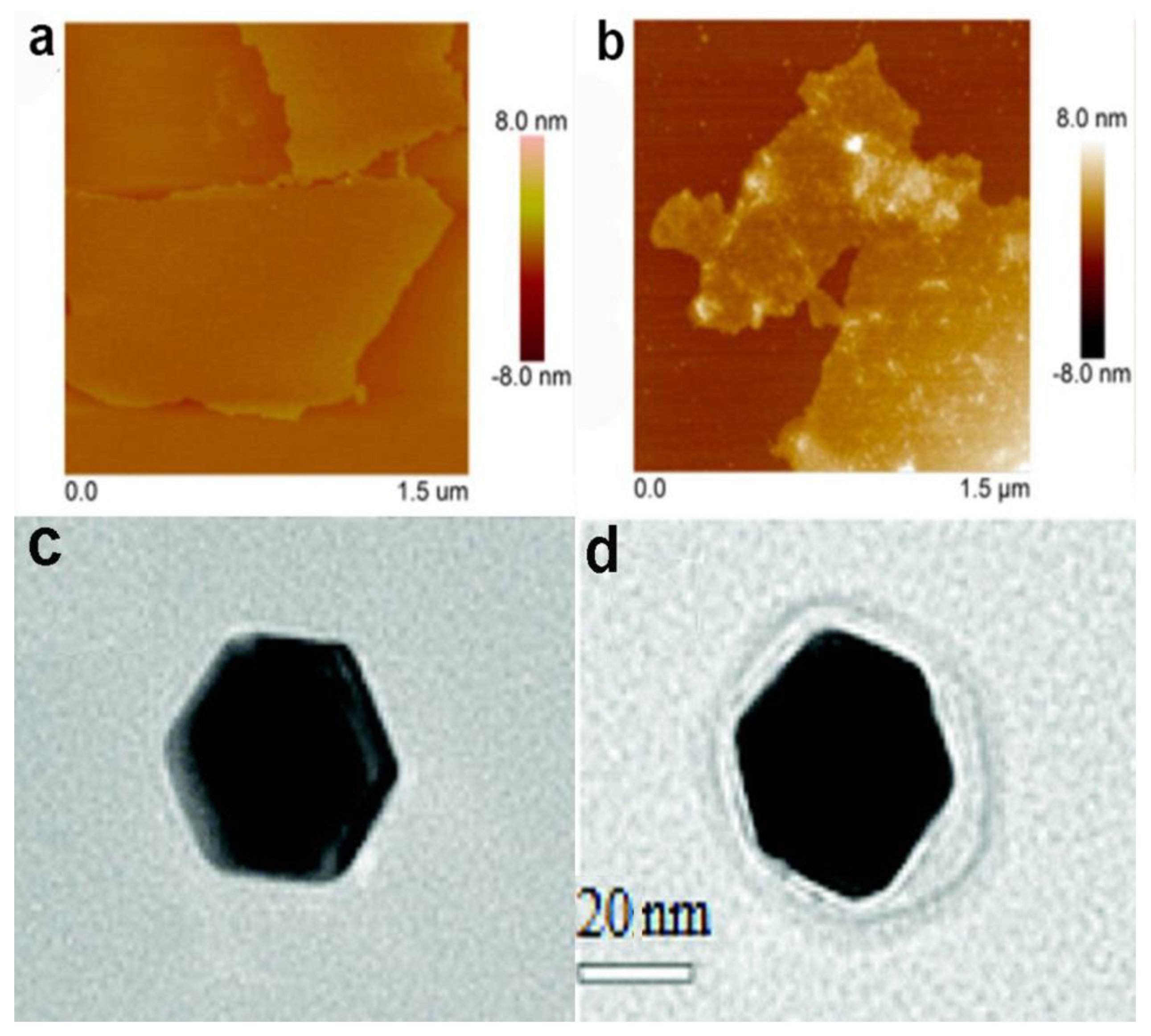
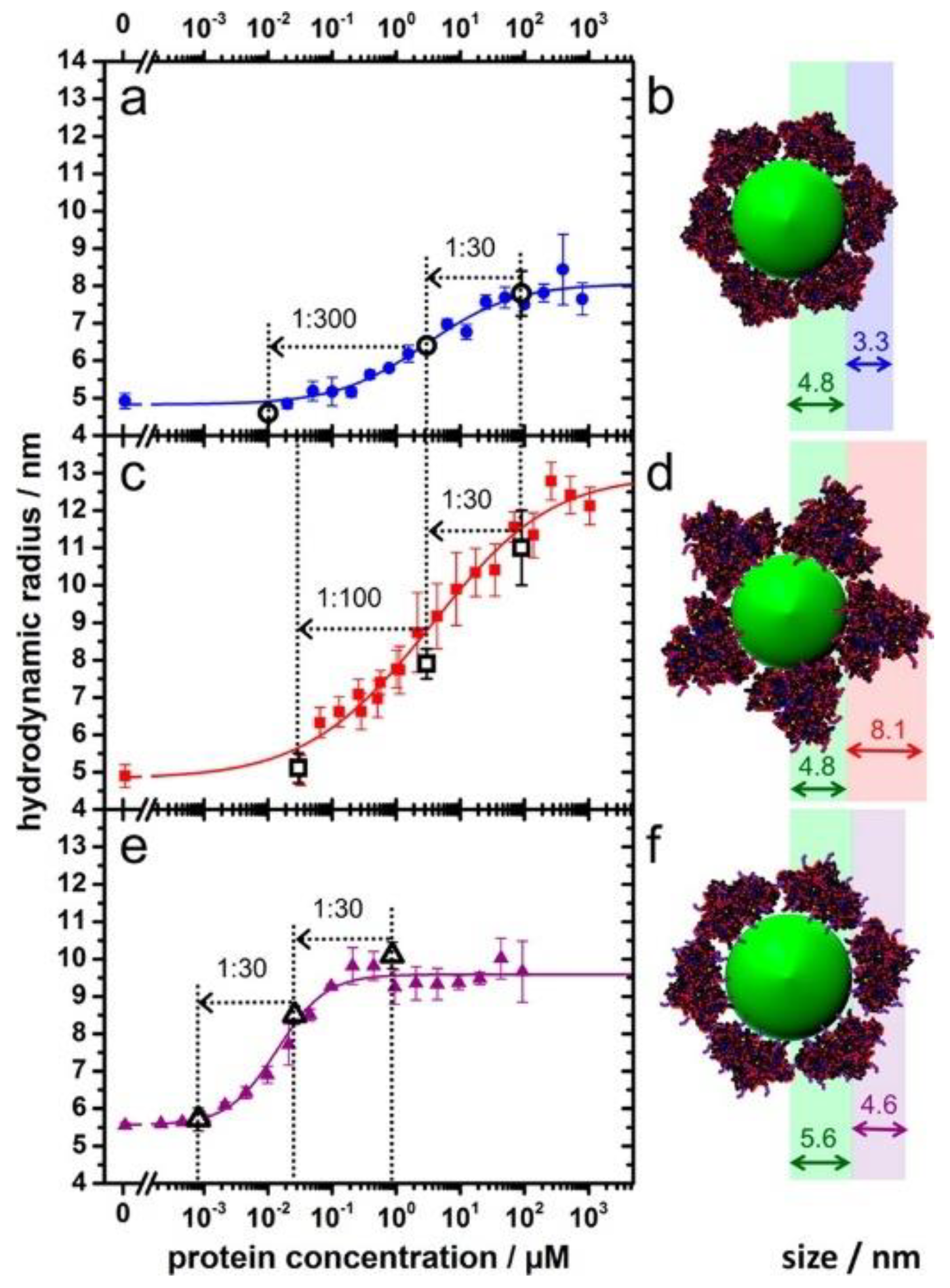
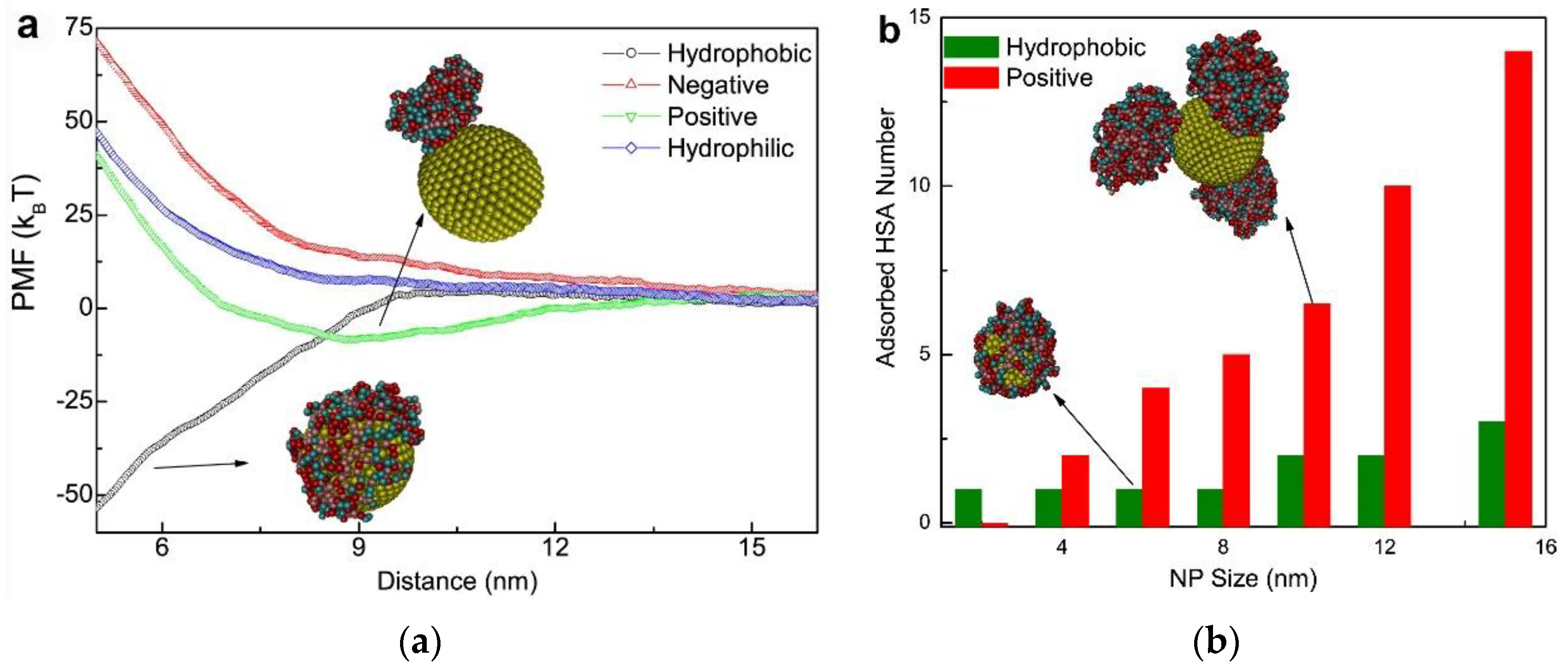
2.1. Nanoparticle–Protein Corona (NP-PC) Size, Shape, and Surface Charge
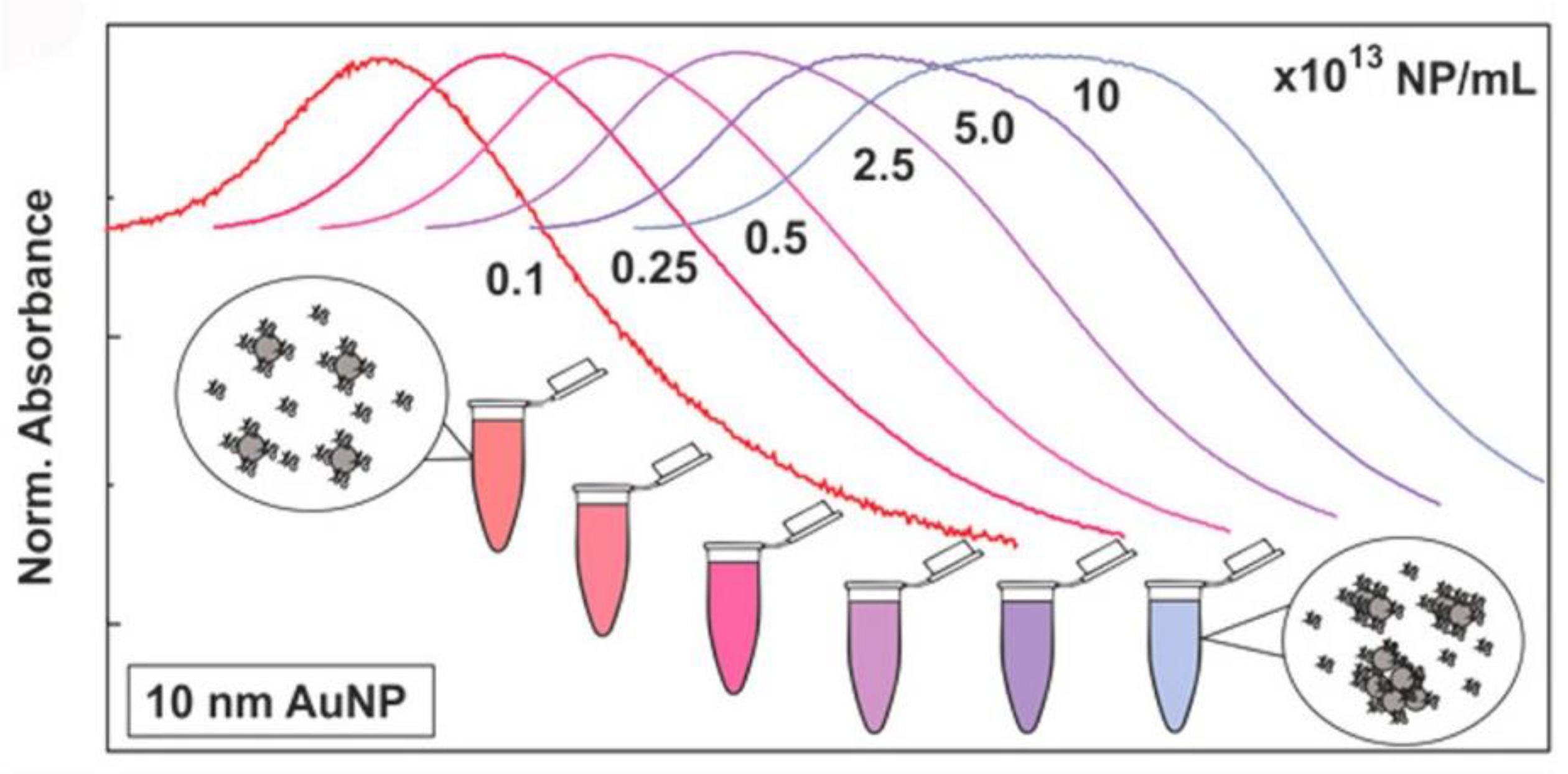
2.2. Binding of Proteins to NPs
2.3. PC Composition
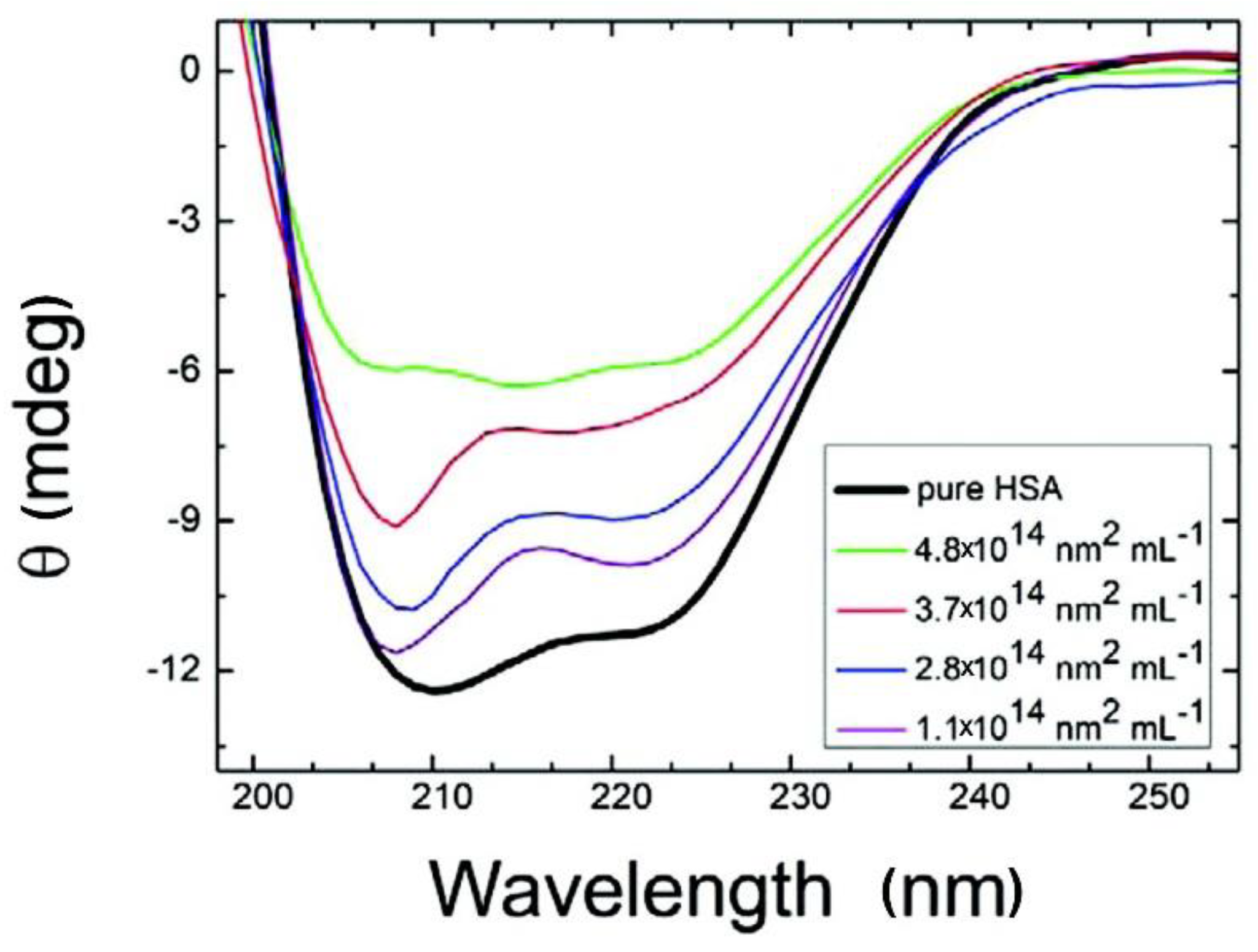
2.4. Conformational Changes in Proteins
3. Applications in Biomedicine
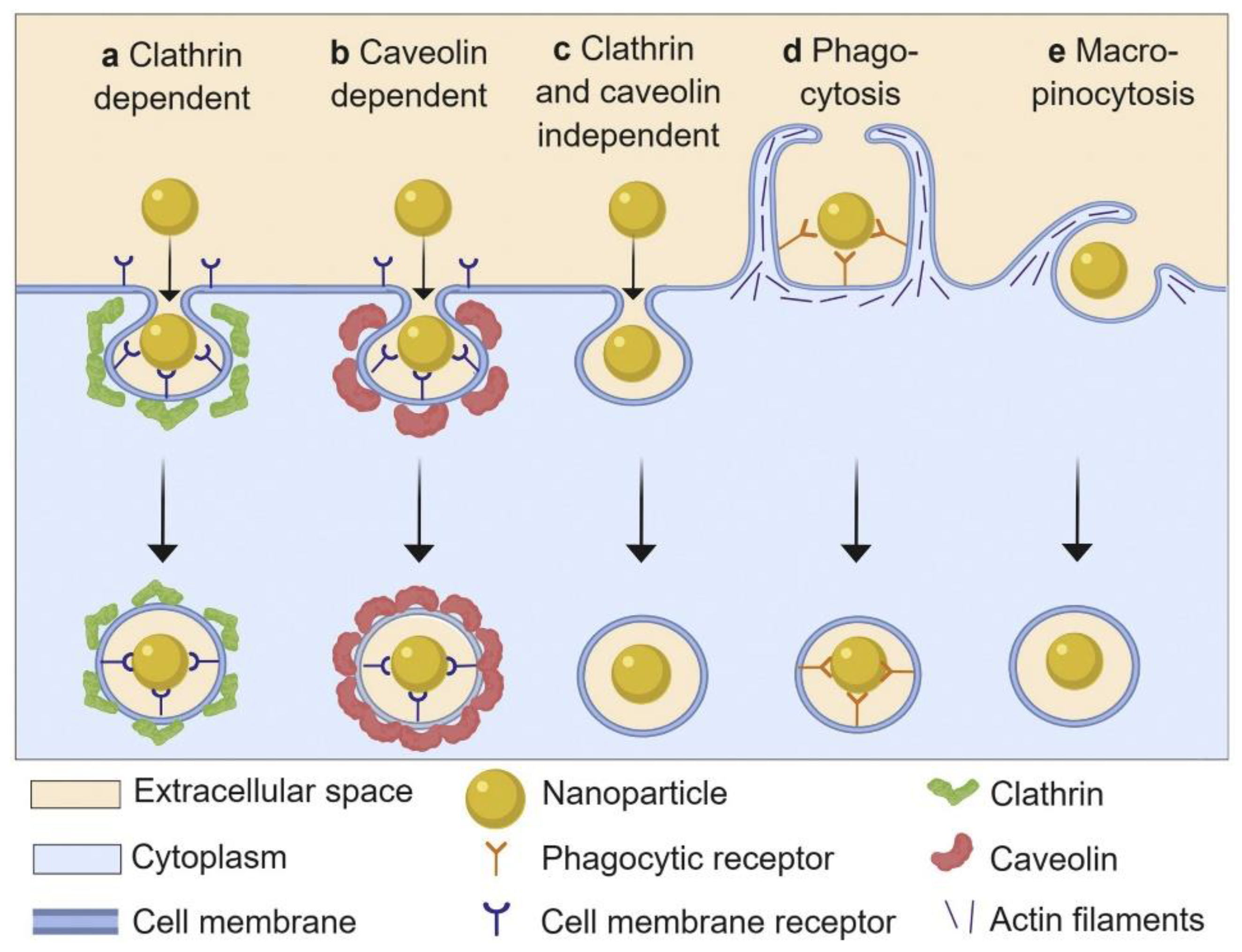
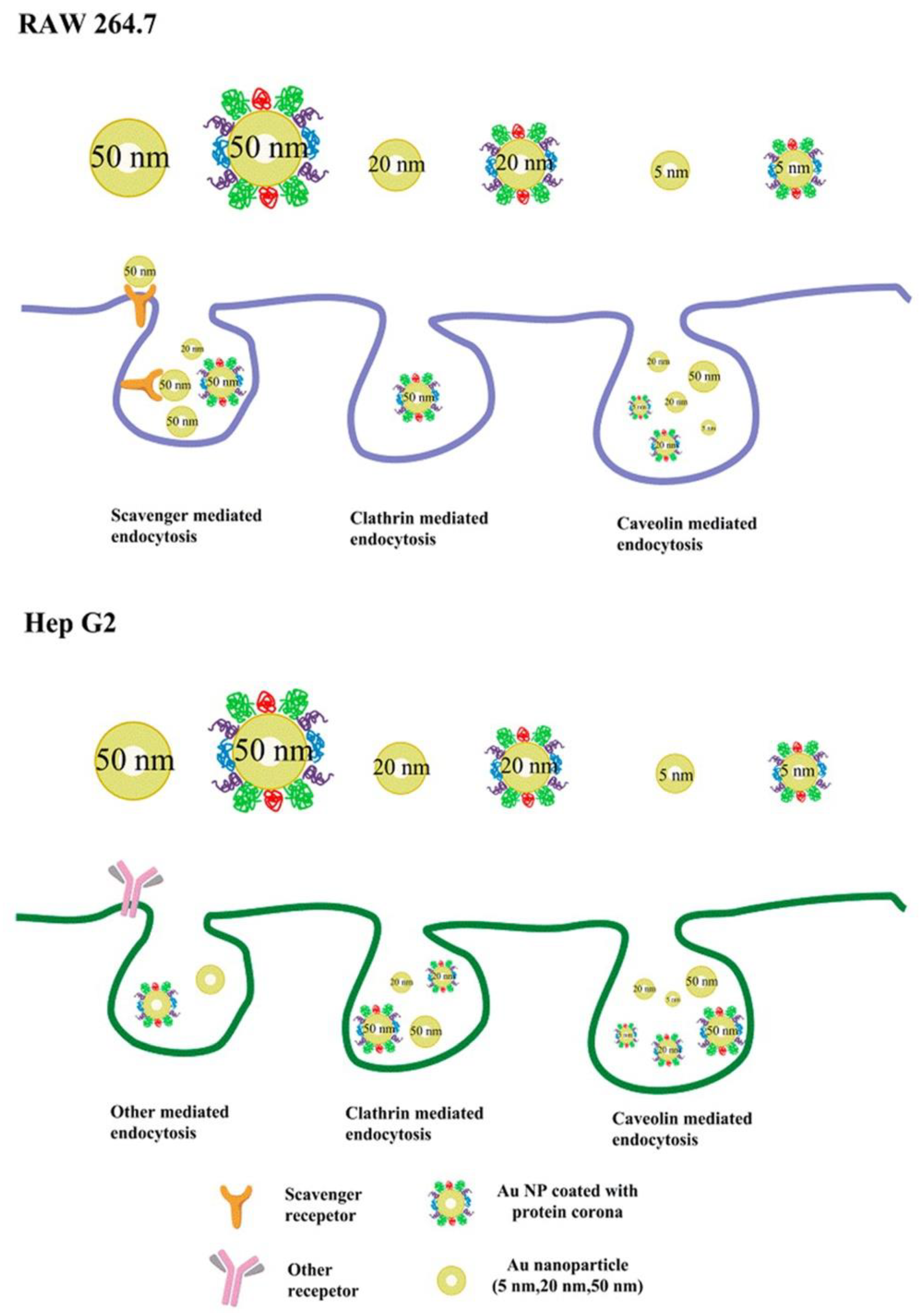
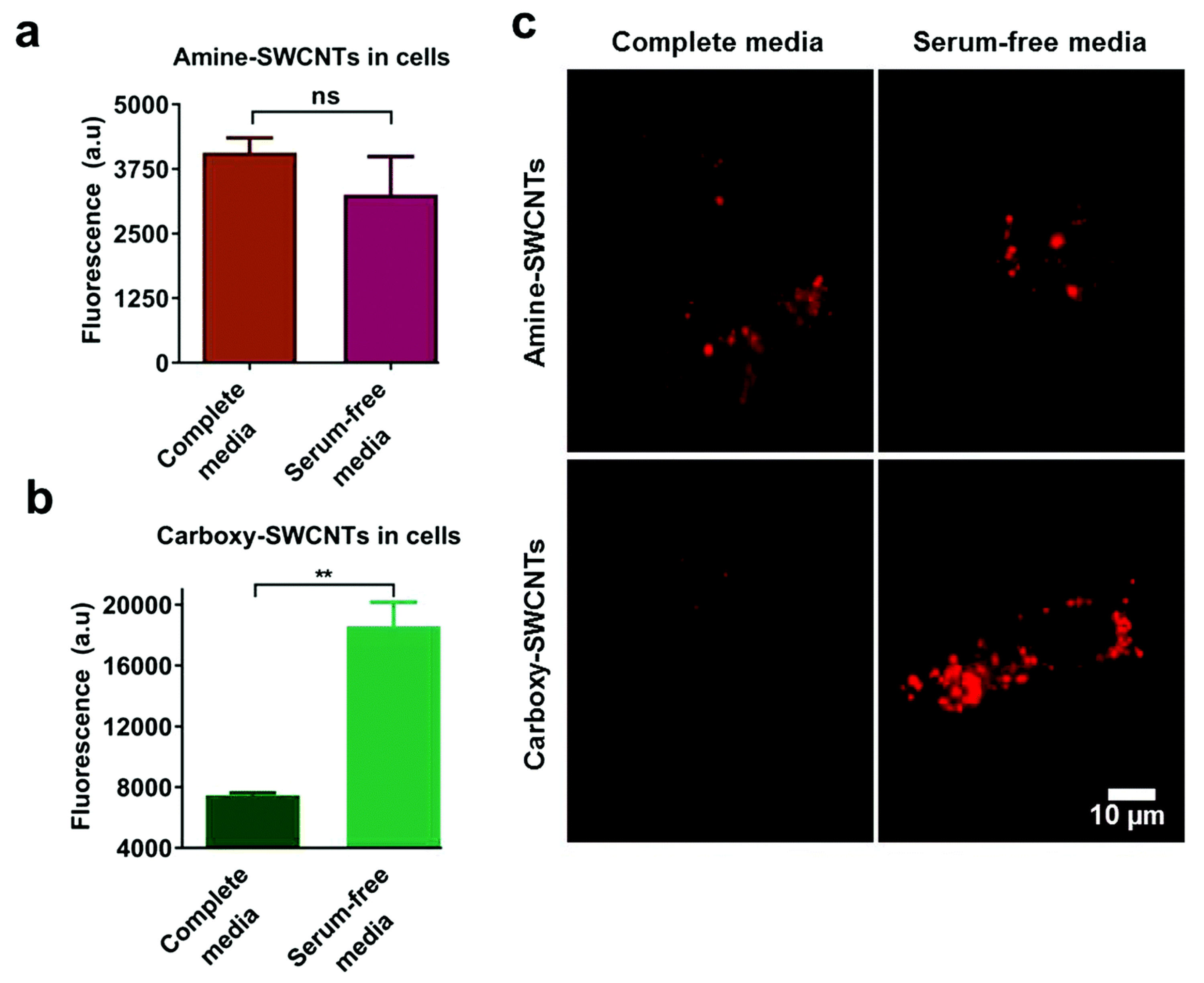
3.1. Regulating Cellular Uptake and Improving Drug Delivery
3.2. Modulating Drug Release
3.3. Modulating Cytotoxicity and Immune Response
3.3.1. Mitigating Bare NP-Mediated Cytotoxicity
3.3.2. Enhancing Bare NP-Mediated Cytotoxicity
3.3.3. Regulating Immune Response
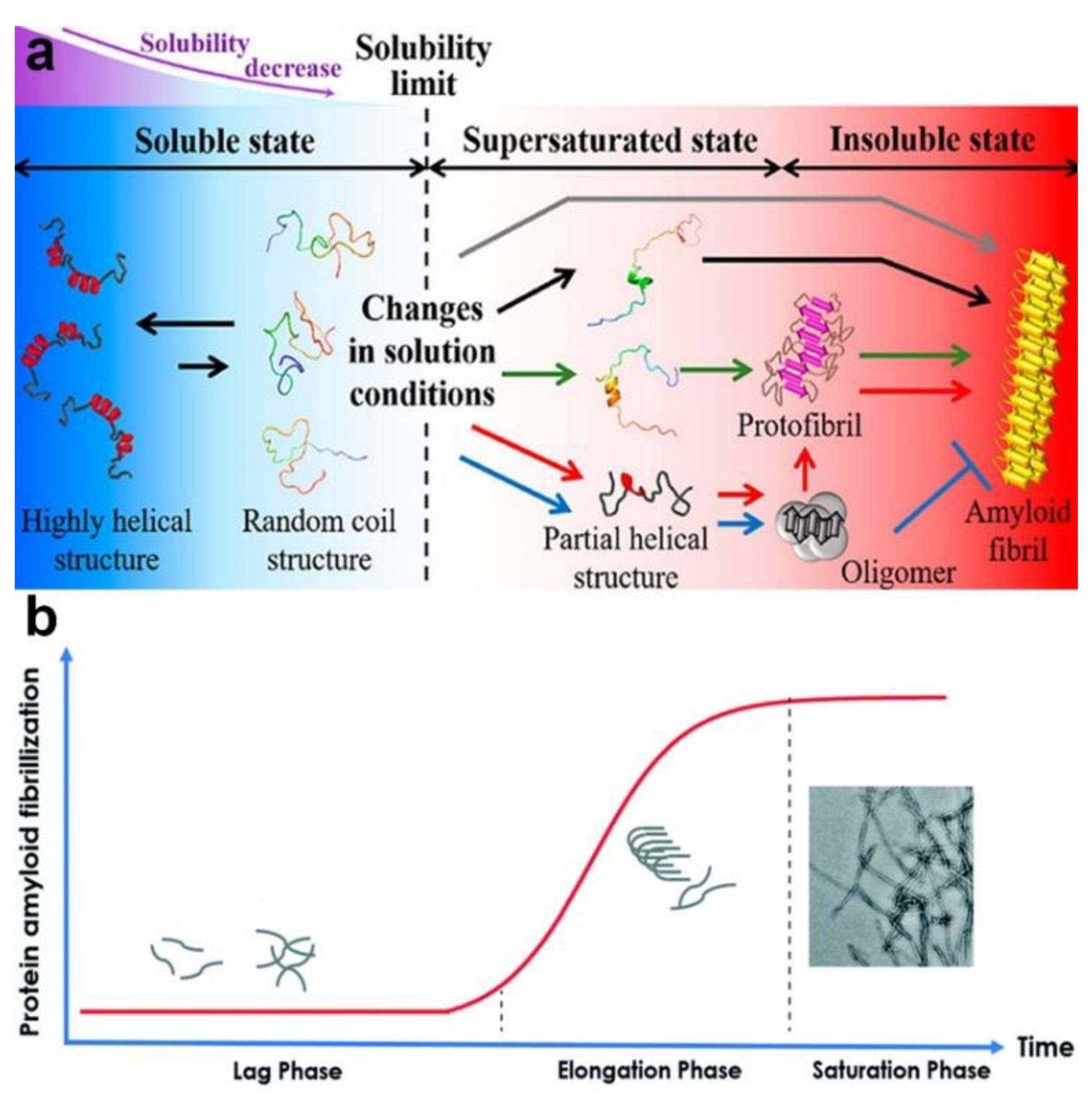
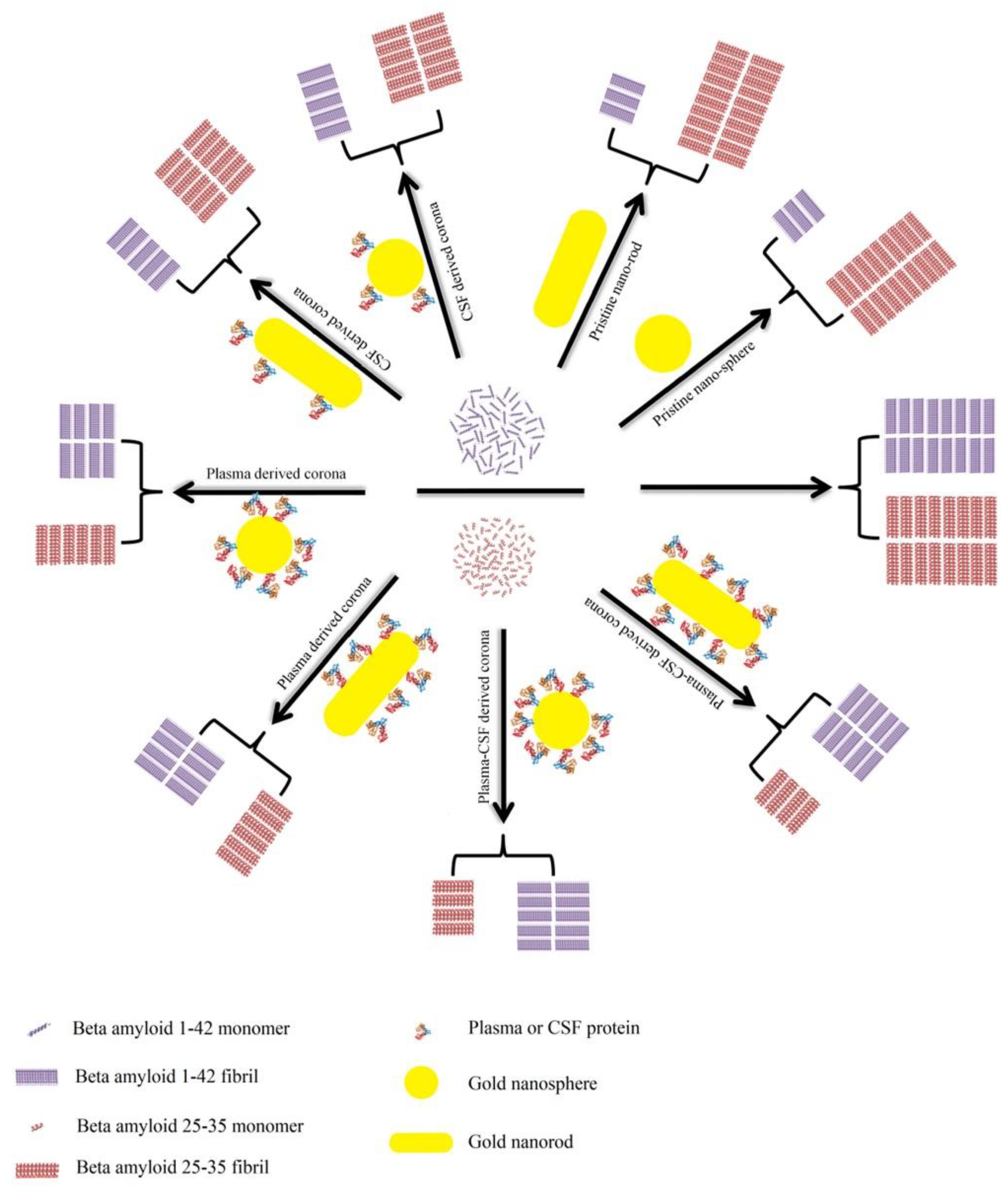
3.4. Regulating Protein Fibrillation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine 2013, 9, 1–14. [Google Scholar] [CrossRef]
- Santi, M.; Maccari, G.; Mereghetti, P.; Voliani, V.; Rocchiccioli, S.; Ucciferri, N.; Luin, S.; Signore, G. Rational design of a transferrin-binding peptide sequence tailored to targeted nanoparticle internalization. Bioconjugate Chem. 2017, 28, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically precise clusters of noble metals: Emerging link between atoms and nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, S.; Thomas, A.; Kotov, N.A.; Haag, R. Functional graphene nanomaterials based architectures: Biointeractions, fabrications, and emerging biological applications. Chem. Rev. 2017, 117, 1826–1914. [Google Scholar] [CrossRef]
- Shirai, H.; Nguyen, M.T.; Cempel, D.; Tsukamoto, H.; Tokunaga, T.; Liao, Y.C.; Yonezawa, T. Preparation of Au/Pd bimetallic nanoparticles by a microwave-induced plasma in liquid process. Bull. Chem. Soc. Jpn. 2017, 90, 279–285. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggard, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Barran-Berdon, A.L.; Pozzi, D.; Caracciolo, G.; Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Riccioli, A.; Palchetti, S.; Lagana, A. Time evolution of nanoparticle-protein corona in human plasma: Relevance for targeted drug delivery. Langmuir 2013, 29, 6485–6494. [Google Scholar] [CrossRef] [PubMed]
- Ritz, S.; Schottler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein corona of nanoparticles: Distinct proteins regulate the cellular uptake. Biomacromolecules 2015, 16, 1311–1321. [Google Scholar] [CrossRef]
- Piella, J.; Bastus, N.G.; Puntes, V. Size-dependent protein-nanoparticle interactions in citrate-stabilized gold nanoparticles: The emergence of the protein corona. Bioconjugate Chem. 2017, 28, 88–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.L.Y.; Lazarovits, J.; Chan, W.C.W. An analysis of the binding function and structural organization of the protein corona. J. Am. Chem. Soc. 2020, 142, 8827–8836. [Google Scholar] [CrossRef] [PubMed]
- Norde, W.; Lyklema, J. Why proteins prefer interfaces. J. Biomater. Sci. Polym. Ed. 1991, 2, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.J. The interaction of proteins with solid surfaces. Curr. Opin. Struc. Biol. 2004, 14, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Dawson, K.A.; Linse, S. Detecting cryptic epitopes created by nanoparticles. Sci. STKE 2006, 2006, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Meckes, B.; Mirkin, C.A. Spherical nucleic acids with tailored and active protein coronae. ACS Cent. Sci. 2019, 5, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.C.; Lin, S.; Parak, W.J.; Davis, T.P.; Caruso, F. A decade of the protein corona. ACS Nano 2017, 11, 11773–11776. [Google Scholar] [CrossRef]
- Deng, Z.J.; Liang, M.T.; Monteiro, M.; Toth, I.; Minchin, R.F. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat. Nanotechnol. 2011, 6, 39–44. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Nanoparticle-cell interactions: Molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- Yan, Y.; Gause, K.T.; Kamphuis, M.M.; Ang, C.S.; O’Brien-Simpson, N.M.; Lenzo, J.C.; Reynolds, E.C.; Nice, E.C.; Caruso, F. Differential roles of the protein corona in the cellular uptake of nanoporous polymer particles by monocyte and macrophage cell lines. ACS Nano 2013, 7, 10960–10970. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Bombelli, F.B.; Dawson, K.A. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Rahimi, M.; Yazdani, M.; Kim, S.T.; Moyano, D.F.; Hou, S.; Das, R.; Mout, R.; Rezaee, F.; Mahmoudi, M.; et al. Regulation of macrophage recognition through the interplay of nanoparticle surface functionality and protein corona. ACS Nano 2016, 10, 4421–4430. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, N.P.; Hurst, G.B.; Wang, W.; Foster, C.M.; Nallathamby, P.D.; Retterer, S.T. Dynamic development of the protein corona on silica nanoparticles: Composition and role in toxicity. Nanoscale 2013, 5, 6372–6380. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Giri, K.; Moyano, D.; Miranda, O.R.; Madden, B.; McCormick, D.J.; Bhattacharya, R.; Rotello, V.M.; Kocher, J.P.; Mukherjee, P. Identifying new therapeutic targets via modulation of protein corona formation by engineered nanoparticles. PLoS ONE 2012, 7, e33650. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Tian, X.; Wu, A.; Li, J.; Tian, J.; Chong, Y.; Chai, Z.; Zhao, Y.; Chen, C.; Ge, C. Protein corona influences cellular uptake of gold nanoparticles by phagocytic and nonphagocytic cells in a size-dependent manner. ACS Appl. Mater. Interfaces 2015, 7, 20568–20575. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, D.; Bombelli, F.B.; Monopoli, M.P.; Lynch, I.; Dawson, K.A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 2010, 132, 5761–5768. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Walkey, C.D.; Olsen, J.B.; Song, F.Y.; Liu, R.; Guo, H.B.; Olsen, D.W.H.; Cohen, Y.; Emili, A.; Chan, W.C.W. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 2014, 8, 2439–2455. [Google Scholar] [CrossRef]
- Winzen, S.; Schoettler, S.; Baier, G.; Rosenauer, C.; Mailaender, V.; Landfester, K.; Mohr, K. Complementary analysis of the hard and soft protein corona: Sample preparation critically effects corona composition. Nanoscale 2015, 7, 2992–3001. [Google Scholar] [CrossRef]
- Pareek, V.; Bhargava, A.; Bhanot, V.; Gupta, R.; Jain, N.; Panwar, J. Formation and characterization of protein corona around nanoparticles: A review. J. Nanosci. Nanotechnol. 2018, 18, 6653–6670. [Google Scholar] [CrossRef]
- Cedervall, T.; Lynch, I.; Foy, M.; Berggard, T.; Donnelly, S.C.; Cagney, G.; Linse, S.; Dawson, K.A. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 5754–5756. [Google Scholar] [CrossRef]
- Rocker, C.; Potzl, M.; Zhang, F.; Parak, W.J.; Nienhaus, G.U. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat. Nanotechnol. 2009, 4, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Vogler, E.A. Protein adsorption in three dimensions. Biomaterials 2012, 33, 1201–1237. [Google Scholar] [CrossRef] [PubMed]
- Huhn, J.; Fedeli, C.; Zhang, Q.; Masood, A.; del Pino, P.; Khashab, N.M.; Papini, E.; Parak, W.J. Dissociation coefficients of protein adsorption to nanoparticles as quantitative metrics for description of the protein corona: A comparison of experimental techniques and methodological relevance. Int. J. Biochem. Cell Biol. 2016, 75, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Patri, A.K.; Zheng, J.; Clogston, J.D.; Ayub, N.; Aggarwal, P.; Neun, B.W.; Hall, J.B.; McNeil, S.E. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomedicine 2009, 5, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Pecora, R. Dynamic light scattering measurement of nanometer particles in liquids. J. Nanoparticle Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef]
- Grafe, C.; Weidner, A.; Luhe, M.V.; Bergemann, C.; Schacher, F.H.; Clement, J.H.; Dutz, S. Intentional formation of a protein corona on nanoparticles: Serum concentration affects protein corona mass, surface charge, and nanoparticle-cell interaction. Int. J. Biochem. Cell Biol. 2016, 75, 196–202. [Google Scholar] [CrossRef]
- Konduru, N.V.; Molina, R.M.; Swami, A.; Damiani, F.; Pyrgiotakis, G.; Lin, P.; Andreozzi, P.; Donaghey, T.C.; Demokritou, P.; Krol, S.; et al. Protein corona: Implications for nanoparticle interactions with pulmonary cells. Part. Fibre Toxicol. 2017, 14, 42. [Google Scholar] [CrossRef]
- Wang, L.; Hartel, N.; Ren, K.X.; Graham, N.A.; Malmstadt, N. Effect of protein corona on nanoparticle-plasma membrane and nanoparticle-biomimetic membrane interactions. Environ. Sci. Nano 2020, 7, 963–974. [Google Scholar] [CrossRef]
- Gupta, M.N.; Roy, I. How corona formation impacts nanomaterials as drug carriers. Mol. Pharmaceutics 2019, 17, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Bergese, P.; Hamad-Schifferli, K. Nanomaterial Interfaces in Biology: Methods and Protocols; Humana Press: New York, NY, USA, 2013; pp. 141–157. [Google Scholar]
- Radauer-Preiml, I.; Andosch, A.; Hawranek, T.; Luetz-Meindl, U.; Wiederstein, M.; Horejs-Hoeck, J.; Himly, M.; Boyles, M.; Duschl, A. Nanoparticle-allergen interactions mediate human allergic responses: Protein corona characterization and cellular responses. Part. Fibre Toxicol. 2016, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Kokkinopoulou, M.; Simon, J.; Landfester, K.; Mailander, V.; Lieberwirth, I. Visualization of the protein corona: Towards a biomolecular understanding of nanoparticle-cell-interactions. Nanoscale 2017, 9, 8858–8870. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.H.; Liu, R.X.; Deng, Z.Y.; Ge, G.L.; Liu, Y.; Xie, L.M. Quantitative study of protein coronas on gold nanoparticles with different surface modifications. Nano Res. 2014, 7, 345–352. [Google Scholar] [CrossRef]
- Johnston, B.D.; Kreyling, W.G.; Pfeiffer, C.; Schaffler, M.; Sarioglu, H.; Ristig, S.; Hirn, S.; Haberl, N.; Thalhammer, S.; Hauck, S.M.; et al. Colloidal stability and surface chemistry are key factors for the composition of the protein corona of inorganic gold nanoparticles. Adv. Funct. Mater. 2017, 27, 1701956. [Google Scholar] [CrossRef]
- Pyrgiotakis, G.; Blattmann, C.O.; Pratsinis, S.; Demokritou, P. Nanoparticle-nanoparticle interactions in biological media by atomic force microscopy. Langmuir 2013, 29, 11385–11395. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhang, F.; Yu, S. Probing the binding affinity of plasma proteins adsorbed on Au nanoparticles. Nanoscale 2017, 9, 4787–4792. [Google Scholar] [CrossRef]
- Vergaro, V.; Pisano, I.; Grisorio, R.; Baldassarre, F.; Mallamaci, R.; Santoro, A.; Suranna, G.P.; Papadia, P.; Fanizzi, F.P.; Ciccarella, G. CaCO3 as an environmentally friendly renewable material for drug delivery systems: Uptake of HSA-CaCO3 nanocrystals conjugates in cancer cell lines. Materials 2019, 12, 1481. [Google Scholar] [CrossRef]
- Natte, K.; Friedrich, J.F.; Wohlrab, S.; Lutzki, J.; von Klitzing, R.; Osterle, W.; Orts-Gil, G. Impact of polymer shell on the formation and time evolution of nanoparticle-protein corona. Colloids Surf. B 2013, 104, 213–220. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Abdelmonem, A.M.; Behzadi, S.; Clement, J.H.; Dutz, S.; Ejtehadi, M.R.; Hartmann, R.; Kantner, K.; Linne, U.; Maffre, P.; et al. Temperature: The “ignored” factor at the nanobio interface. ACS Nano 2013, 7, 6555–6562. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Yang, Z.; Garate, J.A.; Gu, Z.; Weber, J.K.; Liu, J.; Zhou, R. Reduced cytotoxicity of graphene nanosheets mediated by blood-protein coating. ACS Nano 2015, 9, 5713–5724. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Gupta, M.N. Protein aggregates: Forms, functions and applications. Int. J. Biol. Macromol. 2017, 97, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, F.; Ceccone, G.; Moretti, P.; Campanella, G.; Ferrero, C.; Combet, S.; Ojeajimenez, I.; Ghigna, P. Structural and thermodynamic properties of nanoparticle-protein complexes: A combined SAXS and SANS study. Langmuir 2017, 33, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yadav, I.; Aswal, V.K.; Kohlbrecher, J. Structure and interaction of nanoparticle-protein complexes. Langmuir 2018, 34, 5679–5695. [Google Scholar] [CrossRef]
- Gillespie, C.; Halling, P.; Edwards, D. Monitoring of particle growth at a low concentration of a poorly water soluble drug using the NanoSight LM20. Colloids Surf. A 2011, 384, 233–239. [Google Scholar] [CrossRef]
- Jedlovszky-Hajdu, A.; Bombelli, F.B.; Monopoli, M.P.; Tombacz, E.; Dawson, K.A. Surface coatings shape the protein corona of spions with relevance to their application in vivo. Langmuir 2012, 28, 14983–14991. [Google Scholar] [CrossRef]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Lundqvist, M.; Sethson, I.; Jonsson, B.H. Protein adsorption onto silica nanoparticles: Conformational changes depend on the particles’ curvature and the protein stability. Langmuir 2004, 20, 10639–10647. [Google Scholar] [CrossRef]
- Delfino, I.; Cannistraro, S. Optical investigation of the electron transfer protein azurin-gold nanoparticle system. Biophys. Chem. 2009, 139, 1–7. [Google Scholar] [CrossRef]
- Furumoto, K.; Ogawara, K.; Nagayama, S.; Takakura, Y.; Hashida, M.; Higaki, K.; Kimura, T. Important role of serum proteins associated on the surface of particles in their hepatic disposition. J. Control. Release 2002, 83, 89–96. [Google Scholar] [CrossRef]
- Vilanova, O.; Mittag, J.J.; Kelly, P.M.; Milani, S.; Dawson, K.A.; Radler, J.O.; Franzese, G. Understanding the kinetics of protein-nanoparticle corona formation. ACS Nano 2016, 10, 10842–10850. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Medina, S.; Chen, S.S.; Blankenburg, J.; Swanglap, P.; Landes, C.F.; Link, S. Measuring the hydrodynamic size of nanoparticles using fluctuation correlation spectroscopy. Annu. Rev. Phys. Chem. 2016, 67, 489–514. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moro, M.; Di Silvio, D.; Moya, S.E. Fluorescence correlation spectroscopy as a tool for the study of the intracellular dynamics and biological fate of protein corona. Biophys. Chem. 2019, 253, 106218. [Google Scholar] [CrossRef]
- Treuel, L.; Brandholt, S.; Maffre, P.; Wiegele, S.; Shang, L.; Nienhaus, G.U. Impact of protein modification on the protein corona on nanoparticles and nanoparticle-cell interactions. ACS Nano 2014, 8, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S.H.; Glomm, W.R.; Johnson, M.C.; Knag, M.K.; Franzen, S. Probing BSA binding to citrate-coated gold nanoparticles and surfaces. Langmuir 2005, 21, 9303–9307. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, E.D.; Belyea, J.; Johnson, M.C.; Nicholson, Z.M.; Ricks, J.L.; Shah, P.K.; Bayless, M.; Pettersson, T.; Feldoto, Z.; Blomberg, E.; et al. Probing protein adsorption onto mercaptoundecanoic acid stabilized gold nanoparticles and surfaces by quartz crystal microbalance and zeta-potential measurements. Langmuir 2007, 23, 6053–6062. [Google Scholar] [CrossRef]
- Huang, R.; Carney, R.P.; Ikuma, K.; Stellacci, F.; Lau, B.L. Effects of surface compositional and structural heterogeneity on nanoparticle-protein interactions: Different protein configurations. ACS Nano 2014, 8, 5402–5412. [Google Scholar] [CrossRef]
- Sanchez-Moreno, P.; Buzon, P.; Boulaiz, H.; Peula-Garcia, J.M.; Ortega-Vinuesa, J.L.; Luque, I.; Salvati, A.; Marchal, J.A. Balancing the effect of corona on therapeutic efficacy and macrophage uptake of lipid nanocapsules. Biomaterials 2015, 61, 266–278. [Google Scholar] [CrossRef]
- Calvaresi, M.; Arnesano, F.; Bonacchi, S.; Bottoni, A.; Calo, V.; Conte, S.; Falini, G.; Fermani, S.; Losacco, M.; Montalti, M.; et al. C60@Lysozyme: Direct observation by nuclear magnetic resonance of a 1:1 fullerene protein adduct. ACS Nano 2014, 8, 1871–1877. [Google Scholar] [CrossRef]
- Carril, M.; Padro, D.; Del Pino, P.; Carrillo-Carrion, C.; Gallego, M.; Parak, W.J. In situ detection of the protein corona in complex environments. Nat. Commun. 2017, 8, 1542. [Google Scholar] [CrossRef]
- Brancolini, G.; Bellucci, L.; Maschio, M.C.; Di Felice, R.; Corni, S. The interaction of peptides and proteins with nanostructures surfaces: A challenge for nanoscience. Curr. Opin. Colloid Interface Sci. 2019, 41, 86–94. [Google Scholar] [CrossRef]
- Ding, H.M.; Ma, Y.Q. Computer simulation of the role of protein corona in cellular delivery of nanoparticles. Biomaterials 2014, 35, 8703–8710. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Hall, C.K. Protein adsorption on nanoparticles: Model development using computer simulation. J. Phys. Condens. Matter 2016, 28, 414019. [Google Scholar] [CrossRef] [PubMed]
- Zhdanov, V.P. Formation of a protein corona around nanoparticles. Curr. Opin. Colloid Interface Sci. 2019, 41, 95–103. [Google Scholar] [CrossRef]
- Strojan, K.; Leonardi, A.; Bregar, V.B.; Krizaj, I.; Svete, J.; Pavlin, M. Dispersion of nanoparticles in different media importantly determines the composition of their protein corona. PLoS ONE 2017, 12, e0169552. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Augustsson, C.; Lilja, M.; Lundkvist, K.; Dahlback, B.; Linse, S.; Cedervall, T. The nanoparticle protein corona formed in human blood or human blood fractions. PLoS ONE 2017, 12, e0175871. [Google Scholar] [CrossRef]
- Partikel, K.; Korte, R.; Mulac, D.; Humpf, H.U.; Langer, K. Serum type and concentration both affect the protein-corona composition of PLGA nanoparticles. Beilstein J. Nanotech. 2019, 10, 1002–1015. [Google Scholar] [CrossRef]
- Ali, N.; Mattsson, K.; Rissler, J.; Karlsson, H.M.; Svensson, C.R.; Gudmundsson, A.; Lindh, C.H.; Jonsson, B.A.G.; Cedervall, T.; Karedal, M. Analysis of nanoparticle-protein coronas formed in vitro between nanosized welding particles and nasal lavage proteins. Nanotoxicology 2016, 10, 226–234. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Blunk, T.; Hochstrasser, D.F.; Sanchez, J.C.; Muller, B.W.; Muller, R.H. Colloidal carriers for intravenous drug targeting: Plasma protein adsorption patterns on surface-modified latex particles evaluated by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis 1993, 14, 1382–1387. [Google Scholar] [CrossRef]
- Goppert, T.M.; Muller, R.H. Plasma protein adsorption of Tween 80- and poloxamer 188-stabilized solid lipid nanoparticles. J. Drug Target. 2003, 11, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gessner, A.; Lieske, A.; Paulke, B.R.; Muller, R.H. Functional groups on polystyrene model nanoparticles: Influence on protein adsorption. J. Biomed. Mater. Res. A 2003, 65, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, S.; Ogawara, K.; Fukuoka, Y.; Higaki, K.; Kimura, T. Time-dependent changes in opsonin amount associated on nanoparticles alter their hepatic uptake characteristics. Int. J. Pharm. 2007, 342, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, H.; You, C.C.; Rotello, V.M.; Knapp, M.J. Facial control of nanoparticle binding to Cytochrome c. J. Am. Chem. Soc. 2007, 129, 2732–2733. [Google Scholar] [CrossRef] [PubMed]
- Buijs, J.; Ramstrom, M.; Danfelter, M.; Larsericsdotter, H.; Hakansson, P.; Oscarsson, S. Localized changes in the structural stability of myoglobin upon adsorption onto silica particles, as studied with hydrogen/deuterium exchange mass spectrometry. J. Colloid Interf. Sci. 2003, 263, 441–448. [Google Scholar] [CrossRef]
- Shrivastava, S.; Nuffer, J.H.; Siegel, R.W.; Dordick, J.S. Position-specific chemical modification and quantitative proteomics disclose protein orientation adsorbed on silica nanoparticles. Nano Lett. 2012, 12, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Diederichs, J.E. Plasma protein adsorption patterns on liposomes: Establishment of analytical procedure. Electrophoresis 1996, 17, 607–611. [Google Scholar] [CrossRef]
- Thode, K.; Luck, M.; Semmler, W.; Muller, R.H.; Kresse, M. Determination of plasma protein adsorption on magnetic iron oxides: Sample preparation. Pharm. Res. 1997, 14, 905–910. [Google Scholar] [CrossRef]
- Bayburt, T.H.; Grinkova, Y.V.; Sligar, S.G. Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2002, 2, 853–856. [Google Scholar] [CrossRef]
- Fan, R.; Chew, S.W.; Cheong, V.V.; Orner, B.P. Fabrication of gold nanoparticles inside unmodified horse spleen apoferritin. Small 2010, 6, 1483–1487. [Google Scholar] [CrossRef]
- Li, Y.; Lee, J.S. Staring at protein-surfactant interactions: Fundamental approaches and comparative evaluation of their combinations. Anal. Chim. Acta. 2019, 1063, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Chauhan, N.; Othman, S.F.; Khalilzad-Sharghi, V.; Ebeling, M.C.; Khan, S.; Jaggi, M.; Chauhan, S.C. Implications of protein corona on physico-chemical and biological properties of magnetic nanoparticles. Biomaterials 2015, 46, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ban, D.K.; Paul, S. Protein corona over silver nanoparticles triggers conformational change of proteins and drop in bactericidal potential of nanoparticles: Polyethylene glycol capping as preventive strategy. Colloids Surf. B 2016, 146, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Mao, X.Y.; Wang, Y.F.; Li, D.D.; Du, Z.Y.; Wu, W.H.; Jiang, L.; Yang, J.; Li, J.J. Study on the interaction of graphene oxide silver nanocomposites with bovine serum albumin and the formation of nanoparticle-protein corona. Int. J. Biol. Macromol. 2018, 116, 492–501. [Google Scholar] [CrossRef]
- Ge, C.C.; Du, J.F.; Zhao, L.N.; Wang, L.M.; Liu, Y.; Li, D.H.; Yang, Y.L.; Zhou, R.H.; Zhao, Y.L.; Chai, Z.F.; et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. USA 2011, 108, 16968–16973. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wang, M.Z.; Lei, R.; Zhu, S.F.; Zhao, Y.L.; Chen, C.Y. Chiral surface of nanoparticles determines the orientation of adsorbed transferrin and its interaction with receptors. ACS Nano 2017, 11, 4606–4616. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Li, J.Y.; Pan, J.; Jiang, X.M.; Ji, Y.L.; Li, Y.F.; Qu, Y.; Zhao, Y.L.; Wu, X.C.; Chen, C.Y. Revealing the binding structure of the protein corona on gold nanorods using synchrotron radiation-based techniques: Understanding the reduced damage in cell membranes. J. Am. Chem. Soc. 2013, 135, 17359–17368. [Google Scholar] [CrossRef]
- Gebauer, J.S.; Malissek, M.; Simon, S.; Knauer, S.K.; Maskos, M.; Stauber, R.H.; Peukert, W.; Treuel, L. Impact of the nanoparticle-protein corona on colloidal stability and protein structure. Langmuir 2012, 28, 9673–9679. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Aboul-Enein, H.Y.; Hoang, V.D. Raman spectroscopy for protein analysis. Appl. Spectrosc. Rev. 2015, 50, 377–386. [Google Scholar] [CrossRef]
- Zhang, D.M.; Neumann, O.; Wang, H.; Yuwono, V.M.; Barhoumi, A.; Perham, M.; Hartgerink, J.D.; Wittung-Stafshede, P.; Halas, N.J. Gold nanoparticles can induce the formation of protein-based aggregates at physiological pH. Nano Lett. 2009, 9, 666–671. [Google Scholar] [CrossRef]
- Cao, Y.C.; Jin, R.C.; Nam, J.M.; Thaxton, C.S.; Mirkin, C.A. Raman dye-labeled nanoparticle probes for proteins. J. Am. Chem. Soc. 2003, 125, 14676–14677. [Google Scholar] [CrossRef] [PubMed]
- Mudunkotuwa, I.A.; Al Minshid, A.; Grassian, V.H. ATR-FTIR spectroscopy as a tool to probe surface adsorption on nanoparticles at the liquid-solid interface in environmentally and biologically relevant media. Analyst 2014, 139, 870–881. [Google Scholar] [CrossRef]
- Mbeh, D.A.; Javanbakht, T.; Tabet, L.; Merhi, Y.; Maghni, K.; Sacher, E.; Yahia, L. Protein corona formation on magnetite nanoparticles: Effects of culture medium composition, and its consequences on superparamagnetic nanoparticle cytotoxicity. J. Biomed. Nanotechnol. 2015, 11, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Varnamkhasti, B.S.; Hosseinzadeh, H.; Azhdarzadeh, M.; Vafaei, S.Y.; Esfandyari-Manesh, M.; Mirzaie, Z.H.; Amini, M.; Ostad, S.N.; Atyabi, F.; Dinarvand, R. Protein corona hampers targeting potential of MUC1 aptamer functionalized SN-38 core-shell nanoparticles. Int. J. Pharm. 2015, 494, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Faklaris, O.; Joshi, V.; Irinopoulou, T.; Tauc, P.; Sennour, M.; Girard, H.; Gesset, C.; Arnault, J.C.; Thorel, A.; Boudou, J.P.; et al. Photoluminescent diamond nanoparticles for cell labeling: Study of the uptake mechanism in mammalian cells. ACS Nano 2009, 3, 3955–3962. [Google Scholar] [CrossRef]
- Krpetic, Z.; Porta, F.; Caneva, E.; Dal Santo, V.; Scari, G. Phagocytosis of biocompatible gold nanoparticles. Langmuir 2010, 26, 14799–14805. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Lin, J.B.; Hens, S.C.; Hussain, S.M. Temporal and mechanistic tracking of cellular uptake dynamics with novel surface fluorophore-bound nanodiamonds. Nanoscale 2011, 3, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-dependent endocytosis of nanoparticles. Adv. Mater. 2009, 21, 419–424. [Google Scholar] [CrossRef]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Ryman-Rasmussen, J.P.; Riviere, J.E.; Monteiro-Riviere, N.A. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol. Sci. 2006, 91, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.L.L.; Cheah, J.U.J.; Thong, P.S.P.; Soo, K.C.; Kah, J.C.Y. Gold nanorods coated with apolipoprotein e protein corona for drug delivery. Acs Appl. Nano Mater. 2019, 2, 6220–6229. [Google Scholar] [CrossRef]
- Guo, L.L.; Feng, Z.Q.; Cai, L.F.; Xi, Z.J.; Mou, X.B.; Wang, T.; He, N.Y. Effects of a protein-corona on the cellular uptake of ferroferric oxide nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 7125–7128. [Google Scholar] [CrossRef]
- Oh, J.Y.; Kim, H.S.; Palanikumar, L.; Go, E.M.; Jana, B.; Park, S.A.; Kim, H.Y.; Kim, K.; Seo, J.K.; Kwak, S.K.; et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat. Commun. 2018, 9, 4548. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Muller, L.K.; Kokkinopoulou, M.; Lieberwirth, I.; Morsbach, S.; Landfester, K.; Mailander, V. Exploiting the biomolecular corona: Pre-coating of nanoparticles enables controlled cellular interactions. Nanoscale 2018, 10, 10731–10739. [Google Scholar] [CrossRef]
- Huang, X.H.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostic and therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef]
- Bodelon, G.; Costas, C.; Perez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzan, L.M. Gold nanoparticles for regulation of cell function and behavior. Nano Today 2017, 13, 40–60. [Google Scholar] [CrossRef]
- Li, Y.; Monteiro-Riviere, N.A. Mechanisms of cell uptake, inflammatory potential and protein corona effects with gold nanoparticles. Nanomedicine 2016, 11, 3185–3203. [Google Scholar] [CrossRef]
- Ortega, M.T.; Riviere, J.E.; Choi, K.; Monteiro-Riviere, N.A. Biocorona formation on gold nanoparticles modulates human proximal tubule kidney cell uptake, cytotoxicity and gene expression. Toxicol. Vitro 2017, 42, 150–160. [Google Scholar] [CrossRef]
- Choi, K.; Riviere, J.E.; Monteiro-Riviere, N.A. Protein corona modulation of hepatocyte uptake and molecular mechanisms of gold nanoparticle toxicity. Nanotoxicology 2017, 11, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Meghani, N.M.; Amin, H.H.; Tran, T.T.D.; Tran, P.H.L.; Park, C.; Lee, B.J. Modulation of serum albumin protein corona for exploring cellular behaviors of fattigation-platform nanoparticles. Colloids Surf. B 2018, 170, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Schottler, S.; Klein, K.; Landfester, K.; Mailander, V. Protein source and choice of anticoagulant decisively affect nanoparticle protein corona and cellular uptake. Nanoscale 2016, 8, 5526–5536. [Google Scholar] [CrossRef] [PubMed]
- Binnemars-Postma, K.A.; ten Hoopen, H.W.M.; Storm, G.; Prakash, J. Differential uptake of nanoparticles by human M1 and M2 polarized macrophages: Protein corona as a critical determinant. Nanomedicine 2016, 11, 2889–2902. [Google Scholar] [CrossRef]
- Ho, Y.T.; Kamm, R.D.; Kah, J.C.Y. Influence of protein corona and caveolae-mediated endocytosis on nanoparticle uptake and transcytosis. Nanoscale 2018, 10, 12386–12397. [Google Scholar] [CrossRef]
- Beck, M.; Mandal, T.; Buske, C.; Linden, M. Serum protein adsorption enhances active leukemia stem cell targeting of mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 18566–18574. [Google Scholar] [CrossRef]
- Budhathoki-Uprety, J.; Harvey, J.D.; Isaac, E.; Williams, R.M.; Galassi, T.V.; Langenbacher, R.E.; Heller, D.A. Polymer cloaking modulates the carbon nanotube protein corona and delivery into cancer cells. J. Mater. Chem. B 2017, 5, 6637–6644. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, M.; Yao, Y.; Ma, Y.; Pu, Y.P. MWCNT interactions with protein: Surface-induced changes in protein adsorption and the impact of protein corona on cellular uptake and cytotoxicity. Int. J. Nanomed. 2019, 14, 993–1009. [Google Scholar] [CrossRef]
- Mosquera, J.; Garcia, I.; Henriksen-Lacey, M.; Martinez-Calvo, M.; Dhanjani, M.; Mascarenas, J.L.; Liz-Marzan, L.M. Reversible control of protein corona formation on gold nanoparticles using host–guest interactions. ACS Nano 2020, 14, 5382–5391. [Google Scholar] [CrossRef]
- Schottler, S.; Becker, G.; Winzen, S.; Steinbach, T.; Mohr, K.; Landfester, K.; Mailander, V.; Wurm, F.R. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat. Nanotechnol. 2016, 11, 372–377. [Google Scholar] [CrossRef]
- Piloni, A.; Wong, C.K.; Chen, F.; Lord, M.; Walther, A.; Stenzel, M.H. Surface roughness influences the protein corona formation of glycosylated nanoparticles and alter their cellular uptake. Nanoscale 2019, 11, 23259–23267. [Google Scholar] [CrossRef] [PubMed]
- Suchanova, J.Z.; Hejtmankova, A.; Neburkova, J.; Cigler, P.; Forstova, J.; Spanielova, H. The protein corona does not influence receptor-mediated targeting of virus-like particles. Bioconjugate Chem. 2020, 31, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Wei, X.Q.; Yang, Q.; Zhang, S.; Zhang, T.; Shao, X.R.; Cai, X.X.; Zhang, Z.R.; Lin, Y.F. Enhanced biostability of nanoparticle-based drug delivery systems by albumin corona. Nanomedicine 2015, 10, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.L.L.; Thong, P.S.P.; Soo, K.C.; Kah, J.C.Y. Protein corona in drug delivery for multimodal cancer therapy in vivo. Nanoscale 2018, 10, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Khandelia, R.; Jaiswal, A.; Ghosh, S.S.; Chattopadhyay, A. Gold nanoparticle-protein agglomerates as versatile nanocarriers for drug delivery. Small 2013, 9, 3494–3505. [Google Scholar] [CrossRef]
- Meng, H.A.; Liong, M.; Xia, T.A.; Li, Z.X.; Ji, Z.X.; Zink, J.I.; Nel, A.E. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano 2010, 4, 4539–4550. [Google Scholar] [CrossRef]
- Yoo, H.S.; Park, T.G. Biodegradable polymeric micelles composed of doxorubicin conjugated PLGA-PEG block copolymer. J. Control. Release 2001, 70, 63–70. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M.; Mahmoudi, N. The effect of protein corona on doxorubicin release from the magnetic mesoporous silica nanoparticles with polyethylene glycol coating. J. Nanoparticle Res. 2015, 17, 197. [Google Scholar] [CrossRef]
- Chakraborty, D.; Tripathi, S.; Ethiraj, K.R.; Chandrasekaran, N.; Mukherjee, A. Human serum albumin corona on functionalized gold nanorods modulates doxorubicin loading and release. New J. Chem. 2018, 42, 16555–16563. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chauhan, P.; Kumar, S.; Chaudhary, S.; Chandrasekaran, N.; Mukherjee, A.; Ethiraj, K.R. Utilizing corona on functionalized selenium nanoparticles for loading and release of doxorubicin payload. J. Mol. Liq. 2019, 296, 111864. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Podila, R.; Brown, J.M. A hyperspectral and toxicological analysis of protein corona impact on silver nanoparticle properties, intracellular modifications, and macrophage activation. Int. J. Nanomed. 2015, 10, 6509–6521. [Google Scholar]
- Pisani, C.; Rascol, E.; Dorandeu, C.; Gaillard, J.C.; Charnay, C.; Guari, Y.; Chopineau, J.; Armengaud, J.; Devoisselle, J.M.; Prat, O. The species origin of the serum in the culture medium influences the in vitro toxicity of silica nanoparticles to HepG2 cells. PLoS ONE 2017, 12, e0182906. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.K.H.; Chiu, J.F.; Che, C.M. Silver nanoparticles: Partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y.B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biot. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Podila, R.; Aldossari, A.A.; Emerson, H.; Powell, B.A.; Ke, P.C.; Rao, A.M.; Brown, J.M. Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicol. Sci. 2015, 143, 136–146. [Google Scholar] [CrossRef]
- Liu, J.Y.; Wang, Z.Y.; Liu, F.D.; Kane, A.B.; Hurt, R.H. Chemical transformations of nanosilver in biological environments. ACS Nano 2012, 6, 9887–9899. [Google Scholar] [CrossRef]
- Singh, R.P.; Ramarao, P. Cellular uptake, intracellular trafficking and cytotoxicity of silver nanoparticles. Toxicol. Lett. 2012, 213, 249–259. [Google Scholar] [CrossRef]
- Nayak, P.S.; Borah, S.M.; Gogoi, H.; Asthana, S.; Bhatnagar, R.; Jha, A.N.; Jha, S. Lactoferrin adsorption onto silver nanoparticle interface: Implications of corona on protein conformation, nanoparticle cytotoxicity and the formulation adjuvanticity. Chem. Eng. J. 2019, 361, 470–484. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H.J. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323. [Google Scholar]
- Sharma, S.; Naskar, S.; Kuotsu, K. A review on carbon nanotubes: Influencing toxicity and emerging carrier for platinum based cytotoxic drug application. J. Drug Deliv. Sci. Tec. 2019, 51, 708–720. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of carbon nanotubes: A review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.Y.; Mandel, A.; Seifalian, A.M. A concise review of carbon nanotube’s toxicology. Nano Rev. 2013, 4, 21521. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Qu, R.J.; Liu, J.Q.; Wei, Z.B.; Wang, L.S.; Yang, S.G.; Huang, Q.G.; Wang, Z.Y. Effect of different carbon nanotubes on cadmium toxicity to Daphnia magna: The role of catalyst impurities and adsorption capacity. Environ. Pollut. 2016, 208, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Lu, D.W.; Hao, F.; Liu, R.T. Exploring the diameter and surface dependent conformational changes in carbon nanotube-protein corona and the related cytotoxicity. J. Hazard. Mater. 2015, 292, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Li, X.Q.; Kang, Y.; Ding, Y.H.; Gu, Z.P.; Cao, Y. Internalization, cytotoxicity, oxidative stress and inflammation of multi-walled carbon nanotubes in human endothelial cells: Influence of pre-incubation with bovine serum albumin. RSC Adv. 2018, 8, 9253–9260. [Google Scholar] [CrossRef]
- Lu, N.H.; Sui, Y.H.; Tian, R.; Peng, Y.Y. Adsorption of plasma proteins on single-walled carbon nanotubes reduced cytotoxicity and modulated neutrophil activation. Chem. Res. Toxicol. 2018, 31, 1061–1068. [Google Scholar] [CrossRef]
- Yin, H.; Chen, R.; Casey, P.S.; Ke, P.C.; Davis, T.P.; Chen, C.Y. Reducing the cytotoxicity of ZnO nanoparticles by a pre-formed protein corona in a supplemented cell culture medium. RSC Adv. 2015, 5, 73963–73973. [Google Scholar] [CrossRef]
- Li, X.Q.; Fang, X.; Ding, Y.H.; Li, J.; Cao, Y. Toxicity of ZnO nanoparticles (NPs) with or without hydrophobic surface coating to THP-1 macrophages: Interactions with BSA or oleate-BSA. Toxicol. Mech. Methods 2018, 28, 520–528. [Google Scholar] [CrossRef]
- Chen, M.M.; Zuo, X.S.; Xu, Q.Q.; Wang, R.; Fan, S.H.; Wu, H. Investigating the interaction of nanodiamonds with human serum albumin and induced cytotoxicity. J. Spectrosc. 2019, 2019, 4503137. [Google Scholar] [CrossRef]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.P.; Humm, J.; Gonen, M.; Kalaigian, H.; Schoder, H.; et al. Clinical translation of an ultrasmall inorganic optical-pet imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [PubMed]
- Boselli, L.; Polo, E.; Castagnola, V.; Dawson, K.A. Regimes of biomolecular ultrasmall nanoparticle interactions. Angew. Chem. Int. Edit. 2017, 56, 4215–4218. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.D.; Jin, S.B.; Ma, X.W.; Xue, X.D.; Yang, K.N.; Kumar, A.; Wang, P.C.; Zhang, J.C.; Hu, Z.B.; Liang, X.J. Ultrasmall gold nanoparticles as carriers for nucleus-based gene therapy due to size-dependent nuclear entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Wang, M.; Zhang, Y.M.; Li, F.; Yu, S.N.; Zhu, L.; Guo, Y.M.; Yang, L.; Yang, S.N. Conformational-transited protein corona regulated cell-membrane penetration and induced cytotoxicity of ultrasmall Au nanoparticles. Rsc Adv. 2019, 9, 4435–4444. [Google Scholar] [CrossRef]
- Barbalinardo, M.; Caicci, F.; Cavallini, M.; Gentili, D. Protein corona mediated uptake and cytotoxicity of silver nanoparticles in mouse embryonic fibroblast. Small 2018, 14, 1801219. [Google Scholar] [CrossRef]
- Persaud, I.; Shannahan, J.H.; Raghavendra, A.J.; Alsaleh, N.B.; Podila, R.; Brown, J.M. Biocorona formation contributes to silver nanoparticle induced endoplasmic reticulum stress. Ecotoxicol. Environ. Saf. 2019, 170, 77–86. [Google Scholar] [CrossRef]
- Wu, G.Z.; Jiang, C.J.; Zhang, T. FcgammaRiib receptor-mediated apoptosis in macrophages through interplay of cadmium sulfide nanomaterials and protein corona. Ecotoxicol. Environ. Saf. 2018, 164, 140–148. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem. Soc. Rev. 2013, 42, 5552–5576. [Google Scholar] [CrossRef]
- Neagu, M.; Piperigkou, Z.; Karamanou, K.; Engin, A.B.; Docea, A.O.; Constantin, C.; Negrei, C.; Nikitovic, D.; Tsatsakis, A. Protein bio-corona: Critical issue in immune nanotoxicology. Arch. Toxicol. 2017, 91, 1031–1048. [Google Scholar] [CrossRef]
- Escamilla-Rivera, V.; Uribe-Ramirez, M.; Gonzalez-Pozos, S.; Lozano, O.; Lucas, S.; De Vizcaya-Ruiz, A. Protein corona acts as a protective shield against Fe3O4-PEG inflammation and ROS-induced toxicity in human macrophages. Toxicol. Lett. 2016, 240, 172–184. [Google Scholar] [CrossRef]
- Mo, J.B.; Xie, Q.Y.; Wei, W.; Zhao, J. Revealing the immune perturbation of black phosphorus nanomaterials to macrophages by understanding the protein corona. Nat. Commun. 2018, 9, 2480. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, V.; Cornaghi, L.; Gianazza, E.; Potenza, M.A.; Donetti, E.; Marinovich, M.; Corsini, E. In vitro assessment of silver nanoparticles immunotoxicity. Food Chem. Toxicol. 2018, 112, 363–374. [Google Scholar] [CrossRef]
- Cabaleiro-Lago, C.; Quinlan-Pluck, F.; Lynch, I.; Lindman, S.; Minogue, A.M.; Thulin, E.; Walsh, D.M.; Dawson, K.A.; Linse, S. Inhibition of amyloid beta protein fibrillation by polymeric nanoparticles. J. Am. Chem. Soc. 2008, 130, 15437–15443. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro-Lago, C.; Lynch, I.; Dawson, K.A.; Linse, S. Inhibition of IAPP and IAPP((20-29)) fibrillation by polymeric nanoparticles. Langmuir 2010, 26, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Chang, Y.J.; Yoshiike, Y.; Chang, Y.C.; Chen, Y.R. Negatively charged gold nanoparticles inhibit alzheimer’s amyloid-beta fibrillization, induce fibril dissociation, and mitigate neurotoxicity. Small 2012, 8, 3631–3639. [Google Scholar] [CrossRef]
- Dubey, K.; Anand, B.G.; Badhwar, R.; Bagler, G.; Navya, P.N.; Daima, H.K.; Kar, K. Tyrosine- and tryptophan-coated gold nanoparticles inhibit amyloid aggregation of insulin. Amino Acids 2015, 47, 2551–2560. [Google Scholar] [CrossRef]
- Bellucci, L.; Ardevol, A.; Parrinello, M.; Lutz, H.; Lu, H.; Weidner, T.; Corni, S. The interaction with gold suppresses fiber-like conformations of the amyloid beta (16-22) peptide. Nanoscale 2016, 8, 8737–8748. [Google Scholar] [CrossRef]
- Wang, M.Y.; Kakinen, A.; Pilkington, E.H.; Davis, T.P.; Ke, P.C. Differential effects of silver and iron oxide nanoparticles on IAPP amyloid aggregation. Biomater. Sci. 2017, 5, 485–493. [Google Scholar] [CrossRef]
- Lin, Y.X.; Sahoo, B.R.; Ozawa, D.; Kinoshita, M.; Kang, J.; Lim, M.H.; Okumura, M.; Huh, Y.H.; Moon, E.; Jang, J.H.; et al. Diverse structural conversion and aggregation pathways of alzheimer’s amyloid-beta (1-40). ACS Nano 2019, 13, 8766–8783. [Google Scholar] [CrossRef]
- Wang, B.; Pilkington, E.H.; Sun, Y.X.; Davis, T.P.; Ke, P.C.; Ding, F. Modulating protein amyloid aggregation with nanomaterials. Environ. Sci. Nano 2017, 4, 1772–1783. [Google Scholar] [CrossRef]
- Mirsadeghi, S.; Dinarvand, R.; Ghahremani, M.H.; Hormozi-Nezhad, M.R.; Mahmoudi, Z.; Hajipour, M.J.; Atyabi, F.; Ghavami, M.; Mahmoudi, M. Protein corona composition of gold nanoparticles/nanorods affects amyloid beta fibrillation process. Nanoscale 2015, 7, 5004–5013. [Google Scholar] [CrossRef] [PubMed]
- Lotfabadi, A.; Hajipour, M.J.; Derakhshankhah, H.; Peirovi, A.; Saffar, S.; Shams, E.; Fatemi, E.; Barzegari, E.; Sarvari, S.; Moakedi, F.; et al. Biomolecular corona dictates a beta fibrillation process. ACS Chem. Neurosci. 2018, 9, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Javed, I.; Sun, Y.X.; Adamcik, J.; Wang, B.; Kakinen, A.; Pilkington, E.H.; Ding, F.; Mezzenga, R.; Davis, T.P.; Ke, P.C. Cofibrillization of pathogenic and functional amyloid proteins with gold nanoparticles against amyloidogenesis. Biomacromolecules 2017, 18, 4316–4322. [Google Scholar] [CrossRef] [PubMed]
| Characterization | Approach | Brief Description |
|---|---|---|
| Size, shape, and surface charge | Dynamic light scattering (DLS) | Size distribution and hydrodynamic sizes |
| Zeta-potential | Surface charge | |
| Differential centrifugal sedimentation (DCS) | Size analysis | |
| Transmission electron microscopy (TEM) or atomic force microscopy (AFM) | Morphology of NPs and thickness of PCs | |
| Small-angle X-ray scattering (SAXS) | Size and shape of particles | |
| NP tracking analysis (NTA) | Particle concentration and size | |
| Binding capacity | UV-vis | Changes in light absorption spectra |
| Fluorescence correlation spectroscopy (FCS) | Changes in diffusion time of fluorescently labelled particles | |
| Quartz crystal microbalancing (QCM) | Mass changes at the oscillating quartz surface | |
| Isothermal titration calorimetry (ITC) | Thermal changes induced by NP-PC binding | |
| Nuclear magnetic resonance (NMR) | Binding site location | |
| Computer simulation | Protein orientation and conformation | |
| PC composition | Gel electrophoresis | Molecular weight estimation |
| Mass spectrometry (MS) | Identification of each protein component | |
| Gel filtration chromatography | Protein separation and kinetic exchange rate calculation | |
| PC conformation | Circular dichroism (CD) | Secondary structural changes |
| Surface-enhanced Raman spectroscopy (SERS) | Tracks molecular vibrations for protein conformation analysis | |
| Fourier-transform infrared spectroscopy (FTIR) | Changes in absorption of infrared light |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Lee, J.-S. Insights into Characterization Methods and Biomedical Applications of Nanoparticle–Protein Corona. Materials 2020, 13, 3093. https://doi.org/10.3390/ma13143093
Li Y, Lee J-S. Insights into Characterization Methods and Biomedical Applications of Nanoparticle–Protein Corona. Materials. 2020; 13(14):3093. https://doi.org/10.3390/ma13143093
Chicago/Turabian StyleLi, Yan, and Jae-Seung Lee. 2020. "Insights into Characterization Methods and Biomedical Applications of Nanoparticle–Protein Corona" Materials 13, no. 14: 3093. https://doi.org/10.3390/ma13143093
APA StyleLi, Y., & Lee, J.-S. (2020). Insights into Characterization Methods and Biomedical Applications of Nanoparticle–Protein Corona. Materials, 13(14), 3093. https://doi.org/10.3390/ma13143093






