Enhancement of Bone Ingrowth into a Porous Titanium Structure to Improve Osseointegration of Dental Implants: A Pilot Study in the Canine Model
Abstract
1. Introduction
2. Materials and Methods
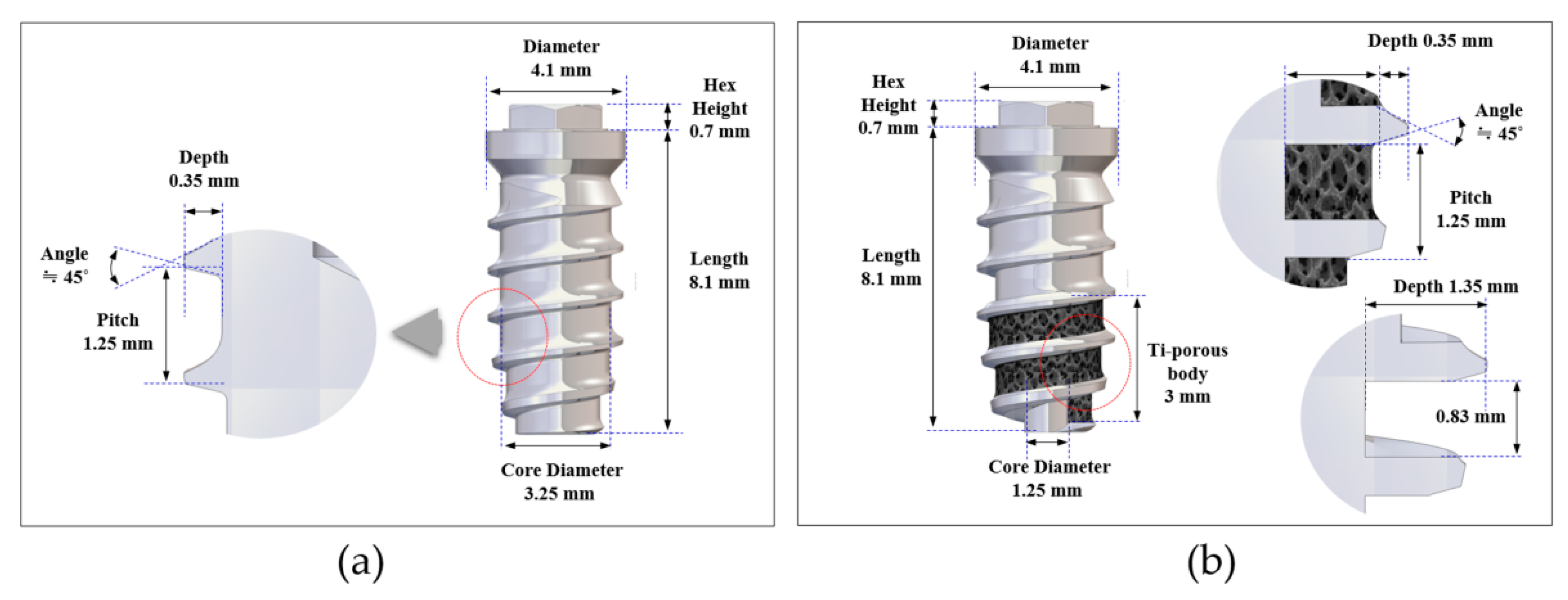
2.1. Design of the Implants
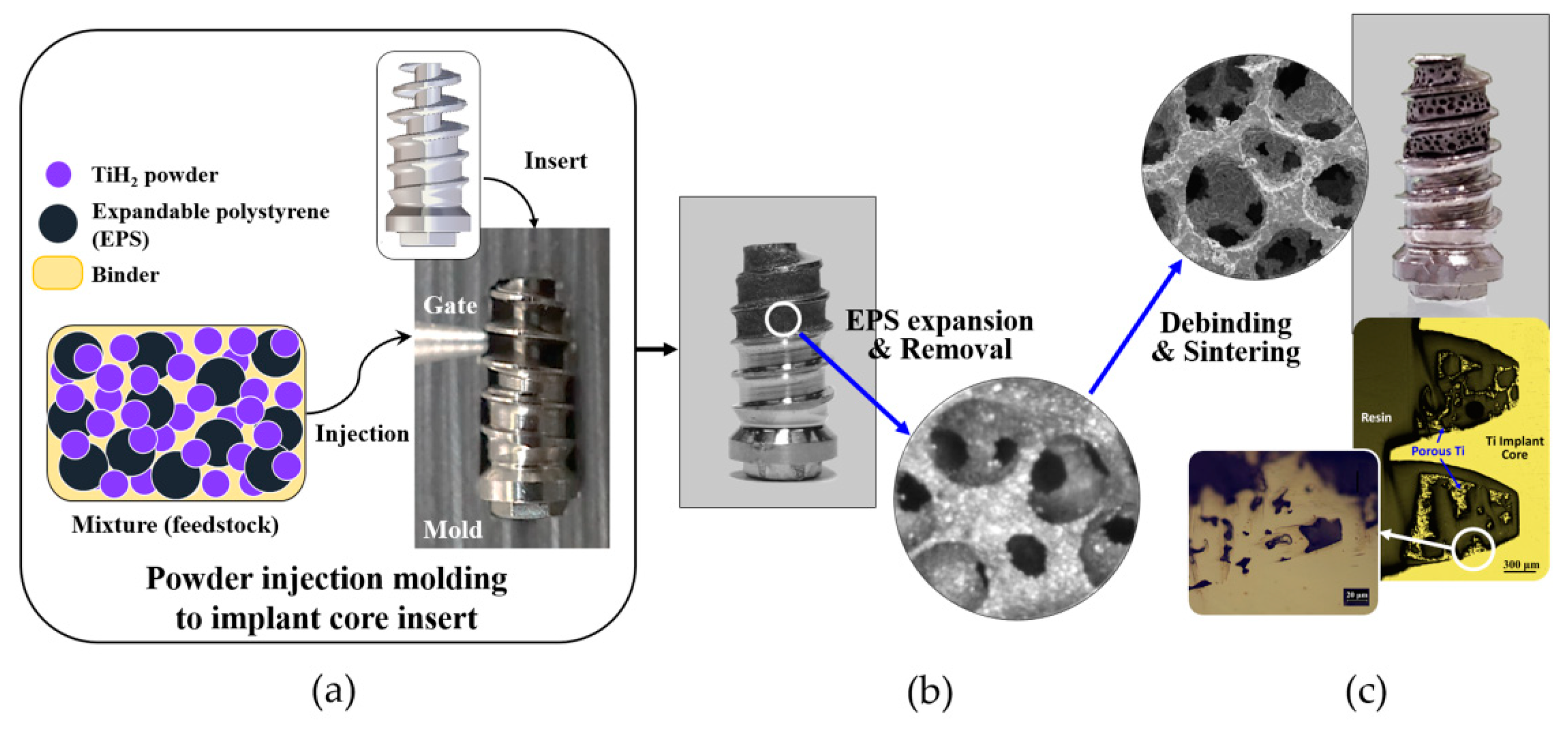
2.2. Fabrication of Porous Titanium Structure
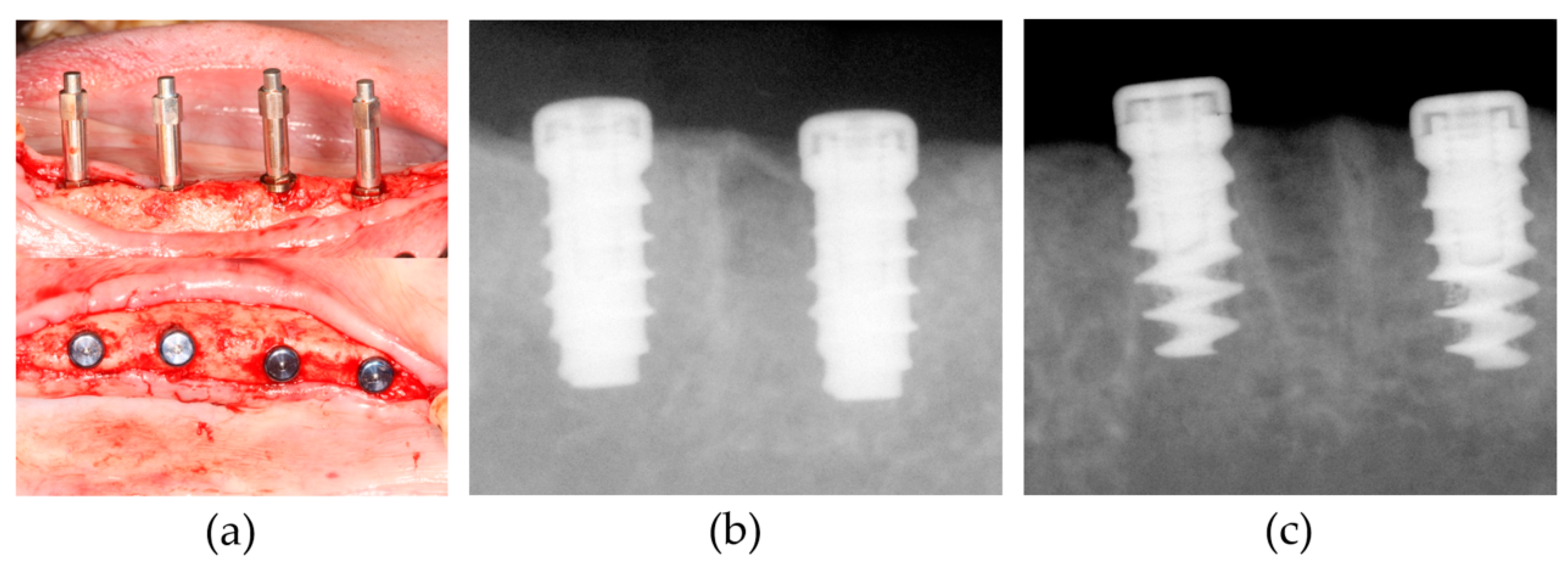
2.3. Animal Experiment
2.4. Resonance Frequency Analysis (RFA), Removal Torque Test and Topographical Analysis
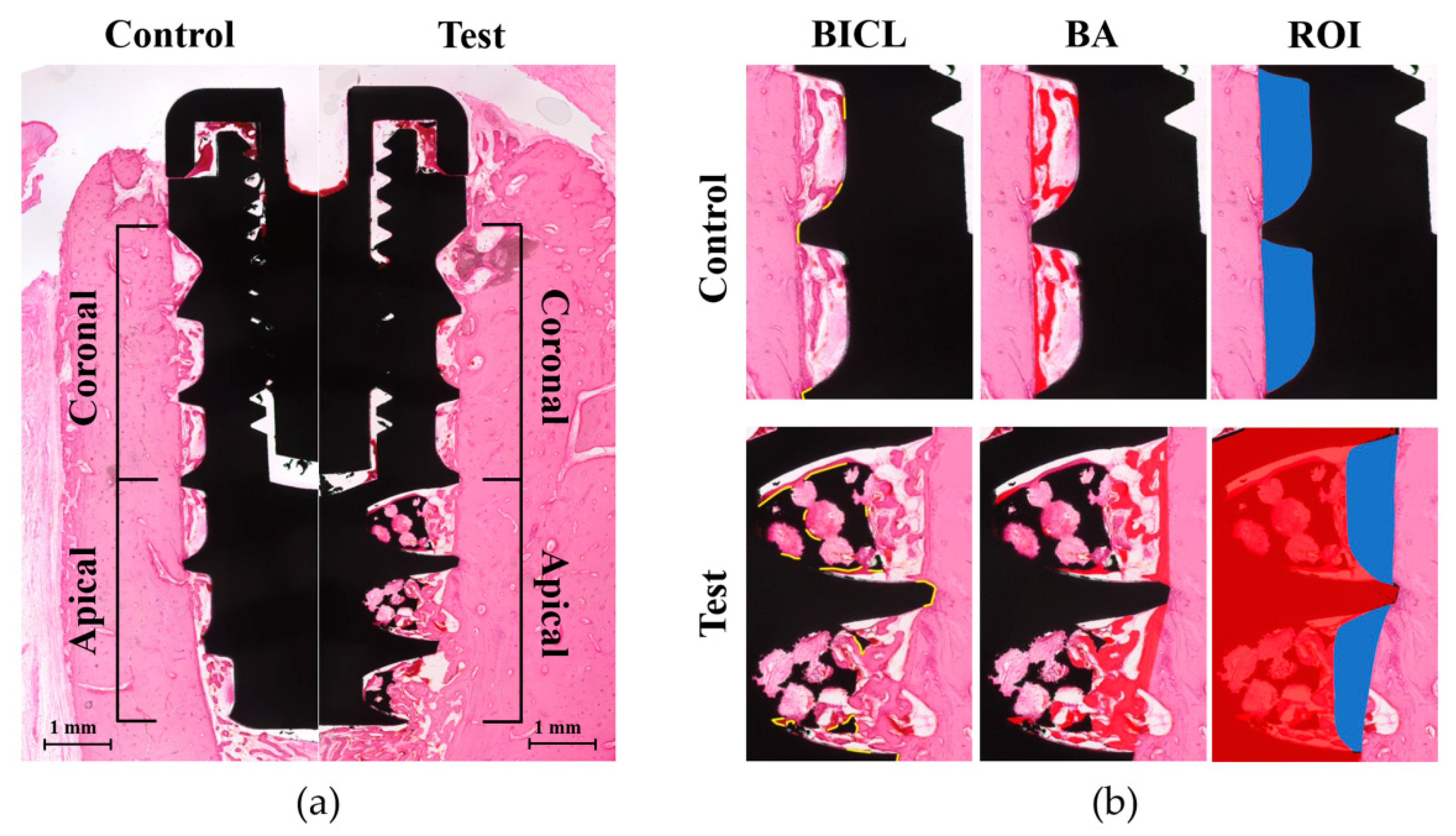
2.5. Histologic and Histometric Analysis
2.6. Statistical Analysis
3. Results
3.1. Clinical Findings
3.2. Resonance Frequency Analysis and Removal Torque Value
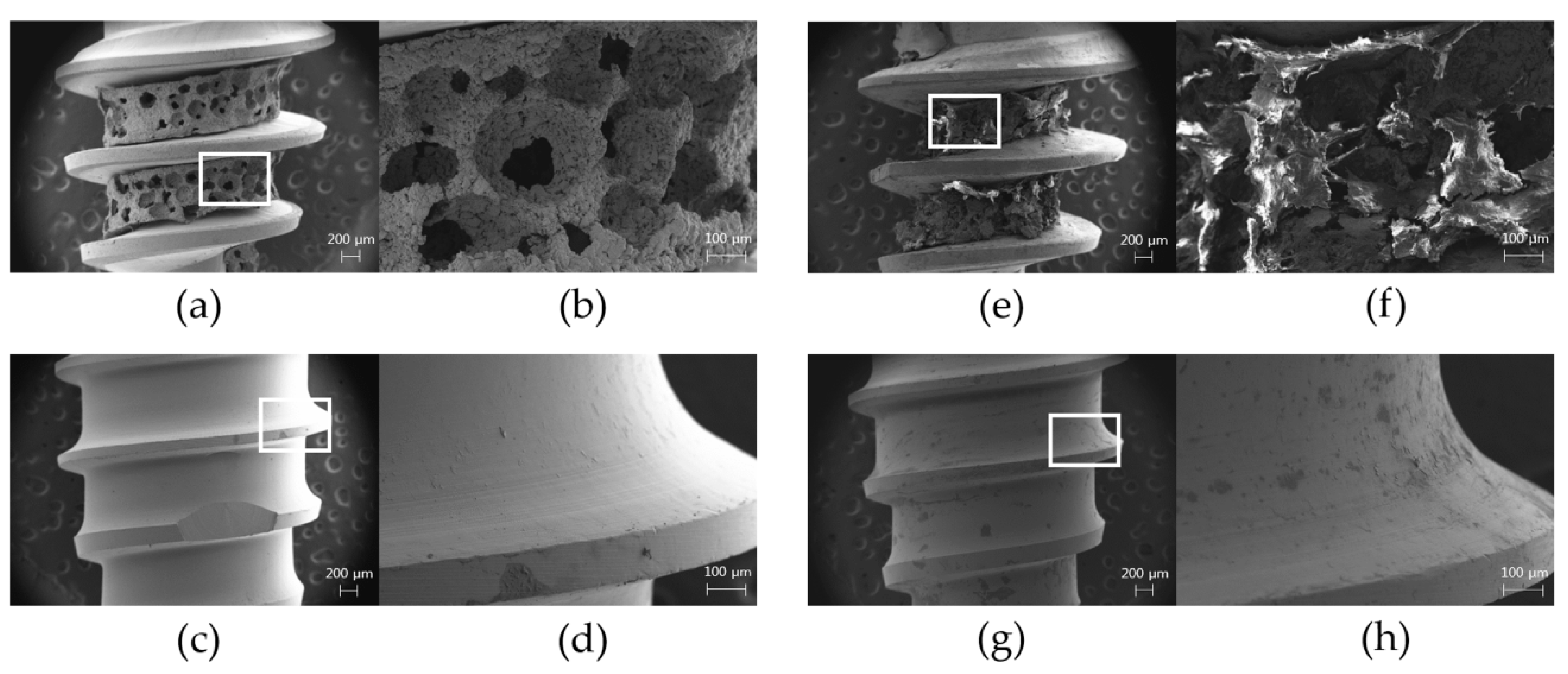
3.3. Surface Topography from FE-SEM Images
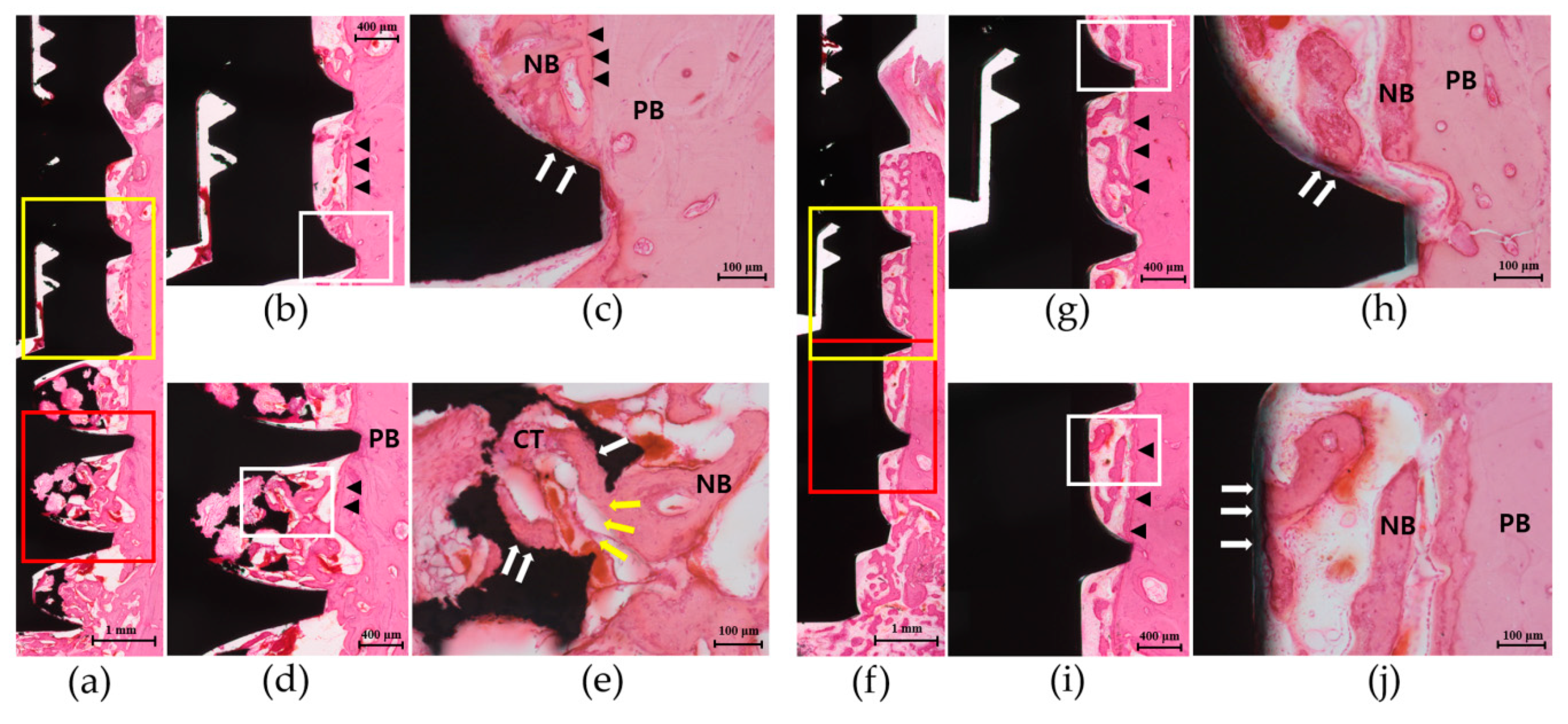
3.4. Histologic Analysis
3.5. Histometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boioli, L.T.; Penaud, J.; Miller, N. A meta-analytic, quantitative assessment of osseointegration establishment and evolution of submerged and non-submerged endosseous titanium oral implants. Clin. Oral Implants Res. 2001, 12, 579–588. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef]
- Moy, P.K.; Medina, D.; Shetty, V.; Aghaloo, T.L. Dental implant failure rates and associated risk factors. Int. J. Oral Maxillofac. Implants 2005, 20, 569–577. [Google Scholar] [PubMed]
- van Steenberghe, D.; Jacobs, R.; Desnyder, M.; Maffei, G.; Quirynen, M. The relative impact of local and endogenous patient-related factors on implant failure up to the abutment stage. Clin. Oral Implants Res. 2002, 13, 617–622. [Google Scholar] [CrossRef]
- Beikler, T.; Flemmig, T.F. Implants in the medically compromised patient. Crit. Rev. Oral Biol. Med. 2003, 14, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Bennardo, F.; Antonelli, A.; Barone, S.; Wagner, F.; Fortunato, L.; Traxler, H. Influence of clinician’s skill on primary implant stability with conventional and piezoelectric preparation techniques: An ex-vivo study. J. Biol. Regul. Homeost. Agents 2020, 34, 739–745. [Google Scholar]
- Shibata, Y.; Tanimoto, Y. A review of improved fixation methods for dental implants. Part I: Surface optimization for rapid osseointegration. J. Prosthodont. Res. 2015, 59, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, P.E.; Borgonovo, A.; Indrieri, M.; Giorgetti, L.; Bongiorno, G.; Carbone, R.; Podestà, A.; Milani, P. The effect of surface nanometre-scale morphology on protein adsorption. PLoS ONE 2010, 5, e11862. [Google Scholar] [CrossRef]
- Pilliar, R.M. Overview of surface variability of metallic endosseous dental implants: Textured and porous surface-structured designs. Imp. Dent. 1998, 7, 305–314. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, B.; Swieszkowski, W.; Godlinski, D.; Kurzydlowski, K.J. Highly porous titanium scaffolds for orthopaedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Kim, H.M.; Kokubo, T.; Ohtsuki, C.; Kato, H.; Nakamura, T. Mechanism of bonelike apatite formation on bioactive tantalum metal in a simulated body fluid. Biomaterials 2002, 23, 827–832. [Google Scholar] [CrossRef]
- Yoo, D. New paradigms in hierarchical porous scaffold design for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1759–1772. [Google Scholar] [CrossRef]
- Karageorgio, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Fujibayashi, S.; Neo, M.; Kim, H.M.; Kokubo, T.; Nakamura, T. Osteoinduction of porous bioactive titanium metal. Biomaterials 2004, 25, 443–450. [Google Scholar] [CrossRef]
- Bencharit, S.; Byrd, W.C.; Altarawneh, S.; Hosseini, B.; Leong, A.; Reside, G.; Morelli, T.; Offenbacher, S. Development and applications of porous tantalum trabecular metal-enhanced titanium dental implants. Clin. Implant. Dent. Relat. Res. 2014, 16, 817–826. [Google Scholar] [CrossRef]
- Pattanayak, D.K.; Fukuda, A.; Matsushita, T.; Takemoto, M.; Fujibayashi, S.; Sasaki, K.; Nishida, N.; Nakamura, T.; Kokubo, T. Bioactive Ti metal analogous to human cancellous bone: Fabrication by selective laser melting and chemical treatments. Acta Biomater. 2011, 7, 1398–1406. [Google Scholar] [CrossRef]
- Lee, J.W.; Wen, H.B.; Gubbi, P.; Romanos, G.E. New bone formation and trabecular bone microarchitecture of highly porous tantalum compared to titanium implant threads: A pilot canine study. Clin. Oral Implants Res. 2018, 29, 164–174. [Google Scholar] [CrossRef]
- Fraser, D.; Funkenbusch, P.; Ercoli, C.; Meirelles, L. Biomechanical analysis of the osseointegration of porous tantalum implants. J. Prosthet. Dent. 2020, 123, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Altman, D.G. Improving bioscience research reporting: ARRIVE-ing at a solution. Lab. Anim. 2010, 44, 377–378. [Google Scholar] [CrossRef]
- Cornell, C.N.; Lane, J.M. Current understanding of osteoconduction in bone regeneration. Clin. Orthop. Relat. Res. 1998, 355, S267–S273. [Google Scholar] [CrossRef]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.; Mendonca, G.; Sartori, E.; Funkenbusch, P.; Ercoli, C.; Meirelles, L. Bone response to porous tantalum implants in a gap-healing model. Clin. Oral Implants Res. 2019, 30, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, A.R.; Patel, D.; Allen, R.K.; Gibson, C.J.; Best, A.M.; Bencharit, S. Retrospective analysis of porous tantalum trabecular metal-enhanced titanium dental implants. J. Prosthet. Dent. 2019, 121, 404–410. [Google Scholar] [CrossRef]
- Dimaira, M. Immediate placement of trabecular implants in sites of failed implants. Int. J. Oral Maxillofac. Implants 2019, 34, e77–e83. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bao, C.; Wismeijer, D.; Wu, G. The physicochemical/biological properties of porous tantalum and the potential surface modification techniques to improve its clinical application in dental implantology. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 323–329. [Google Scholar] [CrossRef]
- Lin, S.I.E. Near-net-shape forming of zirconia optical sleeves by ceramics injection molding. Ceram. Int. 2001, 27, 205–214. [Google Scholar] [CrossRef]
- Park, Y.S.; Chung, S.H.; Shon, W.J. Peri-implant bone formation and surface characteristics of rough surface zirconia implants manufactured by powder injection molding technique in rabbit tibiae. Clin. Oral Implants Res. 2013, 24, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.J.; Yun, J.H. Three-dimensional microstructure of human alveolar trabecular bone: A micro-computed tomography study. J. Periodontal Implant. Sci. 2017, 47, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Bobyn, J.D.; Stackpool, G.J.; Hacking, S.A.; Tanzer, M.; Krygier, J.J. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J. Bone Joint Surg. Br. 1999, 81, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, O.; Cortella, C.A.; Boskovic, M.M.; James, R.A.; Slots, J.; Wikesjö, U.M. Experimental peri-implant tissue breakdown around hydroxyapatite-coated implants. J. Periodontol. 1997, 68, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Tran, D.; Jeng, M.D.; Shen, Y.T. Survival rates of hybrid rough surface implants and their alveolar bone level alteration. J. Periodontol. 2018, 12, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Baggi, L.; Cappelloni, I.; Di Girolamo, M.; Maceri, F.; Vairo, G. The influence of implant diameter and length on stress distribution of osseointegrated implants related to crestal bone geometry: A three-dimensional finite element analysis. J. Prosthet. Dent. 2008, 100, 422–431. [Google Scholar] [CrossRef]
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontology 2000 2008, 47, 51–66. [Google Scholar] [CrossRef]
- Klokkevold, P.R.; Johnson, P.; Dadgostari, S.; Caputo, A.; Davies, J.E.; Nishimura, R.D. Early endosseous integration enhanced by dual acid etching of titanium: A torque removal study in the rabbit. Clin. Oral Implants Res. 2001, 12, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, S.; Giannoni, P.; Bianchini, P.; Sandri, M.; Marotta, R.; Firpo, G.; Valbusa, U.; Tampieri, A.; Diaspro, A.; Bianco, P.; et al. Order versus disorder: In vivo bone formation within osteoconductive scaffolds. Sci. Rep. 2012, 2, 274. [Google Scholar] [CrossRef]
- Chang, B.S.; Lee, C.K.; Hong, K.S.; Youn, H.J.; Ryu, H.S.; Chung, S.S.; Park, K.W. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials 2000, 21, 1291–1298. [Google Scholar] [CrossRef]
- Götz, H.E.; Müller, M.; Emmel, A.; Holzwarth, U.; Erben, R.G.; Stangl, R. Effect of surface finish on the osseointegration of laser-treated titanium alloy implants. Biomaterials 2004, 25, 4057–4064. [Google Scholar] [CrossRef] [PubMed]
| ISQ Value | Test Group (n = 14) | Control Group (n = 14) | |
| Baseline | 66.7 ± 4.0 | 69.5 ± 8.3 | |
| 4 weeks | 67.5 ± 5.0 | 68.4 ± 6.3 | |
| RTV (Ncm) | Test Group (n = 7) | Control Group (n = 7) | |
| 4 weeks | 20.5 ± 6.8 1 | 8.0 ± 3.6 |
| Test Group (n = 7) | Control Group (n = 7) | ||
|---|---|---|---|
| BICL (mm) | Coronal part | 1.88 ± 0.98 | 1.31 ± 0.76 |
| Apical part | 3.43 ± 0.68 1,2 | 1.30 ± 0.78 | |
| Total | 5.31 ± 1.281 | 2.60 ± 1.21 | |
| BICR (%) | Coronal part | 17.83 ± 9.53 | 12.56 ± 7.26 |
| Apical part | 31.70 ± 7.53 1,2 | 12.89 ± 7.57 | |
| Total | 24.83 ± 6.441 | 12.69 ± 5.74 | |
| BA (mm2) | Coronal part | 0.28 ± 0.11 | 0.21 ± 0.08 |
| Apical part | 0.48 ± 0.12 1,2 | 0.25 ± 0.12 | |
| Total | 0.77 ± 0.24 1 | 0.45 ± 0.16 | |
| BAR (%) | Coronal part | 24.9 ± 10.84 | 20.7 ± 7.74 |
| Apical part | 37.2 ± 10.04 1,2 | 23.0 ± 9.75 | |
| Total | 31.5 ± 8.03 1 | 21.8 ± 6.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.-Y.; Ko, S.-Y.; Lee, W.; Chang, Y.-Y.; Kim, S.-H.; Yun, J.-H. Enhancement of Bone Ingrowth into a Porous Titanium Structure to Improve Osseointegration of Dental Implants: A Pilot Study in the Canine Model. Materials 2020, 13, 3061. https://doi.org/10.3390/ma13143061
Hong J-Y, Ko S-Y, Lee W, Chang Y-Y, Kim S-H, Yun J-H. Enhancement of Bone Ingrowth into a Porous Titanium Structure to Improve Osseointegration of Dental Implants: A Pilot Study in the Canine Model. Materials. 2020; 13(14):3061. https://doi.org/10.3390/ma13143061
Chicago/Turabian StyleHong, Ji-Youn, Seok-Yeong Ko, Wonsik Lee, Yun-Young Chang, Su-Hwan Kim, and Jeong-Ho Yun. 2020. "Enhancement of Bone Ingrowth into a Porous Titanium Structure to Improve Osseointegration of Dental Implants: A Pilot Study in the Canine Model" Materials 13, no. 14: 3061. https://doi.org/10.3390/ma13143061
APA StyleHong, J.-Y., Ko, S.-Y., Lee, W., Chang, Y.-Y., Kim, S.-H., & Yun, J.-H. (2020). Enhancement of Bone Ingrowth into a Porous Titanium Structure to Improve Osseointegration of Dental Implants: A Pilot Study in the Canine Model. Materials, 13(14), 3061. https://doi.org/10.3390/ma13143061





