Ultra-High Molecular Weight Polyethylene/Titanium-Hybrid Implant for Bone-Defect Replacement
Abstract
1. Introduction
2. Materials and Methods
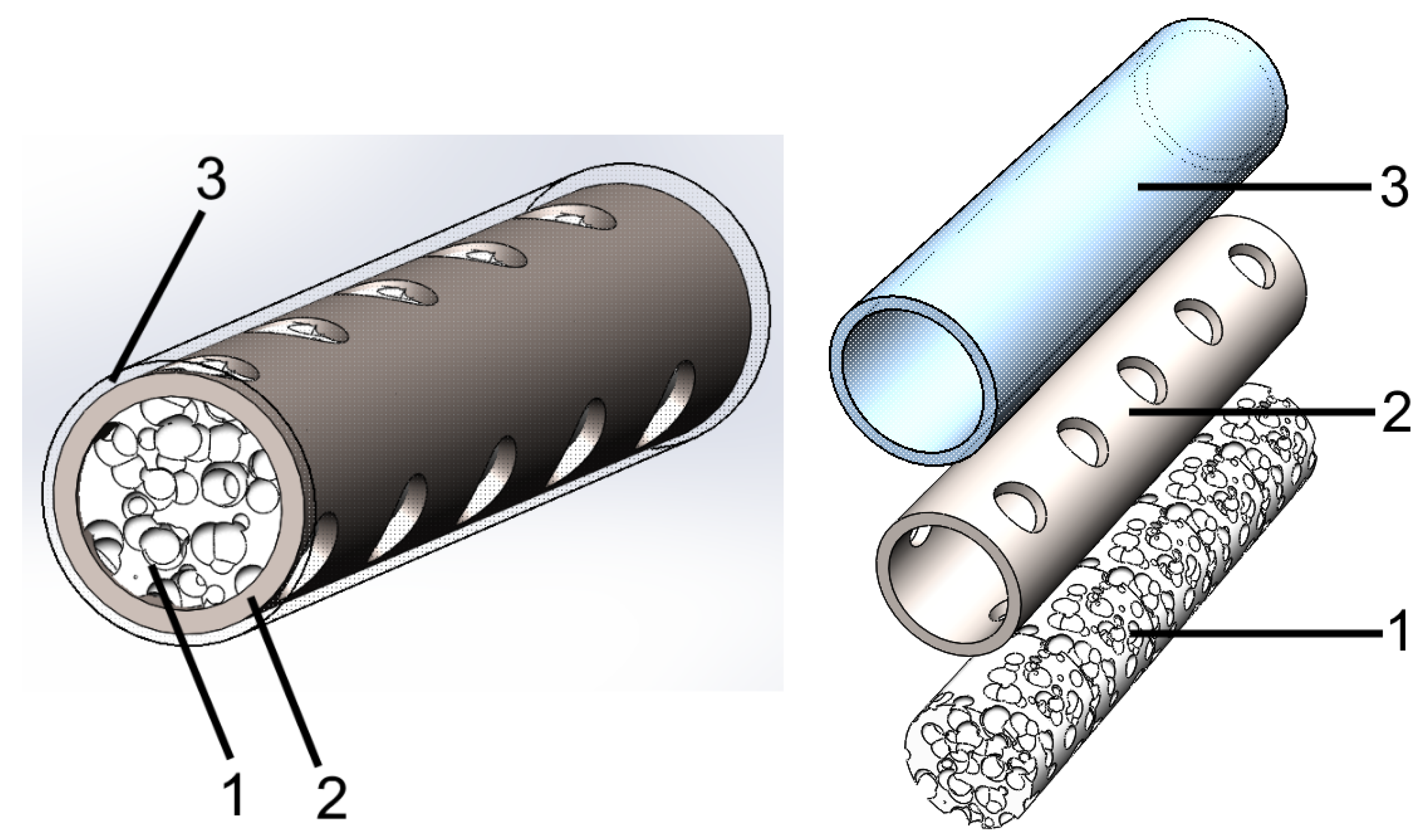
2.1. Description of UHMWPE/Titanium-Hybrid Model
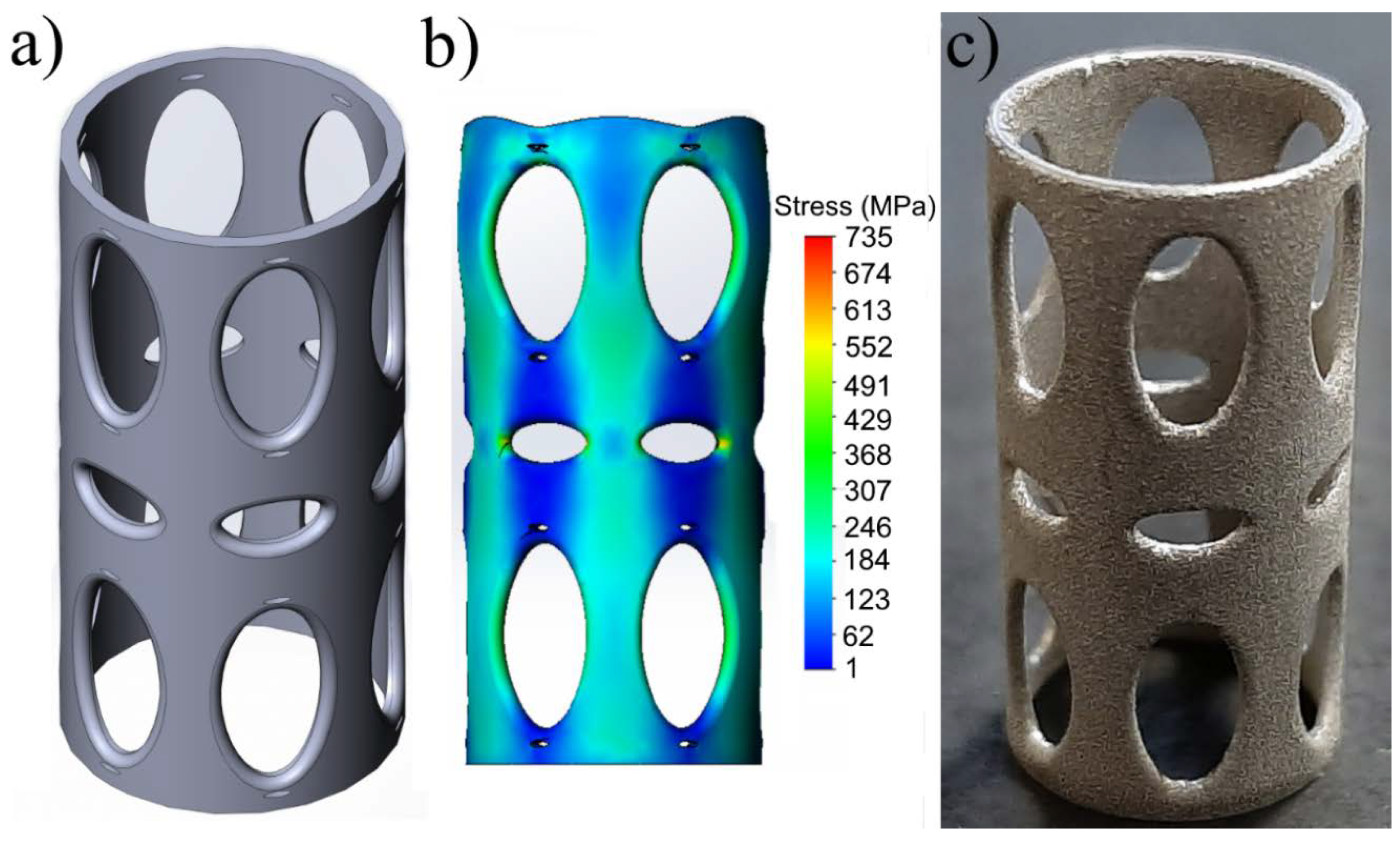
2.2. Three-Dimensional Printing of Titanium Reinforcement
2.3. Three-Dimensional Structural Model of Titanium Reinforcement with Reduced Elastic Modulus
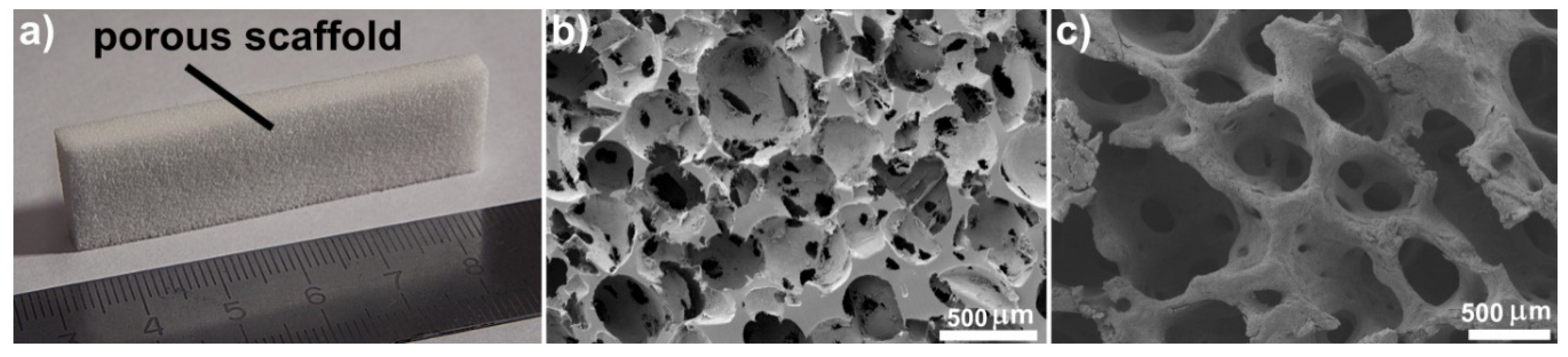
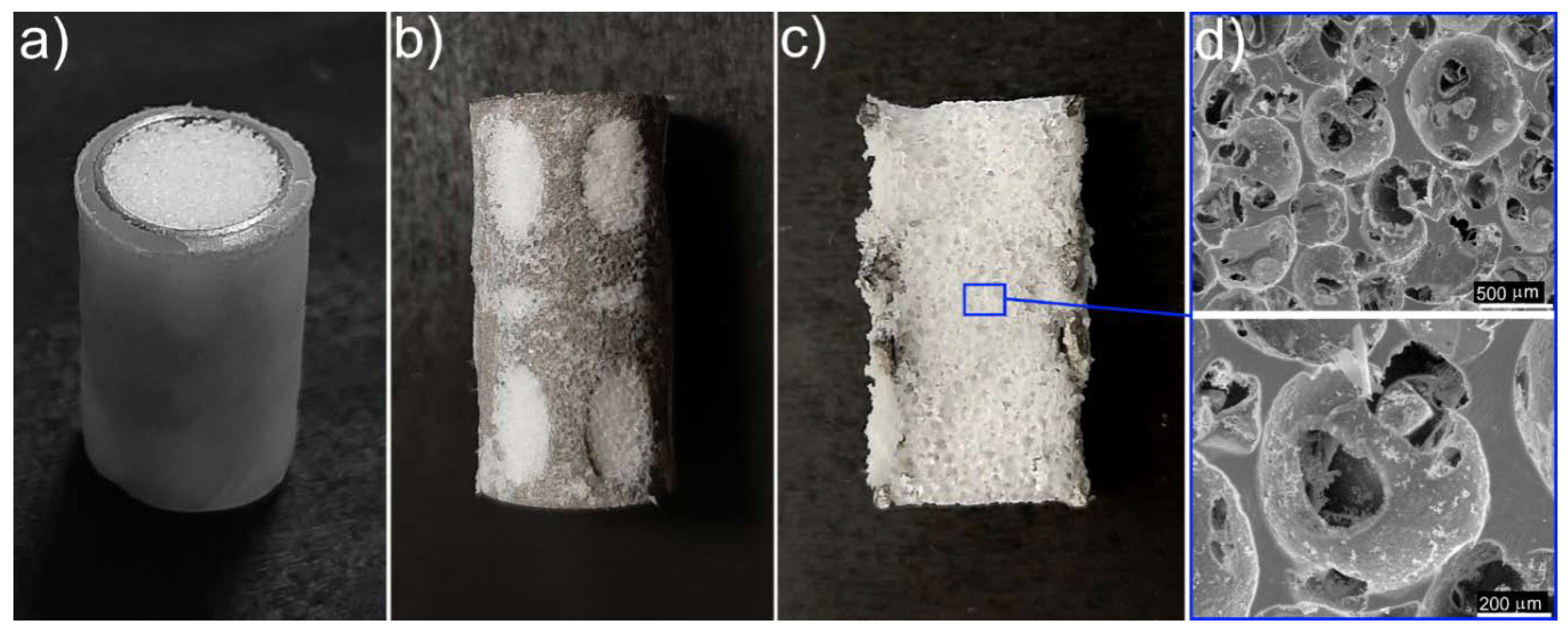
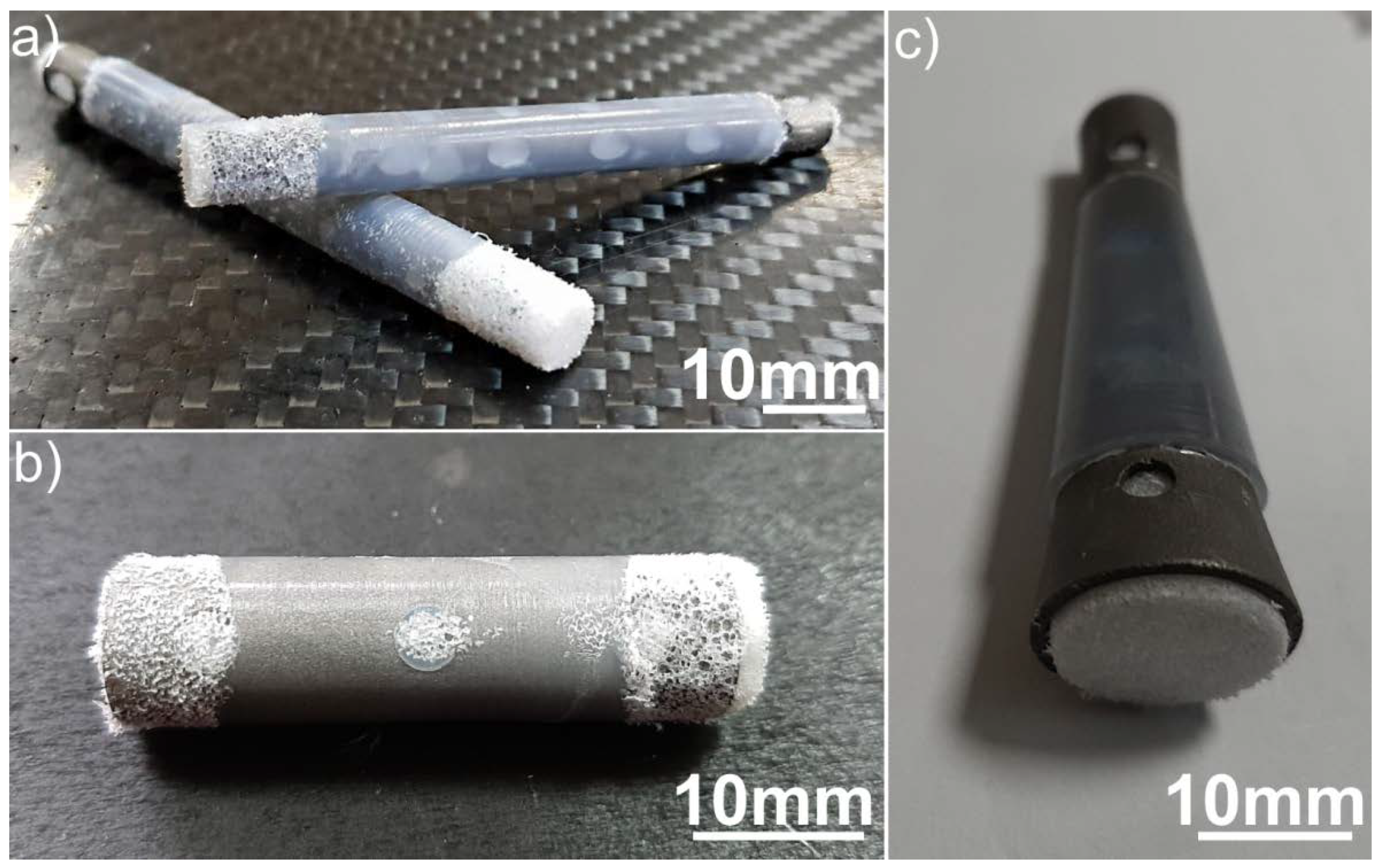
2.4. UHMWPE/Titanium-Hybrid Implant Molding
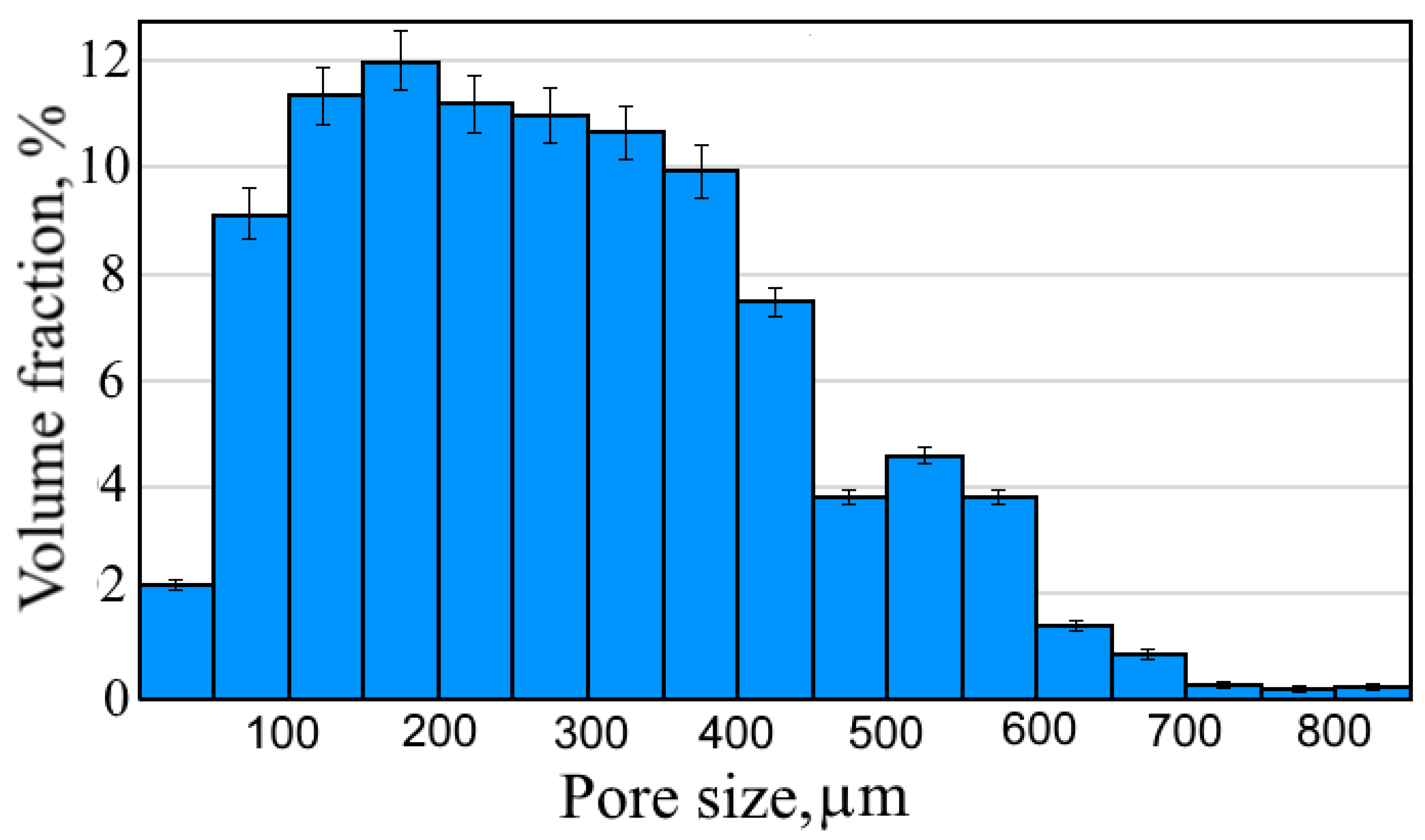
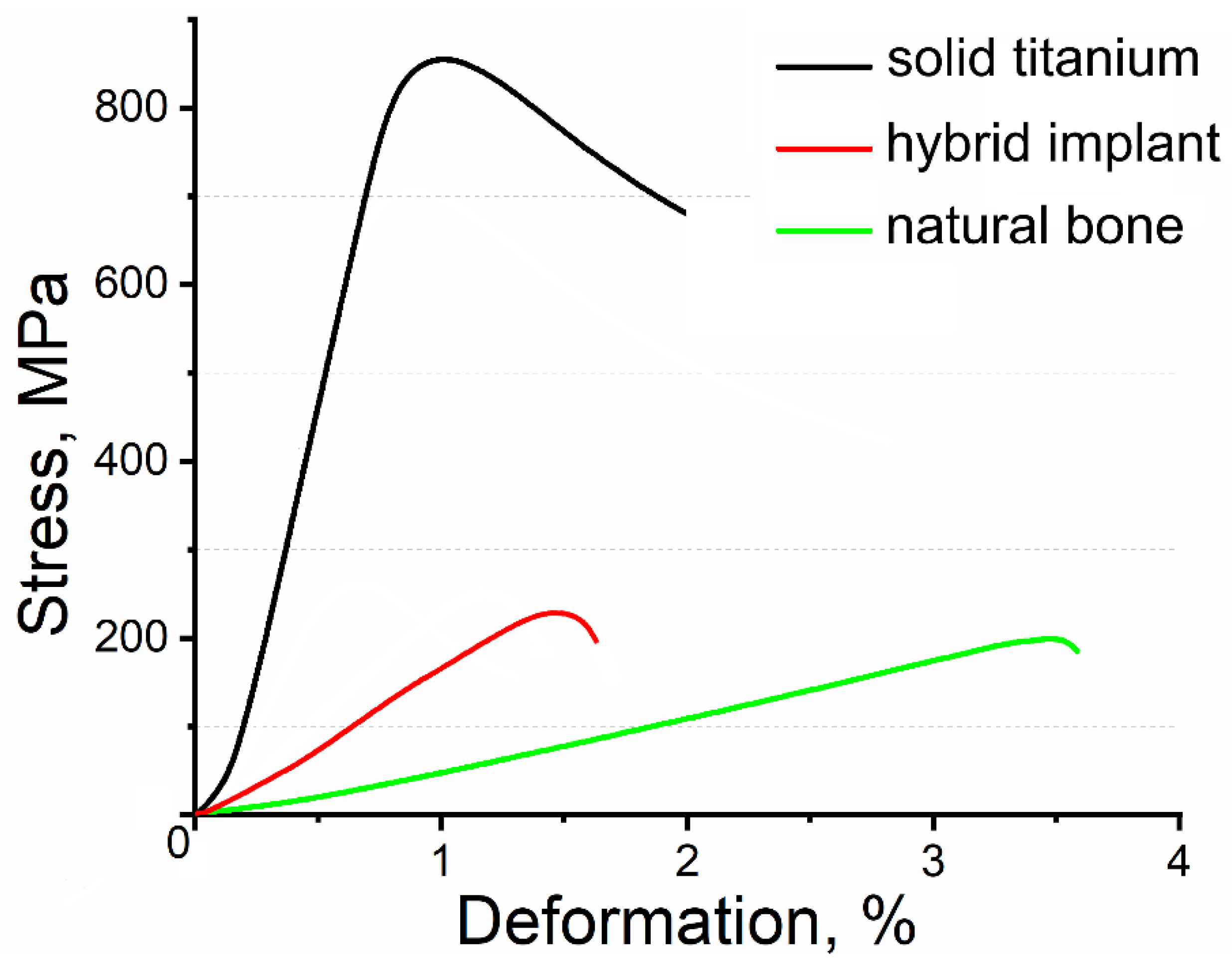
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ridzwan, M.I.Z.; Shuib, S.; Hassan, A.; Shokri, A.A.; Ibrahim, M.N.M. Problem of stress shielding and improvement to the hip implant designs: A review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef]
- Evrard, L. Titanium: A new allergen. In Implant Dentistry—A Rapidly Evolving Practice; Turkyilmaz, I., Ed.; IntechOpen: London, UK, 2011; ISBN 978-953-307-658-4. [Google Scholar] [CrossRef]
- AyodeOtitoju, T.; UgochukwuOkoye, P.; Chen, G.; Li, Y.; OnyekaOkoye, M.; Li, S. Advanced ceramic components: Materials, fabrication, and applications. J. Ind. Eng. Chem. 2020, 85, 34–65. [Google Scholar] [CrossRef]
- Wiederhorn, S.M. Brittle fracture and toughening mechanisms in ceramics. Annu. Rev. Mater. Sci. 1984, 14, 373–403. [Google Scholar] [CrossRef]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common biocompatible polymeric materials for tissue engineering and regenerative medicine. Mater. Chem. Phys. 2019, 122528. [Google Scholar] [CrossRef]
- Banoriya, D.; Purohit, R.; Dwivedi, R.K. Advanced application of polymer based biomaterials. Mater. Today Proc. 2017, 4, 3534–3541. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Bogdał, D.; Matysek, D. 3D hierarchical, nanostructured chitosan/PLA/HA scaffolds doped with TiO2/Au/Pt NPs with tunable properties for guided bone tissue engineering. Polymers 2020, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Rao Kummara, M.; Kamal, T.; Alghyamah, A.-A.A.; Jan Iftikhar, F.; Bano, B.; Khan, N.; AmjidAfridi, M.; Soo Han, S.; et al. Advances in the scaffolds fabrication techniques using biocompatible polymers and their biomedical application: A technical and statistical review. J. Saudi Chem. Soc. 2020, 24, 186–215. [Google Scholar] [CrossRef]
- Safadi, F.F.; Barbe, M.F.; Abdelmagid, S.M.; Rico, M.C.; Aswad, R.A.; Litvin, J.; Popoff, S.N. Bone structure, development and bone biology. In Bone Pathology; Khurana, J.S., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 1–50. ISBN 978-1-59745-347-9. [Google Scholar]
- Wang, A.; Yau, S.-S.; Essner, A.; Herrera, L.; Manley, M.; Dumbleton, J. A highly crosslinked UHMWPE for CR and PS total knee arthroplasties. J. Arthroplast. 2008, 23, 559–566. [Google Scholar] [CrossRef]
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-high-molecular-weight-polyethylene (UHMWPE) as a promising polymer material for biomedical applications: A concise review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Villarraga, M.L.; Ianuzzi, A. The clinical performance of UHMWPE in the spine. In UHMWPE Biomaterials Handbook, 2nd ed.; Kurtz, S.M., Ed.; Academic Press: Boston, MA, USA, 2009; Chapter 12; pp. 171–195. ISBN 978-0-12-374721-1. [Google Scholar]
- Maksimkin, A.V.; Senatov, F.S.; Anisimova, N.Y.; Kiselevskiy, M.V.; Zalepugin, D.Y.; Chernyshova, I.V.; Tilkunova, N.A.; Kaloshkin, S.D. Multilayer porous UHMWPE scaffolds for bone defects replacement. Mater. Sci. Eng. C 2017, 73, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Senatov, F.; Amanbek, G.; Orlova, P.; Bartov, M.; Grunina, T.; Kolesnikov, E.; Maksimkin, A.; Kaloshkin, S.; Poponova, M.; Nikitin, K.; et al. Biomimetic UHMWPE/HA scaffolds with rhBMP-2 and erythropoietin for reconstructive surgery. Mater. Sci. Eng. C 2020, 111, 110750. [Google Scholar] [CrossRef]
- Doktor, T. Pore size distribution of human trabecular bone—comparison of intrusion measurements with image analysis. In Proceedings of the 17th International Conference Engineering Mechanics, Svratka, Czech Republic, 9–12 May 2011. [Google Scholar]
- Porter, M.M.; McKittrick, J. It’s tough to be strong: Advances in bioinspired structural ceramic-based materials. Am. Ceram. Soc. Bull. 2014, 93, 18–24. [Google Scholar]
- Liu, X.; Chen, S.; Tsoi, J.K.H.; Matinlinna, J.P. Binary titanium alloys as dental implant materials—A review. Regen. Biomater. 2017, 4, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Poh, C.K. Titanium Alloys in Orthopedics. Titanium Alloys-Advances in Properties Control; Books on Demand: Norderstedt, Germany, 2013. [Google Scholar] [CrossRef][Green Version]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477. [Google Scholar] [CrossRef]
- Niinomi, M.; Hattori, T.; Morikawa, K.; Kasuga, T.; Suzuki, A.; Fukui, H.; Niwa, S. Development of low rigidity β-type titanium alloy for biomedical applications. Mater. Trans. 2002, 43, 2970–2977. [Google Scholar] [CrossRef]
- Hao, Y.L.; Li, S.J.; Sun, S.Y.; Zheng, C.Y.; Yang, R. Elastic deformation behaviour of Ti–24Nb–4Zr–7.9Sn for biomedical applications. Acta Biomater. 2007, 3, 277–286. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Kuroda, D.; Niinomi, M.; Morinaga, M.; Kato, Y.; Yashiro, T. Design and mechanical properties of new β type titanium alloys for implant materials. Mater. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of beta-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef]
- Wolfe, R.A.; Merion, R.M.; Roys, E.C.; Port, F.K. Trends in organ donation and transplantation in the United States, 1998–2007. Am. J. Transpl. 2009, 9, 869–878. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Hajje, A.; Kolos, E.C.; Wang, J.K.; Maleksaeedi, S.; He, Z.; Wiria, F.E.; Choong, C.; Ruys, A.J. Physical and mechanical characterisation of 3D-printed porous titanium for biomedical applications. J. Mater. Sci. Mater. Med. 2014, 25, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Espana, F.; Balla, V.K.; Bose, S.; Ohgami, Y.; Davies, N.M. Influence of porosity on mechanical properties and in vivo response of Ti6Al4V implants. Acta Biomater. 2010, 6, 1640–1648. [Google Scholar] [CrossRef]
- Teoh, S.H. Engineering Materials for Biomedical Applications; World Scientific: Singapore, 2004; ISBN 978-981-256-222-7. [Google Scholar]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Zioupos, P.; Currey, J.D. Changes in the stiffness, strength, and toughness of human cortical bone with age. Bone 1998, 22, 57–66. [Google Scholar] [CrossRef]
- Maksimkin, A.V.; Kaloshkin, S.D.; Tcherdyntsev, V.V.; Chukov, D.I.; Stepashkin, A.A. Technologies for manufacturing ultrahigh molecular weight polyethylene-based porous structures for bone implants. Biomed. Eng. 2013, 47, 73–77. [Google Scholar] [CrossRef]
- Zalepugin, D.Y.; Maksimkin, A.V.; Senatov, F.S.; Tilkunova, N.A.; Chernyshova, I.V.; Vlasov, M.I. Formation of porous ultrahigh molecular weight polyethylene using subcritical water. Mendeleev Commun. 2017, 27, 527–528. [Google Scholar] [CrossRef]
- Maksimkin, A.; Kaloshkin, S.; Zadorozhnyy, M.; Tcherdyntsev, V. Comparison of shape memory effect in UHMWPE for bulk and fiber state. J. Alloys Compd. 2014, 586, S214–S217. [Google Scholar] [CrossRef]
- Anisimova, N.Y.; Zalepugin, D.Y.; Chernyshova, I.V.; Maksimkin, A.V.; Kiselevskii, M.V.; Senatov, F.S.; Spirina, T.S.; Sitdikova, S.M.; Karaulov, A.V. Antibacterial activity of hybrid polymeric scaffold for reconstruction of tubular bone defects. Bull. Exp. Biol. Med. 2019, 168, 58–61. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimkin, A.V.; Senatov, F.S.; Niaza, K.; Dayyoub, T.; Kaloshkin, S.D. Ultra-High Molecular Weight Polyethylene/Titanium-Hybrid Implant for Bone-Defect Replacement. Materials 2020, 13, 3010. https://doi.org/10.3390/ma13133010
Maksimkin AV, Senatov FS, Niaza K, Dayyoub T, Kaloshkin SD. Ultra-High Molecular Weight Polyethylene/Titanium-Hybrid Implant for Bone-Defect Replacement. Materials. 2020; 13(13):3010. https://doi.org/10.3390/ma13133010
Chicago/Turabian StyleMaksimkin, Aleksey V., Fedor S. Senatov, Kirill Niaza, Tarek Dayyoub, and Sergey D. Kaloshkin. 2020. "Ultra-High Molecular Weight Polyethylene/Titanium-Hybrid Implant for Bone-Defect Replacement" Materials 13, no. 13: 3010. https://doi.org/10.3390/ma13133010
APA StyleMaksimkin, A. V., Senatov, F. S., Niaza, K., Dayyoub, T., & Kaloshkin, S. D. (2020). Ultra-High Molecular Weight Polyethylene/Titanium-Hybrid Implant for Bone-Defect Replacement. Materials, 13(13), 3010. https://doi.org/10.3390/ma13133010









