A Nonenzymatic Glucose Sensor Platform Based on Specific Recognition and Conductive Polymer-Decorated CuCo2O4 Carbon Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
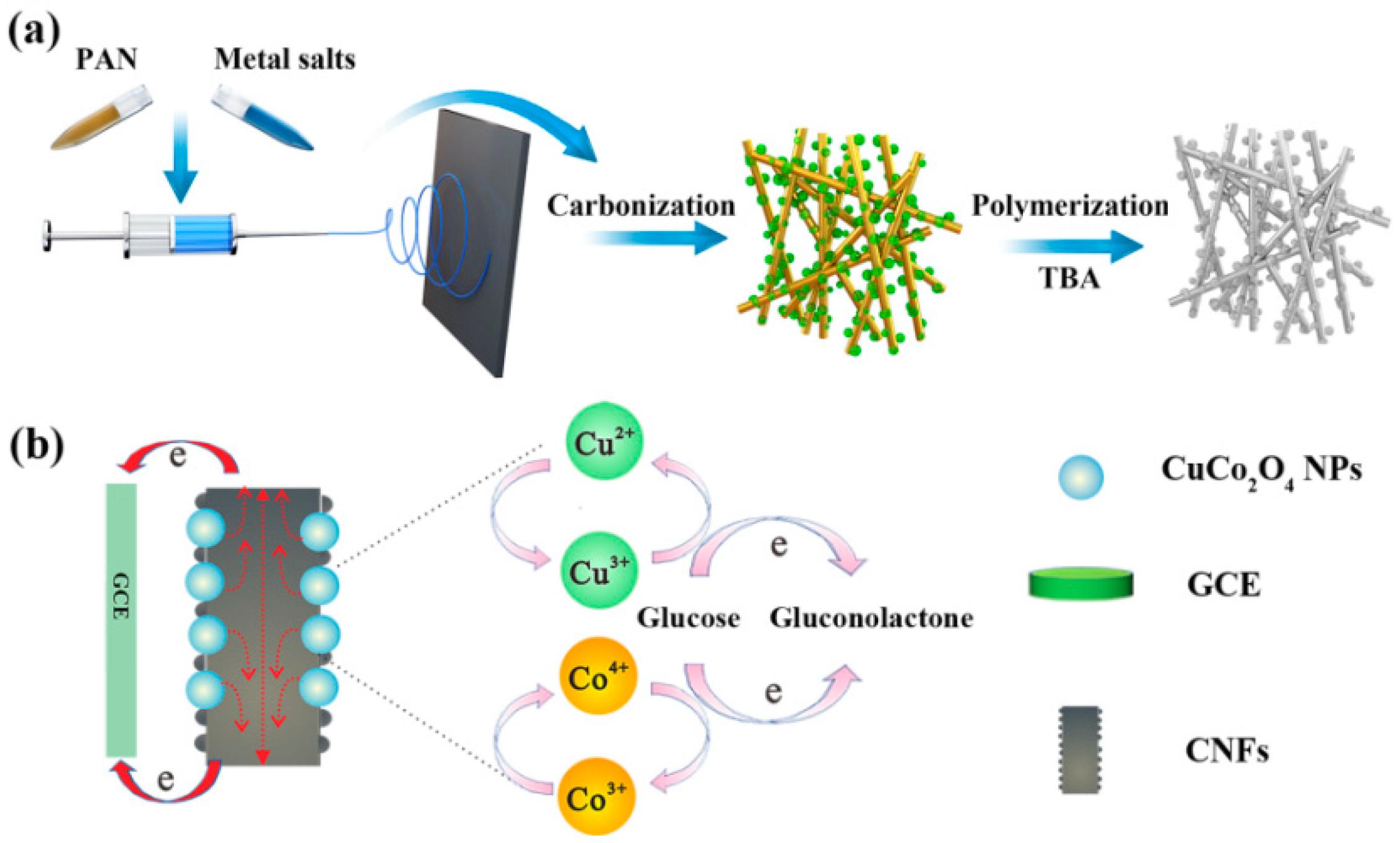
2.2. Preparation of CuCo2O4-Decorated Carbon Nanofibers (CuCo2O4–CNFs)
2.3. Fabrication of Carbon Nanofibers (CNFs) and CuCo2O4–CNF-Modified Electrode
2.4. Fabrication of PTBA/CuCo2O4–CNFs/GCE
2.5. Characterizations
2.6. Electrochemical Characterization
3. Results
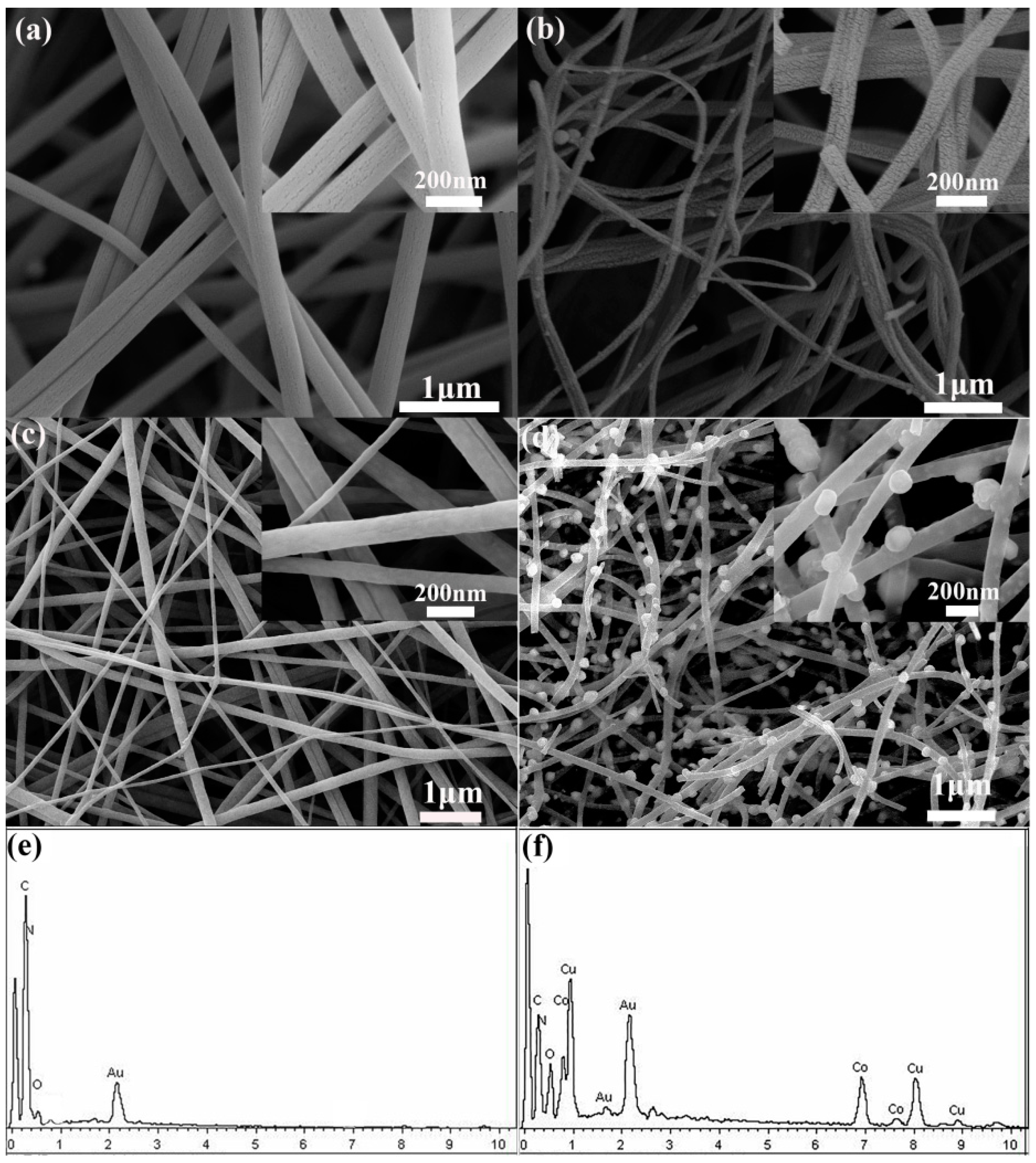
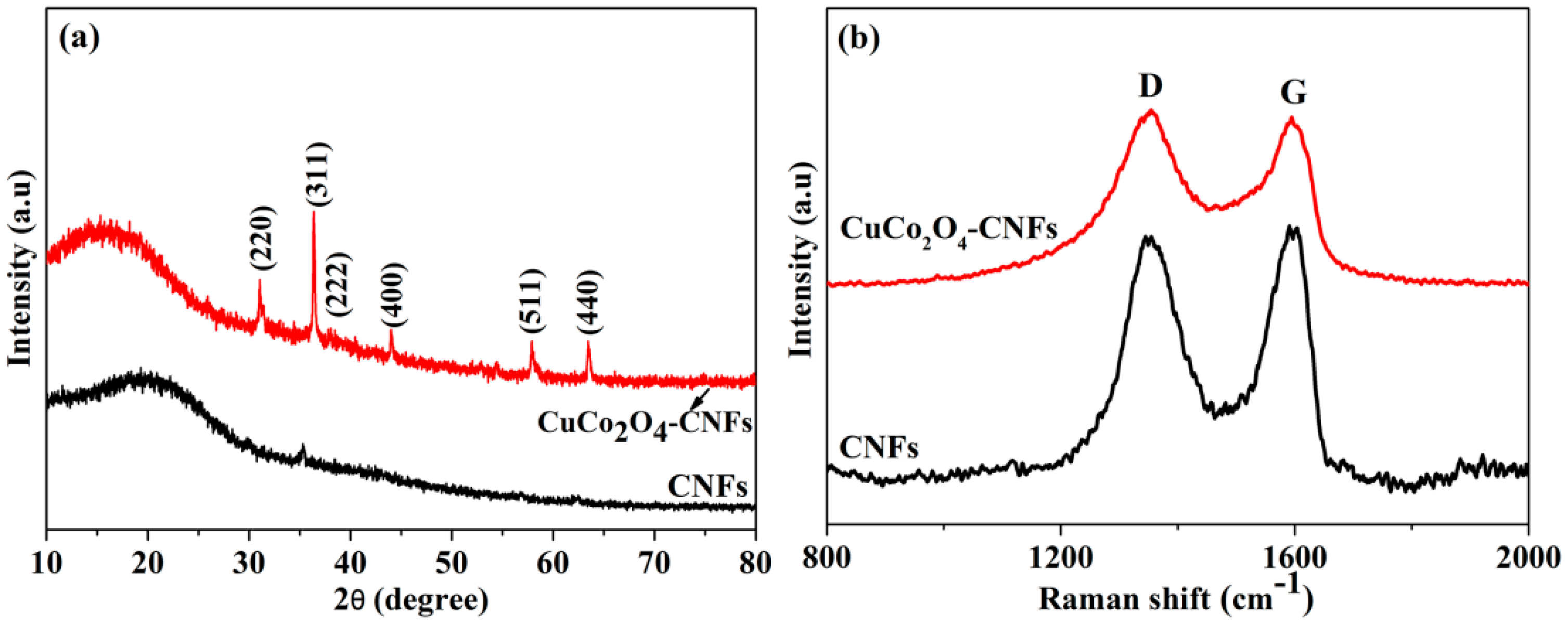
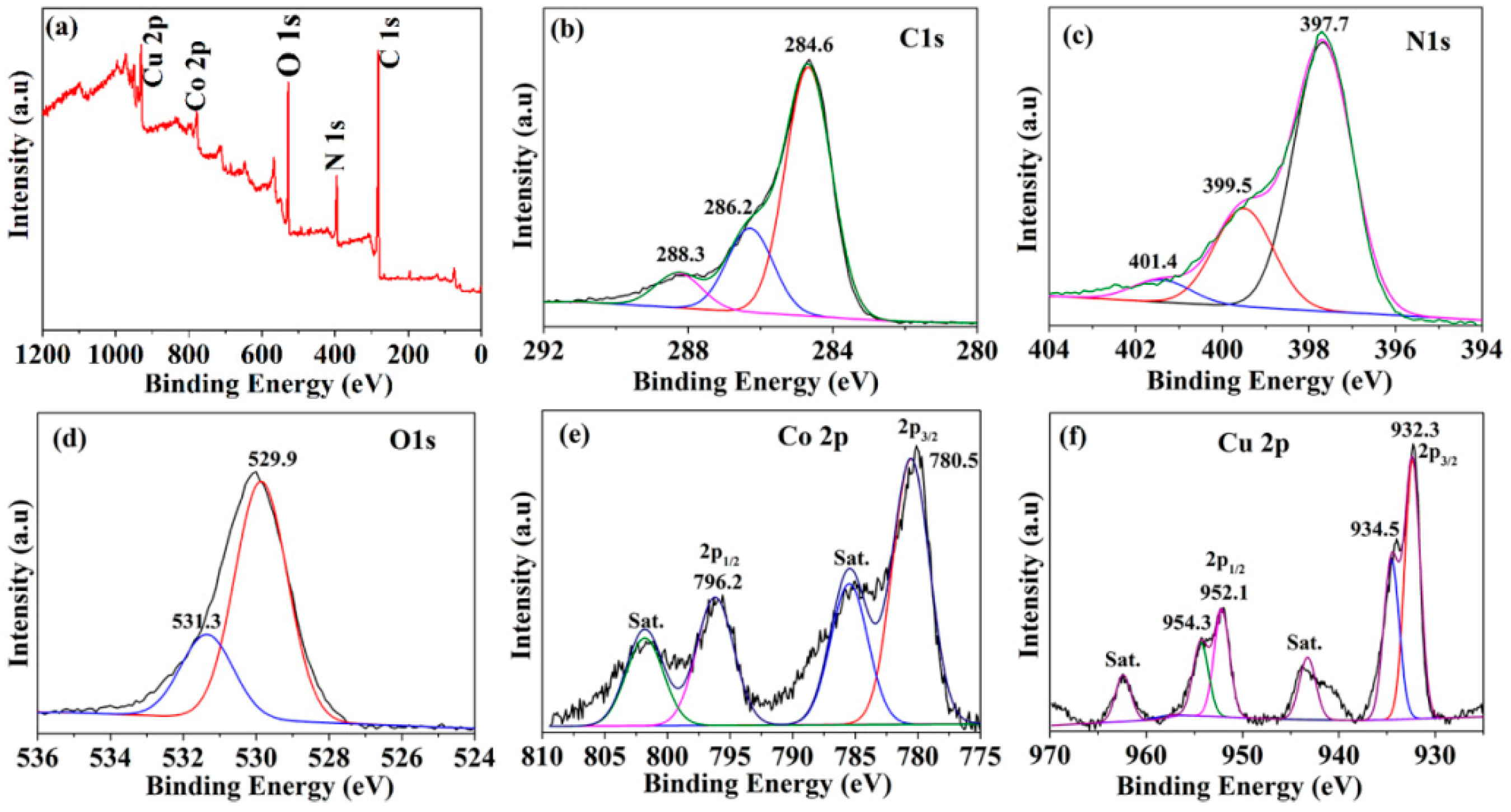
3.1. Structure Characterizations of CNFs and CuCo2O4–CNFs
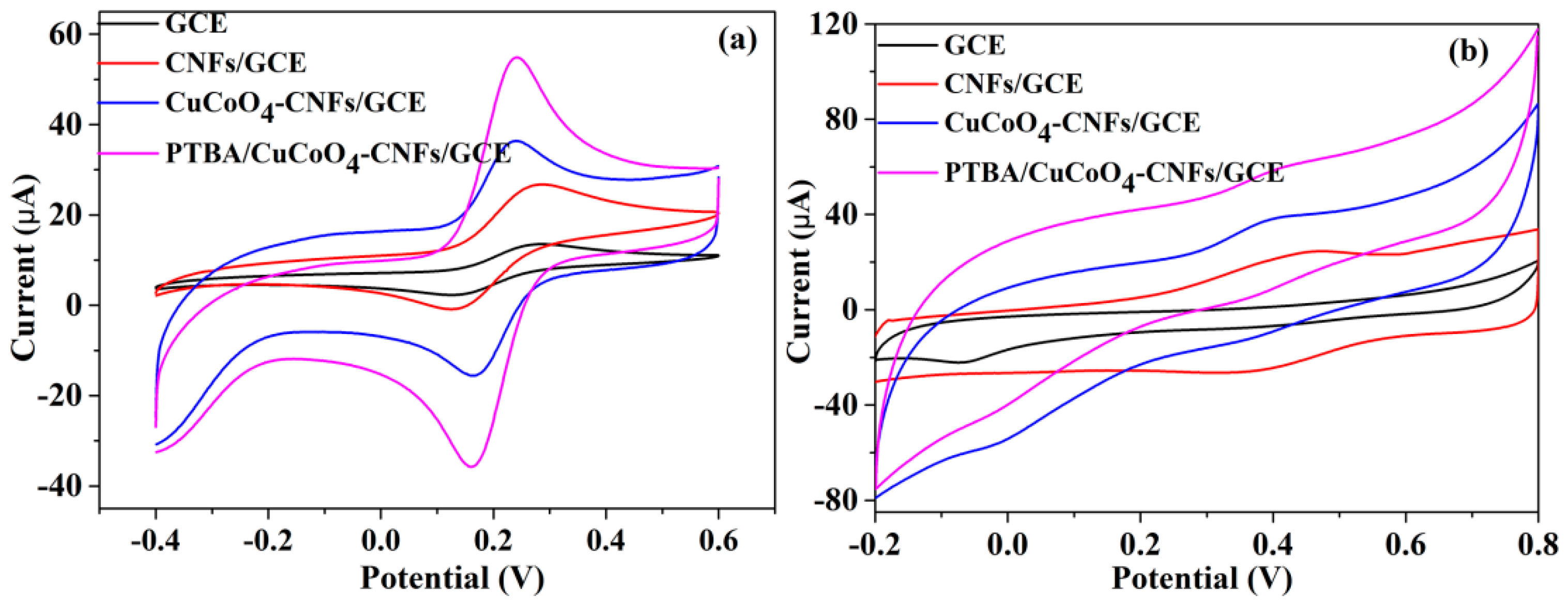
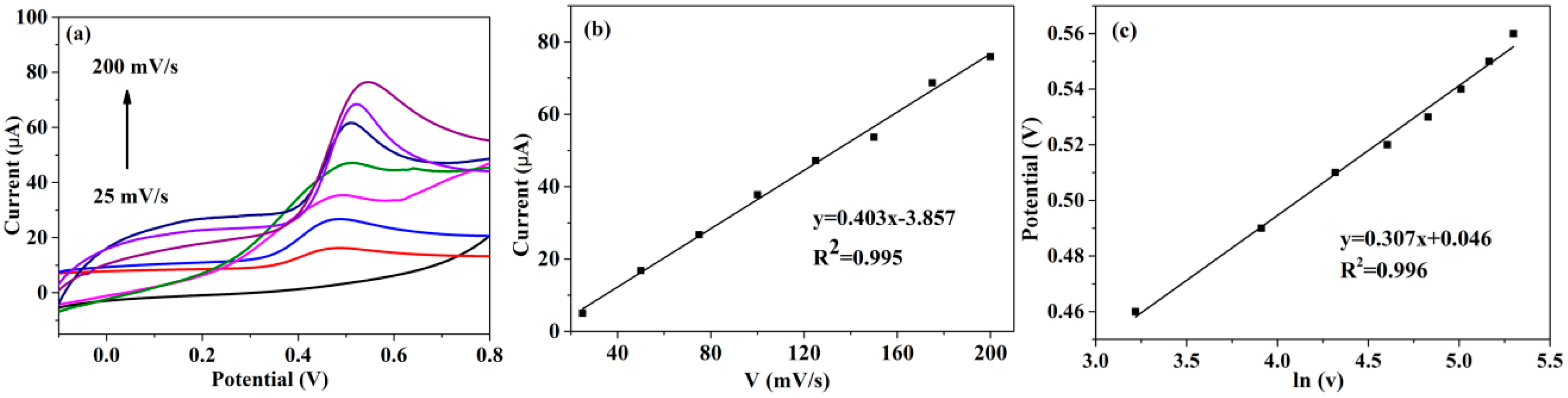
3.2. Electrochemical Characterization of PTBA/CuCo2O4–CNFs/GCE
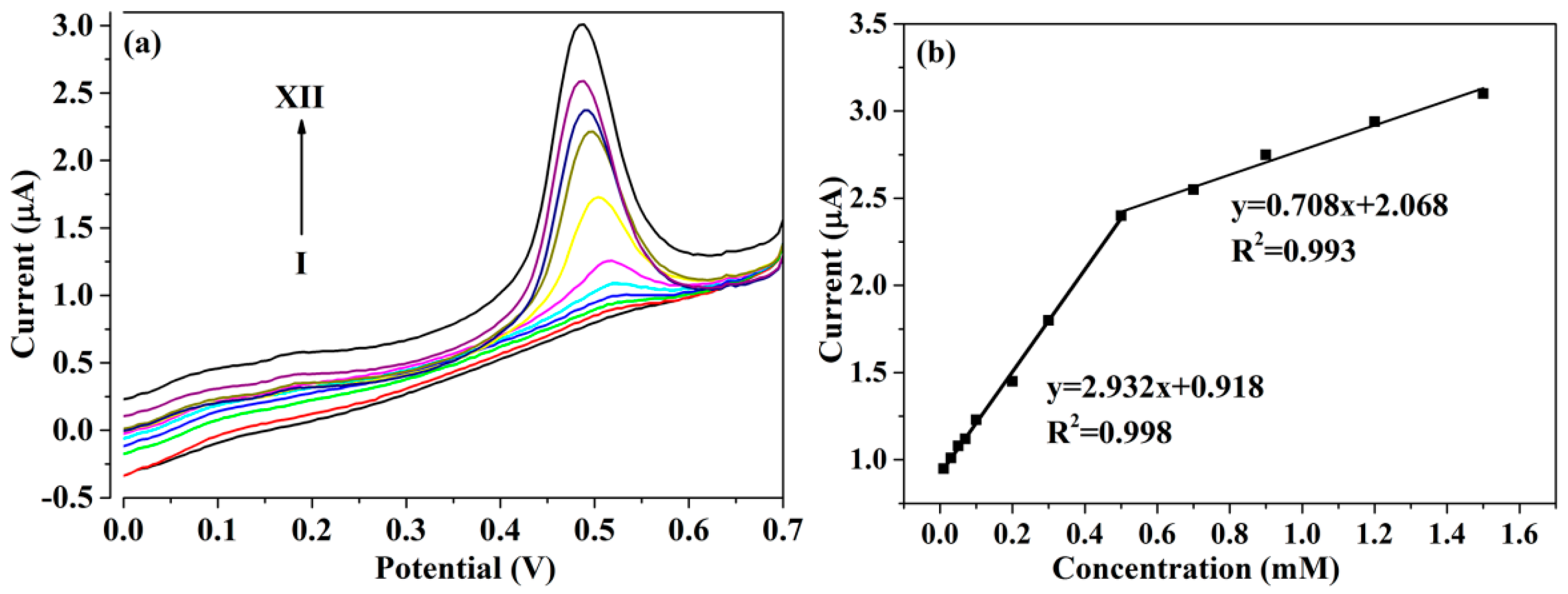
3.3. DPV Responses of PTBA/CuCo2O4–CNFs/GCE toward Glucose
3.4. Interference Studies
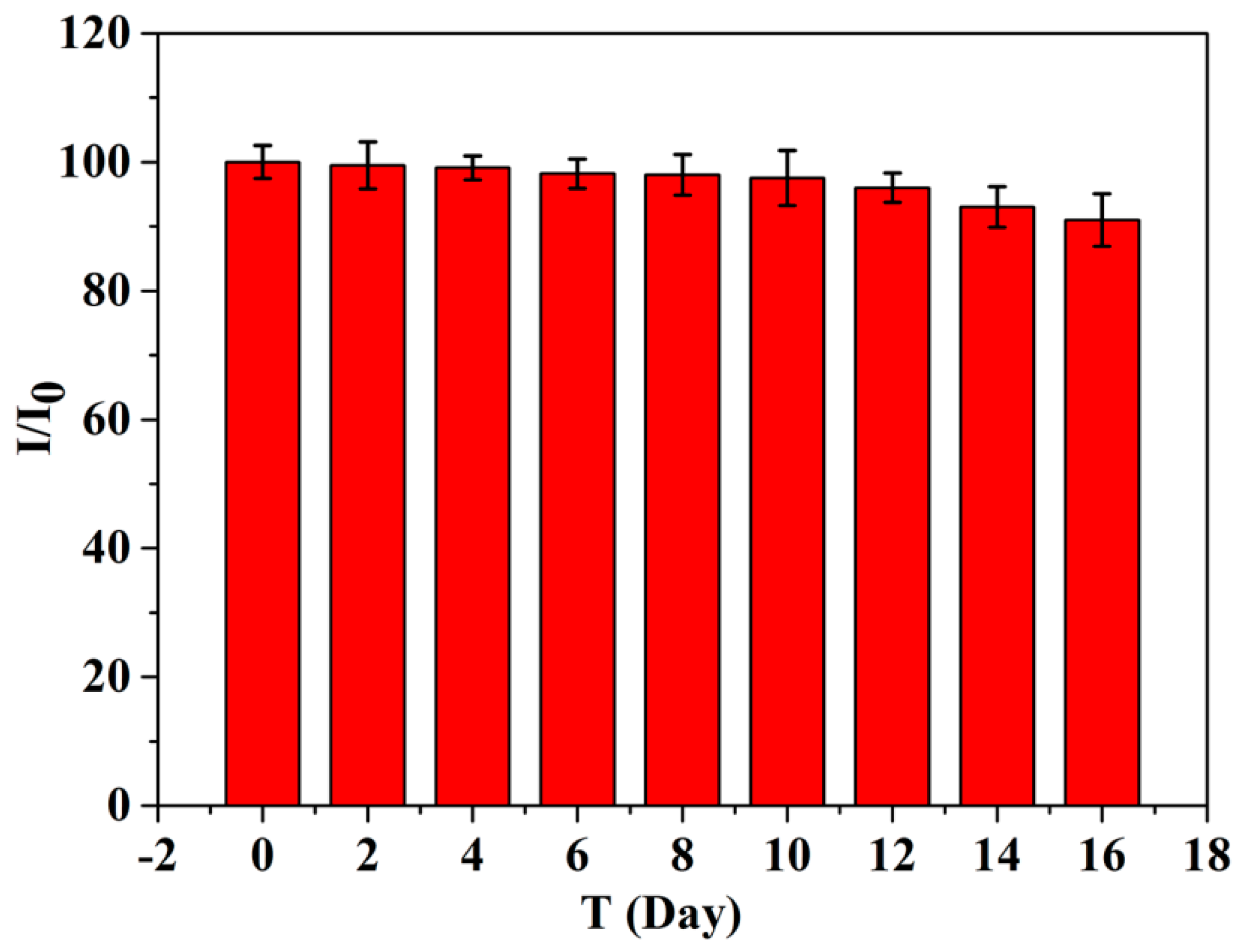
3.5. Reproducibility, Stability, and Application for Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, J.; Lin, Q.; Yin, W.; Jiang, T.; Zhao, D.; Jiang, L. A novel nonenzymatic glucose sensor based on functionalized PDDA-graphene/CuO nanocomposites. Sens. Actuators B-Chem. 2017, 253, 1087–1095. [Google Scholar] [CrossRef]
- Li, M.; Bo, X.; Mu, Z.; Zhang, Y.; Guo, L. Electrodeposition of nickel oxide and platinum nanoparticles on electrochemically reduced graphene oxide film as a nonenzymatic glucose sensor. Sens. Actuators B-Chem. 2014, 192, 261–268. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.-P.; You, T.-Y. Electrochemical Performance of Electrospun Free-Standing Nitrogen-Doped Carbon Nanofibers and Their Application for Glucose Biosensing. ACS Appl. Mater. Interfaces 2014, 6, 6275–6280. [Google Scholar] [CrossRef]
- Zhu, Z.-G.; Garcia-Gancedo, L.; Chen, C.; Zhu, X.-R.; Xie, H.-Q.; Flewitt, A.-J.; Milne, W.-I. Enzyme-free glucose biosensor based on low density CNT forest grown directly on a Si/SiO2 substrate. Sens. Actuators B-Chem. 2013, 178, 586–592. [Google Scholar] [CrossRef]
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-F.; He, X.-P.; Wang, L.-L.; Gu, A.-X.; Huang, Y.; Fang, B.; Geng, B.-Y.; Zhang, X.-J. Non-enzymatic electrochemical sensing of glucose. Microchim. Acta 2012, 180, 161–186. [Google Scholar] [CrossRef]
- Du, H.; Liu, D.; Zhang, X.-D.; Chen, Z.-J.; Xia, H.-B.; Lu, F.-W.; Zhang, Y.-D.; Xia, K.; Jia, R. Effects of Surface Modification on the Catalytic Performances of Nickel Sulfide Nanocatalysts for Residue Hydrocracking: A Monte Carlo Simulation and Experimental Study. ChemCatChem 2017, 9, 1329–1336. [Google Scholar] [CrossRef]
- Du, H.; Liu, D.; Wu, H.; Xia, W.; Zhang, X.-D.; Chen, Z.-J.; Liu, Y.-J.; Liu, H.-L. Surface Modification of Nickel Sulfide Nanoparticles: Towards Stable Ultra-Dispersed Nanocatalysts for Residue Hydrocracking. ChemCatChem 2016, 8, 1543–1550. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Luo, L.-Q.; Zhang, Z.; Ding, Y.-P.; Liu, S.; Deng, D.-M.; Zhao, H.-B.; Chen, Y.-G. Synthesis of MnCo2O4 nanofibers by electrospinning and calcination: Application for a highly sensitive non-enzymatic glucose sensor. J. Mater. Chem. B 2013, 2, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhu, L.; Xu, Y.; Shen, L.; Wang, X.; Ding, Y.; Li, Q.; Deng, D. Hydrogen peroxide biosensor based on horseradish peroxidase immobilized on chitosan-wrapped NiFe2O4 nanoparticles. Microchim. Acta 2011, 174, 55–61. [Google Scholar] [CrossRef]
- Lin, K.-C.; Huang, L.-H.; Chen, S.-M. Electrochemical synthesis of mixed-valence manganese/copper hybrid composite using graphene oxide and multi-walled carbon nanotubes for nonenzymatic glucose sensor. J. Electroanal. Chem. 2014, 735, 36–42. [Google Scholar] [CrossRef]
- Nguyen, N.-S.; Das, G.; Yoon, H.-H. Nickel/cobalt oxide-decorated 3D grapheme nanocomposite electrode for enhanced electrochemical detection of urea. Biosens. Bioelectron. 2016, 77, 372–377. [Google Scholar] [CrossRef]
- Gu, S.; Lou, Z.; Ma, X.; Shen, G. CuCo2O4 nanowires grown on a Ni wire for high-performance, flexible fiber supercapacitors. ChemElectroChem 2015, 2, 1042–1047. [Google Scholar] [CrossRef]
- Ning, R.; Tian, J.; Asiri, A.-M.; Qusti, A.-H.; Al-Youbi, A.-O.; Sun, X. Spinel CuCo2O4 nanoparticles supported on N-doped reduced graphene oxide: A highly active and stable hybrid electrocatalyst for the oxygen reduction reaction. Langmuir 2013, 29, 13146–13151. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Tang, Y.; Li, W.; Li, Z.; Yang, X.; Xu, J.; Lee, C.-S. Porous CuCo2O4 nanocubes wrapped by reduced graphene oxide as high-performance lithium-ion battery anodes. Nanoscale 2014, 6, 6551–6556. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.-R.; Yang, M.; Li, C.-Y.; Tan, H.-Y.; Deng, L.; Xie, S.; Xu, F.-G.; Wang, L.; Song, Y.-H. Preparation of spinel nickel-cobalt oxide nanowrinkles/reduced grapheme oxide hybrid for nonenzymatic glucose detection at physiological level. Electrochim. Acta 2016, 220, 545–553. [Google Scholar] [CrossRef]
- Le, T.-H.; Jin, S.-C.; Hur, S.-H. A highly sensitive enzyme-free glucose sensor based on Co3O4 nanoflowers and 3D graphene oxide hydrogel fabricated via hydrothermal synthesis. Sens. Actuators B-Chem. 2016, 223, 76–82. [Google Scholar]
- Niyogi, S.; Bekyarova, E.; Itkis, M.-E.; Mcwilliams, J.-L.; And, M.-A.-H.; Haddon, R.-C. Solution properties of graphite and grapheme. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef]
- Marini, S.; Mansour, N.B.; Hjiri, M.; Dhahri, R.; Mir, L.E.; Espro, C.; Bonavita, A.; Galvagno, S.; Neri, G.; Leonardi, S.G. Non-enzymatic glucose sensor based on nickel/carbon composite. Electroanalysis 2018, 30, 727–733. [Google Scholar] [CrossRef]
- Malara, A.; Leonardi, S.G.; Bonavita, A.; Fazio, E.; Stelitano, S.; Neri, G.; Neri, F.; Santangelo, S. Origin of the different behavior of some platinum decorated nanocarbons towards the electrochemical oxidation of hydrogen peroxide. Mater. Chem. Phys. 2016, 184, 269–278. [Google Scholar] [CrossRef]
- Frontera, P.; Malara, A.; Stelitano, S.; Leonardi, S.G.; Bonavita, A.; Fazio, E.; Antonucci, P.; Neri, G.; Neri, F.; Santangelo, S. Characterisation and H2O2 sensing properties of TiO2-CNTs/Pt electro-catalysts. Mater. Chem. Phys. 2016, 170, 129–137. [Google Scholar] [CrossRef]
- Huang, J.; Wang, D.; Hou, H.; You, T. Electrospun palladium nanoparticle-loaded carbon nanofibers and their electrocatalytic activities towards hydrogen peroxide and NADH. Adv. Funct. Mater. 2008, 18, 441–448. [Google Scholar] [CrossRef]
- Kanayama, N.; Kitano, H. Interfacial recognition of sugars by boronic acidcarrying self-assembled monolayer. Langmuir 2000, 16, 577–583. [Google Scholar] [CrossRef]
- Moon, J.-M.; Thapliyal, N.; Hussain, K.-K.; Goyal, R.-N.; Shim, Y.-B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef]
- Liu, Y.; Turner, A.-P.-F.; Zhao, M.-J.; Mak, W.-C. Processable enzyme-hybrid conductive polymer composites for electrochemical biosensing. Biosens. Bioelectron. 2018, 100, 374–381. [Google Scholar] [CrossRef]
- Antolini, E.; Cardellini, F. Formation of carbon supported PtRu alloys: An XRD analysis. J. Alloys Compd. 2001, 315, 118–122. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS. Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.-H.; Zhang, X.-J.; Shi, H.-M.; Zeng, W.; Zhang, H.; Liu, Q.; Li, C.-C.; Liu, Q.-H.; Duan, H.G. Porous ultrathin carbon nanobubbles formed carbon nanofiber webs for high-performance flexible supercapacitors. J. Mater. Chem. A 2017, 5, 14801–14810. [Google Scholar] [CrossRef]
- Lu, N.; Shao, C.-L.; Li, X.-H.; Miao, F.-J.; Wang, K.-X.; Liu, Y.-C. CuO nanoparticles/nitrogen-doped carbon nanofibers modified glassy carbon electrodes for non-enzymatic glucose sensors with improved sensitivity. Ceram. Int. 2016, 42, 11285–11293. [Google Scholar] [CrossRef]
- Liu, J.; Bo, X.; Zhao, Z.; Guo, L. Highly exposed Pt nanoparticles supported on porous graphene for electrochemical detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2015, 74, 71–77. [Google Scholar] [CrossRef]
- Chen, S.; Chen, D.; Wang, W.; Quan, H.; Luo, X.; Guo, L. rGO-stabilized MnO/N-doped carbon nanofibers for efficient removal of Pb(II) ion and catalytic degradation of methylene blue. J. Mater. Sci. 2017, 52, 5117–5132. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, X.; Huang, J.; Sun, L.; Li, Q.; Yang, X. Fine Co nanoparticles encapsulated in N-doped porous carbon matrix with superficial N-doped porous carbon nanofibers for efficient oxygen reduction. ACS Appl. Mater. Interfaces 2017, 9, 21747–21755. [Google Scholar] [CrossRef] [PubMed]
- Strelko, V.-V.; Kuts, V.-S.; Thrower, P.-A. On the mechanism of possible influence of heteroatoms of nitrogen, boron and phosphorus in a carbon matrix on the catalytic activity of carbons in electron transfer reactions. Carbon 2000, 38, 1499–1503. [Google Scholar] [CrossRef]
- Tan, G.-Q.; Bao, W.; Yuan, Y.-F.; Liu, Z.; Shahbazian-Yassar, R.; Wu, F.; Amine, K.; Wang, J.; Lu, J. Free standing highly defect nitrogen-enriched carbon nanofibers for lithium ion battery thin-film anodes. J. Mater. Chem. A 2017, 5, 5532–5540. [Google Scholar] [CrossRef]
- Kong, D.; Luo, J.; Wang, Y.; Ren, W.; Yu, T.; Luo, Y.; Yang, Y.; Cheng, C. Three-dimensional Co3O4@MnO2 hierarchical nanoneedle arrays: Morphology control and electrochemical energy storage. Adv. Funct. Mater. 2014, 24, 3815–3826. [Google Scholar] [CrossRef]
- Liu, S.; Hui, K.-S.; Hui, K.-N. Flower-like copper cobaltite nanosheets on graphite paper as high-performance supercapacitor electrodes and enzymeless glucose sensors. ACS Appl. Mater. Interfaces 2016, 8, 3258–3267. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Xie, J.-Q.; Ye, H.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Co-Fe-P nanotubes electrocatalysts derived from metal-organic frameworks for efficient hydrogen evolution reaction under wide pH range. Nano Energy 2019, 56, 225–233. [Google Scholar] [CrossRef]
- Yang, X.; Lu, A.-Y.; Zhu, Y.; Hedhili, M.-N.; Min, S.; Huang, K.-W.; Han, Y.; Li, L.-J. CoP nanosheet assembly grown on carbon cloth: A highly efficient electrocatalyst for hydrogen generation. Nano Energy 2015, 15, 634–641. [Google Scholar] [CrossRef]
- Koninck, M.-D.; Poirier, S.; Marsan, B. CuxCo3-xO4 used as bifunctional electrocatalyst physicochemical properties and electrochemical characterization for the oxygen evolution reaction. J. Electrochem. Soc. 2006, 153, A2103–A2110. [Google Scholar]
- Liu, F.; Kan, X.-W. Conductive imprinted electrochemical sensor for epinephrine sensitive detection and double recognition. J. Electroanal. Chem. 2019, 836, 182–189. [Google Scholar] [CrossRef]
- Dervisevic, M.; Çevik, E.; Şenel, M.; Nergiz, C.; Abasiyanik, M.-F. Amperometric cholesterol biosensor based on reconstituted cholesterol oxidase on boronic acid functional conducting polymers. J. Electroanal. Chem. 2016, 776, 18–24. [Google Scholar] [CrossRef]
- Zhong, X.; Bai, H.-J.; Xu, J.-J.; Chen, H.-Y.; Zhu, Y.-H. A reusable interface constructed by 3-aminophenylboronic acid-functionalized multiwalled carbon nanotubes for cell capture, release, and cytosensing. Adv. Funct. Mater. 2010, 20, 992–996. [Google Scholar] [CrossRef]
- Huang, Y.; Bu, L.-J.; Wang, W.; Qin, X.-L.; Li, Z.; Huang, Z.; Fu, Y.-C.; Su, X.-L.; Xie, Q.-J.; Yao, S.-Z. One-pot preparation of uricase-poly(thiophene-3-boronic acid)-Pt nano composites for high-performance amperometric biosensing of uric acid. Sens. Actuators B-Chem. 2013, 177, 116–123. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Li, J.-J.; Qu, L.-B. Assembly of multi-walled carbon nanotubes-ZnSe quantum dot hybrids for a paeonol electrochemical sensor. Anal. Methods 2014, 6, 3449–3455. [Google Scholar] [CrossRef]
- Jiang, L.-C.; Zhang, W.-D. A highly sensitive nonenzymatic glucose sensor based on CuO nanoparticles-modified carbon nanotube electrode. Biosens. Bioelectron. 2010, 25, 1402–1407. [Google Scholar] [CrossRef]
- Dong, X.-C.; Xu, H.; Wang, X.-W.; Huang, Y.-X.; Chan-Park, M.-B.; Zhang, H.; Wang, L.-H.; Huang, W.; Chen, P. 3D graphene cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 2012, 6, 3206–3213. [Google Scholar] [CrossRef]
- Madhu, R.; Veeramani, V.; Chen, S.-M.; Manikandan, A.; Lo, A.-Y.; Chueh, Y.-L. Honeycomb-like porous carbon-cobalt oxide nanocomposite for high-performance enzymeless glucose sensor and supercapacitor applications. ACS Appl. Mater. Interfaces 2015, 7, 15812–15820. [Google Scholar] [CrossRef]
- Wu, M.; Meng, S.; Wang, Q.; Si, W.; Huang, W.; Dong, X. Nickel-cobalt oxide decorated three-dimensional graphene as an enzyme mimic for glucose and calcium detection. ACS Appl. Mater. Interfaces 2015, 7, 21089–21094. [Google Scholar] [CrossRef]
- Esmaeeli, E.; Ghaffarinejad, A.; Zahedi, A.; Vahidi, O. Copper oxide-polyaniline nanofiber modified fluorine doped tin oxide (FTO) electrode as non-enzymatic glucose sensor. Sens. Actuators B-Chem. 2018, 266, 294–301. [Google Scholar] [CrossRef]
- Liu, L.-J.; Wang, Z.-H.; Yang, J.-H.; Liu, G.-L.; Li, J.-J.; Guo, L.; Chen, S.-L.; Guo, Q.-H. NiCo2O4 nanoneedle-decorated electrospun carbon nanofiber nanohybrids for sensitive non-enzymatic glucose sensors. Sens. Actuators B-Chem. 2018, 258, 920–928. [Google Scholar] [CrossRef]
- Yang, J.; Ye, H.-L.; Zhang, Z.-Q.; Zhao, F.-Q.; Zeng, B.-Z. Metal-organic framework derived hollow polyhedron CuCo2O4 functionalized porous graphene for sensitive glucose sensing. Sens. Actuators B-Chem. 2017, 242, 728–735. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Wang, Y.-Z.; Jia, J.-B.; Wang, J.-G. Nonenzymatic glucose sensor based on graphene oxide and electrospun NiO nanofibers. Sens. Actuators B-Chem. 2012, 171–172, 580–587. [Google Scholar] [CrossRef]
- Yassin, M.-A.; Shrestha, B.-K.; Ahmad, R.; Shrestha, S.; Park, C.-H.; Kim, C.-S. Exfoliated nanosheets of Co3O4 webbed with polyaniline nanofibers: A novel composite electrode material for enzymeless glucose sensing application. J. Ind. Eng. Chem. 2019, 73, 106–117. [Google Scholar] [CrossRef]
- Park, S.; Chung, T.-D.; Kim, H.-C. Nonenzymatic glucose detection using mesoporous platinum. Anal. Chem. 2003, 75, 3046–3049. [Google Scholar] [CrossRef]
| Materials | Method | Sensitivity (µA·cm−2·mM−1) | Linear Range (mM) | Detection Limit (µM) | Reference |
|---|---|---|---|---|---|
| CuO-PANI | Amperometry | 28001359 | 0.00025–0.28 0.28–4.6 | 0.24 | [49] |
| NiCo2O4/ECF | Amperometry | 1947.2 | 0.005–19.175 | 1.5 | [50] |
| CuCo2O4/PrGO | Amperometry | 2426 | 0.0005–3.354 | 0.15 | [51] |
| CuCo2O4 nanosheets | Amperometry | 3.625 | Up to 0.32 | 5 | [36] |
| NiONFs–rGO | Amperometry | 1100 | 0.002–0.6 | 0.77 | [52] |
| Co3O4@PANINFs | Amperometry | 14.25 | 0.1–8 | 60 | [53] |
| PTBA/CuCo2O4–CNFs | DPV | 2932708 | 0.01–0.50.5–1.5 | 0.15 | This work |
| Interference | Concentration (mM) | Current Ratio | RSD (%) |
|---|---|---|---|
| Ascorbic acid (AA) | 2 | 0.99 | 2.35 |
| Uric acid (UA) | 2 | 0.98 | 1.59 |
| Dopamine (DA) | 1 | 0.93 | 3.56 |
| Sodium chloride | 10 | 0.99 | 1.28 |
| l-Lysine | 2 | 0.98 | 2.69 |
| No. | Added (mM) | Founded (mM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 0.3 | 0.31 | 103 | 2.25 |
| 2 | 0.7 | 0.69 | 98.5 | 3.49 |
| 3 | 1.2 | 1.26 | 105 | 2.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Sun, H.; Ren, C.; Zhang, M.; Sun, K. A Nonenzymatic Glucose Sensor Platform Based on Specific Recognition and Conductive Polymer-Decorated CuCo2O4 Carbon Nanofibers. Materials 2020, 13, 2874. https://doi.org/10.3390/ma13122874
Ding Y, Sun H, Ren C, Zhang M, Sun K. A Nonenzymatic Glucose Sensor Platform Based on Specific Recognition and Conductive Polymer-Decorated CuCo2O4 Carbon Nanofibers. Materials. 2020; 13(12):2874. https://doi.org/10.3390/ma13122874
Chicago/Turabian StyleDing, Yongling, Huadong Sun, Chunrong Ren, Mingchen Zhang, and Kangning Sun. 2020. "A Nonenzymatic Glucose Sensor Platform Based on Specific Recognition and Conductive Polymer-Decorated CuCo2O4 Carbon Nanofibers" Materials 13, no. 12: 2874. https://doi.org/10.3390/ma13122874
APA StyleDing, Y., Sun, H., Ren, C., Zhang, M., & Sun, K. (2020). A Nonenzymatic Glucose Sensor Platform Based on Specific Recognition and Conductive Polymer-Decorated CuCo2O4 Carbon Nanofibers. Materials, 13(12), 2874. https://doi.org/10.3390/ma13122874





