Effect of Heat Treatment on Cr2Nb Phase and Properties of Spark Plasma Sintered Cu-2Cr-1Nb Alloy
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
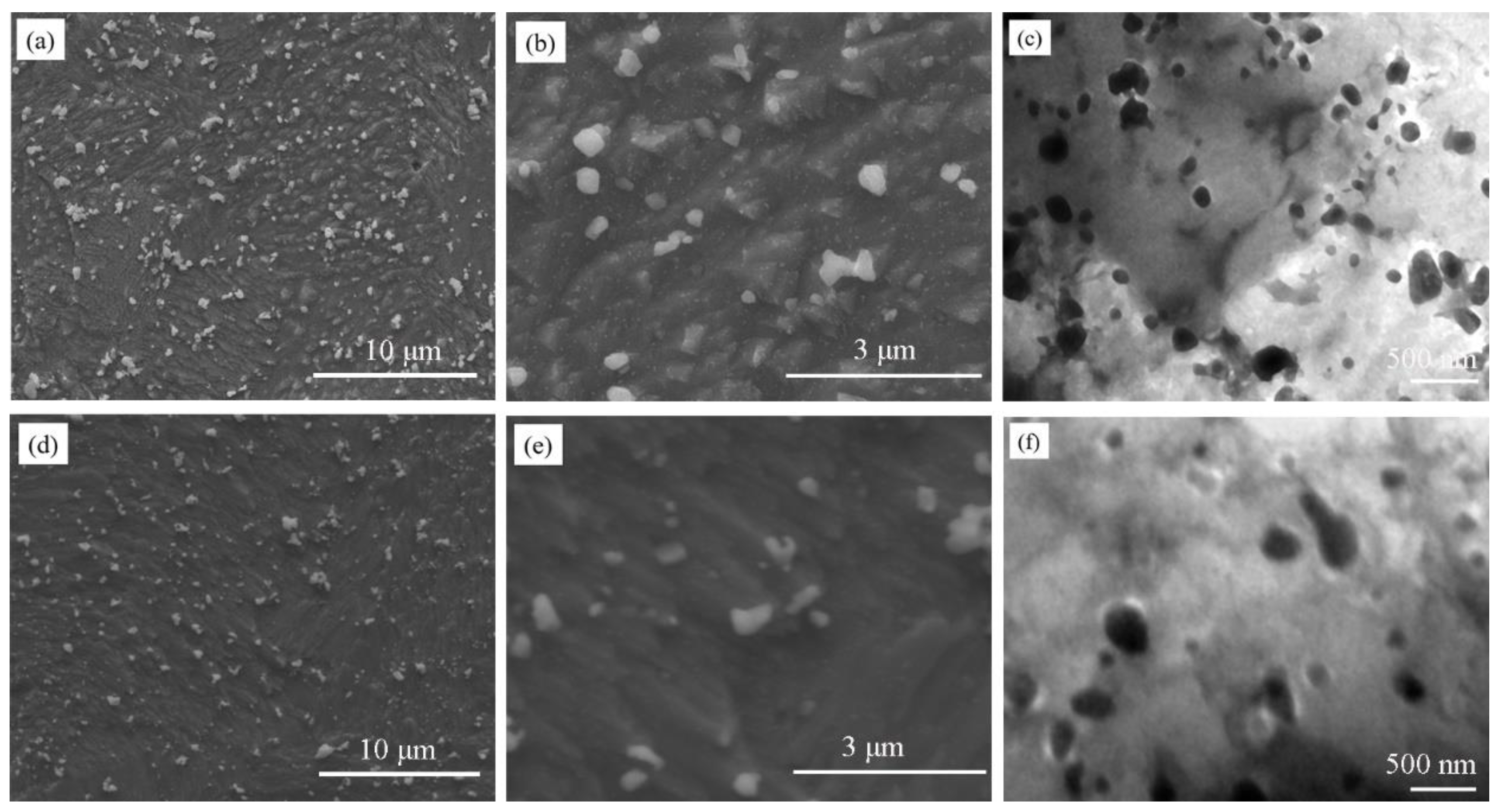
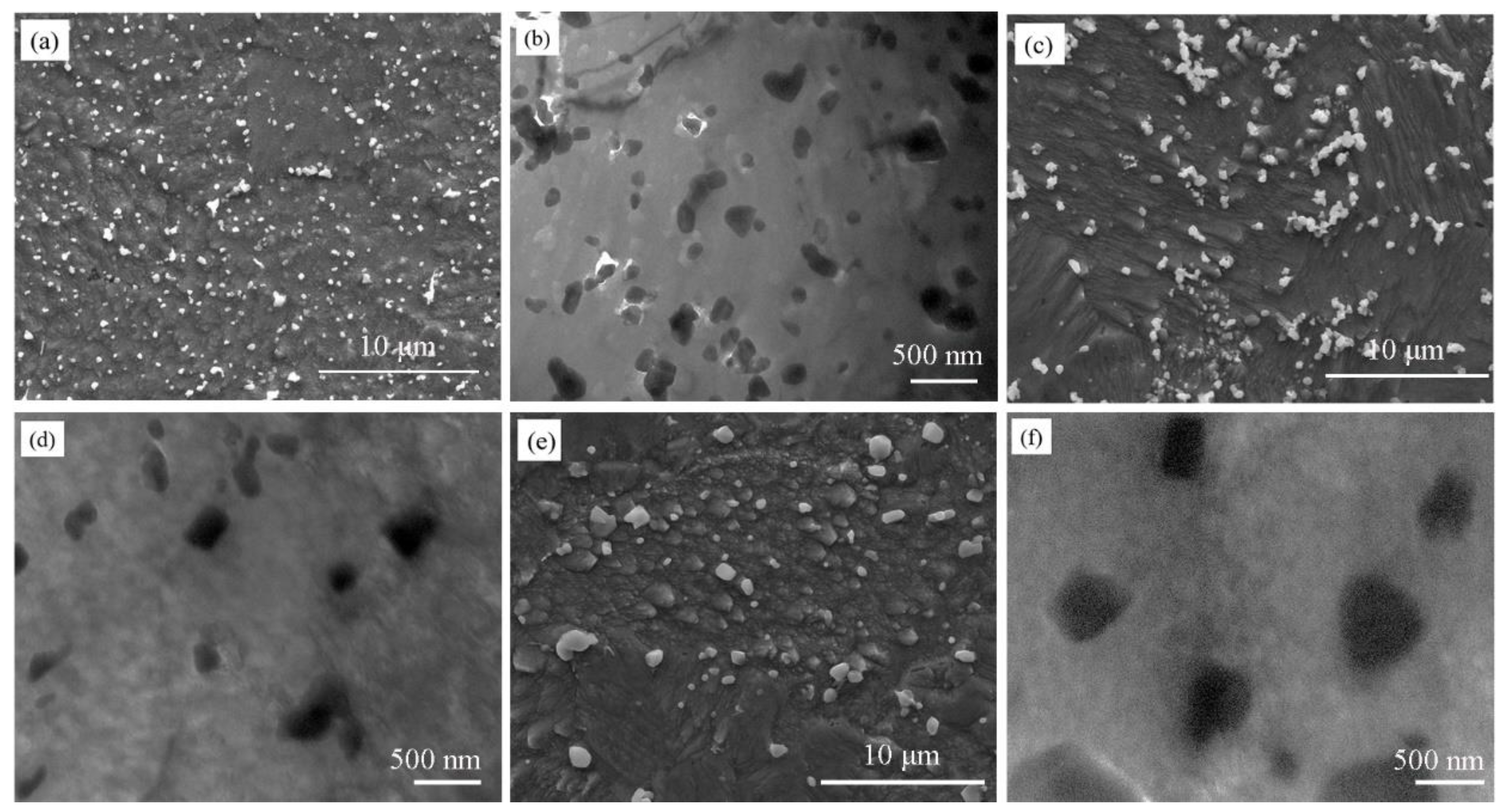
3.1. The Microstructure of the As-SPSed Cu-2Cr-1Nb Alloy
3.2. The Microstructure of the Heat-Treated Cu-2Cr-1Nb Alloy
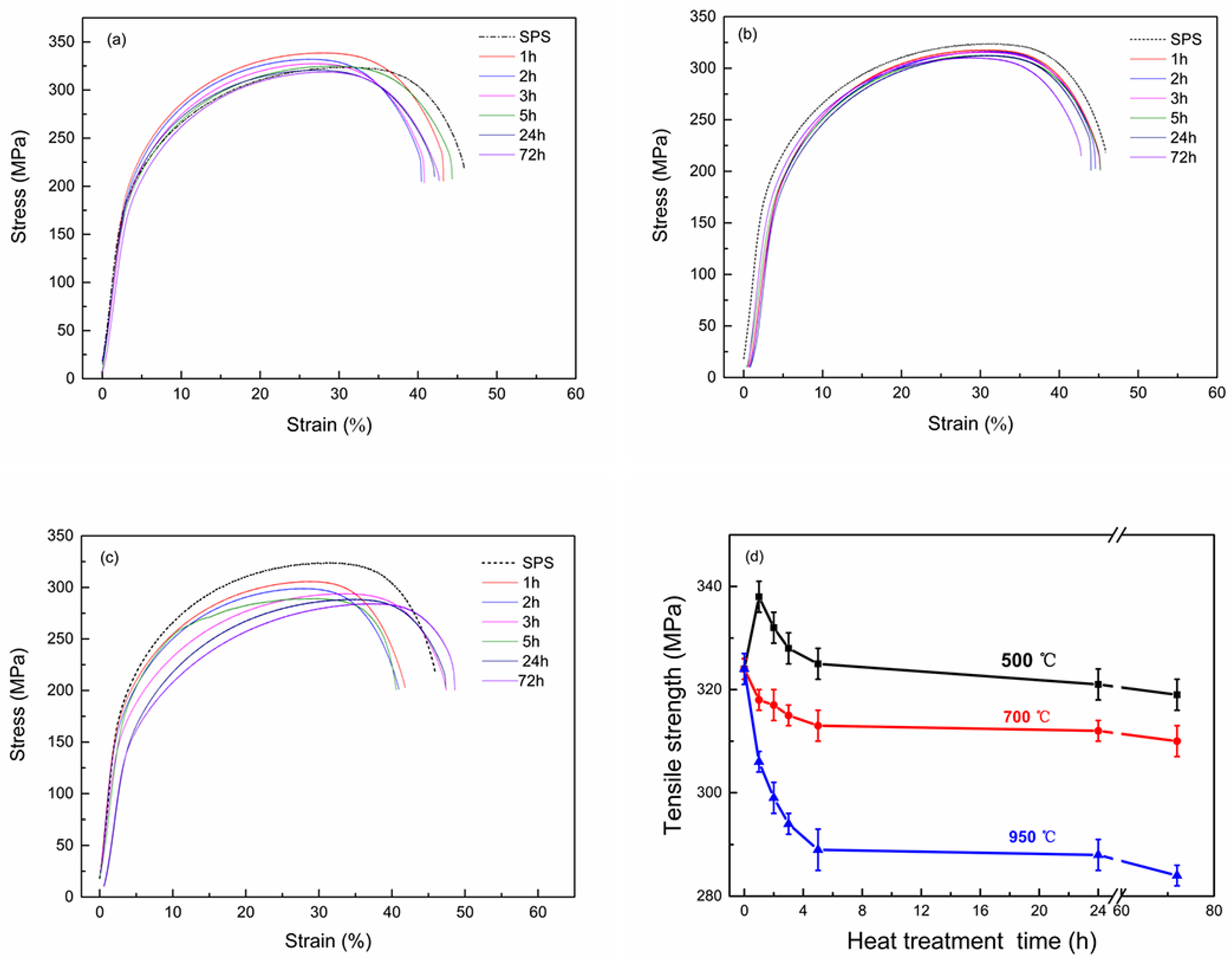
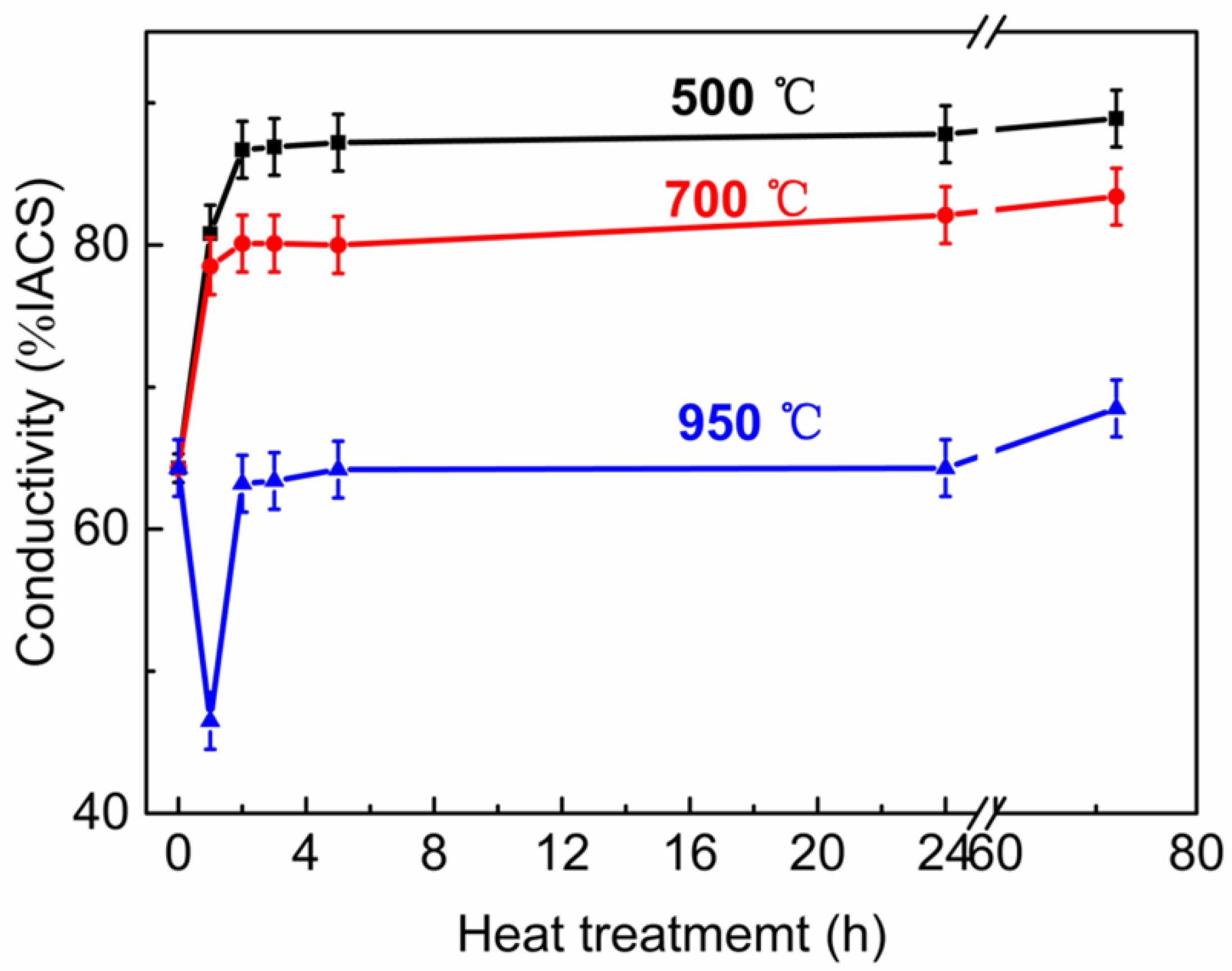
3.3. The Properties of Cu-2Cr-1Nb Alloy
4. Conclusions
- (1)
- The Cr2Nb phase of the Cu-2Cr-1Nb alloy could be regulated by heat treatment. The multi-scale Cr2Nb phase with sizes of 0.10–0.50 μm, 30–100 nm and less than 30 nm was formed after heat treatment at 500 °C for 1 h and 2 h; a small amount of Cr2Nb phase with a size of less than 100 nm was observed in the alloy heat-treated at 500 °C for 72 h. With the increase of heat treatment temperature and time, the Cr2Nb phase coarsened; the average size of Cr2Nb phase of the alloy heat-treated for 72 h at 700 °C and 950 °C increased from 0.33 μm to 0.48 μm and 0.60 μm, and Cr2Nb phase with a size of less than 100 nm was not observed.
- (2)
- The strength and conductivity of the Cu-2Cr-1Nb alloy were simultaneous improved by heat treatment, and they were 332 MPa and 86.7% IACS, respectively, which were 2.5% and 34.8% higher than that of SPSed alloy, and the tensile strength at high temperature 700 °C was 76 MPa, after heat treatment at 500 °C for 2 h. Increasing heat treatment temperature and time, the tensile strength of the alloy was reduced by 1.5%, 4.3% and 12.3% after heat treatment at 500 °C, 700 °C and 950 °C for 72 h.
- (3)
- These suggest that the simultaneous improvement of strength and conductivity of Cu-Cr-Nb alloy can be realized by reducing content of alloying elements Cr and Nb, rapid solidification by close-coupled argon gas atomization, and rapid densification by SPS and heat treatment. The good matching of strength, conductivity and thermal stability of Cu-Cr-Nb alloy can also be achieved.
Author Contributions
Funding
Conflicts of Interest
References
- Zinkle, S.J. Applicability of copper alloys for DEMO high heat flux components. Phys. Scripta 2016, T167, 014004. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Snead, L.; Zinkle, S.J. Development of novel Cu-Cr-Nb-Zr alloys with the aid of computational thermodynamics. Mater. Des. 2018, 156, 370–380. [Google Scholar] [CrossRef]
- Chen, G.; Shen, J.; Zhu, Q.; Yao, S.; Wang, C.; Zhang, P. Tensile deformation and fracture behaviours of cold rolled Cu-3wt.%Ag-0.5wt.%Zr thin sheets with different annealed microstructures. Mater. Sci. Eng. A 2019, 756, 27–34. [Google Scholar] [CrossRef]
- Chenna Krishna, S.; Jha, A.K.; Pant, B.; George, K.M. Achieving higher strength in Cu–Ag–Zr alloy by warm/hot rolling. Rare Metals 2017, 36, 263–267. [Google Scholar] [CrossRef]
- Fu, H.D.; Sheng, X.; Li, W.; Xie, J.X.; Zhao, H.B.; Pan, Z.J. Effect of rolling and aging processes on microstructure and properties of Cu-Cr-Zr alloy. Mater. Sci. Eng. A 2017, 700, 107–115. [Google Scholar] [CrossRef]
- Kulczyk, M.; Pachla, W.; Godek, J.; Smalc-Koziorowska, J.; Skiba, J.; Przybysz, S.; Wróblewska, M.; Przybysz, M. Improved compromise between the electrical conductivity and hardness of the thermo-mechanically treated CuCrZr alloy. Mater. Sci. Eng. A 2018, 724, 45–52. [Google Scholar] [CrossRef]
- Morozova, A.; Borodin, E.; Bratov, V.; Zherebtsov, S.; Belyakov, A.; Kaibyshev, R. Grain Refinement Kinetics in a Low Alloyed Cu-Cr-Zr Alloy Subjected to Large Strain Deformation. Materials 2017, 10, 1394. [Google Scholar] [CrossRef]
- Zhang, D.D.; Bai, F.; Wang, Y.; Wang, J.G.; Wang, W.Q. Grain Refinement and Mechanical Properties of Cu-Cr-Zr Alloys with Different Nano-Sized TiCp Addition. Materials 2017, 10, 919. [Google Scholar] [CrossRef]
- Gao, L.Q.; Yang, X.; Zhang, X.F.; Zhang, Y.; Sun, H.L.; Li, N. Aging behavior and phase transformation of the Cu-0.2 wt%Zr-0.15 wt%Y alloy. Vacuum 2019, 159, 367–373. [Google Scholar] [CrossRef]
- Dobatkin, S.V.; Gubicza, J.; Shangina, D.V.; Bochvar, N.R.; Tabachkova, N.Y. High strength and good electrical conductivity in Cu–Cr alloys processed by severe plastic deformation. Mater. Lett. 2015, 153, 5–9. [Google Scholar] [CrossRef]
- Zhang, S.S.; Zhu, H.H.; Zhang, L.; Zhang, W.Q.; Yang, H.Q.; Zeng, X.Y. Microstructure and properties of high strength and high conductivity Cu-Cr alloy components fabricated by high power selective laser melting. Mater. Lett. 2019, 237, 306–309. [Google Scholar] [CrossRef]
- Zhou, D.S.; Wang, X.K.; Muránsky, O.; Wang, X.R.; Xie, Y.H.; Yang, C.; Zhang, D.L. Heterogeneous microstructure of an Al2O3 dispersion strengthened Cu by spark plasma sintering and extrusion and its effect on tensile properties and electrical conductivity. Mater. Sci. Eng. A 2018, 730, 328–335. [Google Scholar] [CrossRef]
- Anderson, K.R. Effects of Thermal and Mechanical Processing on Microstructures and Desired Properties of Particle-Strengthened Cu-Cr-Nb Alloys; NASA: Washington, WA, USA, 2000. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20000025222.pdf (accessed on 15 December 2019).
- Ellis, D.L.; Michal, G.M. Precipitation Strengthened High Strength, High Conductivity Cu-Cr-Nb Alloys Produced by Chill Block Melt Spinning; NASA: Washington, WA, USA, 1989. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19900002537.pdf (accessed on 15 December 2019).
- Guo, X.L.; Xiao, Z.; Qiu, W.T.; Li, Z.; Zhao, Z.Q.; Wang, X.K.; Jiang, Y.B. Microstructure and properties of Cu-Cr-Nb alloy with high strength, high electrical conductivity and good softening resistance performance at elevated temperature. Mater. Sci. Eng. A 2019, 749, 281–290. [Google Scholar] [CrossRef]
- Shukla, A.K.; Narayana Murty, S.V.S.; Suresh Kumar, R.; Mondal, K. Densification behavior and mechanical properties of Cu–Cr–Nb alloy powders. Mater. Sci. Eng. A 2012, 551, 241–248. [Google Scholar] [CrossRef]
- Asraff, A.K.; Aparna, R.; Kumaresan, D.; Muthukumar, R. Comparison of Creep Properties of Four Copper Alloys and Creep Based Stress Analysis of a Rocket Engine Combustion Chamber. Procedia Eng. 2013, 55, 45–50. [Google Scholar] [CrossRef]
- de Groh, H.C.; Ellis, D.L.; Loewenthal, W.S. Comparison of GRCop-84 to Other Cu Alloys with High Thermal Conductivities. J. Mater. Eng. Perform. 2008, 17, 594–606. [Google Scholar] [CrossRef]
- Ellis, D.L. Mechanical and Thermal Properties of Two Cu-Cr-Nb Alloys and NARloy-Z.; NASA: Washington, WA, USA, 1996. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19970002915.pdf (accessed on 15 December 2019).
- Ellis, D.L. Thermophysical Properties of GRCop-84; NASA: Washington, WA, USA, 2000. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20000064095.pdf (accessed on 15 December 2019).
- Ellis, D.L. GRCop-84:A High-Temperature Copper Alloy. for High.-Heat-Flux Applications; NASA: Washington, WA, USA, 2005. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20050123582.pdf (accessed on 15 December 2019).
- Ellis, D.L. Tensile Properties of GRCop-84; NASA: Washington, WA, USA, 2012. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20120008551.pdf (accessed on 15 December 2019).
- Shukla, A.K.; Sharma, V.M.J.; Murty, S.V.S.N.; Narayanan, P.R.; Sharma, S.C. Integrity of Structural and Thermo-structural Materials for Indian Space Programme. Procedia Eng. 2014, 86, 8–17. [Google Scholar] [CrossRef]
- Tian, B.H.; Song, K.X.; Liu, P. High. Performance Dispersion Strengthened Copper Matrix Composite and Its Preparation Technology, 1st ed.; Science Press: Beijing, China, 2011; pp. 1–30. [Google Scholar]
- Groza, J.R.; Gibeling, J.C. Principles of particles selection for dispersion strengthened copper. Mater. Sci. Eng. A 1993, 171, 115–125. [Google Scholar] [CrossRef]
- Hazzledine, P.M. Direct Versus Indirect Dispersion Hardening. Scripta Metal. Mater. 1992, 26, 57–58. [Google Scholar] [CrossRef]
- Anderson, K.R.; Groza, J.R. Microstructural size effects in high-strength high-conductivity Cu-Cr-Nb alloys. Metall. Mater. Trans. A 2001, 32A, 1211–1224. [Google Scholar] [CrossRef]
- Shukla, A.K.; Narayana Murty, S.V.S.; Sharma, S.C.; Mondal, K. The serrated flow and recrystallization in dispersion hardened Cu–Cr–Nb alloy during hot deformation. Mater. Sci. Eng. A 2016, 673, 135–140. [Google Scholar] [CrossRef]
- Dhokey, N.B.; Sarve, S.N.; Lamsoge, H.A. Development of In-Situ Synthesis of Cr2Nb Reinforced Copper Alloy by Aluminothermic Process. T Indian I Met. 2011, 64, 425–429. [Google Scholar] [CrossRef]
- Dhokey, N.B.; Sarve, S.N.; Lamsoge, H.A. In-situ Synthesis of Cr2Nb Reinforced Copper Alloy by Liquid Metallurgy Route. Mater. Sci. Forum 2012, 710, 143–148. [Google Scholar] [CrossRef]
- Ellis, D.L.; Lerch, B.A. Improvement of GRCop-84 Through the Addition of Zirconium; NASA: Washington, WA, USA, 2012. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20120012545.pdf (accessed on 15 December 2019).
- Shukla, A.K.; Samuel, M.G.; Suresh Kumar, R.; Narayana Murty, S.V.S.; Mondal, K. Effect of powder oxidation on densification and properties of vacuum hot pressed Cu–Cr–Nb alloy. Mater. Sci. Eng. A 2013, 561, 452–459. [Google Scholar] [CrossRef]
- Shukla, A.K.; Suresh Kumar, R.; Narayana Murty, S.V.S.; Mondal, K. Enhancement of high temperature ductility of hot-pressed Cu–Cr–Nb alloy by hot rolling. Mater. Sci. Eng. A 2013, 577, 36–42. [Google Scholar] [CrossRef]
- Shukla, A.K.; Narayana Murty, S.V.S.; Suresh Kumar, R.; Mondal, K. Spark plasma sintering of dispersion hardened Cu–Cr–Nb alloy powders. J. Alloys Compd. 2013, 577, 70–78. [Google Scholar] [CrossRef]
- Li, W.Y.; Guo, X.P.; Verdy, C.; Dembinski, L.; Liao, H.L.; Coddet, C. Improvement of microstructure and property of cold-sprayed Cu–4 at.%Cr–2 at.%Nb alloy by heat treatment. Scripta Mater. 2006, 55, 327–330. [Google Scholar] [CrossRef]
- Anderson, K.R.; Groza, J.R.; Dreshfield, R.L.; Ellis, D. Microstructural evolution and thermal stability of precipitation-strengthened Cu-8Cr-4Nb alloy. Mater. Sci. Eng. A 1993, 169, 167–175. [Google Scholar] [CrossRef]
- Shukla, A.K.; Narayana Murty, S.V.S.; Sharma, S.C.; Mondal, K. Aging behavior and microstructural stability of a Cu–8Cr–4Nb alloy. J. Alloys Compd. 2014, 590, 514–525. [Google Scholar] [CrossRef]
- Anderson, K.R.; Groza, J.R.; Ulme, D.G. Microstructural refinement and strengthening of Cu-4Cr-2-Nb alloy by mechanical milling. Scripta Mater. 1997, 37, 179–185. [Google Scholar] [CrossRef]
- Shukla, A.K.; Narayana Murty, S.V.S.; Suresh Kumar, R.; Mondal, K. Effect of powder milling on mechanical properties of hot-pressed and hot-rolled Cu–Cr–Nb alloy. J. Alloys Compd. 2013, 580, 427–434. [Google Scholar] [CrossRef]
- Lv, X.Q.; Liu, Z.M.; Lei, T.; Li, Q.; Peng, K.; Zhao, F.; Ren, Y.K.; Lu, S.Z.; Nong, B.Z. Microstructure and properties of Cu-Cr-Nb alloy powder prepared by argon gas atomization. Adv. Powder Technol. 2019, 30, 2464–2472. [Google Scholar] [CrossRef]
- Lv, X.Q.; Liu, Z.M.; Lei, T.; Li, Q.; Ji, X.B.; Wen, T.; Ren, Y.K.; Wei, B. Microstructure and properties of Cu-2Cr-1Nb alloy fabricated by spark plasma sintering. Trans. Nonferrous Met. Soc. China 2020. Submitted. [Google Scholar]
- ISO 6892-1:2016. Metallic Materials-Tensile Testing Part 1: Merhod of Test at Room Temperature; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- ISO 6892-2:2018. Metallic Materials—Tensile Testing Part 2: Method of Test at Elevated Temperature; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Butrymowwicz, D.B. Diffusion Rate Data and Mass Transport. Phenomena for Copper Systems, 1st ed.; National Standard Reference Data System: Washington, DC, USA, 1977. [Google Scholar]
- Zheng, Z.Q. Fundamentals of the Materials Science, 2nd ed.; Central South University Press: Changsha, China, 2013; pp. 474–503. [Google Scholar]
- Montes, J.M.; Cuevas, F.G.; Cintas, J. Porosity effect on the electrical conductivity of sintered powder compacts. Appl. Phys. A 2008, 92, 375–380. [Google Scholar] [CrossRef]
- Montes, J.M.; Rodríguez, J.A.; Herrera, E.J. Thermal and electrical conductivities of sintered powder compacts. Powder Metall. 2013, 46, 251–256. [Google Scholar] [CrossRef]
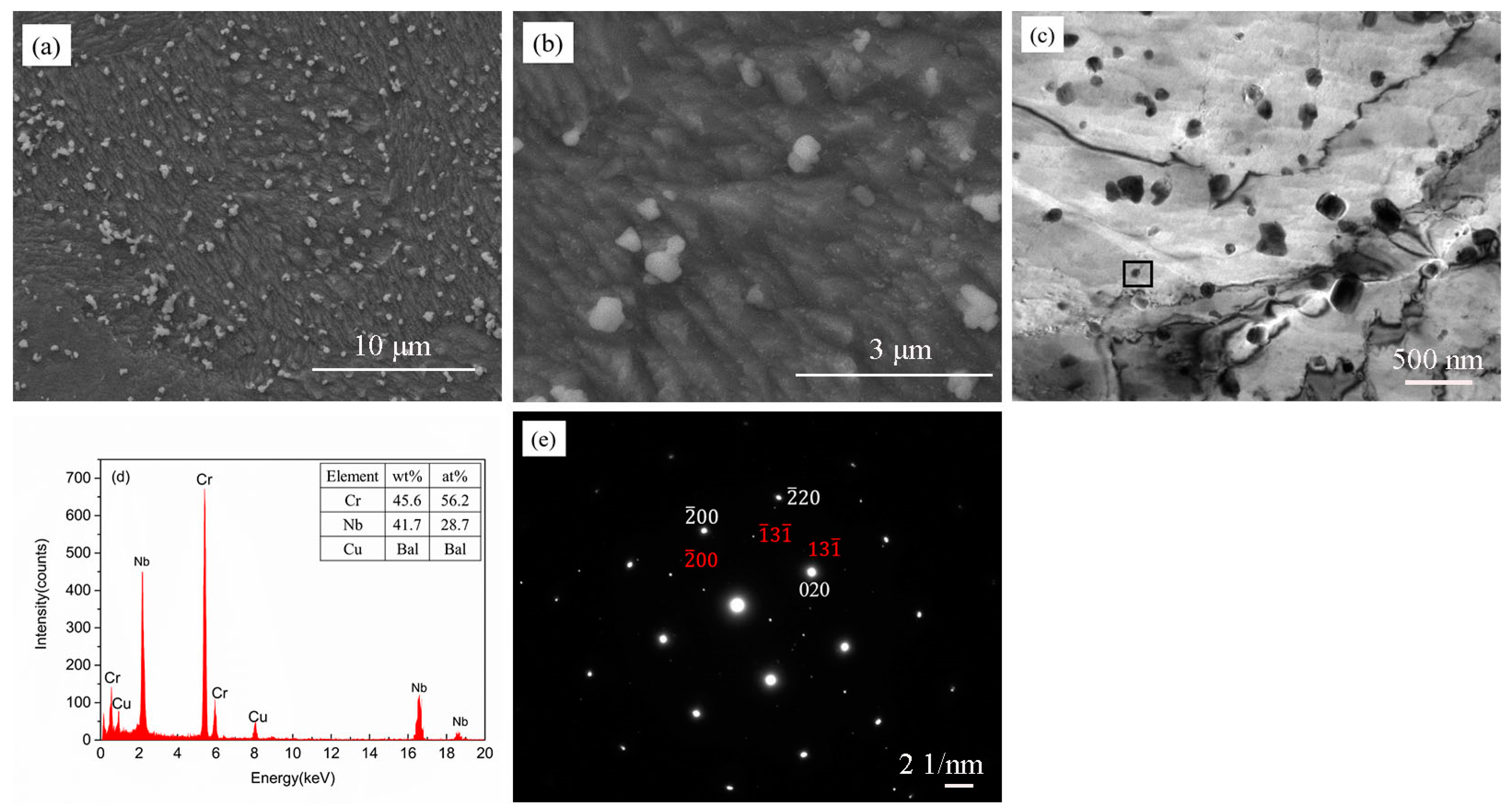
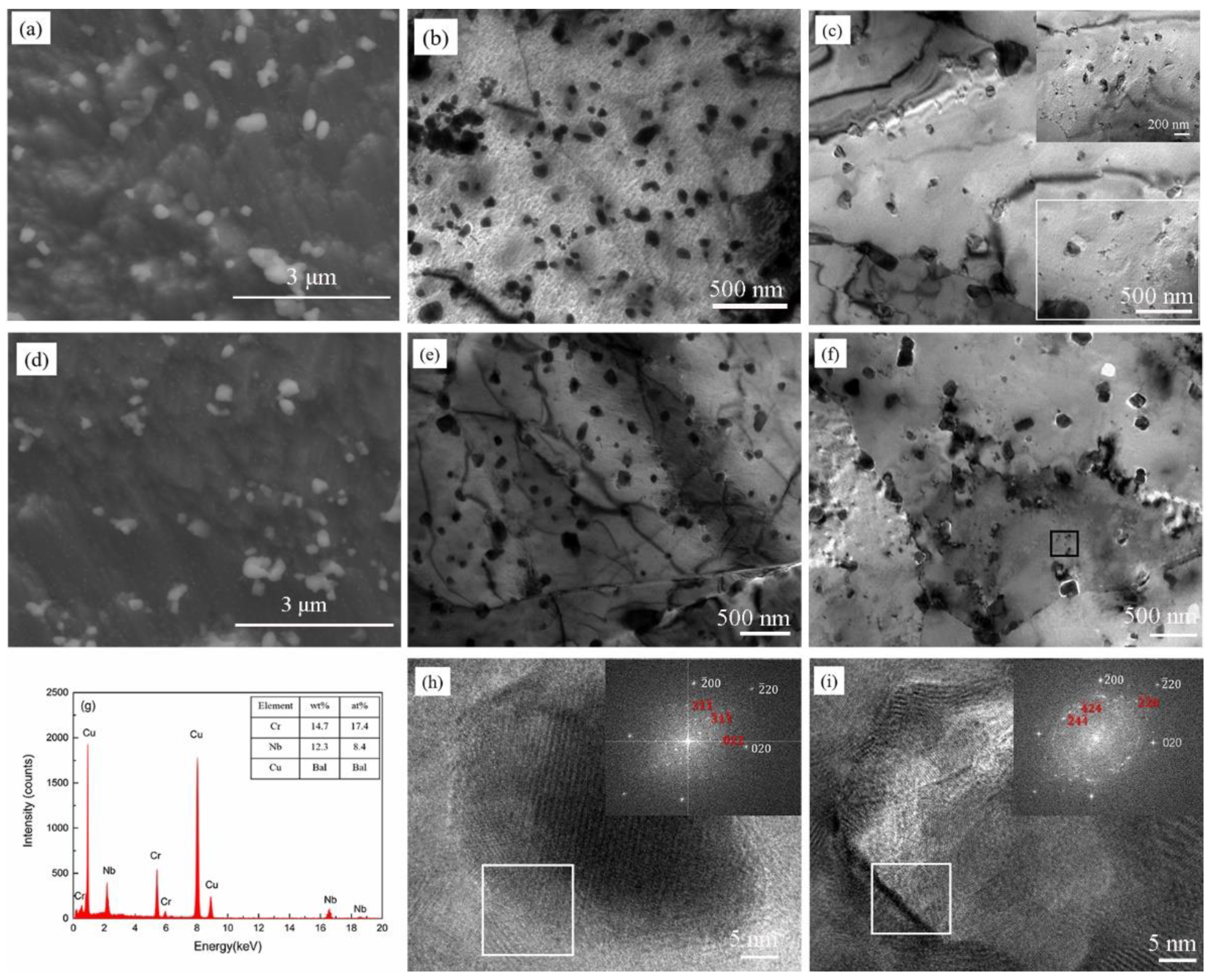
| Element | Cr | Nb | O | Cu |
|---|---|---|---|---|
| Measured composition (wt%/at%) | 1.54/1.89 | 1.26/0.88 | 0.01/0.02 | Bal |
| Alloy | Processing Method | Phase Size (μm) | Tensile Strength (MPa) | Conductivity (% IACS) | Refs. | |
|---|---|---|---|---|---|---|
| 25 °C | 700 °C | |||||
| Cu-0.47Cr-0.16Nb (wt%) | Casting + Homogenization + CR + Aging | 0.70 | 453 | - | 89.1 | [15] |
| Cu-2Cr-1.35Nb-0.15Zr (wt%) | Drop casting + Deformation processing + Heat treatment | 0.3–1 | 385 | - | <60 | [2] |
| Cu-8Cr-4Nb (at%) | Casting | 0.7–7 | - | - | - | [29,30] |
| Atomization + HE + Aging | 0.93 | 426 | 100 | 54 | [13,22] | |
| Atomization + VHP + Aging | 400 | 72 | 75 | [33,37] | ||
| Cu-4Cr-2Nb (at%) | Atomization + HE + Aging | 0.78 | 325 | 75 | 74 | [13] |
| Cu-2Cr-1Nb (at%) | Close-coupled gas atomization + SPS + Aging | <0.33 | 332 | 76 | 86.7 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Liu, Z.; Lei, T.; Li, Q.; Ren, Y.; Zhou, X.; Zhang, Z. Effect of Heat Treatment on Cr2Nb Phase and Properties of Spark Plasma Sintered Cu-2Cr-1Nb Alloy. Materials 2020, 13, 2860. https://doi.org/10.3390/ma13122860
Lv X, Liu Z, Lei T, Li Q, Ren Y, Zhou X, Zhang Z. Effect of Heat Treatment on Cr2Nb Phase and Properties of Spark Plasma Sintered Cu-2Cr-1Nb Alloy. Materials. 2020; 13(12):2860. https://doi.org/10.3390/ma13122860
Chicago/Turabian StyleLv, Xueqian, Zuming Liu, Ting Lei, Quan Li, Yake Ren, Xu Zhou, and Zejie Zhang. 2020. "Effect of Heat Treatment on Cr2Nb Phase and Properties of Spark Plasma Sintered Cu-2Cr-1Nb Alloy" Materials 13, no. 12: 2860. https://doi.org/10.3390/ma13122860
APA StyleLv, X., Liu, Z., Lei, T., Li, Q., Ren, Y., Zhou, X., & Zhang, Z. (2020). Effect of Heat Treatment on Cr2Nb Phase and Properties of Spark Plasma Sintered Cu-2Cr-1Nb Alloy. Materials, 13(12), 2860. https://doi.org/10.3390/ma13122860




