Utilization of Polypropylene in the Production of Metal-Filled Polymer Composites: Development and Characteristics
Abstract
:1. Introduction
2. Experimental
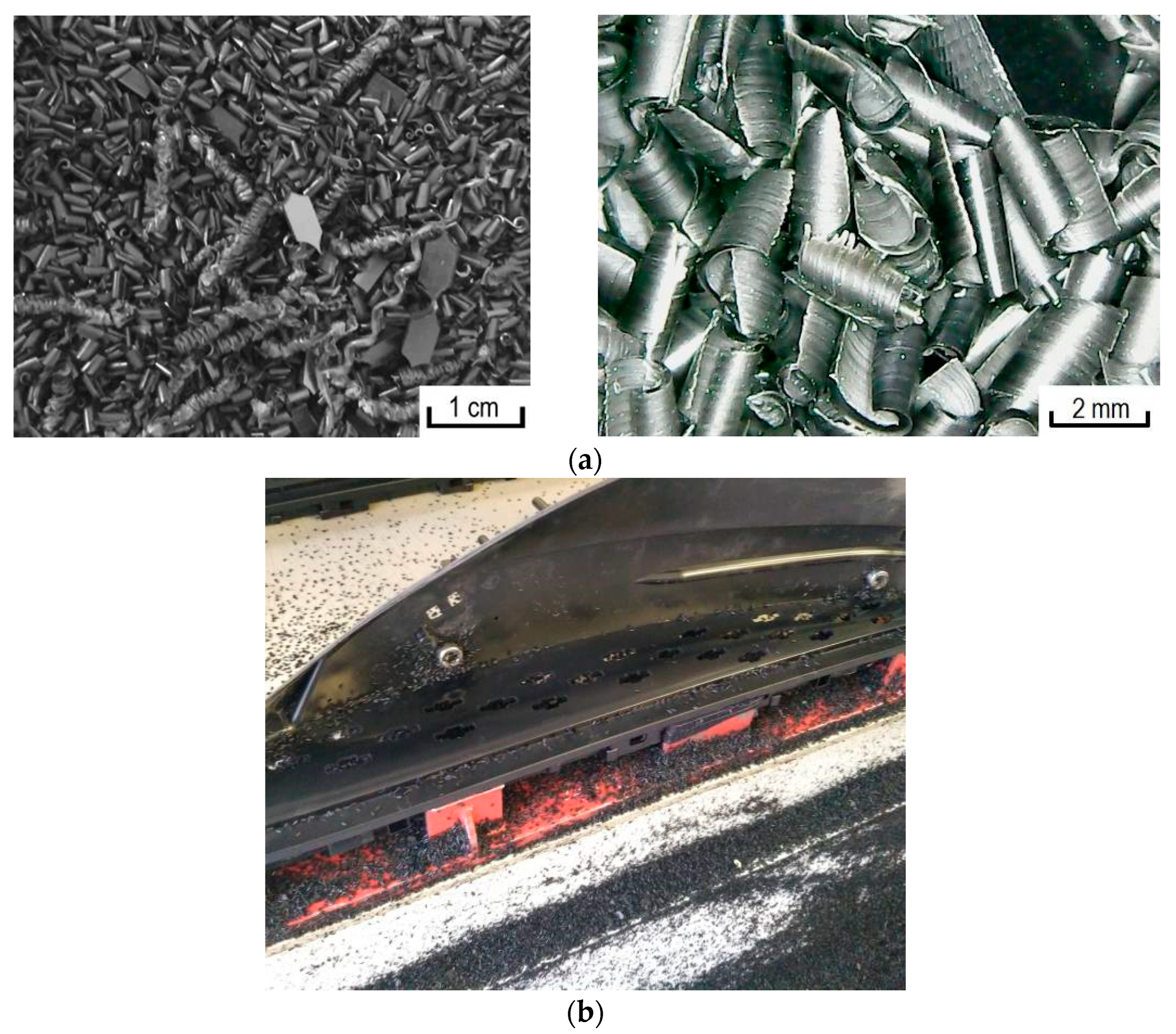
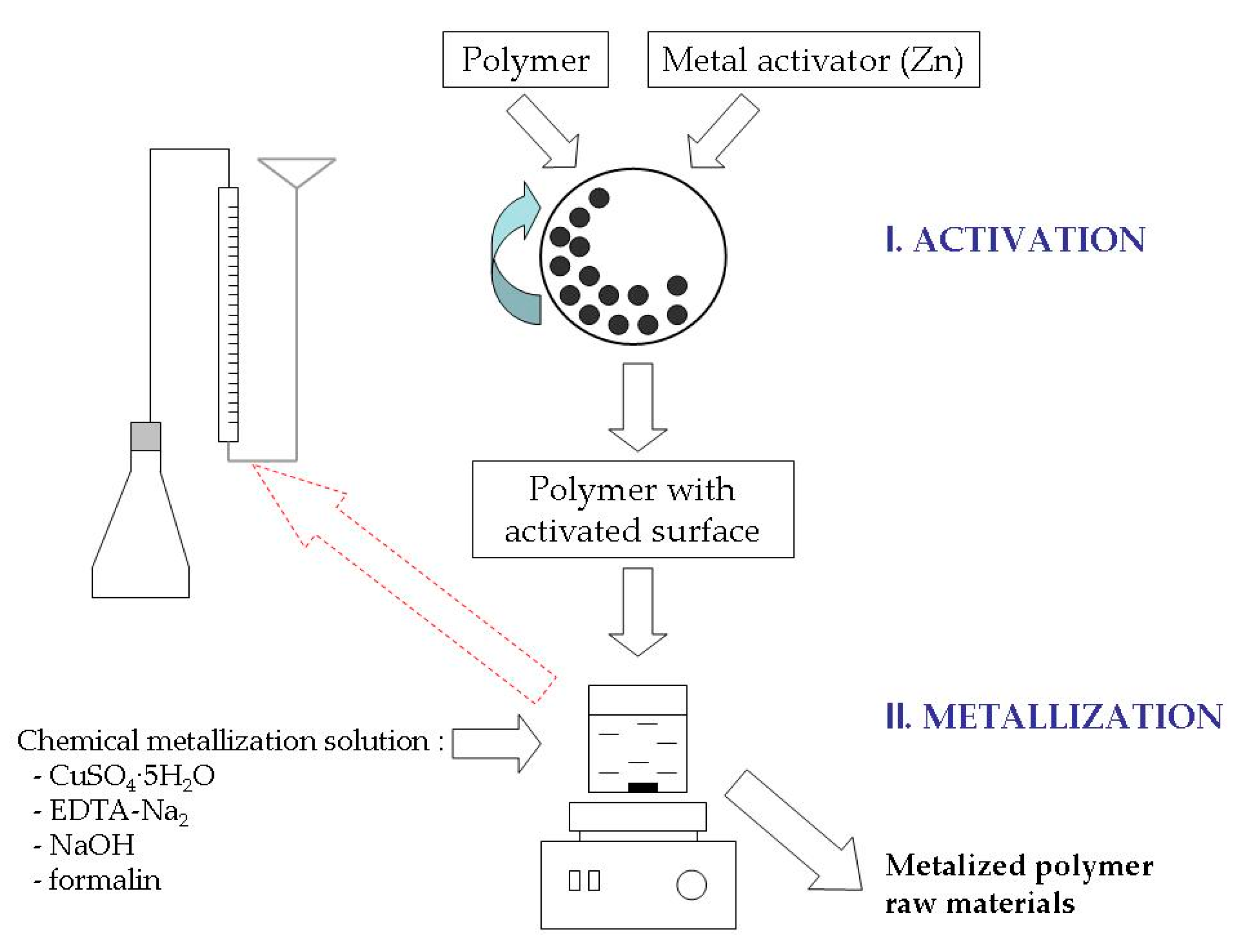
2.1. Materials and Obtaining of Metalized Polymeric Raw Materials
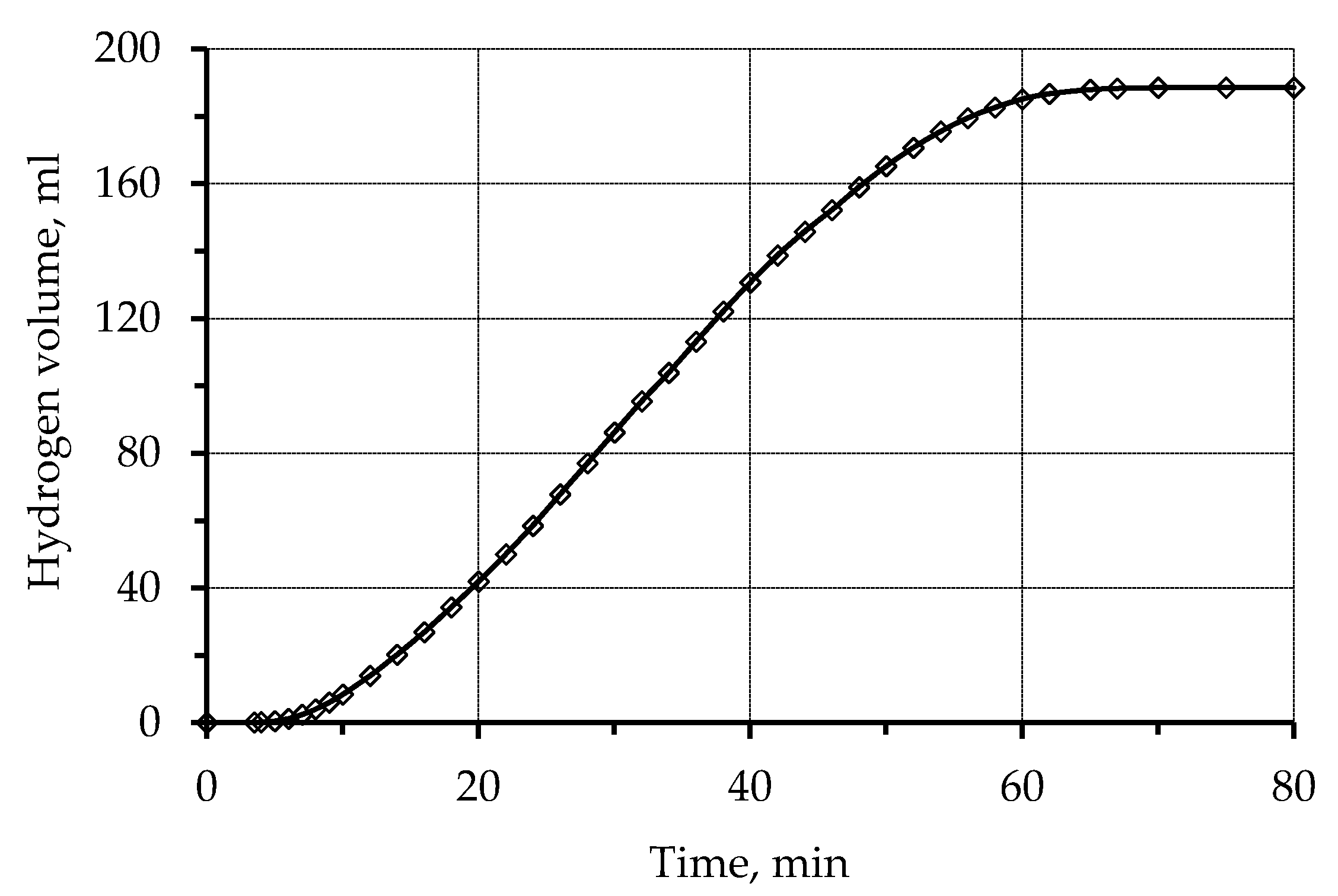
2.2. Kinetics of Metallization
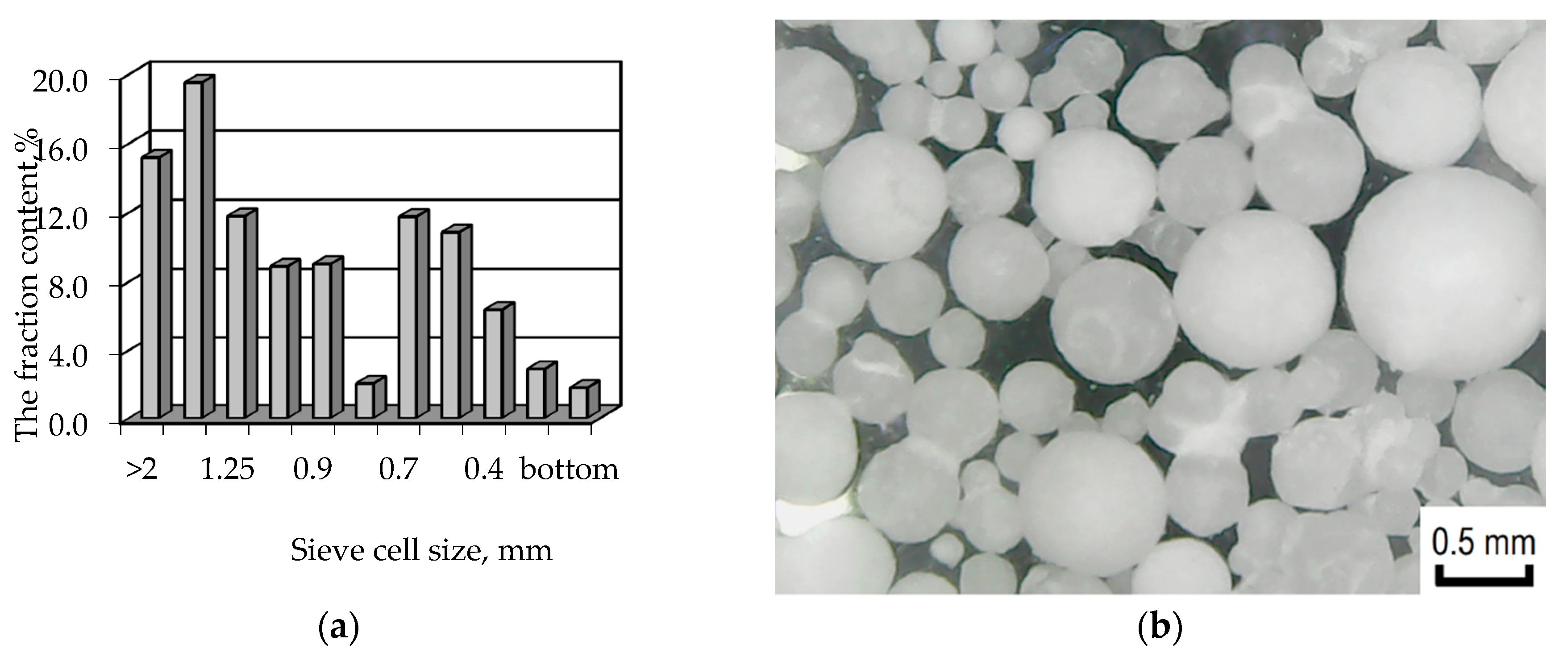
2.3. Sieve Analysis and Calculation of the Polymer Surface Area
2.4. Calculation of Metal Content and Metallization Efficiency
2.5. Obtaining a Metal-Filled Polymer Composite
2.6. Test Methods
3. Results and Discussion
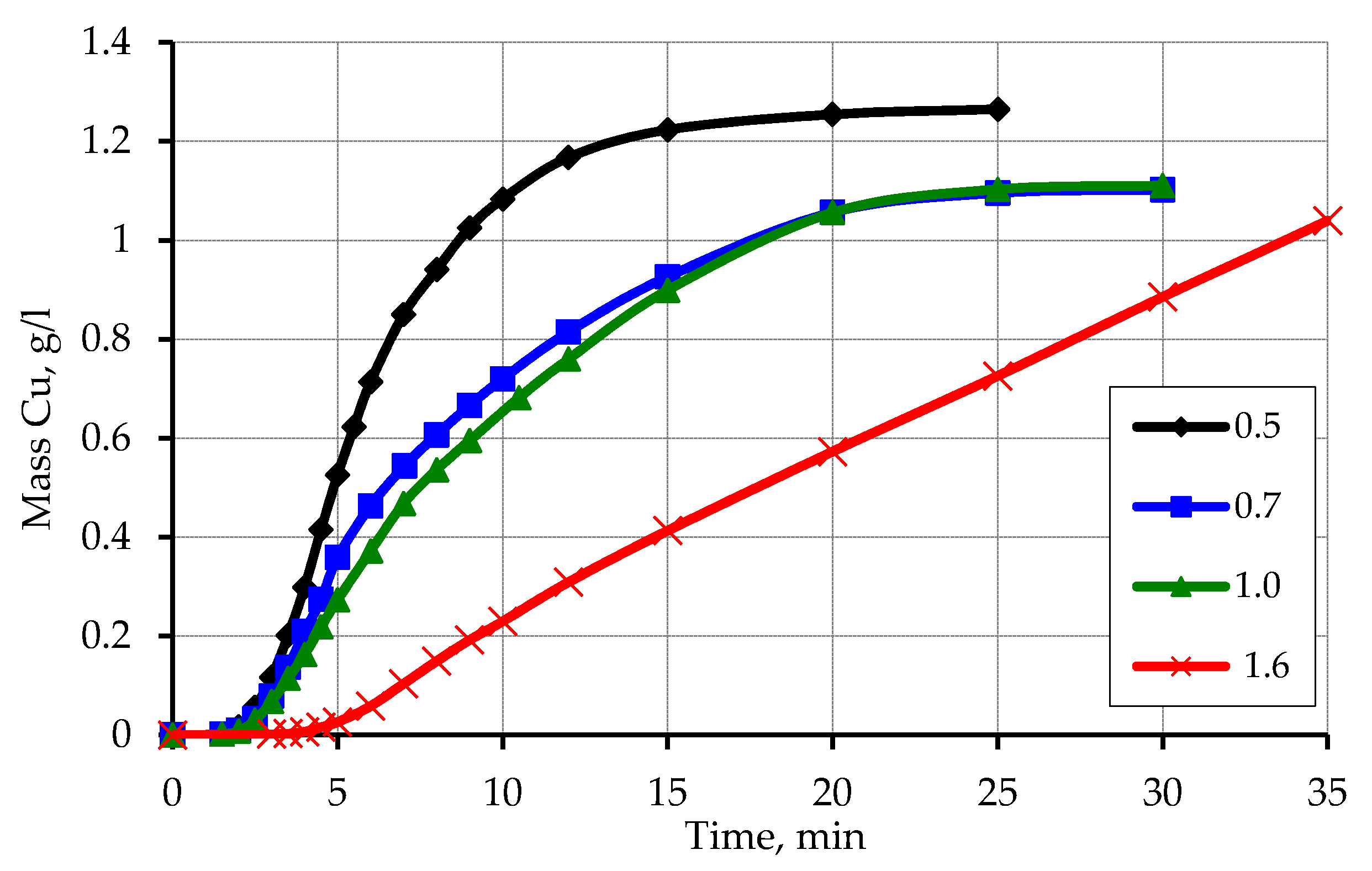
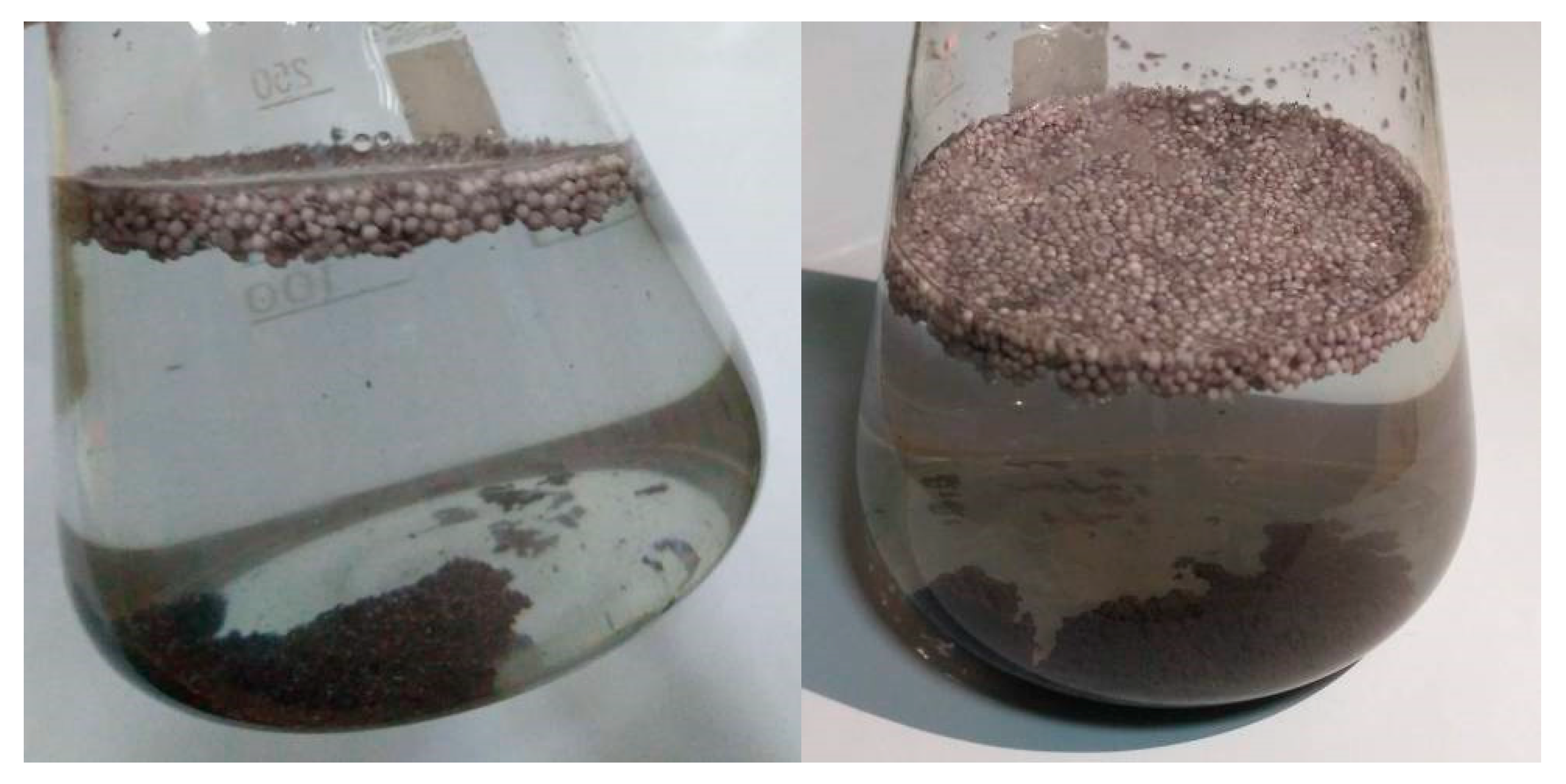
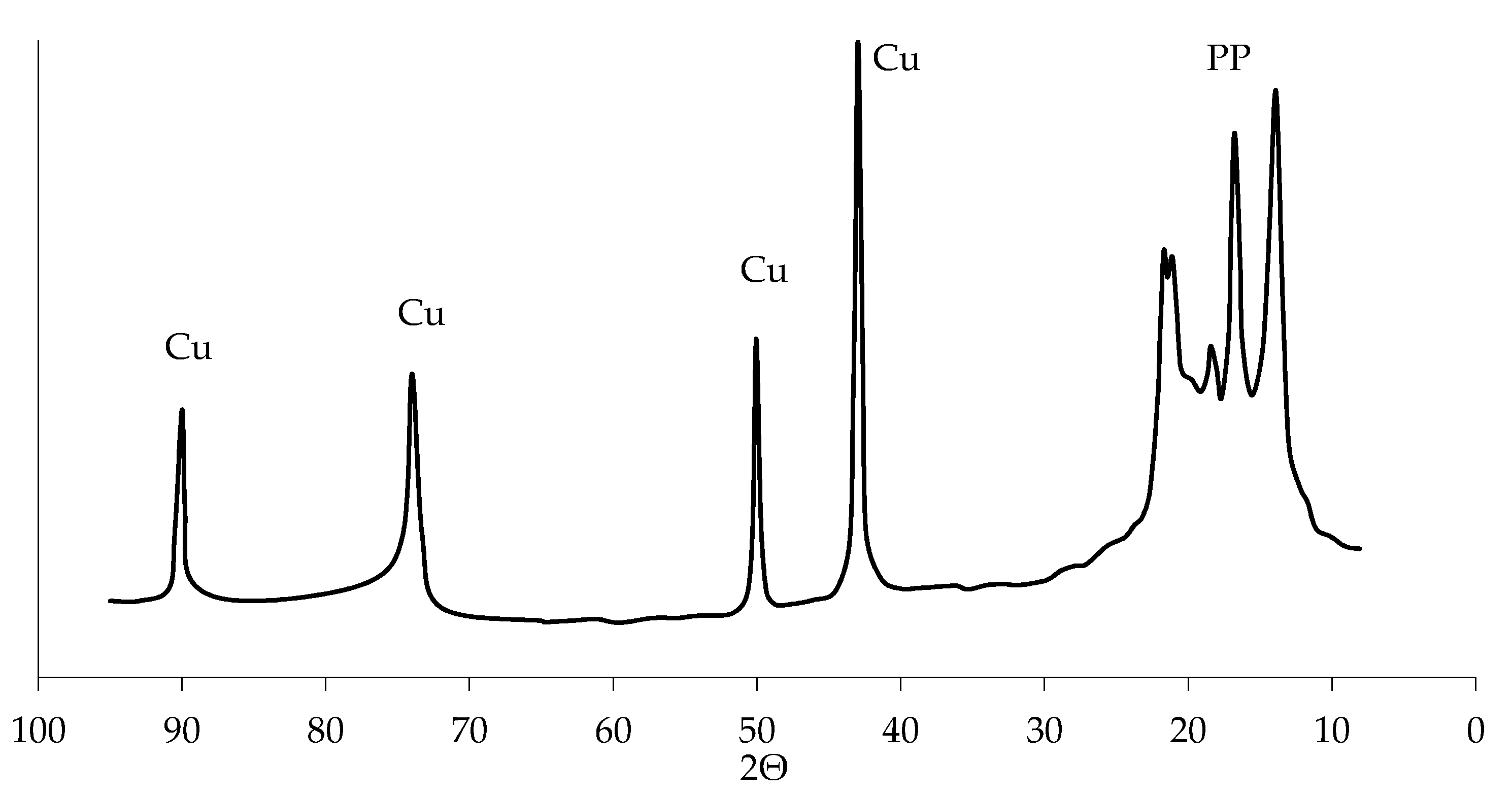
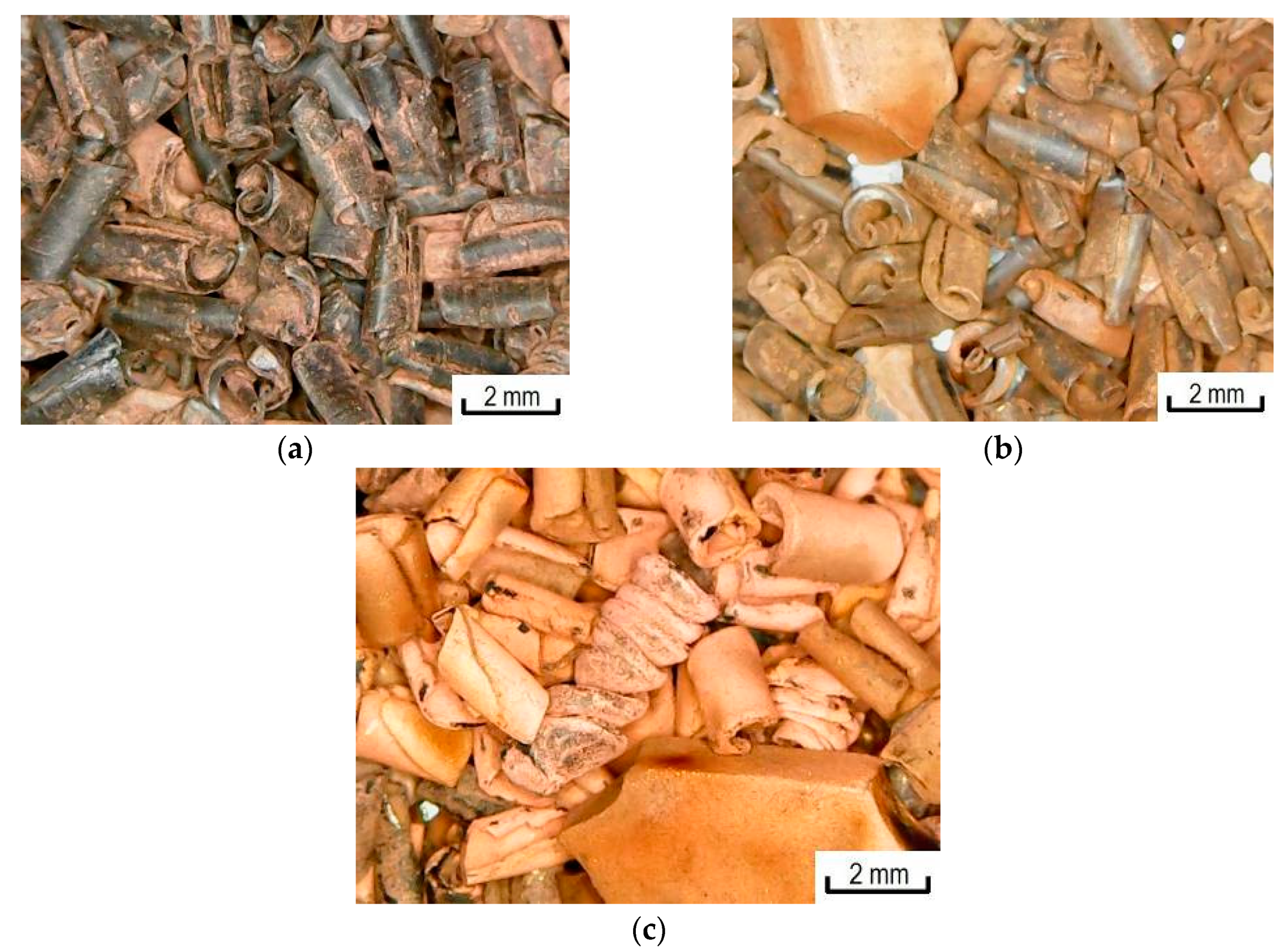
3.1. Metallization of Activated Polypropylene Waste
3.2. Sieve Analysis
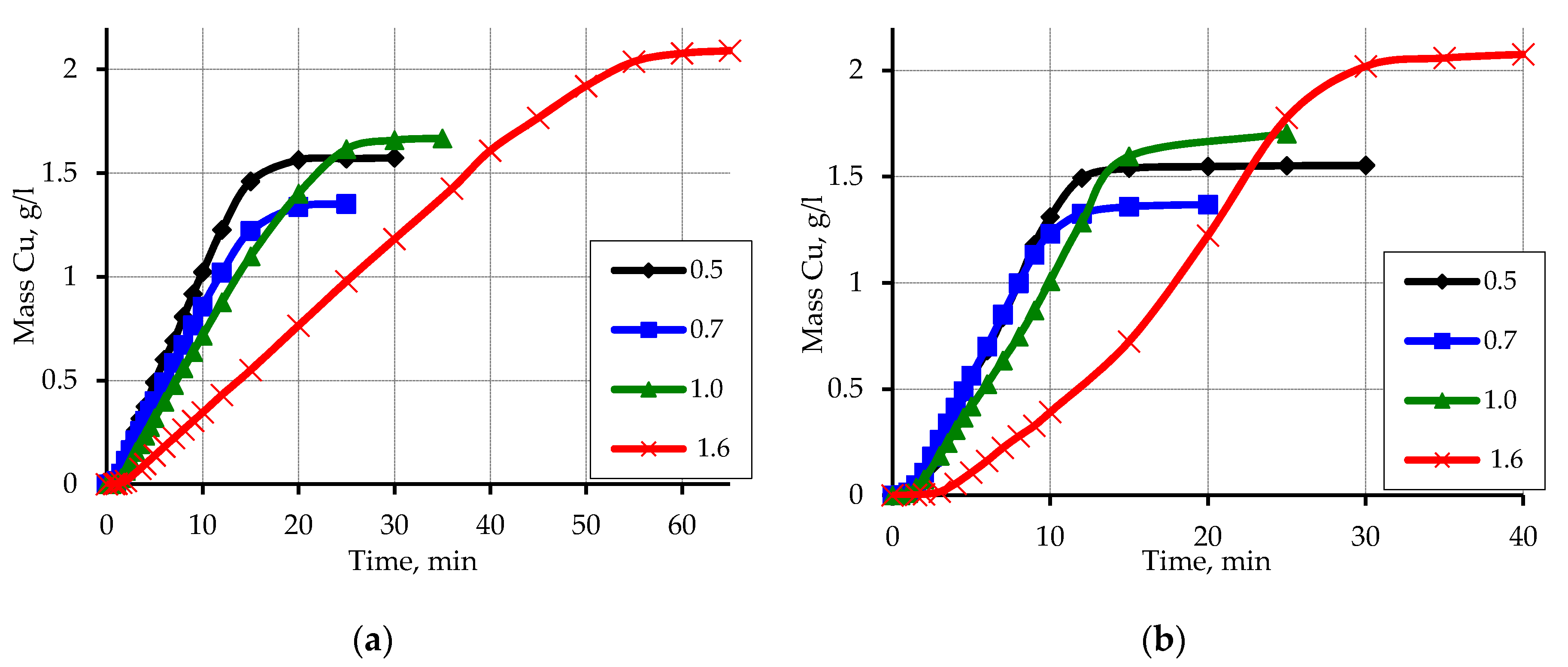
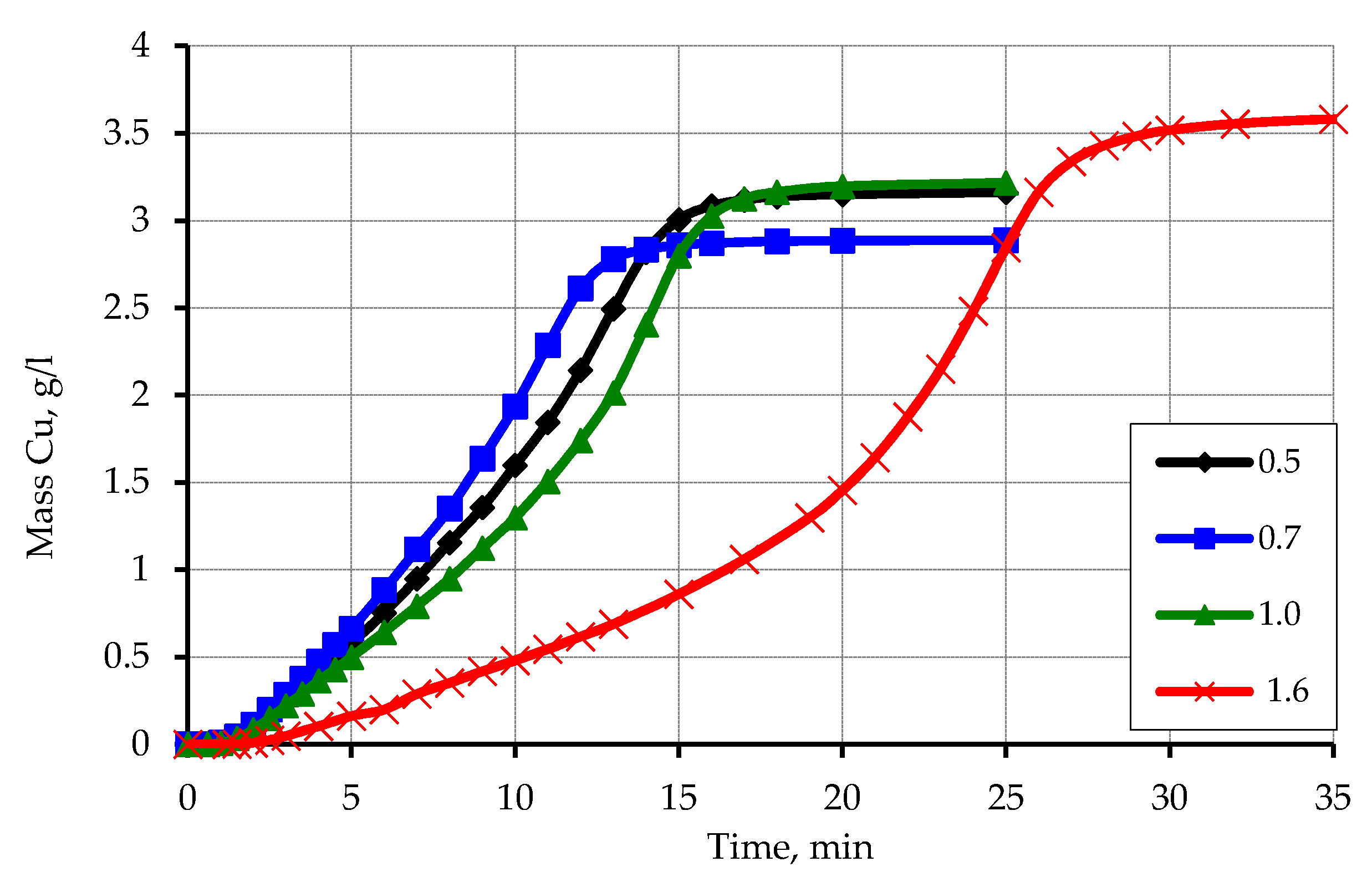
3.3. Metallization of Activated Polypropylene Brand Moplen HF501N
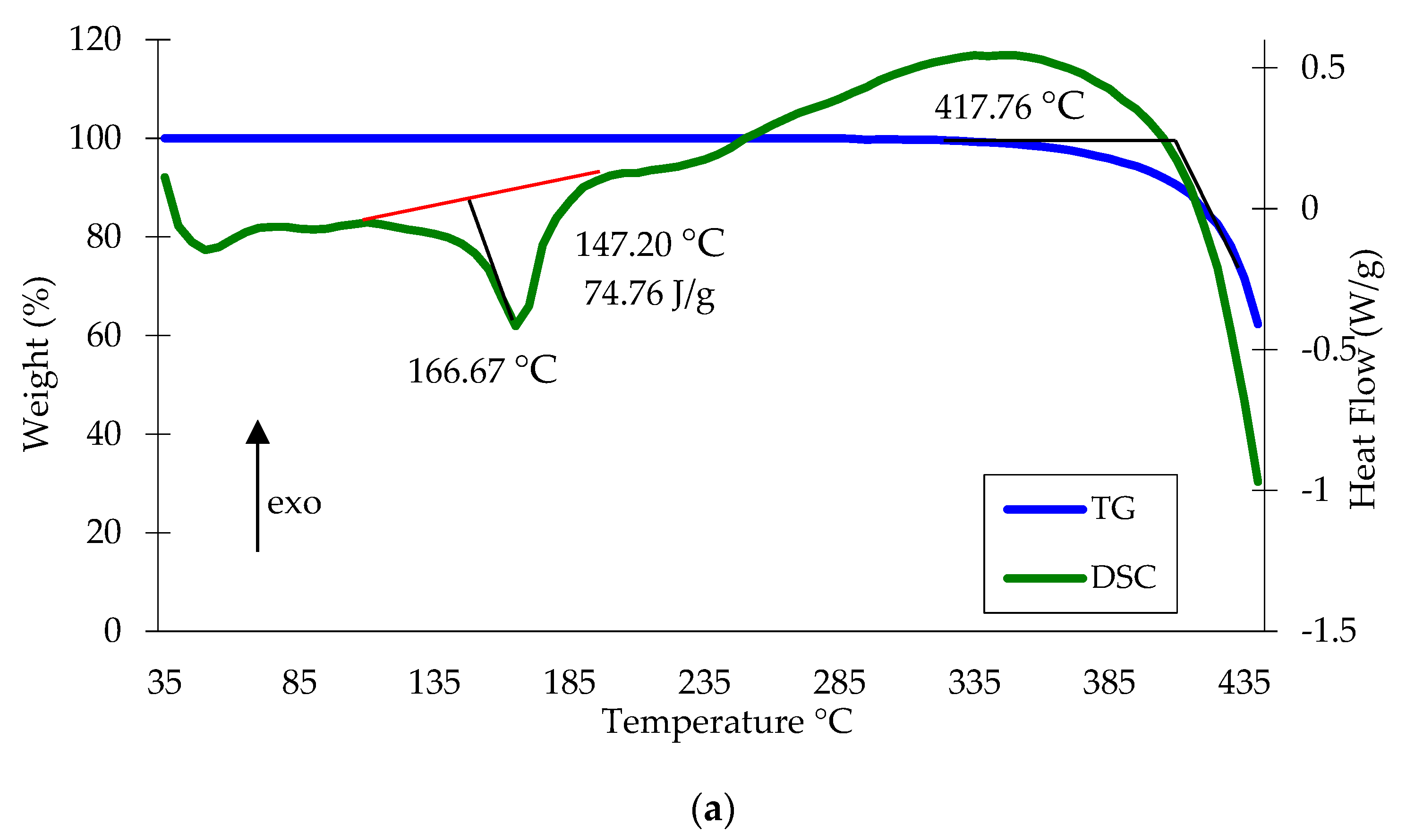
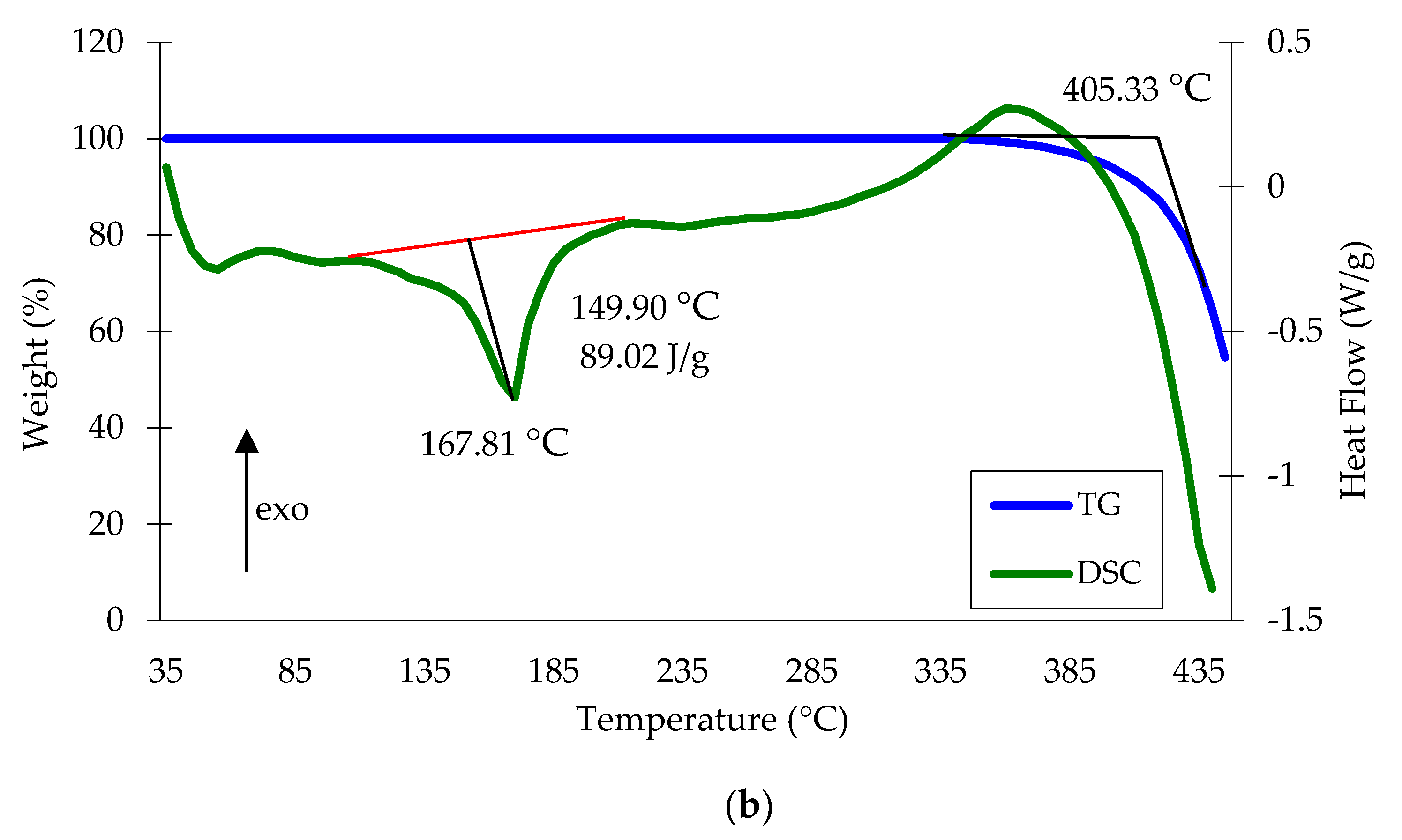
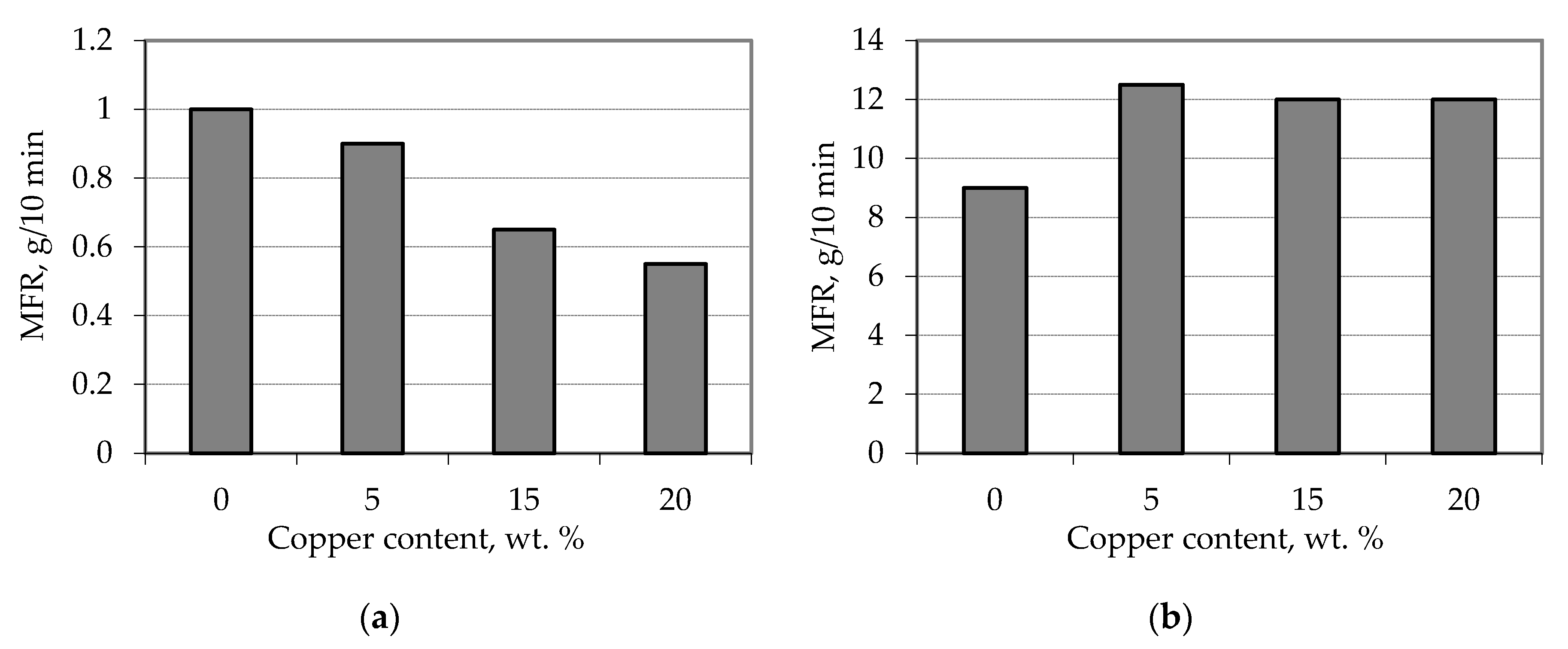
3.4. Properties of Copper waste Polypropylene
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zharin, D.E.; Selivanov, O.Y.; Gumerov, A.F. Constructional Metal-Filled Polymer Composites. Int. Polym. Sci. Technol. 2003, 30, 37–38. [Google Scholar] [CrossRef] [Green Version]
- Krasinskyi, V.; Suberlyak, O.; Dulebová, L.; Antoniuk, V. Nanocomposites on the Basis of Thermoplastics and Montmorillonite Modified by Polyvinylpyrrolidone. Key Eng. Mat. 2017, 756, 3–10. [Google Scholar] [CrossRef]
- Krasinskyi, V.; Kochubei, V.; Klym, Y.; Suberlyak, O. Thermogravimetric research into composites based on the mixtures of polypropylene and modified polyamide. East.-Eur. J. Enterp. Technol. 2017, 4, 44–50. [Google Scholar] [CrossRef]
- Haq, M.; Gang, Z. Ionic polymer–metal composite applications. Emerg. Mater. Resear. 2016, 5, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Grytsenko, O.; Gajdoš, I.; Spišák, E.; Krasinskyi, V.; Suberlyak, O. Novel Ni/pHEMA-gr-PVP Composites Obtained by Polymerization with Simultaneous Metal Deposition: Structure and Properties. Materials 2019, 12, 1956. [Google Scholar] [CrossRef] [Green Version]
- Bashtyk, Y.; Fechan, A.; Grytsenko, O.; Hotra, Z.; Kremer, I.; Suberlyak, O.; Aksimentyeva, O.; Horbenko, Y.; Kotsarenko, M. Electrical elements of the optical systems based on hydrogel-electrochromic polymer composites. Mol. Cryst. Liq. Cryst. 2018, 672, 150–158. [Google Scholar] [CrossRef]
- Grytsenko, O.; Spišák, E.; Dulebová, L.; Moravskii, V.; Suberlyak, O. Sorption Capable Film Coatings with Variable Conductivity. Mater. Sci. Forum. 2015, 818, 97–100. [Google Scholar] [CrossRef]
- Levytskyi, V.Y.; Masyuk, A.S.; Suberlyak, O.V. Preparation and properties of polymer-silicate composites based on hydrophilic polymers. Vopr. Khimii Khimicheskoi Tekhnol. 2017, 6, 68–74. [Google Scholar]
- Khatri, B.; Lappe, K.; Noetzel, D.; Pursche, K.; Hanemann, T. A 3D-Printable Polymer-Metal Soft-Magnetic Functional Composite-Development and Characterization. Materials 2018, 11, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaman, K.; Taga, O. Thermal and Electrical Conductivity of Unsaturated Polyester Resin Filled with Copper Filler Composites. Intern. J. Polym. Sci. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Levyts’kyi, V.E.; Katruk, D.S.; Kochubei, V.V.; Humenets’kyi, T.V.; Bilyi, L.M.; Masyuk, A. Influence of Polyvinylchloride on the Chemical and Thermal Resistance of Highly Filled Polyester Composites. Mater. Sci. 2017, 53, 385–391. [Google Scholar] [CrossRef]
- Levyts’kyi, V.E.; Masyuk, A.S.; Samoilyuk, D.S.; Bilyi, L.M.; Humenets’kyi, T.V. Morphology and Properties of Polymer–Silicate Composites. Mater. Sci. 2016, 52, 17–24. [Google Scholar] [CrossRef]
- Pavlovic, M.M.; Janković, Z.; Nikolić, N.D. Novel Biodegradable Composites Based on Lignocellulose and Electrodeposited Copper Powders. In Metals and Metal-Based Electrocatalytic Materials for Alternative Energy Sources and Electronics; Stevanovic, J., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2018; pp. 199–240. [Google Scholar]
- Jankovic, Z.; Pavlovic, M.M.; Pantovic Pavlovic, M.R.; Pavlovic, M.G.; Nikolic, N.D.; Stevanovic, J.S.; Prsic, S. Electrical and Thermal Properties of Poly(methylmetacrylate) Composites Filled With Electrolytic Copper Powder. Int. J. Electrochem. Sci. 2018, 13, 45–57. [Google Scholar] [CrossRef]
- Krupa, I.; Cecen, V.; Boudenne, A.; Prokeš, J.; Novák, I. The mechanical and adhesive properties of electrically and thermally conductive polymeric composites based on high density polyethylene filled with nickel powder. Mater. Des. 2013, 51, 620–628. [Google Scholar] [CrossRef]
- Levytskyi, V.; Masyuk, A.; Katruk, D.; Bratychak, M. Regularities of obtaining, morphology and properties of metal-containing polymer-silicate materials and polyester composites on their basis. Chem. Chem. Technol. 2016, 10, 35–40. [Google Scholar] [CrossRef]
- Sikora, J.W.; Gajdoš, I.; Puszka, A. Polyethylene-Matrix Composites with Halloysite Nanotubes with Enhanced Physical/Thermal Properties. Polymers 2019, 11, 787. [Google Scholar] [CrossRef] [Green Version]
- Krasinskyi, V.; Moravskyi, V.; Suberlyak, O.; Sikora, J.W.; Jachowicz, T. Effect of nature and amount of polypropylene composite Filler on the productivity of extruder with the cylinder equipped with grooves. Adv. Sci. Technol. Res. J. 2019, 13, 264–269. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G. Utilization of Recycled Polystyrene and Aluminum Wastes in the Development of Conductive Plastic Composites: Evaluation of Electrical Properties. In Handbook of Environmental Materials Management; Hussain, C., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–9. [Google Scholar] [CrossRef]
- Abdulkareem, S.; Adeniyi, A.G. Recycling Copper and Polystyrene from Solid Waste Stream in Developing Conductive Composites. J. Solid Waste Tech. Manag. 2019, 45, 39–44. [Google Scholar] [CrossRef]
- Pargi, M.N.F.; Teh, P.L.; Hussiensyah, S.; Yeoh, C.K.; Abd Ghani, S. Recycled-copper-filled epoxy composites: The effect of mixed particle size. Int. J. Mech. Mater. Eng. 2015, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Ji, K.; Xu, Y.; Zhang, J.; Chen, J.; Dai, Z. Foamed-metal-reinforced composites: Tribological behavior of foamed copper filled with epoxy-matrix polymer. Mater. Des. 2014, 61, 109–116. [Google Scholar] [CrossRef]
- Misiura, A.I.; Mamunya, Y.P.; Kulish, M.P. Metal-Filled Epoxy Composites: Mechanical Properties and Electrical/Thermal Conductivity. J. Macrom. Sci. B 2019, 59, 121–136. [Google Scholar] [CrossRef]
- Zeng, J.L.; Sun, L.X.; Xu, F.; Tan, Z.C.; Zhang, Z.H.; Zhang, J.; Zhang, T. Study of a PCM based energy storage system containing Ag nanoparticles. J. Therm. Anal. Calorim. 2007, 87, 371–375. [Google Scholar] [CrossRef]
- Zayed, M.E.; Zhao, J.; Li, W.; Elsheikh, A.H.; Elbanna, A.M.; Jing, L.; Geweda, A.E. Recent progress in phase change materials storage containers: Geometries, design considerations and heat transfer improvement methods. J. Energy Storage 2020, 30, 101341. [Google Scholar] [CrossRef]
- Navarro, L.; Barreneche, C.; Castell, A.; Redpath, D.A.G.; Griffiths, P.W.; Cabeza, L.F. High density polyethylene spheres with PCM for domestic hot water applications: Water tank and laboratory scale study. J. Energy Storage 2017, 13, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Noël, J.A.; Kahwaji, S.; Desgrosseilliers, L.; Groulx, D.; White, M.A. Phase Change Materials. In Storing Energy: With Special Reference to Renewable Energy Sources; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 249–272. [Google Scholar]
- Bechiri, M.; Mansouri, K.; Saleem, S. Study of heat sink effects during melting of constrained phase change material inside a spherical enclosure. J. Energy Storage 2020, 27, 101151. [Google Scholar] [CrossRef]
- Garnier, B.; Agoudjil, B.; Boudenne, A. Metallic Particle-Filled Polymer Microcomposites. In Polymer Composites, 1st ed.; Thomas, S., Joseph, K., Malhotra, S.K., Goda, K., Sreekala, M.S., Eds.; Wiley-VCH Verlag GmbH & Co: Hoboken, NJ, USA, 2012; pp. 575–612. [Google Scholar] [CrossRef]
- Osman, A.F.; Mariatti, M. Properties of Aluminum Filled Polypropylene Composites. Polym. Polym. Comp. 2006, 14, 623–634. [Google Scholar] [CrossRef]
- Moravskyi, V.; Dziaman, I.; Suberliak, S.; Kuznetsova, M.; Tsimbalista, T.; Dulebova, L. Research into kinetic patterns of chemical metallization of powder-like polyvinylchloride. East.-Eur. J. Enterp. Technol. 2017, 4, 50–57. [Google Scholar] [CrossRef]
- Moravskyi, V.; Dziaman, I.; Suberliak, S.; Grytsenko, O.; Kuznetsova, M. Features of the production of metal-filled composites by metallization of polymeric raw materials. In Proceedings of the 7th International Conference Nanomaterials: Application & Properties (NAP), Zatoka, Ukraine, 10–15 September 2017; IEEE: Odessa, Ukraine, 2017. [Google Scholar] [CrossRef]
- Moravskyi, V.; Kucherenko, A.; Kuznetsova, M.; Dziaman, I.; Grytsenko, O.; Dulebova, L. Studying the effect of concentration factors on the process of chemical metallization of powdered polyvinylchloride. East.-Eur. J. Enterp. Technol. 2018, 3, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Kucherenko, A.; Moravskyi, V.; Kuznetsova, M.; Grytsenko, O.; Masyuk, A.; Dulebova, L. Regularities of Obtaining Metal-filled Polymer Composites. In Nanomaterials in Biomedical Application and Biosensors; Pogrebnjak, A., Pogorielov, M., Viter, R., Eds.; Springer Nature Singapore: Singapore, 2020; pp. 59–66. [Google Scholar] [CrossRef]
- Shalkauskas, M.; Vashkyalis, A. Chemical Metallization of Plastics; Khimiya: Leningrad, Russia, 1985. (In Russian) [Google Scholar]
- Moravskyi, W.; Dziaman, I.; Baran, N.; Kucherenko, A.; Dulebova, L. Activation efficiency study of powdered polyvinyl chloride. Bull. Lviv Nat. Polyt. Univ. 2017, 868, 413–418. (In Ukrainian) [Google Scholar]


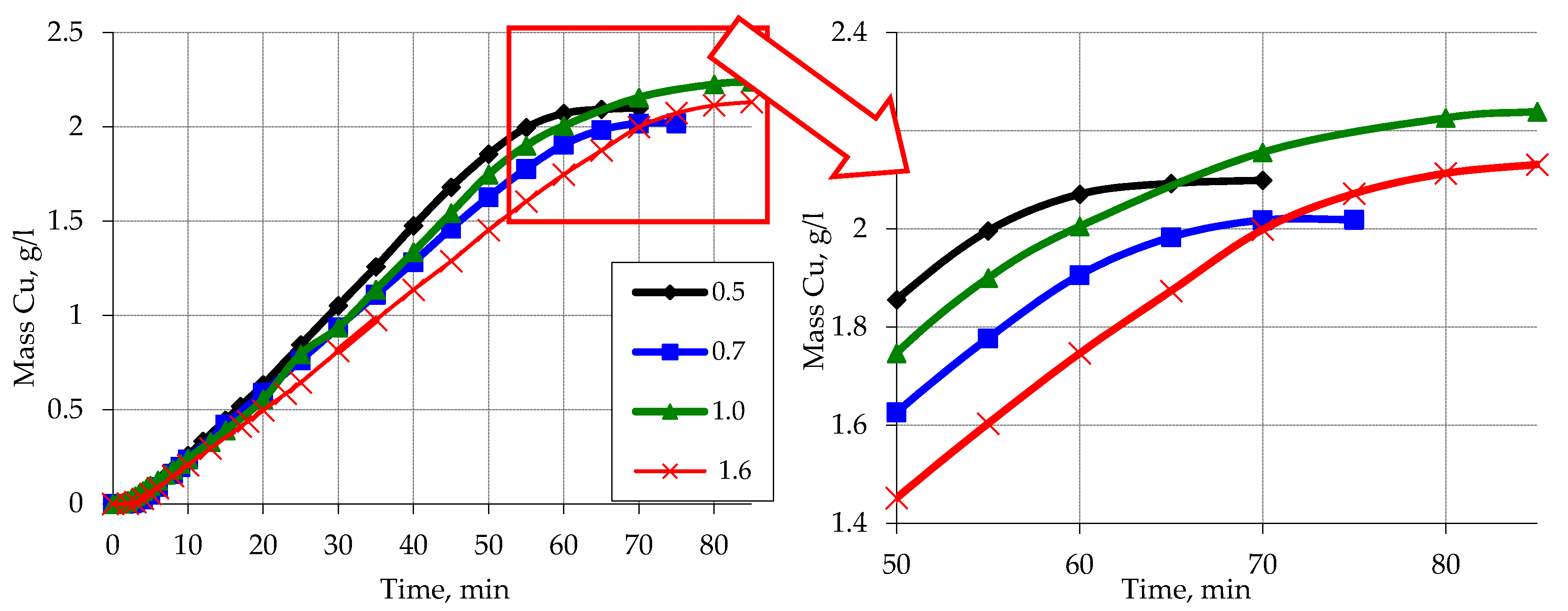
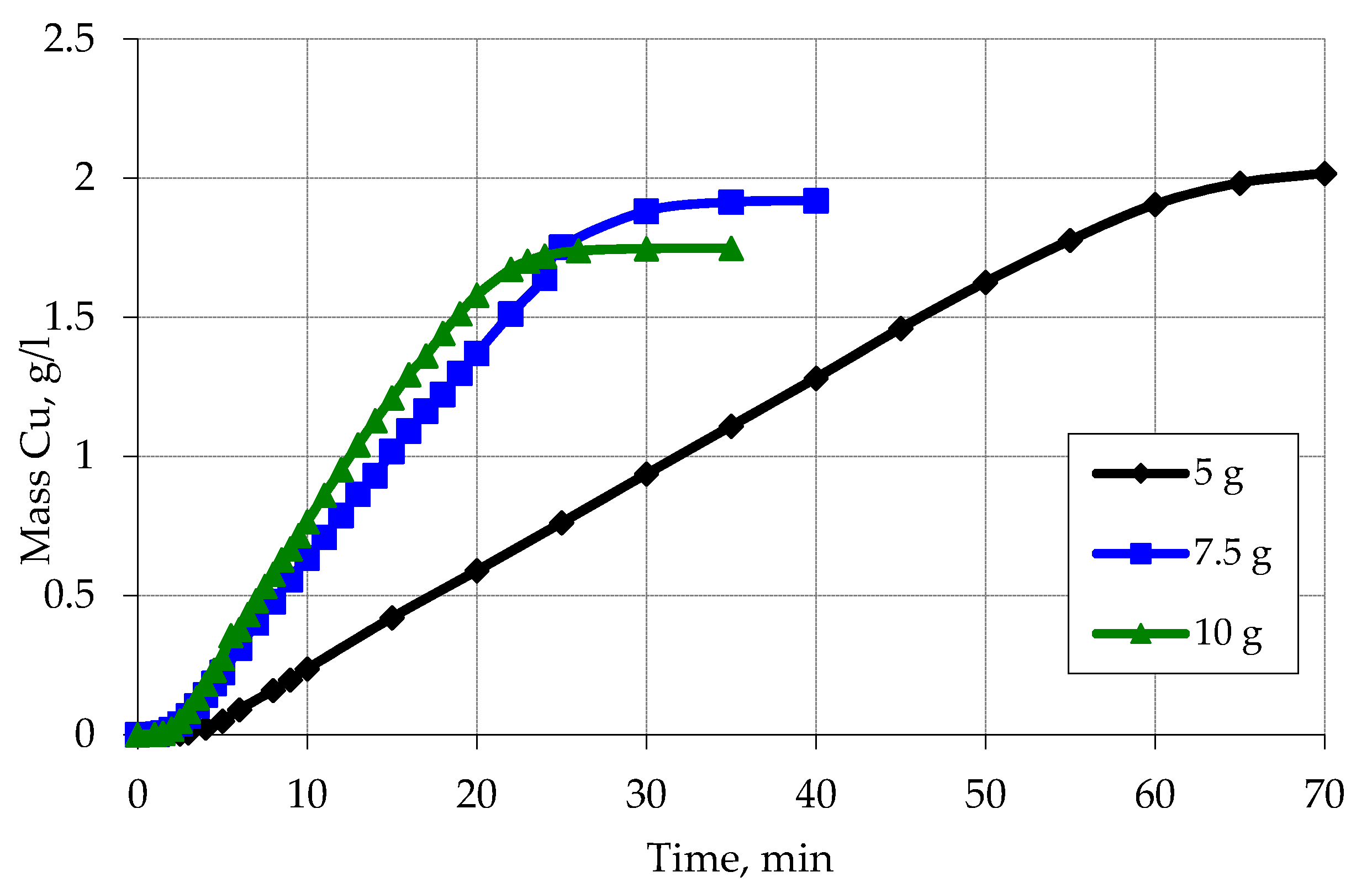
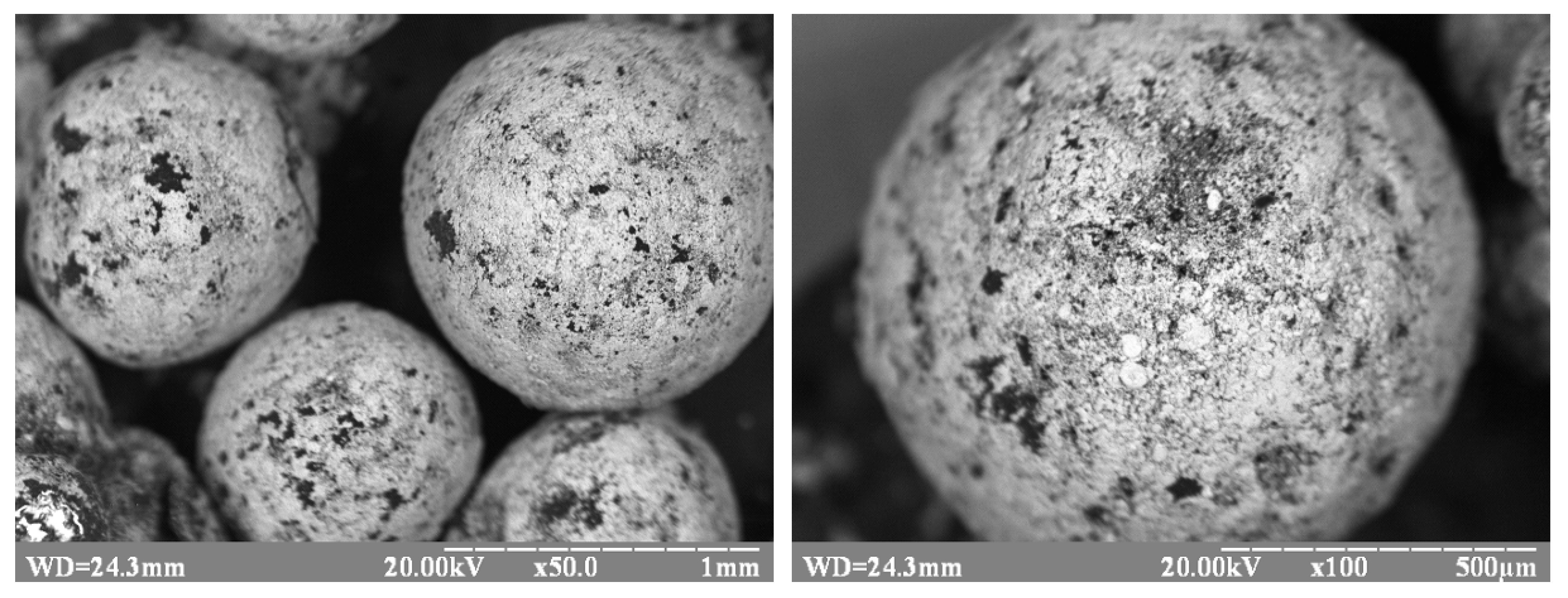
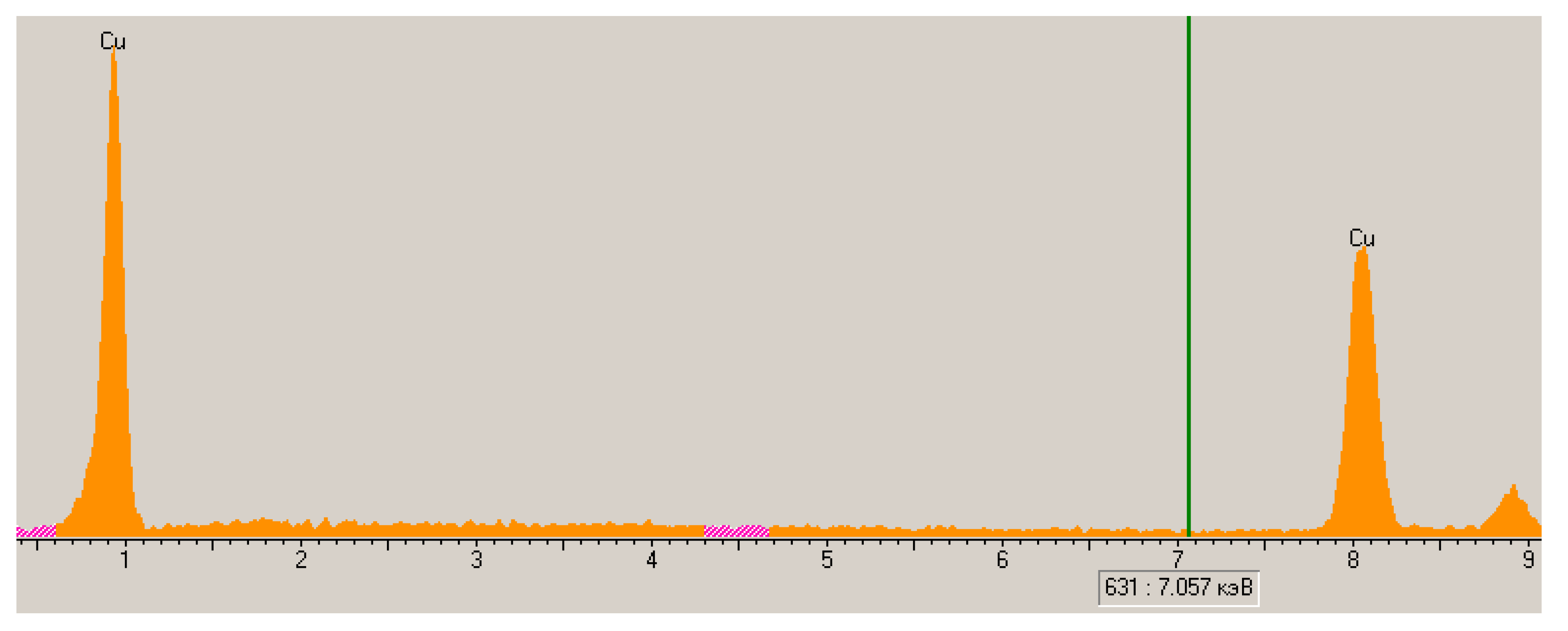

| Sieve Cell Size (mm) | The Average Particle Diameter of the PP on the Sieve (mm) | The Surface Area of Polypropylene (cm2) |
|---|---|---|
| 1.6 | 1.8 | 185 |
| 1.0 | 1.125 | 290 |
| 0.7 | 0.75 | 440 |
| 0.5 | 0.6 | 550 |
| PP Fraction on a Sieve | Concentration (mol/L) | Metallization Efficiency (%) | |
|---|---|---|---|
| NaOH | CuSO4 | ||
| 0.7 | 0.25 | 48 | 27.0 |
| 0.7 | 0.56 | 48 | 11.5 |
| 0.7 | 0.56 | 80 | 34.8 |
| 0.5 | 1.68 | 144 | 35.8 |
| PP Fraction on a Sieve | 0.5 | 1.0 | 1.6 |
|---|---|---|---|
| The content of Zn (wt.%) | Activated polypropylene | ||
| 6.6 | 5.4 | 1.8 | |
| Washed activated polypropylene | |||
| 0.9 | 0.5 | 0.6 | |
| PP Fraction on a Sieve | Concentration (mol/L) | Metallization Efficiency (%) | |
|---|---|---|---|
| NaOH | CuSO4 | ||
| 0.7 | 0.56 | 48 | 98.2 |
| The Copper Content (% mass) | Ultimate Tensile Strength (MPa) | Strain at Strength (%) | Tensile Stress at Break (MPa) | Strain at Break (%) |
|---|---|---|---|---|
| 0 | 57.5 | 30 | 51.4 | 37 |
| 5 | 57.2 | 30 | 48.7 | 35 |
| 15 | 57.5 | 28 | 55.1 | 32 |
| 20 | - | - | 55.0 | 32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moravskyi, V.; Kucherenko, A.; Kuznetsova, M.; Dulebova, L.; Spišák, E.; Majerníková, J. Utilization of Polypropylene in the Production of Metal-Filled Polymer Composites: Development and Characteristics. Materials 2020, 13, 2856. https://doi.org/10.3390/ma13122856
Moravskyi V, Kucherenko A, Kuznetsova M, Dulebova L, Spišák E, Majerníková J. Utilization of Polypropylene in the Production of Metal-Filled Polymer Composites: Development and Characteristics. Materials. 2020; 13(12):2856. https://doi.org/10.3390/ma13122856
Chicago/Turabian StyleMoravskyi, Volodymyr, Anastasiia Kucherenko, Marta Kuznetsova, Ludmila Dulebova, Emil Spišák, and Janka Majerníková. 2020. "Utilization of Polypropylene in the Production of Metal-Filled Polymer Composites: Development and Characteristics" Materials 13, no. 12: 2856. https://doi.org/10.3390/ma13122856
APA StyleMoravskyi, V., Kucherenko, A., Kuznetsova, M., Dulebova, L., Spišák, E., & Majerníková, J. (2020). Utilization of Polypropylene in the Production of Metal-Filled Polymer Composites: Development and Characteristics. Materials, 13(12), 2856. https://doi.org/10.3390/ma13122856






