Crossover from Ferroelectric to Relaxor Behavior in Ba1−xCaxTiO3 (x = 0.17) System
Abstract
1. Introduction
2. Materials and Methods
2.1. Ceramics Preparation
2.2. XRD (X-Ray Diffraction) Research
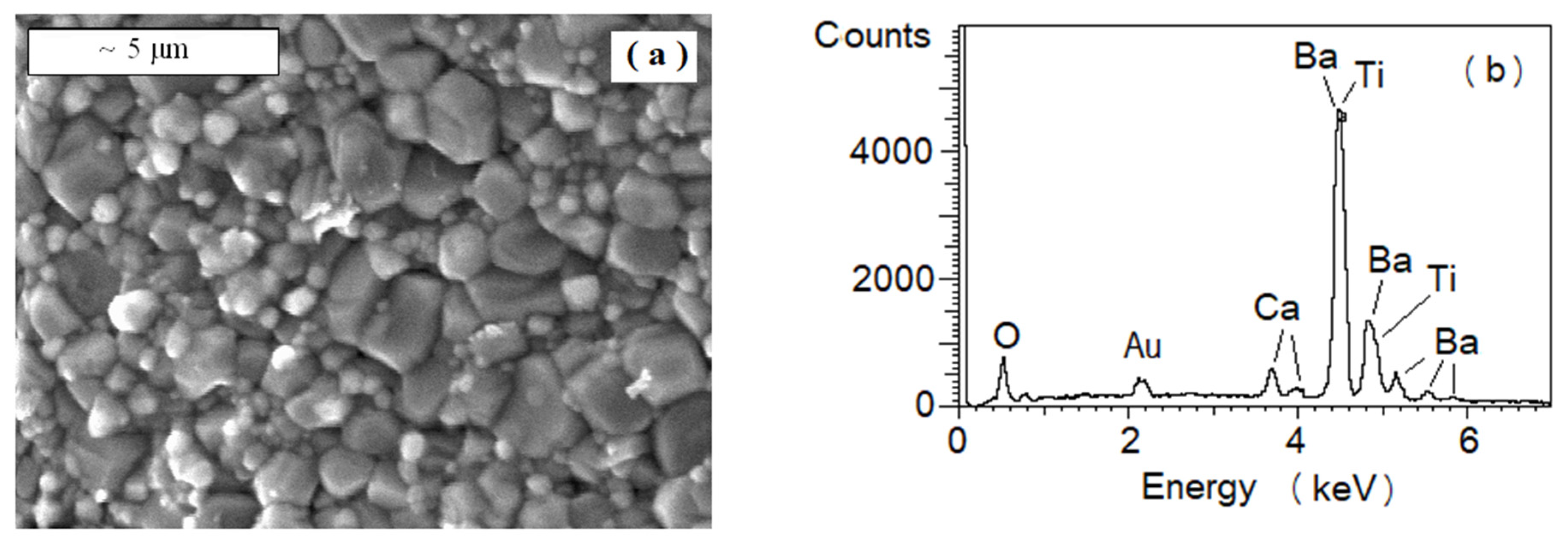
2.3. Microanalysis
2.4. BDS (Broadband Dielectric Spectroscopy)
3. Results
3.1. XRD
3.2. SEM (Scanning Electron Microscope)
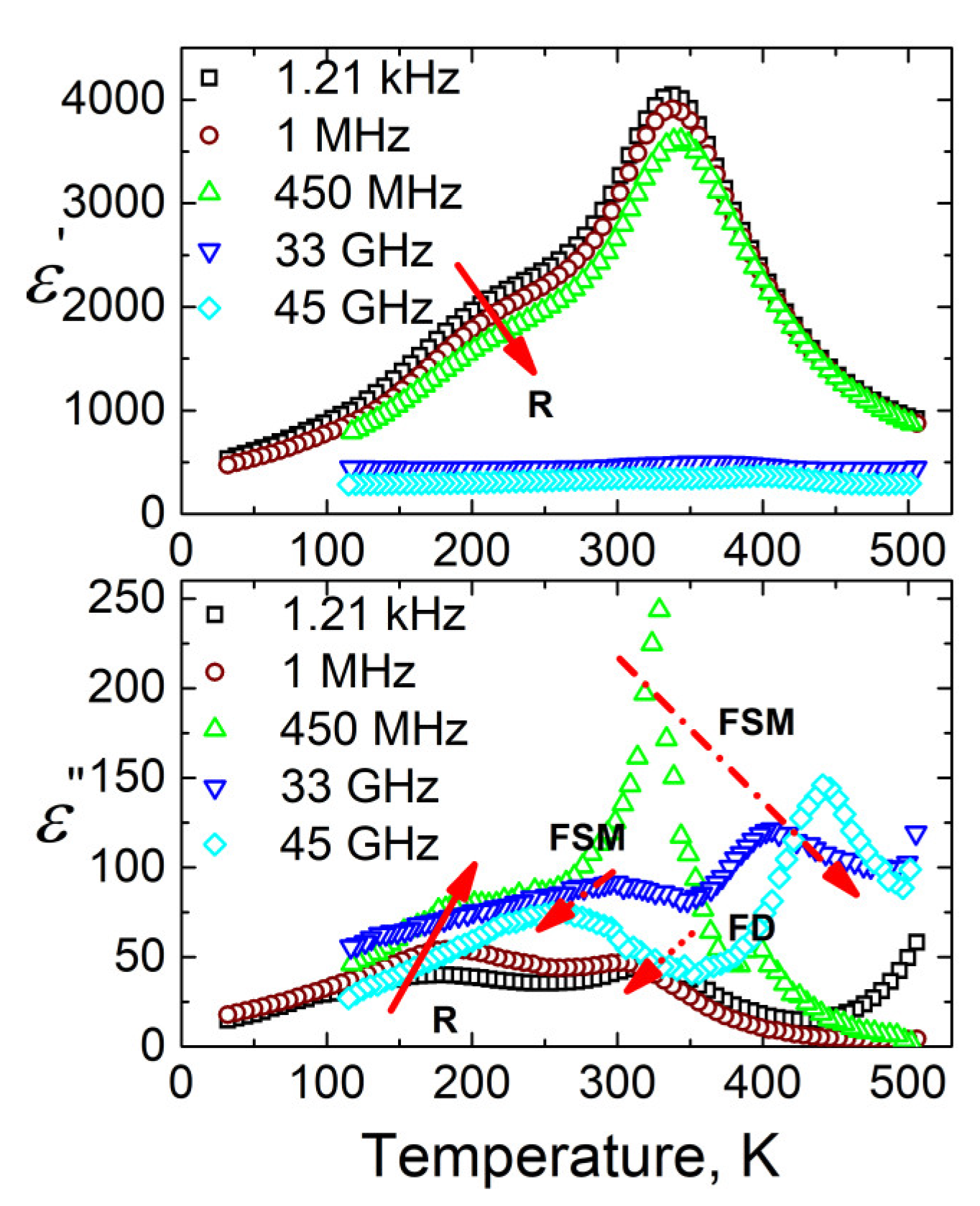
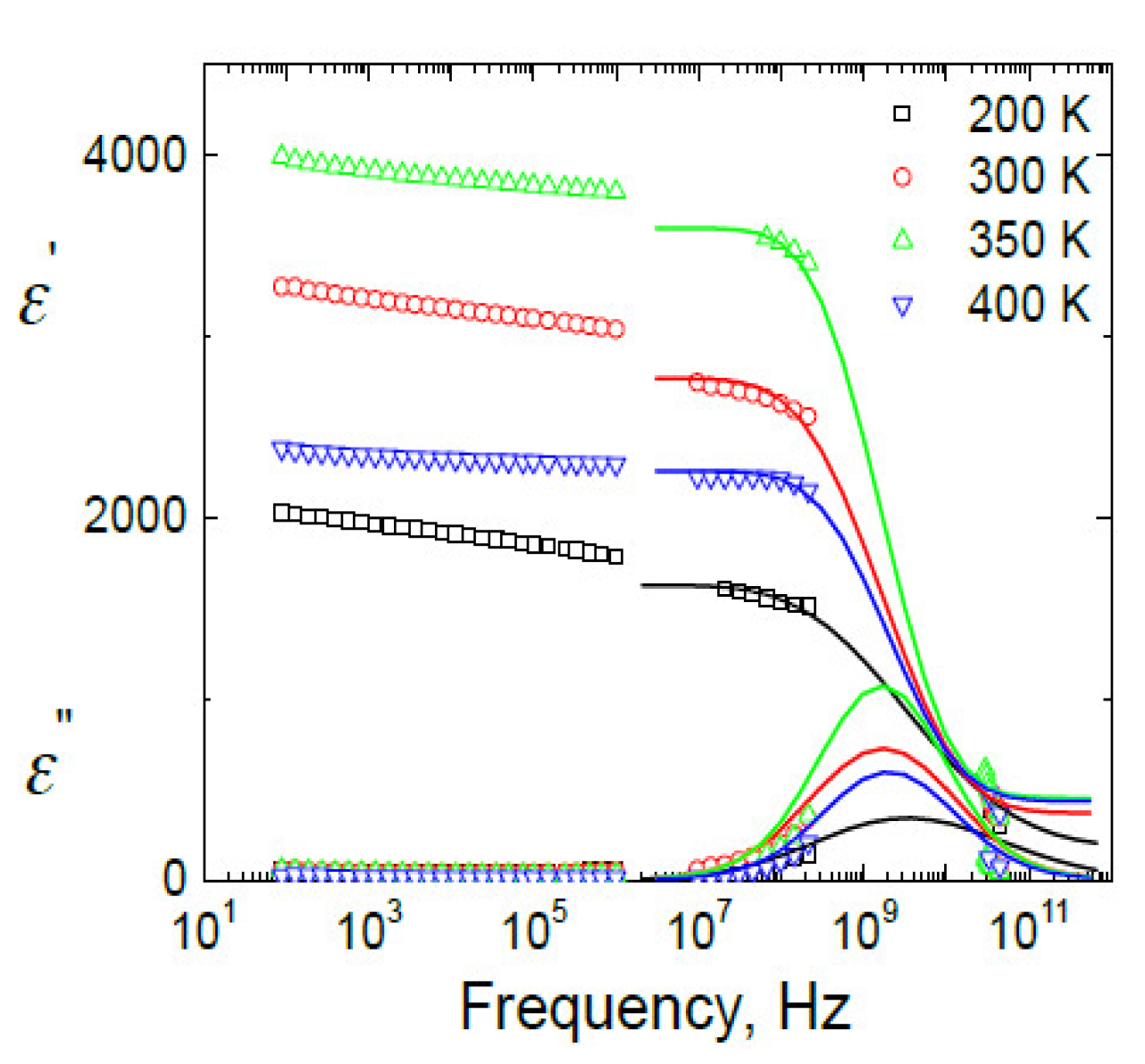
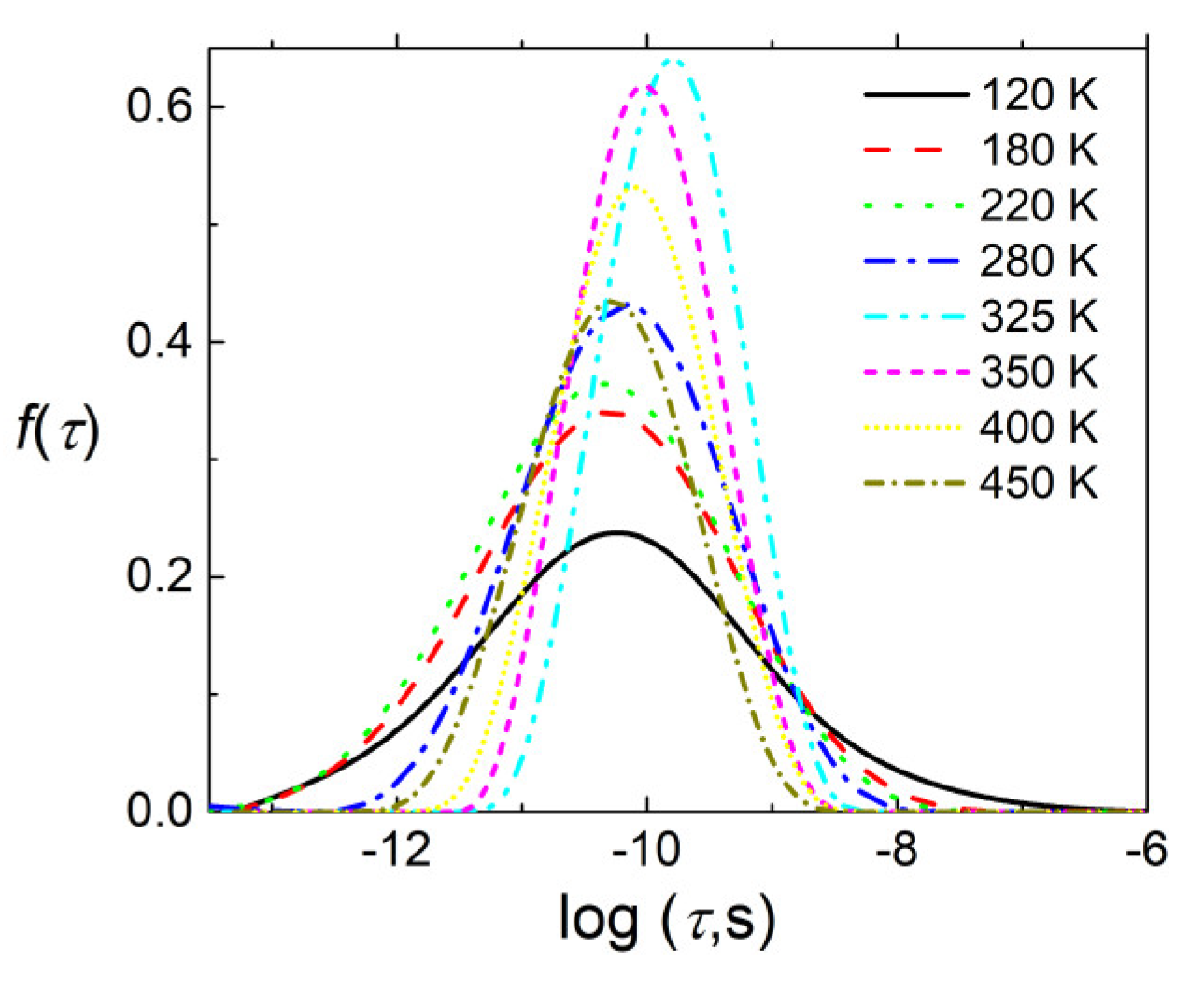
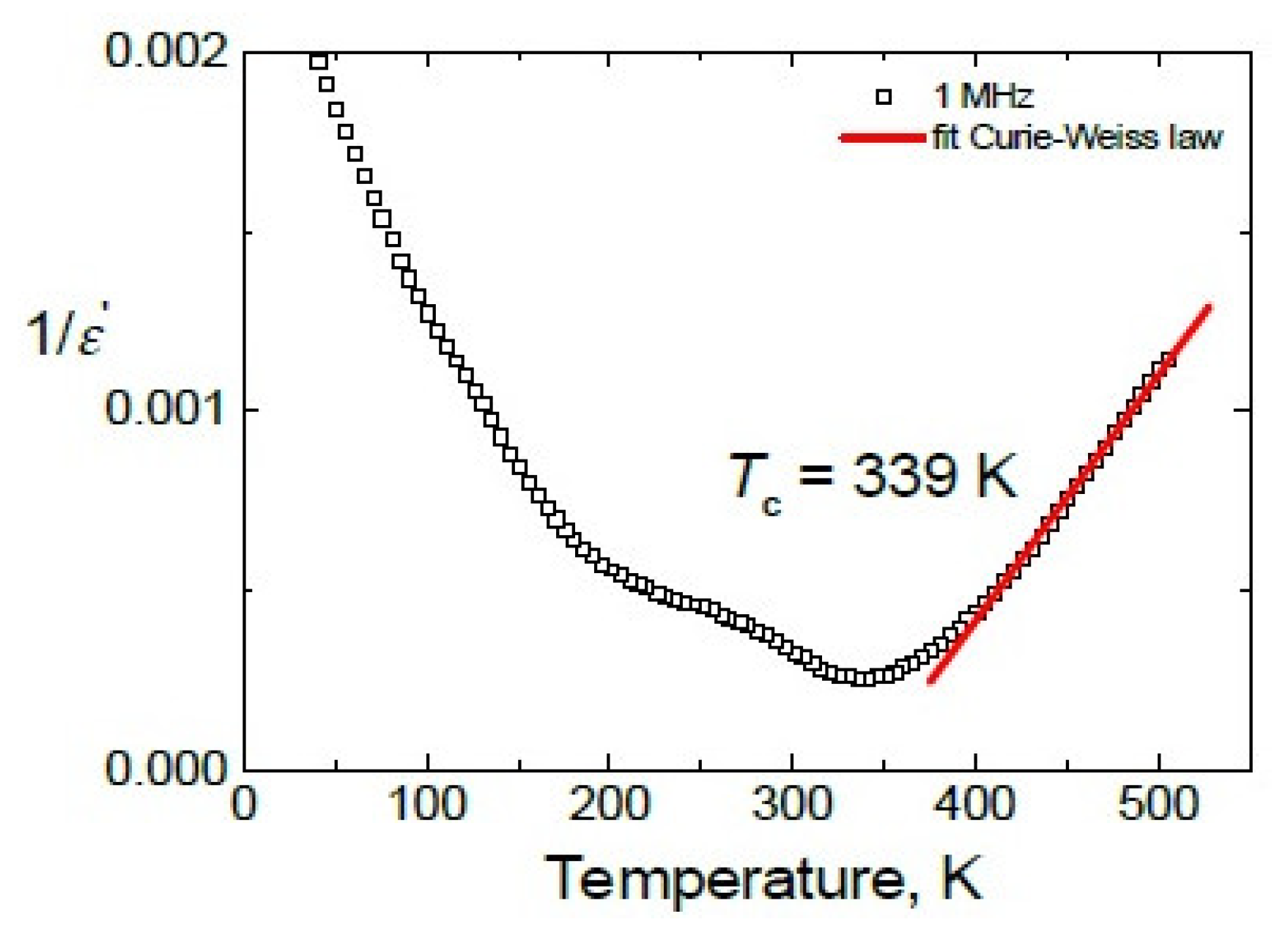
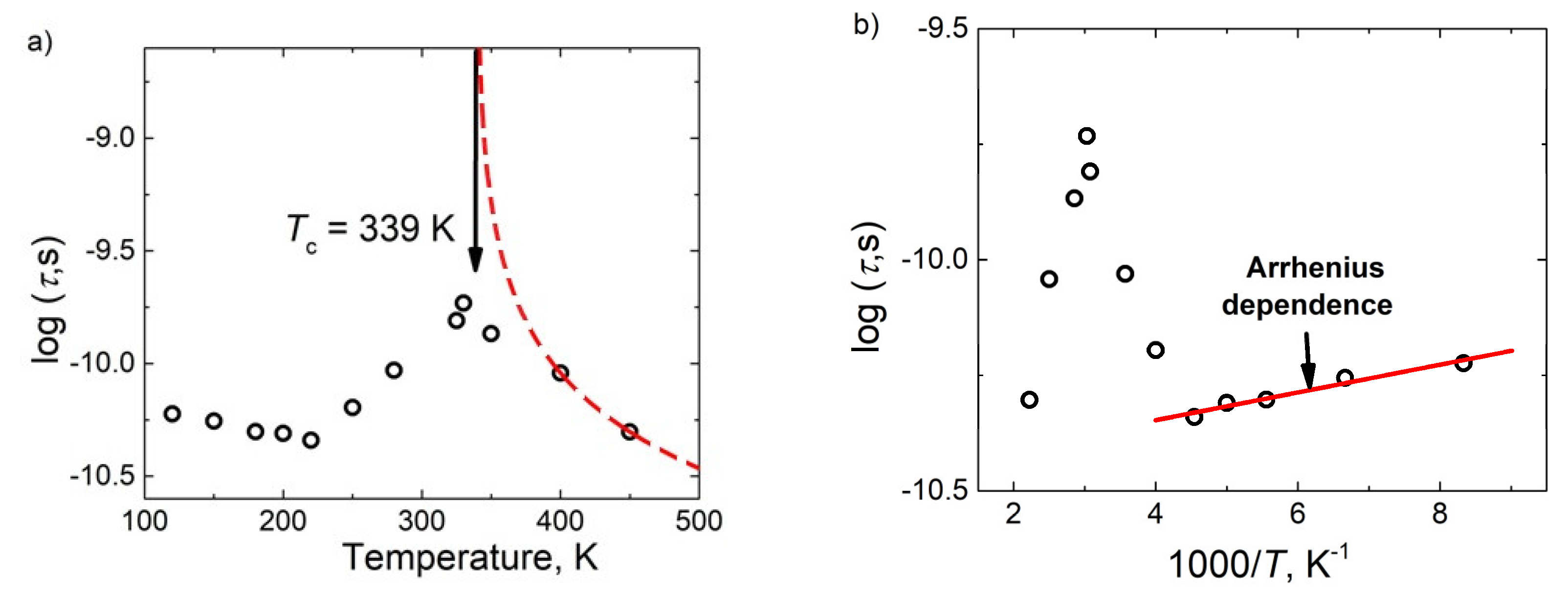
3.3. BDS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chan, N.H.; Sharma, R.K.; Smyth, D.M. Nonstoichometry in undoped BaTiO3. J. Am. Ceram. Soc. 1981, 64, 556–562. [Google Scholar] [CrossRef]
- Jona, F.; Shirane, G. Ferroelectric Crystals; Pergamon: Elmsford, NY, USA, 1962; pp. 1–402. [Google Scholar]
- Hlinka, J.; Ospachuk, T.; Nuzhnyy, D.; Petzelt, J.; Kuzel, P.; Kadlec, C.; Vanek, P.; Ponomareva, I.; Bellaiche, L. Coexistence of the phonon and relaxation soft modes in the terahertz dielectric response of tetragonal BaTiO3. Phys. Rev. Lett. 2008, 101, 167401. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.; Novak, N.; Rojas, V.; Patel, S.; Vaish, R.; Koruza, J.; Rossetti, G.A.; Rodel, J. BaTiO3-based piezoelectrics: Fundamentals, current status and perspectives. Appl. Phys. Rev. 2017, 4, 04130. [Google Scholar] [CrossRef]
- Kozielski, K.; Wilk, A.; Bucko, M.M.; Banys, J. A large piezoelectric strain recorded in BCT ceramics obtained by modified pechini method. Materials 2020, 13, 1620. [Google Scholar] [CrossRef] [PubMed]
- He, L.Q.; Ji, Y.C.; Ren, S.; Zhao, L.; Luo, H.Y.; Liu, C.; Hao, Y.S.; Zhang, L.; Zhang, L.X.; Ren, X.B. Large piezoelectric coefficient with enhanced thermal stability in Nb5+ doped Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramics. Ceram. Int. 2020, 46, 3236–3241. [Google Scholar] [CrossRef]
- Zheng, T.; Wu, J.G.; Xiao, D.Q.; Zhu, J.G. Recent development in lead-free piezoelectric bulk materials. Prog. Mater. Sci. 2018, 98, 552–624. [Google Scholar] [CrossRef]
- Shvartsman, V.V.; Dec, J.; Xu, Z.K.; Banys, J.; Keburis, P.; Kleemann, W. Crossover from ferroelectric to relaxor behavior in BaTi1−xSnxO3 solid solutions. Phase Transit. 2008, 81, 1013–1021. [Google Scholar] [CrossRef]
- Nuzhnyy, D.; Petzelt, J.; Savinov, M.; Ostapchuk, T.; Bovtun, V.; Kempa, M.; Hlinka, J.; Buscaglia, V.; Buscaglia, M.T.; Nanni, P. Broadband dielectric response of Ba(Zr,Ti)O3 ceramics: From incipient via relaxor and diffuse up to classical ferroelectric behavior. Phys. Rev. B 2012, 86, 014106. [Google Scholar] [CrossRef]
- Canu, G.; Confalonieri, G.; Deluca, M.; Curecheriu, L.; Buscaglia, M.T.; Asandulesa, M.; Horchidan, N.; Dapiaggi, M.; Mitoseriu, L.; Buscaglia, V. Structure-property correlations and origin of relaxor behaviour in BaCexTi1−xO3. Acta Mater. 2018, 152, 258–268. [Google Scholar] [CrossRef]
- Filipic, C.; Kutnjak, Z.; Pirc, R.; Canu, G. BaZr0.5Ti0.5O3: Lead free relaxor ferroelectric or dipolar glass. Phys. Rev. B 2016, 93, 224105. [Google Scholar] [CrossRef]
- Lemanov, V.V.; Sotnikov, A.V.; Smirnova, E.P.; Weihnacht, M.; Kunze, R. Perovskite CaTiO3 as an incipient ferroelectric. Solid State Commun. 1999, 110, 611–614. [Google Scholar] [CrossRef]
- Pullar, R.C.; Zhang, Y.; Chen, L.; Yang, S.; Evans, J.R.G.; Salak, A.N.; Kiselev, D.A.; Kholkin, A.L.; Fierra, V.M.; Alford, N.M. Dielectric measurements on a novel Ba1−xCaxTiO3 (BCT) bulk ceramic combinatorial library. J. Electroceram. 2009, 22, 245–251. [Google Scholar] [CrossRef]
- Liu, F.W.; Ren, X.B. Large piezoelectric effect in Pb-free ceramics. Phys. Rev. Lett. 2009, 103, 257602–257604. [Google Scholar] [CrossRef] [PubMed]
- Feliksik, K.; Kozielski, L.; Szafraniak-Wiza, I.; Goryczka, T.; Adamczyk-Habrajska, M. Dielectric and impedance studies of (Ba,Ca)TiO3 ceramics obtained from mechanically synthesized powders. Materials 2019, 12, 4036. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-F.; Lin, J.-L.; Hu, C.-T.; Lin, I.-N. The microstructure developments and electrical properties of calcium-modified barium titanate ceramics. J. Mater. Sci. 1991, 26, 491–496. [Google Scholar] [CrossRef]
- Zhu, X.N.; Zhang, W.; Chen, X.M. Enhanced dielectric and ferroelectric characteristics in Ca-modified BaTiO3 ceramics. AIP Adv. 2013, 3, 082125. [Google Scholar] [CrossRef]
- Suresh, P.; Mathiyalagan, P.; Srikanth, K.S. Structural, ferroelectric and photocatalysis performance of Ba1−xCaxTiO3 ceramics. Ferroelectrics 2020, 555, 74–87. [Google Scholar] [CrossRef]
- Sharma, P.; Berwal, N.; Ahlawat, N.; Maan, A.S.; Punia, R. Study of structural, dielectric, ferroelectric and magnetic properties of vanadium doped BCT ceramics. Ceram. Int. 2019, 45, 20368–20378. [Google Scholar] [CrossRef]
- Krayzman, V.; Levin, I.; Woicik, J.C.; Bridges, F.; Nelson, E.J.; Sinclair, D.C. Ca K-edge X-ray absorption fine structure in BaTiO3-CaTiO3 solid solutions. J. Appl. Phys. 2013, 113, 044106. [Google Scholar] [CrossRef]
- Panigrahi, M.R.; Panigrahi, S. Diffuse phase transition and dielectric study in Ba0.95Ca0.05TiO3 ceramic. Phys. B Condens. Matter 2010, 405, 2556–2559. [Google Scholar] [CrossRef]
- Fu, D.; Itoh, M.; Koshihara, S.; Kosugi, T.; Tsuneyuki, S. Anomalous Phase Diagram of Ferroelectric (Ba,Ca) TiO3 Single Crystals with Giant Electromechanical Respons. Phys. Rev. Lett. 2008, 100, 227601. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, M.; Rai, R.; Zeb, A.; Kumar, S. Modification of structural and electrical properties of Ca element on barium titanate nano-material synthesized by hydrothermal method. Ferroelectrics 2017, 520, 93–109. [Google Scholar] [CrossRef]
- Tiwari, V.S.; Pandey, D.; Groves, P. The influence of a powder processing technique on chemical homogeneity and the diffuse phase transition behaviour of Ba0.9Ca0.1TiO3 ceramics. J. Phys. D. Appl. Phys. 1989, 22, 837–843. [Google Scholar] [CrossRef]
- Wada, S.; Tabata, K.; Kakemoto, H.; Tsurumi, T. Anomalous tructure and dielectric properties of BaTiO3-CaTiO3 system ceramic composites. J. Ceram. Soc. Jpn. 2004, 112, S773–S779. [Google Scholar]
- Ellisade, C.; Ravez, J. Ferroelectric ceramics: Defects and dielectric relaxations. J. Mater. Chem. 2001, 11, 1957–1967. [Google Scholar] [CrossRef]
- Lee, S.; Randall, C.A. A modified Vegard’s law for multisite occupancy of Ca in BaTiO3-CaTiO3 solid solutions. Appl. Phys. Lett. 2008, 92, 111904. [Google Scholar] [CrossRef]
- Levin, I.; Krayzman, V.; Woicik, J.C. Local-structure origins of the sustained Curie temperature in (Ba,Ca)TiO3 ferroelectrics. Appl. Phys. Lett. 2013, 102, 162906. [Google Scholar] [CrossRef]
- Macutkevic, J.; Banys, J.; Grigalaitis, R.; Vysochanskii, Y. Asymmetric phase diagram of CuInP2(SxSe1−x)6 crystals. Phys. Rev. B 2008, 78, 064101. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29 (Suppl. S2), S13–S18. [Google Scholar] [CrossRef]
- Grigas, J. Microwave Dielectric Spectroscopy of Ferroelectrics and Related Materials; Gordon and Breach Science Publishers: New York, NY, USA; OPA Amsterdam: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Szot, K.; Pawelczyk, M.; Herion, J.; Freiburg, C.; Albers, J.; Waser, R.; Hulliger, J.; Kwapulinski, J.; Dec, J. Nature of the surface layer in ABO3-type perovskites at elevated temperatures. Appl. Phys. A 1996, 62, 335–343. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.M.; Chen, T.C.S. Surface chemical states of barium titanate: Influence of sample processing. J. Mater. Res. 1995, 10, 1502–1507. [Google Scholar] [CrossRef]
- Molak, A.; Paluch, M.; Pawlus, S.; Klimontko, J.; Ujma, Z.; Gruszka, I. Electric modulus approach to analysis of the electric relaxation in highly conducting (Na0.75Bi0.25)(Mn0.25Nb0.75)O3 ceramics. J. Phys. D Appl. Phys. 2005, 38, 1450–1460. [Google Scholar] [CrossRef]
- Schafer, E.; Sternin, R.; Stannarius, M.; Arndt, M.; Kremer, F. Novel approach to the analysis of broadband dielectric spectra. Phys. Rev. Lett. 1996, 76, 2177. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, A.N. Ill-posed problem of linear algebra and stable methods for solving them. Dokl. Akad. Nauk SSSR 1965, 163, 591–594. [Google Scholar]
- Macutkevic, J.; Banys, J.; Matulis, A. Determination of distribution of the relaxation times from dielectric spectra. Nonlinear Anal. Model. Control 2004, 9, 75–88. [Google Scholar] [CrossRef]

| Ca Content, x | 0.17 | 0.18 | 0.20 |
|---|---|---|---|
| a = b (Å) | 3.98710(6) | 3.98700(6) | 3.98700(6) |
| c (Å) | 4.00300(9) | 4.00300(9) | 4.00300(9) |
| α =β = γ (°) | 90 | 90 | 90 |
| V (Å3) | 63.63555 | 63.63241 | 63.63237 |
| Content | Polished | Un-Polished | |||
|---|---|---|---|---|---|
| Area | Area | Area | Large Grain | Small Grain | |
| High Ti | Similar Ba and Ti | High Ba | High Ba | High Ba | |
| Ba | 39.1 | 45.9 | 49.1 | 46.5 | 47.1 |
| Ca | 6.3 | 9.1 | 7.8 | 9.3 | 9.3 |
| Ti | 54.6 | 45.0 | 43.1 | 43.9 | 43.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaimiene, E.; Macutkevic, J.; Banys, J.; Winiarski, A.; Gruszka, I.; Koperski, J.; Molak, A. Crossover from Ferroelectric to Relaxor Behavior in Ba1−xCaxTiO3 (x = 0.17) System. Materials 2020, 13, 2854. https://doi.org/10.3390/ma13122854
Palaimiene E, Macutkevic J, Banys J, Winiarski A, Gruszka I, Koperski J, Molak A. Crossover from Ferroelectric to Relaxor Behavior in Ba1−xCaxTiO3 (x = 0.17) System. Materials. 2020; 13(12):2854. https://doi.org/10.3390/ma13122854
Chicago/Turabian StylePalaimiene, Edita, Jan Macutkevic, Juras Banys, Antoni Winiarski, Irena Gruszka, Janusz Koperski, and Andrzej Molak. 2020. "Crossover from Ferroelectric to Relaxor Behavior in Ba1−xCaxTiO3 (x = 0.17) System" Materials 13, no. 12: 2854. https://doi.org/10.3390/ma13122854
APA StylePalaimiene, E., Macutkevic, J., Banys, J., Winiarski, A., Gruszka, I., Koperski, J., & Molak, A. (2020). Crossover from Ferroelectric to Relaxor Behavior in Ba1−xCaxTiO3 (x = 0.17) System. Materials, 13(12), 2854. https://doi.org/10.3390/ma13122854







