A Novel Microstructural Evolution Model for Growth of Ultra-Fine Al2O3 Oxides from SiO2 Silica Ceramic Decomposition during Self-Propagated High-Temperature Synthesis
Abstract
1. Introduction
2. Materials and Methods
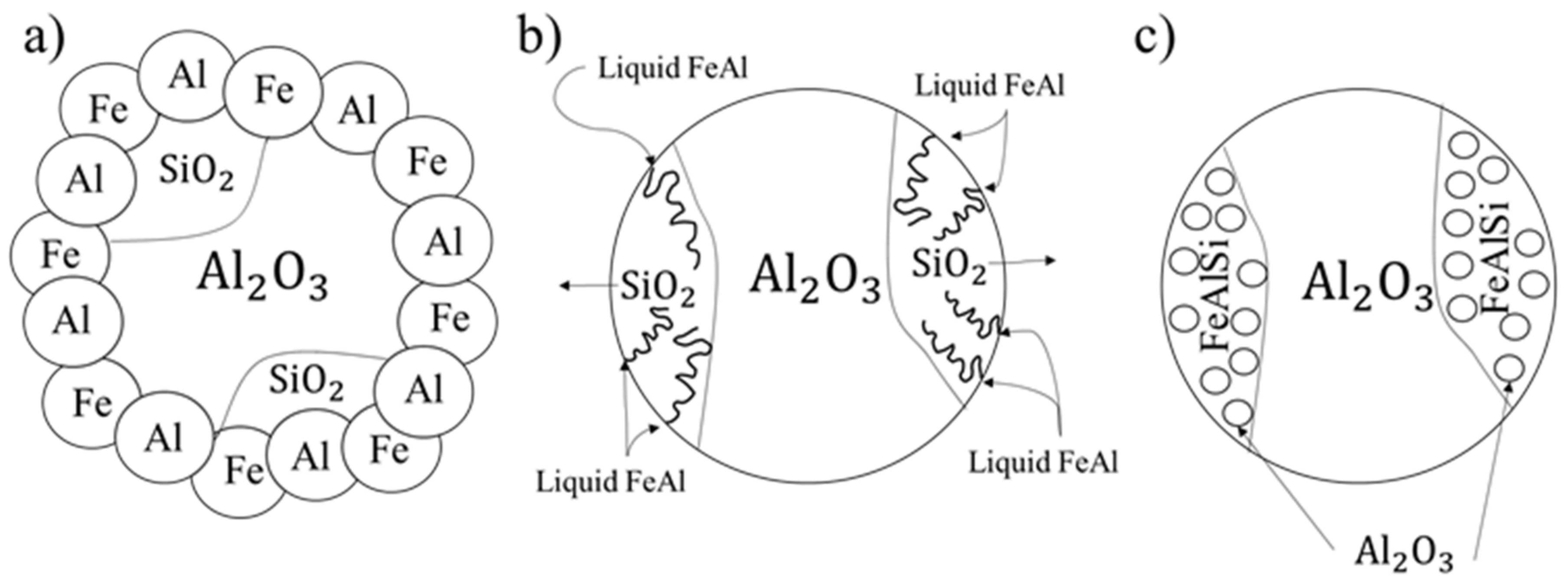
3. Introduction of Microstructure Evolution Model for Growth of Ultra-Fine Al2O3 Oxides from SiO2 Silica Ceramic Decomposition
4. Results and Discussion
4.1. Optimization of Sintering Temperature for Investigated Powder Mixtures
4.2. Microstructural Characterization of Sintered Material and Experimental Verification of the Model
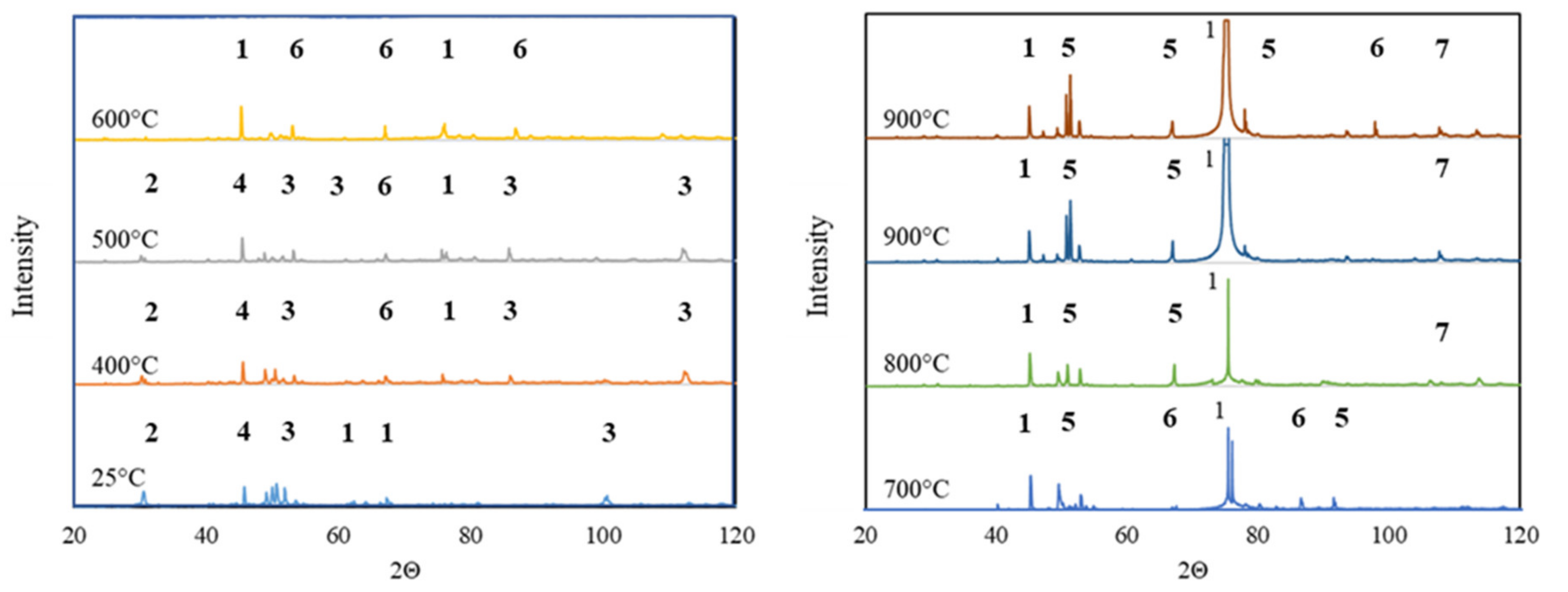
4.3. X-ray Phase Analysis of Sintered Material
- At temperatures up to 500 °C, the material mainly consisted of Al2O3, SiO2, Fe2Al9 and Al2FeSi phases. Such phase compositions indicated that the temperature was too low to initiate phase transformations.
- In the temperature range of 500 to 600 °C, the material mainly consisted of the Al2O3 phase and Al2Fe3Si3 and Fe2Al9 phases, which were presumably indirect products of the phase transformations initiated.
- At the temperature of 700 °C, an intense peak of Al2O3 was observed. An occurrence of such a peak was associated with the formation of new, fine precipitates of this oxide.
- At temperatures higher than 700 °C, the sinter phase composition stabilized. It consisted of the Al4.5FeSi phase and new Al2O3. The homogenization at the temperature of 900 °C did not affect the phase structure of the material significantly because only one additional peak of the Al2Fe3Si3 phase was observed.
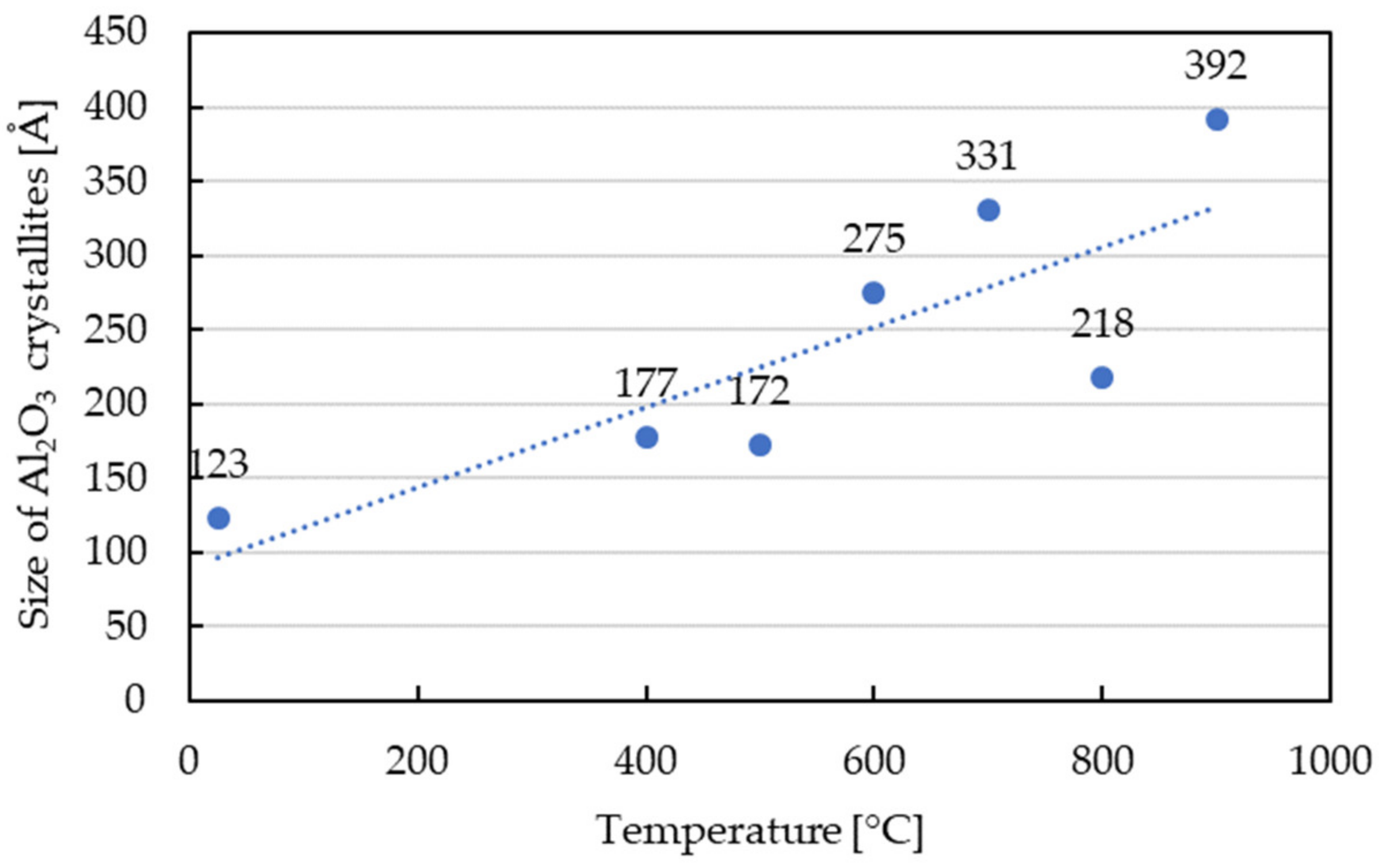
- The Halder–Wagner (HW) method was used to determine crystallite size in the function of temperature (Figure 6). The size of Al2O3 crystallites increased with the temperature, and the average value of 392 Å was obtained after heating to 900 °C.
5. Conclusions
- The proposed model of composite fabrication, based on the decomposition of mullite ceramics infiltrated with a metallic liquid, allows for obtainment of a composite material with an intermetallic matrix reinforced with Al2O3 ceramic particles derived from the defragmentation of primary alundum ceramics and newly formed fine-grained spherical ceramics resulting from SiO2 decomposition.
- The parameters of the sintering process and subsequent homogenization are significantly affected by the participation of silicon, which significantly increases the fluidity of the metallic liquid formed during sintering and the final phase structure of the sintered matrix.
- The correctness of the assumed model must be supported by using proper sintering conditions that would lead to satisfactory cohesion between the matrix and reinforcement. Future promising sintering technology seems to be hot isostatic pressing that should guarantee fabrication of IMC composites with satisfactory performance.
Author Contributions
Funding
Conflicts of Interest
References
- Kainer, K.U. Basics of Metal Matrix Composites; WileyVCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Rosso, M. Ceramic and metal matrix composites: Route and properties. J. Mater. Process. Technol. 2006, 175, 364–375. [Google Scholar] [CrossRef]
- Sritharan, T.; Murali, S.; Hing, P. Exothermic reactions in powder mixtures of Al, Fe and Si. Mater. Lett. 2001, 51, 455–460. [Google Scholar] [CrossRef]
- Murali, S.; Sritharan, T.; Hing, P. Self-propagating high temperature synthesis of AlFeSi intermetallic compound. Intermetallics 2003, 11, 279–281. [Google Scholar] [CrossRef]
- Gialanella, S.; Camana, G.; Kazior, J.; Lutterotti, L.; Pieczonka, T.; Molinari, A. Reaction sintering of Fe-Al-Si alloys. J. Phase Equilib. 2002, 23, 72. [Google Scholar] [CrossRef]
- Gupta, S.P. Intermetallic compound formation in Fe-Al-Si ternary system: Part I. Mater. Charact. 2003, 49, 269–291. [Google Scholar] [CrossRef]
- Maitra, T.; Gupta, S.P. Intermetallic compound formation in Fe-Al-Si ternary system: Part II. Mater. Charact. 2003, 49, 293–311. [Google Scholar] [CrossRef]
- Novák, P.; Zelinková, M.; Serák, J.; Michalcová, A.; Novák, M.; Vojt, D. Oxidation resistance of SHS Fe–Al–Si alloys at 800°C in air. Intermetallics 2011, 19, 1306–1312. [Google Scholar] [CrossRef]
- Novák, P.; Průša, F.; Knotek, V.; Šerák, J.; Vojtěch, D. Properties of intermetallic phases prepared by reactive sintering. In Proceedings of the 19th International Conference on Metallurgy and Materials 2010, Rožnov pod Radhoštěm, Czech Republic, 18–20 May 2010. [Google Scholar]
- Sritharan, T.; Murali, S. Synthesis of ternary intermetallics by exothermic reaction. J. Mater. Process. Technol. 2001, 113, 469–473. [Google Scholar] [CrossRef]
- Chen, K.; Liu, G.; Li, J. Combustion synthesis of refractory and hard materials: A review. Int. J. Refract. Met Hard Mater. 2013, 39, 90–102. [Google Scholar] [CrossRef]
- Yadav, T.P.; Yadav, R.M.; Singh, D.P. Mechanical milling: A top down approach for the synthesis of nanomaterials and nanocomposites. J. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef]
- Halldearn, R.D.; Xiao, P. Mechanisms of the aluminium-iron oxide thermite reaction. Scr. Mater. 1999, 41, 541–548. [Google Scholar] [CrossRef]
- Nash, J.M.; Williams, J.C.; Breslin, M.C.; Daehn, G.S. Co-continuous composites for high-temperature applications. Mat Sci Eng A 2007, 463, 115–121. [Google Scholar] [CrossRef]
- Han, C.Z.; Brown, I.W.M.; Zhang, D.L. Microstructure development and properties of alumina—Ti aluminide interpenetrating composites. Curr. Appl. Phys. 2006, 6, 444–447. [Google Scholar] [CrossRef]
- Siqueira, J.R.R.; Simões, A.Z.; Stojanovic, B.D.; Paiva-Santos, C.O.; Santos, L.P.S.; Longo, E.; Varela, J.A. Influence of milling time on mechanically assisted synthesis of Pb0.91Ca0.1TiO3 powders. Cer. Inter. 2007, 33, 937–941. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, H.; Fu, Z.; Fang, S.; Zhu, D. Reactive hot pressing and mechanical properties of TiAl 3-Ti3AlC2-Al2O3. Mater. Des. 2013, 49, 929–934. [Google Scholar] [CrossRef]
- Inoue, M.; Nagao, H.; Suganuma, K.; Niihara, K. Fracture properties of Fe 40 at % Al matrix composites reinforced with ceramic particles and fibers. Mat. Sci. Eng. A 1998, 258, 298–305. [Google Scholar] [CrossRef]
- Avraham, S.; Beyer, P.; Janssen, R.; Claussen, N.; Kaplan, W.D. Characterization of Al2O3–(Al–Si)3Ti composites. J. Eur. Ceram. Soc. 2006, 26, 2719–2726. [Google Scholar] [CrossRef]
- Zhu, H.X.; Abbaschian, R. In-situ processing of NiAl–alumina composites by thermite reaction. Mat Sci Eng A 2000, 282, 1–7. [Google Scholar] [CrossRef]
- Bauer, R.S.; Bachrach, R.Z.; Brillson, L.J. Au and Al interface reactions with SiO2. Appl. Phys. Lett. 1980, 1006, 35–38. [Google Scholar] [CrossRef]
- Liu, W.; Kiister, U. Criteria for formation of interpenetrating oxide/metal-composites by immersing sacrificial Oxide preforms in molten metals. Scr. Mater. 1996, 35, 35–40. [Google Scholar] [CrossRef]
- Wagner, F.; Garcia, D.E.; Krupp, A.; Claussen, N. Interpenetrating Al2O3-TiAl3 alloys produced by reactive infiltration. J. Eur. Ceram. Soc. 1999, 19, 2449–2453. [Google Scholar] [CrossRef]
- Fan, R.; Sun, K.; Wang, W.; Yi, X. Kinetics of thermite reaction in Al-Fe2O3 system. Thermochim. Acta 2006, 440, 129–131. [Google Scholar] [CrossRef]
- Manfredi, D.; Pavese, M.; Biamino, S.; Fino, P.; Badini, C. Preparation and properties of NiAl(Si)/Al2O3 co-continuous composites obtained by reactive metal penetration. Compos. Sci. Technol. 2009, 69, 1777–1782. [Google Scholar] [CrossRef]
- Schicker, S.; Garcia, D.E.; Bruhn, J.; Janssen, R.; Claussen, N. Reaction synthesized A1203-based Intermetallic composites. Acta Mater. 1998, 46, 2485–2492. [Google Scholar] [CrossRef]
- Bhatt, J.; Balachander, N.; Shekher, S.; Karthikeyan, R.; Peshwe, D.R.; Murty, B.S. Synthesis of nanostructured Al-Mg-SiO2 metal matrix composites using high-energy ball milling and spark plasma sintering. J. Alloys Compd. 2012, 536, S35–S40. [Google Scholar] [CrossRef]
- Missiaen, J. Modelling of sintering: recent developments and perspectives. Rev. Met. Paris 2002, 12, 1009–1019. [Google Scholar] [CrossRef]
- Exner, H.; Kraft, T. Review on computer simulations of sintering processes. Powder Metall. World Congr. 1998, 2, 278–283. [Google Scholar]
- Pan, J. Modelling sintering at different length scales. Int. Mater. Rev. 2003, 2, 69–85. [Google Scholar] [CrossRef]
- Parhami, F.; McMeeking, R. A network model for initial stage sintering. Mech. Mater. 1998, 27, 111–124. [Google Scholar] [CrossRef]
- Martin, C.; Schneider, L.; Olmos, L.; Bouvard, D. Discrete element modeling of metallic powder sintering. Scr. Mater. 2006, 55, 425–428. [Google Scholar] [CrossRef]
- Park, M.S.; Arroyave, R. Early stages of intermetallic compound formation and growth during lead-free soldering. Acta Mater. 2010, 58, 4900–4910. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y.C. Simulation and experimental studies on microstructure evolution of resolidified dendritic TiCx in laser direct deposited Ti-TiC composite. Mater. Des. 2018, 159, 212–223. [Google Scholar] [CrossRef]
- Roehling, J.D.; Perron, A.; Fattebert, J.-L.; Haxhimali, T.; Guss, G.; Li, T.T.; Bober, D.; Stokes, A.W.; Clarke, A.J.; Turchi, P.E.A.; et al. Rapid solidification in bulk Ti-Nb alloys by single-track laser melting. JOM J. Miner. Met. Mater. Soc. 2018, 70, 1589–1597. [Google Scholar] [CrossRef]
- Keller, T.; Lindwall, G.; Ghosh, S. Application of finite element, phase-field, and CALPHAD-based methods to additive manufacturing of Ni-based superalloys. Acta Mater. 2017, 139, 244–253. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Paradela, K.G.; Junior, P.F.; Júnior, Z.A.; Garcia, A. Phase-field simulation of microsegregation and dendritic growth during solidification of hypoeutectic Al-Cu alloys. Mater. Res. 2017, 20, 423–429. [Google Scholar] [CrossRef][Green Version]
- Bhaskar, M.S. Quantitative phase field modelling of precipitate coarsening in Ni-Al-Mo alloys. Comput. Mater. Sci. 2018, 146, 102–111. [Google Scholar] [CrossRef]
- Aristizabal, K.; Katzensteiner, A.; Bachmaier, A. Microstructural evolution during heating of CNT/Metal Matrix Composites processed by Severe Plastic Deformation. Sci. Rep. 2020, 10, 857. [Google Scholar] [CrossRef]
- Długosz, P.; Darłak, P.; Purgert, R.M.; Sobczak, J.J. Technological aspects of the synthesis of metal matrix composites reinforced with cenospheres. Prace Instytutu Odlewnictwa 2011, 51, 35–44. [Google Scholar]
- Raghavan, V. Al-Fe-Si (Aluminum-Iron-Silicon). J. Phase Equilib. 2002, 23, 362–366. [Google Scholar] [CrossRef]
- Siemiaszko, D.; Jóźwiak, S.; Czarnecki, M.; Bojar, Z. Influence of temperature during pressure-assisted induction sintering (PAIS) on structure and properties of the Fe40Al intermetallic phase. Intermetallics 2013, 41, 16–21. [Google Scholar] [CrossRef]
- Pocheć, E.; Jóżwiak, S.; Karczewski, K.; Bojar, Z. Fe–Al phase formation around SHS reactions under isothermal conditions. J. Alloys Compd. 2011, 509, 1124–1128. [Google Scholar] [CrossRef]
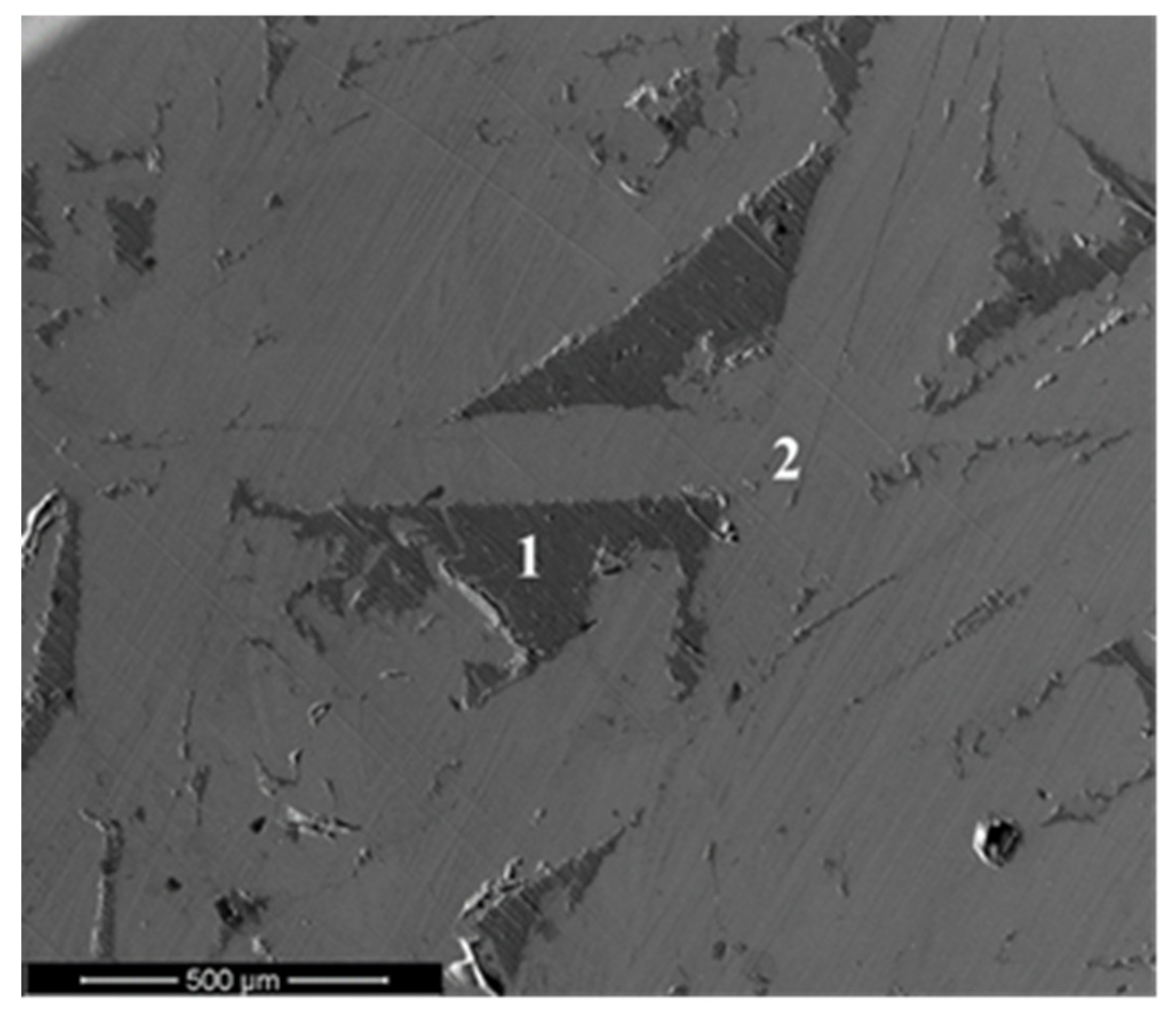
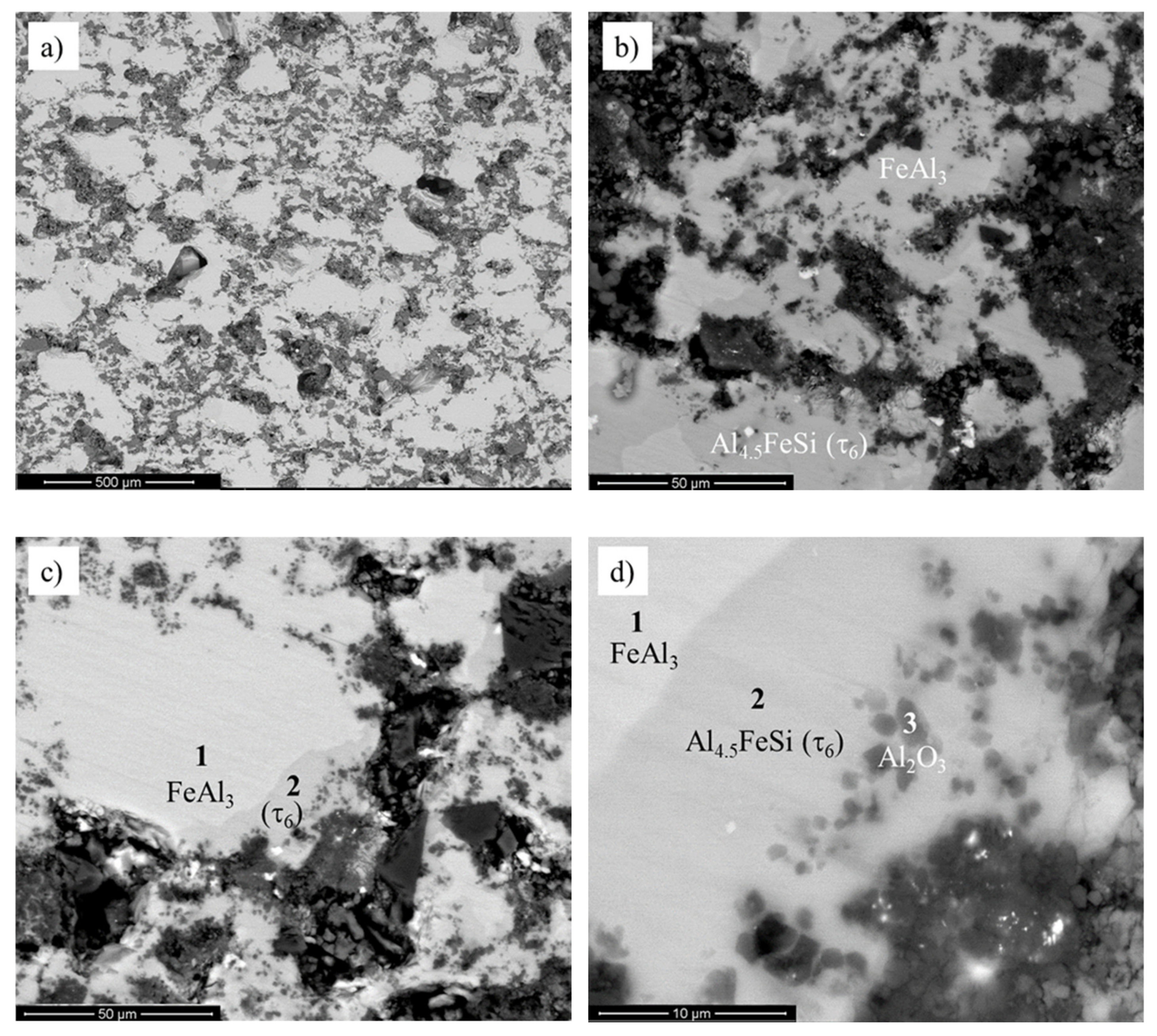

| Wt.% | Al | Si | Fe |
|---|---|---|---|
| 1 | 87.95 | 8.92 | 3.13 |
| 2 | 76.18 | 1.91 | 21.94 |
| Wt.% | Al | Si | Fe | O |
|---|---|---|---|---|
| 1 | 62.39 | 2.93 | 34.68 | - |
| 2 | 55.69 | 14.98 | 29.33 | - |
| 3 | 61.27 | - | 8.73 | 30.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopec, M.; Jóźwiak, S.; Kowalewski, Z.L. A Novel Microstructural Evolution Model for Growth of Ultra-Fine Al2O3 Oxides from SiO2 Silica Ceramic Decomposition during Self-Propagated High-Temperature Synthesis. Materials 2020, 13, 2821. https://doi.org/10.3390/ma13122821
Kopec M, Jóźwiak S, Kowalewski ZL. A Novel Microstructural Evolution Model for Growth of Ultra-Fine Al2O3 Oxides from SiO2 Silica Ceramic Decomposition during Self-Propagated High-Temperature Synthesis. Materials. 2020; 13(12):2821. https://doi.org/10.3390/ma13122821
Chicago/Turabian StyleKopec, Mateusz, Stanisław Jóźwiak, and Zbigniew L. Kowalewski. 2020. "A Novel Microstructural Evolution Model for Growth of Ultra-Fine Al2O3 Oxides from SiO2 Silica Ceramic Decomposition during Self-Propagated High-Temperature Synthesis" Materials 13, no. 12: 2821. https://doi.org/10.3390/ma13122821
APA StyleKopec, M., Jóźwiak, S., & Kowalewski, Z. L. (2020). A Novel Microstructural Evolution Model for Growth of Ultra-Fine Al2O3 Oxides from SiO2 Silica Ceramic Decomposition during Self-Propagated High-Temperature Synthesis. Materials, 13(12), 2821. https://doi.org/10.3390/ma13122821







