Chitosan-Based Bioactive Glass Gauze: Microstructural Properties, In Vitro Bioactivity, and Biological Tests
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Glass Powders
2.2. Chitosan-Based Bioactive Glass Wound Dressings Preparation
2.3. Microstructural Characterization
2.4. Swelling Tests
2.5. In Vitro Bioactivity
2.6. Biological Tests
2.6.1. Culture of NIH 3T3
2.6.2. Samples’ Eluates Preparation
2.6.3. NR Uptake Assay at 24 and 72 h
2.6.4. MTT Assay at 24 and 72 h
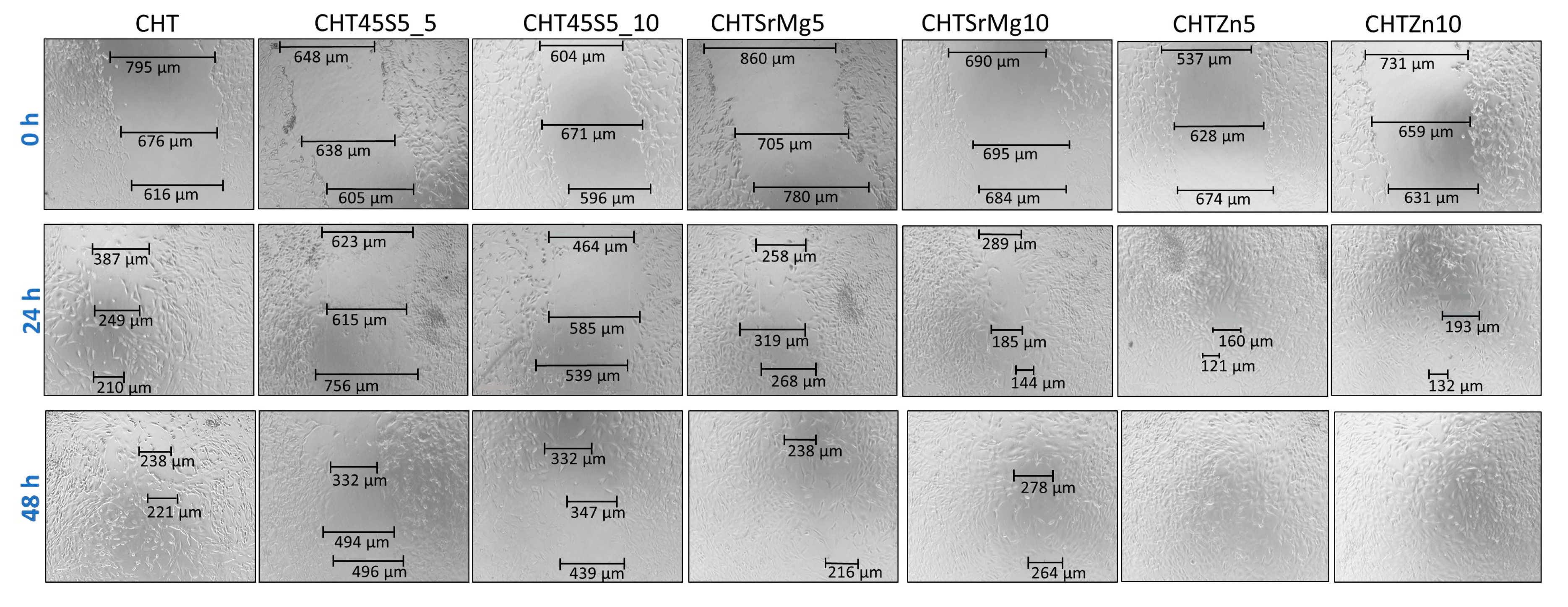
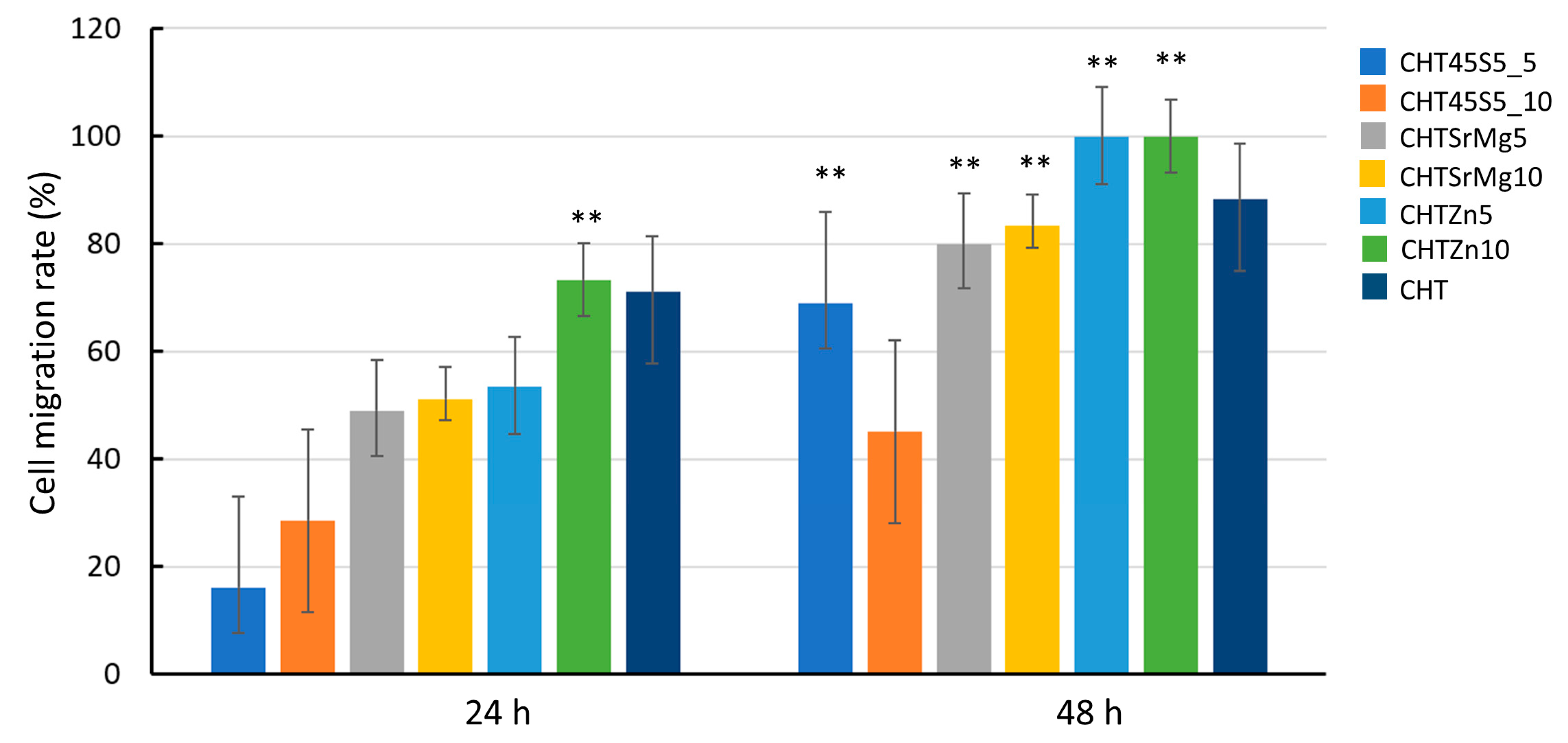
2.6.5. Scratch Test
2.7. Statistical Analysis
3. Results and Discussion
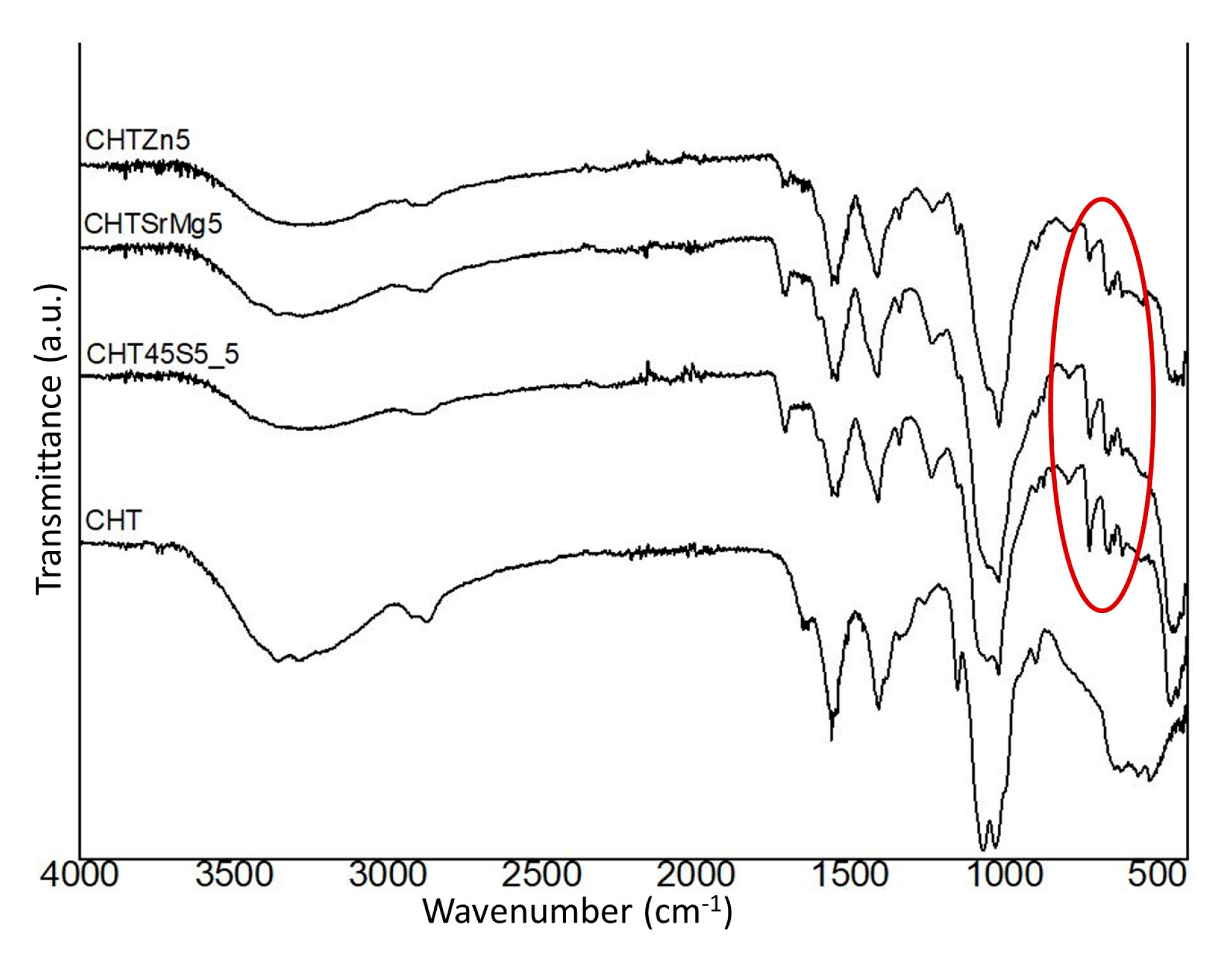
3.1. Microstructural Characterization
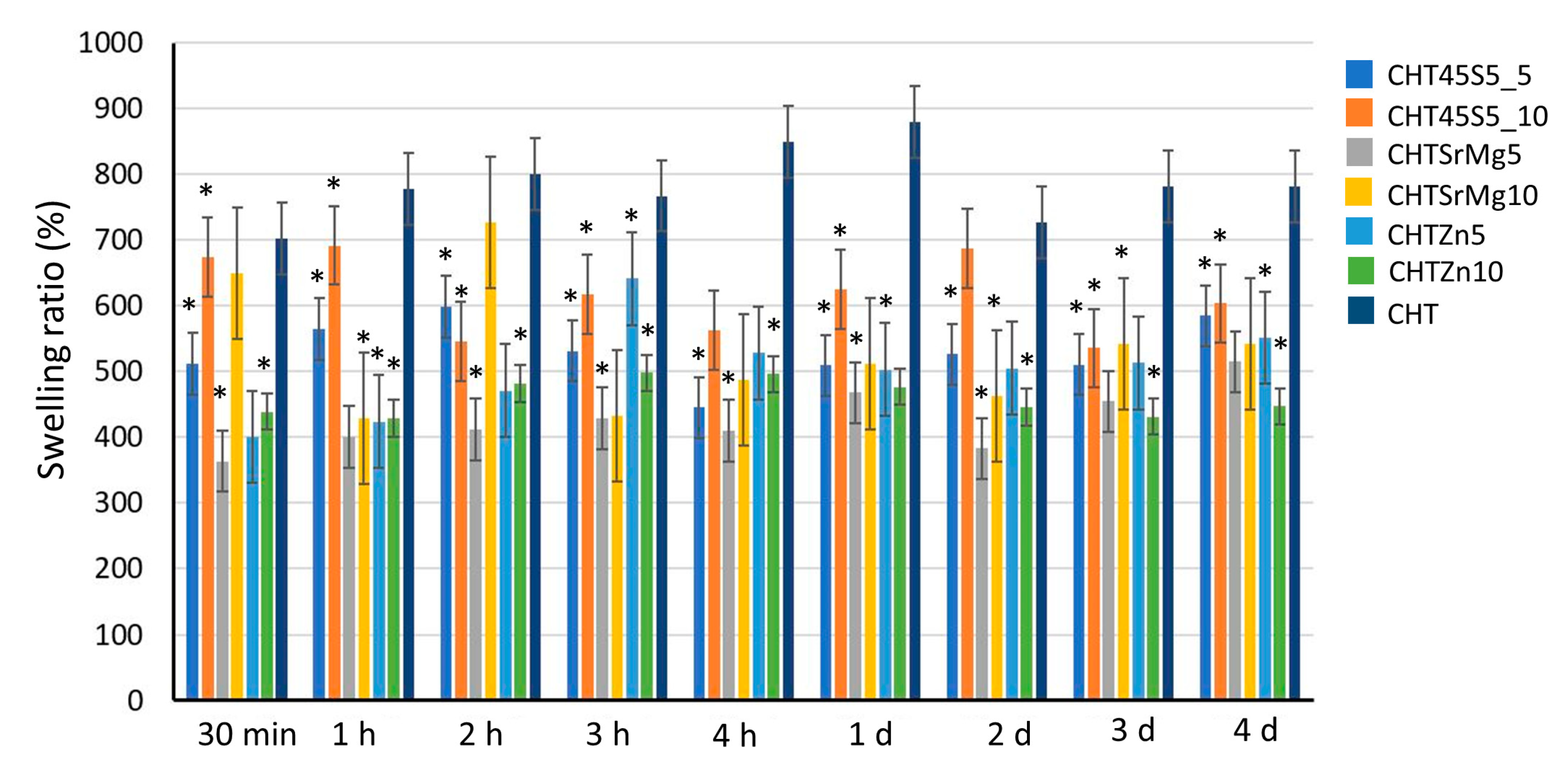
3.2. Swelling Property
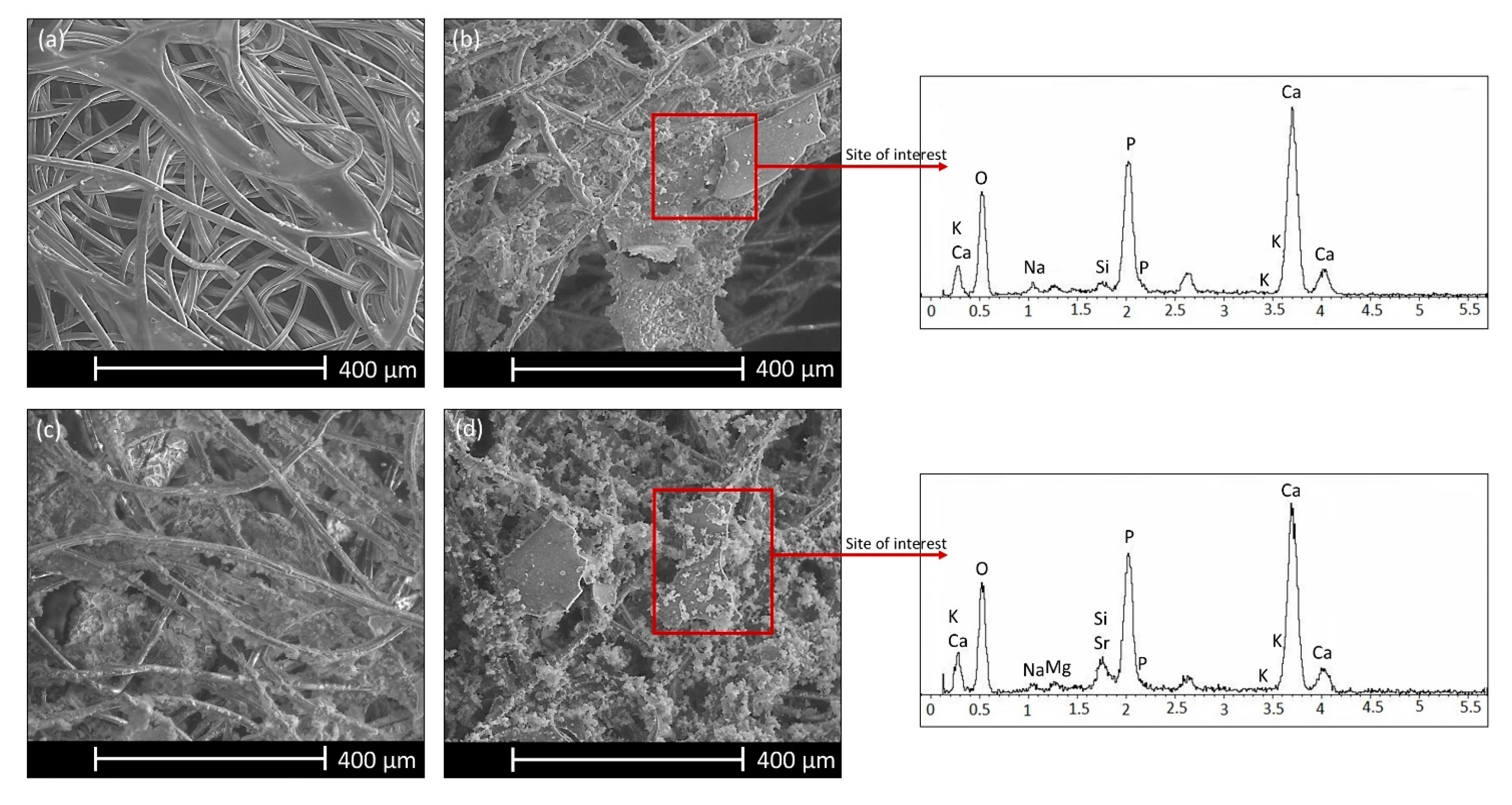
3.3. In vitro Bioactivity Investigation
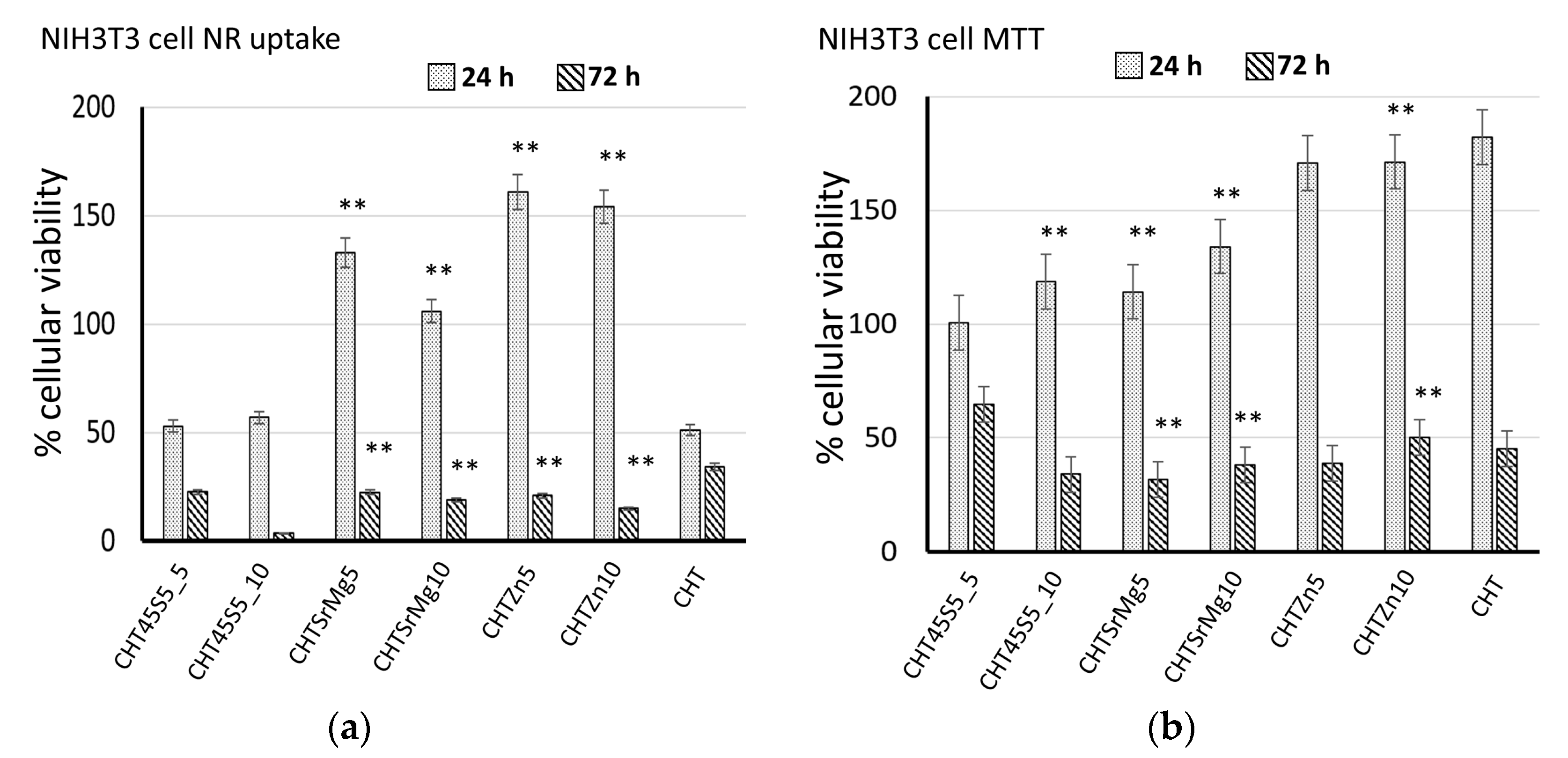
3.4. Biological Investigations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef]
- Antunes, B.P.; Moreira, A.F.; Gaspar, V.M.; Correia, I.J. Chitosan/arginine-chitosan polymer blends for assembly of nanofibrous membranes for wound regeneration. Carbohydr. Polym. 2015, 130, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef]
- Rajabian, M.H.; Ghorabi, G.H.; Geramizadeh, B.; Sameni, S.; Ayatollahi, M. Evaluation of bone marrow derived mesenchymal stem cells for full-thickness wound healing in comparison to tissue engineered chitosan scaffold in rabbit. Tissue Cell 2017, 49, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Sivashankari, P.R.; Prabaharan, M. Prospects of chitosan-based scaffolds for growth factor release in tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Thiruvoth, F.; Mohapatra, D.; Sivakumar, D.; Chittoria, R.; Nandhagopal, V. Current concepts in the physiology of adult wound healing. Plast. Aesthet. Res. 2015, 2, 250. [Google Scholar]
- Schulz, C.; Perdiguero, E.G.; Chorro, L.; Szabo-Rogers, H.; Cagnard, N.; Kierdorf, K.; Prinz, M.; Wu, B.; Jacobsen, S.E.W.; Pollard, J.W.; et al. A lineage of myeloid cells independent of myb and hematopoietic stem cells. Science 2012, 335, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Childs, D.R.; Murthy, A.S. Overview of Wound Healing and Management. Surg. Clin. N. Am. 2017, 97, 189–207. [Google Scholar]
- Morgado, P.I.; Aguiar-Ricardo, A.; Correia, I.J. Asymmetric membranes as ideal wound dressings: An overview on production methods, structure, properties and performance relationship. J. Memb. Sci. 2015, 490, 139–151. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.E. The physiology of wound healing. Surgery (Oxford) 2017, 35, 473–477. [Google Scholar]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh, K.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Tang, F.; Lv, L.; Lu, F.; Rong, B.; Li, Z.; Lu, B.; Yu, K.; Liu, J.; Dai, F.; Wu, D.; et al. Preparation and characterization of N-chitosan as a wound healing accelerator. Int. J. Biol. Macromol. 2016, 93, 1295–1303. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Khoshakhlagh, P.; Rabiee, S.M.; Kiaee, G.; Heidari, P.; Miri, A.K.; Moradi, R.; Moztarzadeh, F.; Ravarian, R. Development and characterization of a bioglass/chitosan composite as an injectable bone substitute. Carbohydr. Polym. 2017, 157, 1261–1271. [Google Scholar] [CrossRef]
- Correia, C.O.; Leite, Á.J.; Mano, J.F. Chitosan/bioactive glass nanoparticles scaffolds with shape memory properties. Carbohydr. Polym. 2015, 123, 39–45. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abdelgawad, A.M.; Elsherbiny, D.A.; El-shazly, W.A.; Ghazanfari, S.; Abdel-Aziz, M.S. Bioactive Wound Dressing Gauze Loaded with Silver Nanoparticles Mediated by Acacia Gum. J. Clust. Sci. 2019, 3, 1–14. [Google Scholar] [CrossRef]
- Abbasipour, M.; Mirjalili, M.; Khajavi, R.; Majidi, M.M. Coated Cotton Gauze with Ag/ZnO/chitosan Nanocomposite as a Modern Wound Dressing. J. Eng. Fiber. Fabr. 2014, 9, 124–130. [Google Scholar] [CrossRef]
- Eremenko, A.M.; Petrik, I.S.; Smirnova, N.P.; Rudenko, A.V.; Marikvas, Y.S. Antibacterial and antimycotic activity of cotton fabrics, impregnated with silver and binary silver/copper nanoparticles. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Malafaya, P.; Oliveira, J.; Mano, J.; Boesel, L.; Neves, N.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R Soc. Int. Erface. 2007, 4, 999–1030. [Google Scholar]
- Chaturvedi, A.; Dowling, M.B.; Gustin, J.P.; Scalea, T.M.; Raghavan, S.R.; Pasley, J.D.; Mayur, N. Hydrophobically modified chitosan gauze: A novel topical hemostat. J. Surg. Res. 2017, 207, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Pogorielov, M.; Kalinkevich, O.; Deineka, V.; Garbuzova, V.; Solodovnik, A.; Kalinkevich, A.; Mayur, N. Haemostatic chitosan coated gauze: In vitro interaction with human blood and in-vivo effectiveness. Biomater. Res. 2015, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Cannillo, V. A novel bioactive glass containing strontium and magnesium with ultra-high crystallization temperature. Mater. Lett 2018, 213, 67–70. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Salvatori, R.; Maisetta, G.; Batoni, G.; Cannillo, V. Zinc containing bioactive glasses with ultra-high crystallization temperature, good biological performance and antibacterial effects. Mater. Sci. Eng. C 2019, 104, 109910. [Google Scholar] [CrossRef]
- Luz, G.M.; Mano, J.F. Chitosan/bioactive glass nanoparticles composites for biomedical applications. Biomed. Mater. 2012, 7, 054104. [Google Scholar] [CrossRef]
- Alves, M.; Zea, D.; Caridade, S.G.; Merino, E.G.; Boccaccini, A.R. Chitosan membranes containing micro or nano-size bioactive glass particles: Evolution of biomineralization followed by in situ dynamic mechanical analysis. J. Mech. Behav. F Biomed. Mater. 2013, 20, 173–183. [Google Scholar]
- Bellucci, D.; Salvatori, R.; Anesi, A.; Chiarini, L.; Cannillo, V. SBF assays, direct and indirect cell culture tests to evaluate the biological performance of bioglasses and bioglass-based composites: Three paradigmatic cases. Mater. Sci. Eng. C 2019, 96, 757–764. [Google Scholar] [CrossRef]
- Bellucci, D.; Salvatori, R.; Giannatiempo, J.; Anesi, A.; Bortolini, S.; Cannillo, V. A New Bioactive Glass/Collagen Hybrid Composite for Applications in Dentistry. Materials 2019, 12, 2079. [Google Scholar] [CrossRef]
- Bellucci, D.; Veronesi, E.; Strusi, V.; Petrachi, T.; Murgia, A.; Mastrolia, I.; Dominici, M.; Cannillo, V. Human Mesenchymal Stem Cell Combined with a New Strontium-Enriched Bioactive Glass: An ex-vivo Model for Bone Regeneration. Materials 2019, 21, 3633. [Google Scholar] [CrossRef]
- Bellucci, D.; Veronesi, E.; Dominici, M.; Cannillo, V. On the in vitro biocompatibility testing of bioactive glasses. Materials 2020, 13, 1816. [Google Scholar] [CrossRef] [PubMed]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, L.; Jiang, L.; Xiong, C. Synthesis of Mg-substituted hydroxyapatite nanopowders: Effect of two different magnesium sources. Mater. Lett. 2013, 106, 246–249. [Google Scholar] [CrossRef]
- Aydin, H. Magnesium supplementation and bone. Mag. Hum. Health Dis. 2013, 57, 149–157. [Google Scholar]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of Bioactive Glasses on Angiogenesis: A Review of In Vitro and In Vivo Evidences. Tissue Eng. Part. B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef]
- Yu, H.; Peng, J.; Xu, Y.; Chang, J.; Li, H. Bioglass Activated Skin Tissue Engineering Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 703–715. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Miguez-pacheco, V.; Boccaccini, A.R. Vitale-brovarone C. Bioactive glasses: Special applications outside the skeletal system. J. Non Cryst. Solids 2016, 432, 15–30. [Google Scholar] [CrossRef]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 2001, 14, 331–341. [Google Scholar] [CrossRef]
- MacDonald, R. The role of Zinc in Growth and Cell Proliferation. J. Nutr. 2000, 130, 1488–1492. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Noizet, G.; Morlière, C.; Bolzinger, M.A. Antimicrobial activity of zinc oxide particles on five micro-organisms of the Challenge Tests related to their physicochemical properties. Int. J. Pharm. 2014, 460, 92–100. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Bolzinger, M.A. Zinc oxide as a new antimicrobial preservative of topical products: Interactions with common formulation ingredients. Int. J. Pharm. 2015, 479, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Bioact. Mater. 2018, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Cannillo, V.; Sola, A. A new potassium-based bioactive glass: Sintering behaviour and possible applications for bioceramic scaffolds. Ceram. Int. 2011, 37, 145–157. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V.; Sola, A. Calcium and potassium addition to facilitate the sintering of bioactive glasses. Mater. Lett. 2011, 65, 1825–1827. [Google Scholar] [CrossRef]
- Bellucci, D.; Cannillo, V.; Ciardelli, G.; Gentile, P.; Sola, A. Potassium based bioactive glass for bone tissue engineering. Ceram. Int. 2010, 36, 2449–2453. [Google Scholar] [CrossRef]
- Bolelli, G.; Bellucci, D.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Müller, P.; Sola, A. Comparison between Suspension Plasma Sprayed and High Velocity Suspension Flame Sprayed bioactive coatings. Surf. Coat. Technol. 2015, 280, 232–249. [Google Scholar] [CrossRef]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Wu, J.; Wang, S.; Huang, M.; Liu, X.; Zhang, R. Construction of silver sulfadiazine loaded chitosan composite sponges as potential wound dressings. Carbohydr. Polym. 2017, 157, 1963–1970. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Tamer, T.M.; El-Meligy, M.A.; Mohy Eldin, M.S. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. ArabJ. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Alam, M.M.; Mina, M.F.; Akhtar, F. Swelling and hydration properties of acrylamide hydrogel in distilled water. Polym. Plast. Technol. Eng. 2003, 42, 533–542. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 1: Evaluation and testing within a risk management process; ISO 10993-1; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; ISO 10993-12; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 5: Tests for in vitro Cytotoxicity; ISO 10993-5; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Todaro, G.J.; Lazar, G.K.; Green, H. The initiation of cell division in a contact-inhibited mammalian cell line. J. Cell. Comp. Physiol. 1965, 66, 325–333. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Caroline, A.; Schneider, K.; Wayne, S. Rasband and KWE. ImageJ. Fundam Digit. Imaging Med. 2010, 9, 185–188. [Google Scholar]
- Braz, E.M.; De, A.; Silva, S.C.C.C.; Da Silva, D.A.; Carvalho, F.A.; De, A.; Barreto, H.M.; Santos Júnior, L.D.; Da Silva Filho, E.C. Modified chitosan-based bioactive material for antimicrobial application: Synthesis and characterization. Int. J. Biol. Macromol. 2018, 117, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Y.; Cheng, S.; Huang, N.; Leng, Y. Syntheses of Novel Chitosan Derivative with Excellent Solubility, Anticoagulation, and Antibacterial Property by Chemical Modification. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Microwave Preparation and Properties of O-Crosslinked Maleic Acyl Chitosan Adsorbent for Pb21 and Cu2. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar]
- Silva, S.M.L.; Braga, C.R.C.; Fook, M.V.L.; Raposo, C.M.O.; Carvalho, L.H.; Canedo, E.L. Application of Infrared Spectroscopy to Analysis of Chitosan/Clay Nanocomposites. Infrared Spectrosc. Mater. Sci. Eng. Technol. 2011, 7, 43–62. [Google Scholar]
- Boschetto, F.; Doan, H.N.; Vo, P.P.; Zanocco, M.; Zhu, W.; Sakai, W. Antibacterial and Osteoconductive Effects of Chitosan/Polyethylene Oxide (PEO)/Bioactive Glass Nanofibers for Orthopedic Applications. Appl. Sci. 2020, 10, 2360. [Google Scholar] [CrossRef]
- Queiroz, M.; Sassaki, G.L.; Alexandre, H.; Rocha, O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation. Marine. Drugs 2015, 1, 141–158. [Google Scholar]
- Inta, O.; Yoksan, R.; Limtrakul, J. Hydrophobically modified chitosan: A bio-based material for antimicrobial active film. Mater. Sci. Eng. C 2014, 42, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Suárez, M.; López-Quintela, M.A.; Lazzari, M. Preparation and characterization of crosslinked chitosan/gelatin scaffolds by ice segregation induced self-assembly. Carbohydr. Polym. 2016, 141, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. Chitosan-PVP-nano silver oxide wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2015, 73, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Yu, N.; Caridade, S.G.; Luz, G.M.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Walboomers, X.F.; Mano, J.F. Chitosan/bioactive glass nanoparticle composite membranes for periodontal regeneration. Acta Biomater. 2012, 8, 4173–4180. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, C.; Lode, A.; Gelinsky, M. Hierarchical mesoporous bioactive glass/alginate composite scaffolds fabricated by three-dimensional plotting for bone tissue engineering. Biofabrication 2013, 5, 1–13. [Google Scholar] [CrossRef]
- Jagan Mohini, G.; Krishnamacharyulu, N.; Sahaya Baskaran, G.; Venkateswara Rao, P.; Veeraiah, N. Studies on influence of aluminium ions on the bioactivity of B2O3–SiO2–P2O5–Na2O–CaO glass system by means of spectroscopic studies. Appl. Surf. Sci. 2013, 287, 46–53. [Google Scholar] [CrossRef]
- Shahrabi, S.; Hesaraki, S.; Moemeni, S.; Khorami, M. Structural discrepancies and in vitro nanoapatite formation ability of sol-gel derived glasses doped with different bone stimulator ions. Ceram. Int. 2011, 37, 2737–2746. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Luz, G.M.; Boesel, L.; Campo Del, A.; Mano, J.F. Micropatterning of bioactive glass nanoparticles on chitosan membranes for spatial controlled biomineralization. Langmuir 2012, 28, 6970–6977. [Google Scholar] [CrossRef]
- Harini, B.; Shadamarshan, R.P.K.; Rao, S.H.; Selvamurugan, N.; Balagangadharan, K. Natural and synthetic Polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macromol. 2017, 110, 88–96. [Google Scholar] [CrossRef]
- Humeau, J.; Bravo-San Pedro, J.M.; Vitale, I.; Nuñez, L.; Villalobos, C.; Kroemer, G. Calcium signaling and cell cycle: Progression or death. Cell Calcium. 2018, 70, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Massera, J.; Kokkari, A.; Närhi, T.; Hupa, L. The influence of SrO and CaO in silicate and phosphate bioactive glasses on human gingival fibroblasts. J. Mater. Sci. Mater. Med. 2015, 26, 196. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Meng, G.; Wang, S.; Wu, F.; Huang, W.; Gu, Z. Zn and Sr incorporated 64S bioglasses: Material characterization, in-vitro bioactivity and mesenchymal stem cell responses. Mater. Sci. Eng. C 2015, 52, 242–250. [Google Scholar] [CrossRef]
- Badr-Mohammadi, M.-R.; Hesaraki, S.; Zamanian, A. Mechanical properties and in vitro cellular behavior of zinc- containing nano-bioactive glass doped biphasic calcium phosphate bone substitutes. J. Mater. Sci. Mater. Med. 2013, 25, 185–197. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Putney, J.W. Calcium Signaling, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 1–540. [Google Scholar]
- Mao, L.; Xia, L.; Chang, J.; Liu, J.; Jiang, L.; Wu, C.; Fang, B. The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomater. 2017, 61, 217–232. [Google Scholar] [CrossRef]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Maret, W. Metals on the move: Zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals 2011, 24, 411–418. [Google Scholar] [CrossRef]
- Maywald, M.; Wessels, I.; Rink, L. Zinc signals and immunity. Int. J. Mol. Sci. 2017, 18, 2222. [Google Scholar] [CrossRef]
- Liu, J.; Kang, Y.; Zheng, W.; Song, B.; Wei, L.; Chen, L.; Shao, L. Ion-Shedding Zinc Oxide Nanoparticles Induce Microglial BV2 Cell Proliferation via the ERK and Akt Signaling Pathways. Toxicol. Sci. 2017, 156, 167–178. [Google Scholar]
- Hasan, R.; Rink, L.; Haase, H. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun. 2013, 19, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Wren, A.W.; Towler, M.R.; Boyd, D. The effect of ionic dissolution products of Ca-Sr-Na-Zn-Si bioactive glass on in vitro cytocompatibility. J. Mater. Sci. Mater. Med. 2010, 21, 2827–2834. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Kikuchi, M.; Saito, Y.; Yura, H.; Matsui, T. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef]
- Ueno, H.; Yamada, H.; Tanaka, I.; Kaba, N.; Matsuura, M.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterial 1999, 20, 1407–1414. [Google Scholar] [CrossRef]


| Oxides | Composition (mol%) | ||
|---|---|---|---|
| 45S5 | BGMS10 [24] | BGMS_2Zn [25] | |
| SiO2 | 45 | 47.2 | 47.2 |
| P2O5 | 6 | 2.6 | 2.6 |
| Na2O | 24.5 | 2.3 | 2.3 |
| K2O | 0 | 2.3 | 2.3 |
| CaO | 24.5 | 25.6 | 25.6 |
| MgO | 0 | 10 | 8 |
| SrO | 0 | 10 | 10 |
| ZnO | 0 | 0 | 2 |
| Amount (wt.%/Vtot) | |||||||
|---|---|---|---|---|---|---|---|
| CHT | CHT45S5_5 | CHT45S5_10 | CHTSrMg5 | CHTSrMg10 | CHTZn5 | CHTZn10 | |
| Chitosan | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Acetic Acid | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 45S5 | 0 | 5 | 10 | 0 | 0 | 0 | 0 |
| BGMS10 [24] | 0 | 0 | 0 | 5 | 10 | 0 | 0 |
| BGMS_2Zn [25] | 0 | 0 | 0 | 0 | 0 | 5 | 10 |
| Wavenumber (cm−1) | CHT45S5_5 | CHTSrMg5 | CHTZn5 | Assignment | Abbreviation | Ref. |
|---|---|---|---|---|---|---|
| 3366–3295 | 3307 | 3291 | Overlapping OH & NH stretching vibration | υ (OH & NH) | [59] | |
| 2926–2876 | 2886 | 2931-2868 | 2923–2881 | Symmetric and asymmetric stretching C–H | υas & υs (C–H) | [62] |
| 1063 | 1055 | 1051 | 1055 | C–O symmetric and asymmetric stretching | υs & υas (C–O) | [63,64] |
| 1156 | 1151 | 1151 | 1151 | Stretching β-glycosidic bond between the carbon 1 and 4 of chitosan | υ C 1 & C 4 | [59] |
| 1403–1339 | Deformation of CH2 & CH3 | δ CH2 & CH3 | [59,63] | |||
| 1557 | 1557 | 1553 | 1541 | Amide | [63] | |
| / | 1231 | 1235 | 1235 | Asymmetric stretching Si–O–Si with bonded oxygen | υas Si–O–Si (BO) | [17] |
| / | 1017 | 1017 | 1017 | Asymmetric stretching Si–O–Si | υas Si–O–Si | [17] |
| / | 724–465 | 721–451 | 791–465 | Symmetric stretching & bending Si–O–Si | υs Si–O–Si & δ Si–O–Si | [17] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergi, R.; Bellucci, D.; Salvatori, R.; Cannillo, V. Chitosan-Based Bioactive Glass Gauze: Microstructural Properties, In Vitro Bioactivity, and Biological Tests. Materials 2020, 13, 2819. https://doi.org/10.3390/ma13122819
Sergi R, Bellucci D, Salvatori R, Cannillo V. Chitosan-Based Bioactive Glass Gauze: Microstructural Properties, In Vitro Bioactivity, and Biological Tests. Materials. 2020; 13(12):2819. https://doi.org/10.3390/ma13122819
Chicago/Turabian StyleSergi, Rachele, Devis Bellucci, Roberta Salvatori, and Valeria Cannillo. 2020. "Chitosan-Based Bioactive Glass Gauze: Microstructural Properties, In Vitro Bioactivity, and Biological Tests" Materials 13, no. 12: 2819. https://doi.org/10.3390/ma13122819
APA StyleSergi, R., Bellucci, D., Salvatori, R., & Cannillo, V. (2020). Chitosan-Based Bioactive Glass Gauze: Microstructural Properties, In Vitro Bioactivity, and Biological Tests. Materials, 13(12), 2819. https://doi.org/10.3390/ma13122819








