Luminescent Studies on Germanate Glasses Doped with Europium Ions for Photonic Applications
Abstract
:1. Introduction
2. Material and Methods
3. Results and Discussion
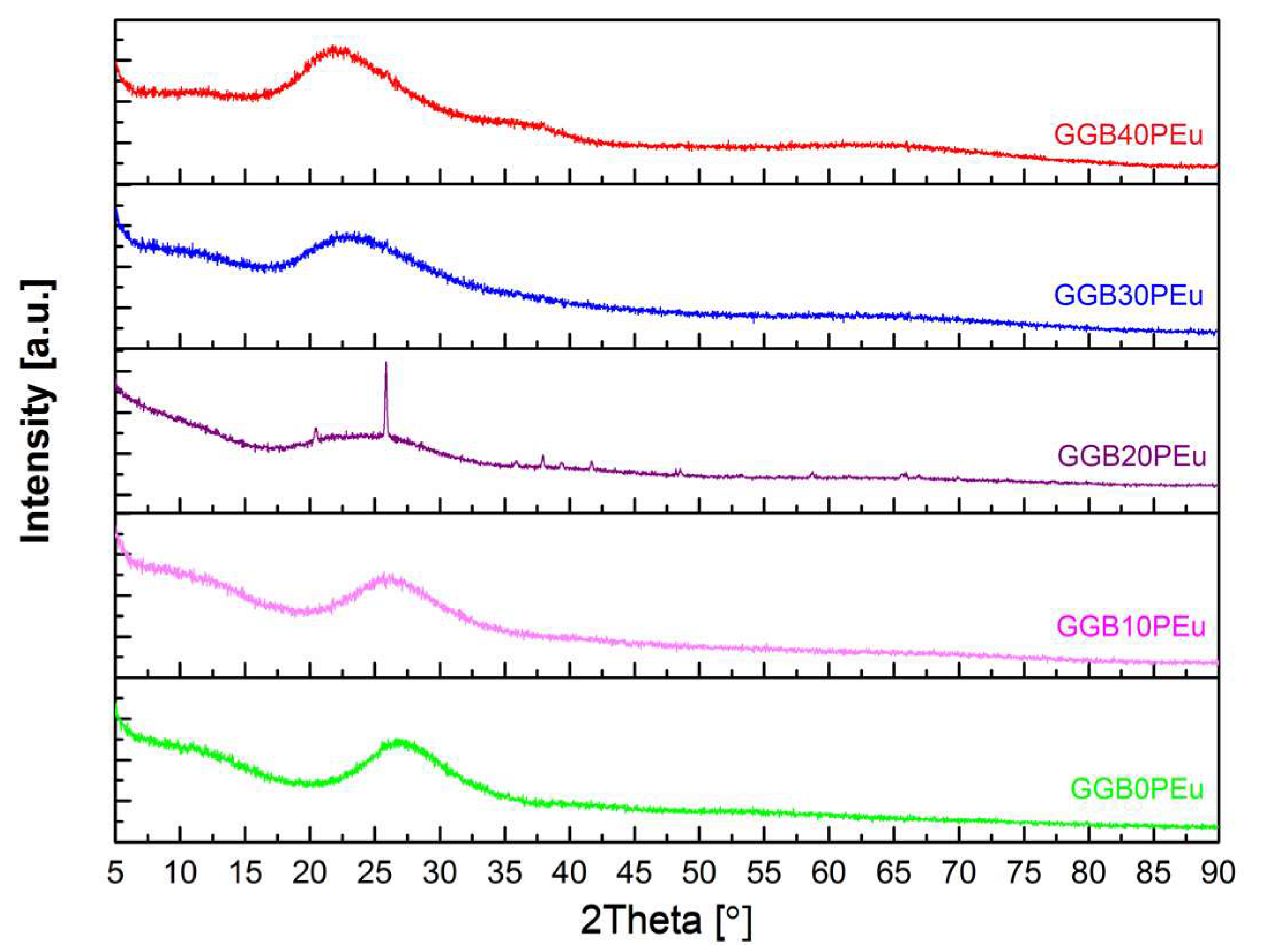
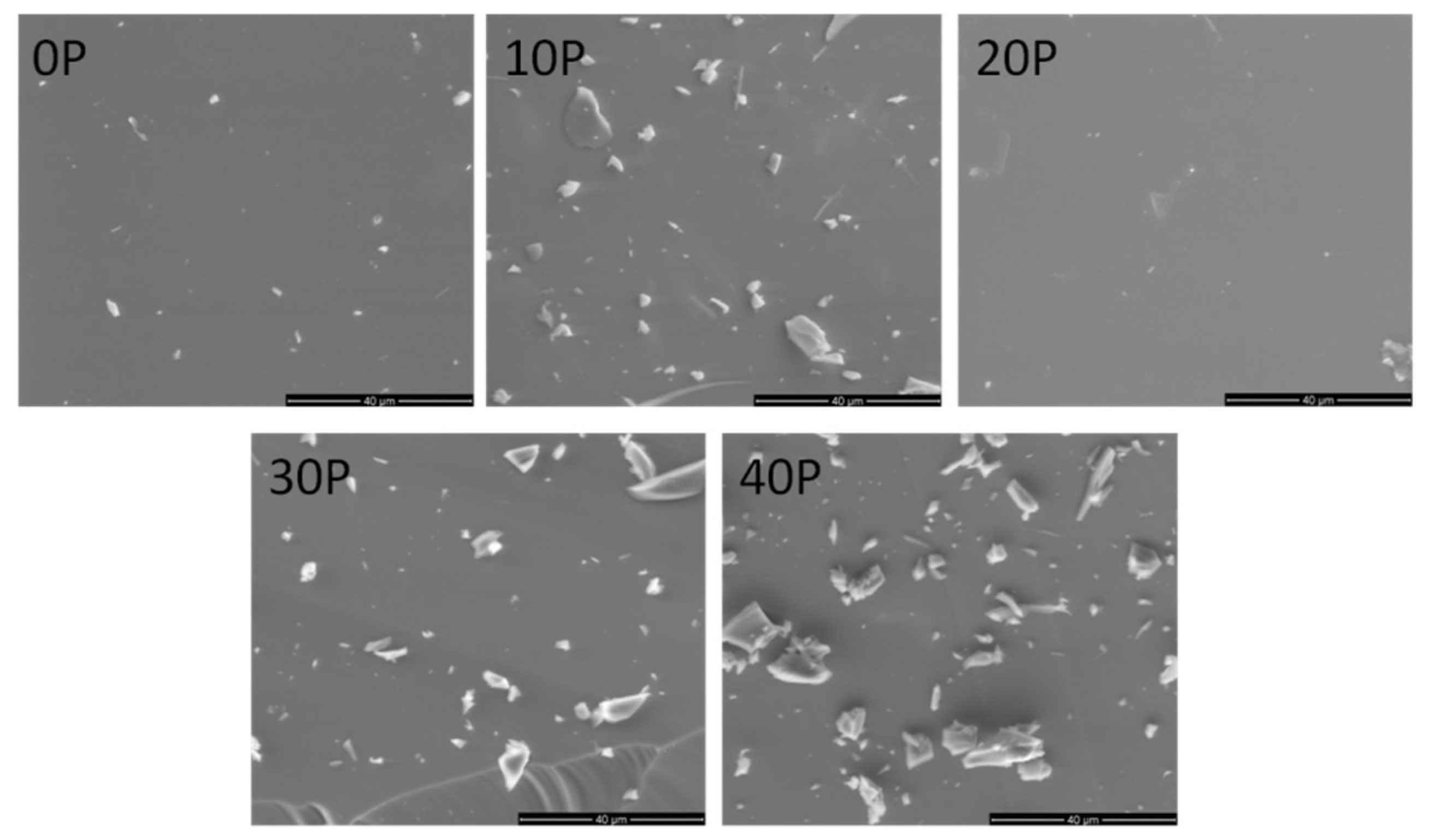
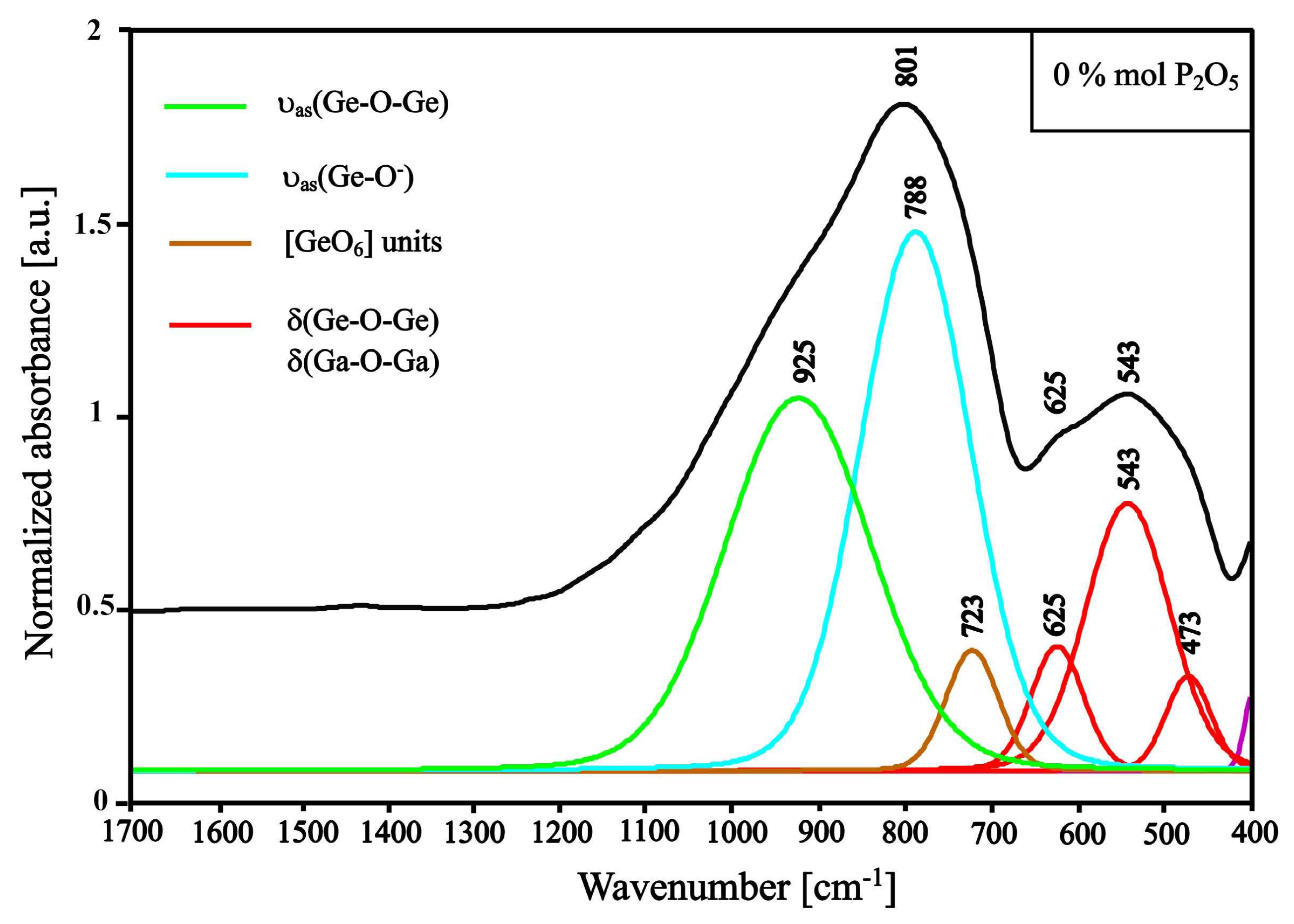
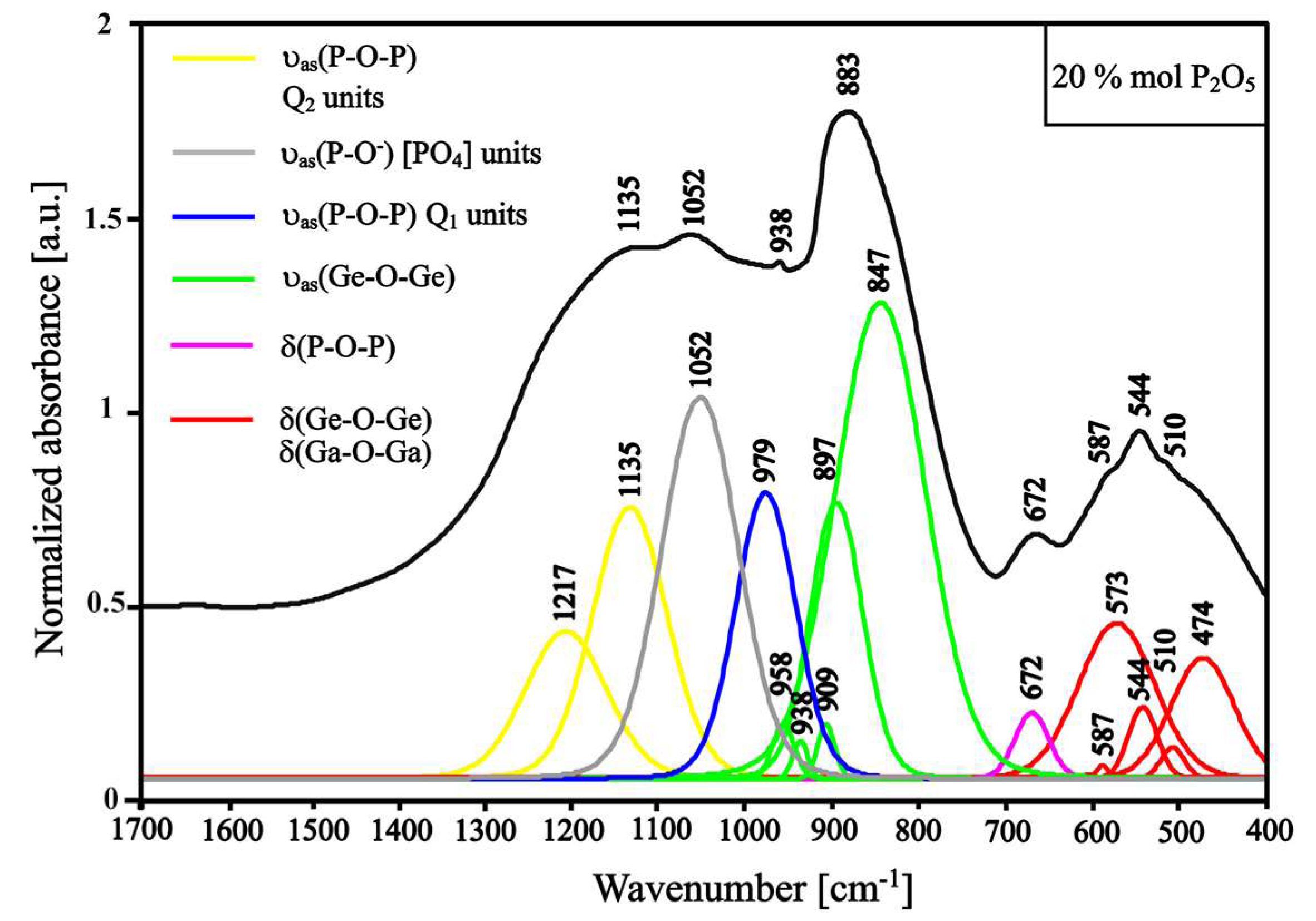
3.1. Structural Studies
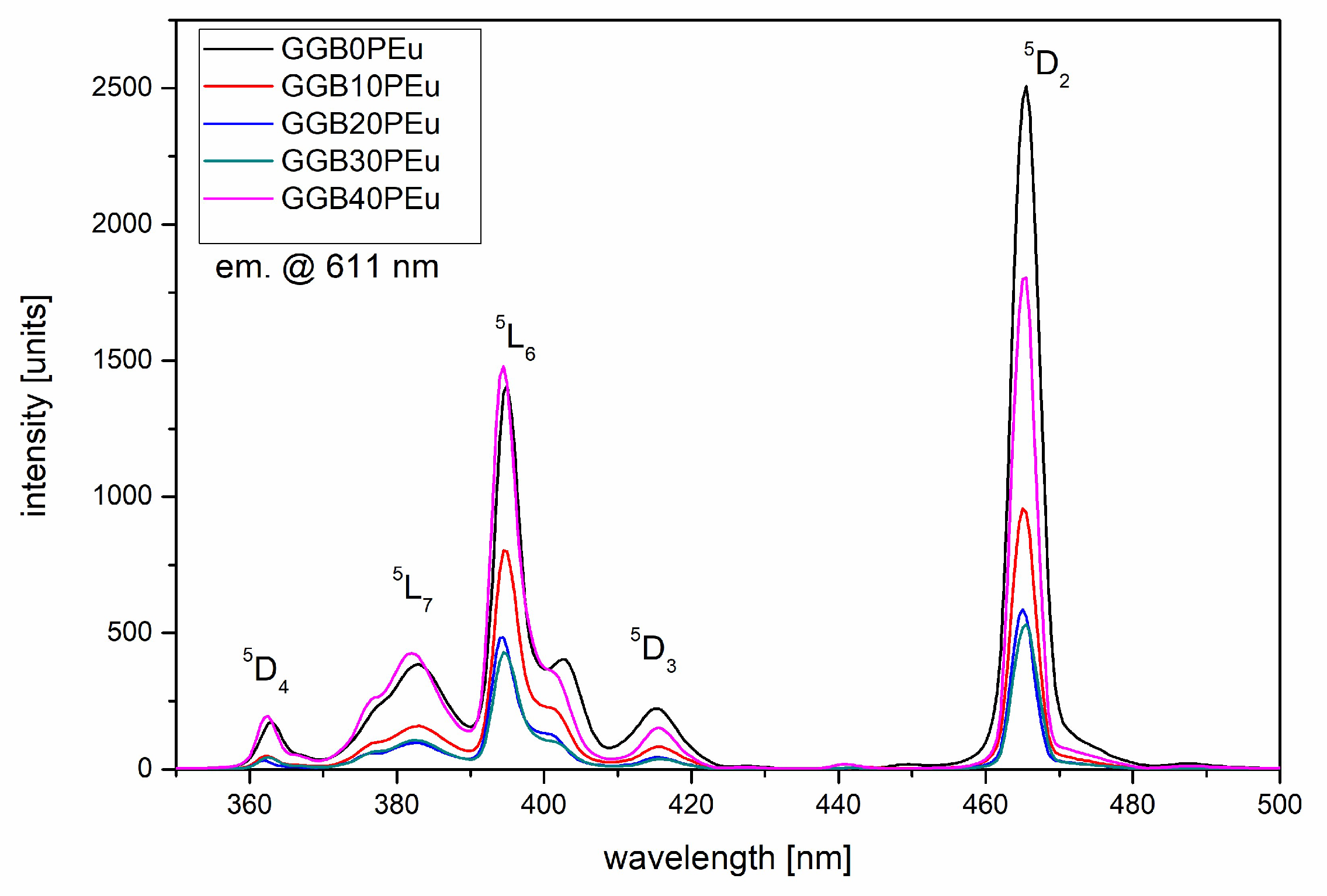
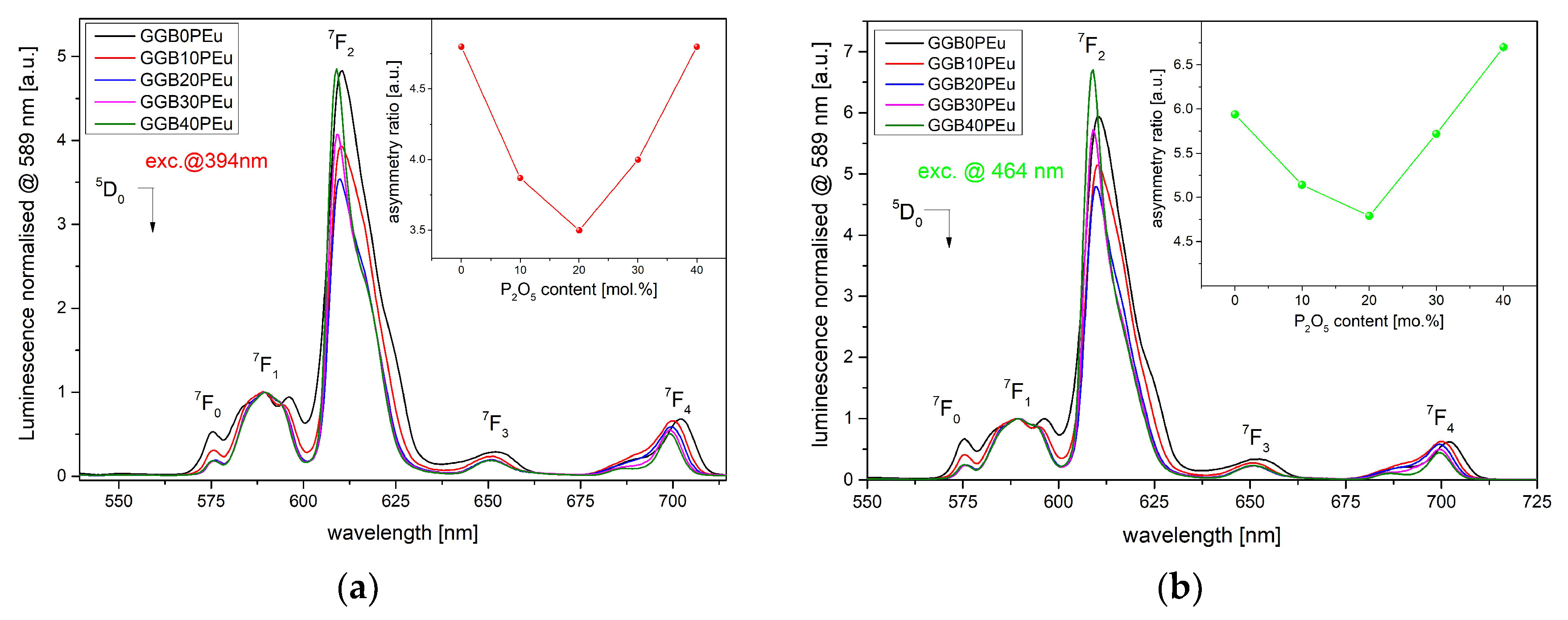
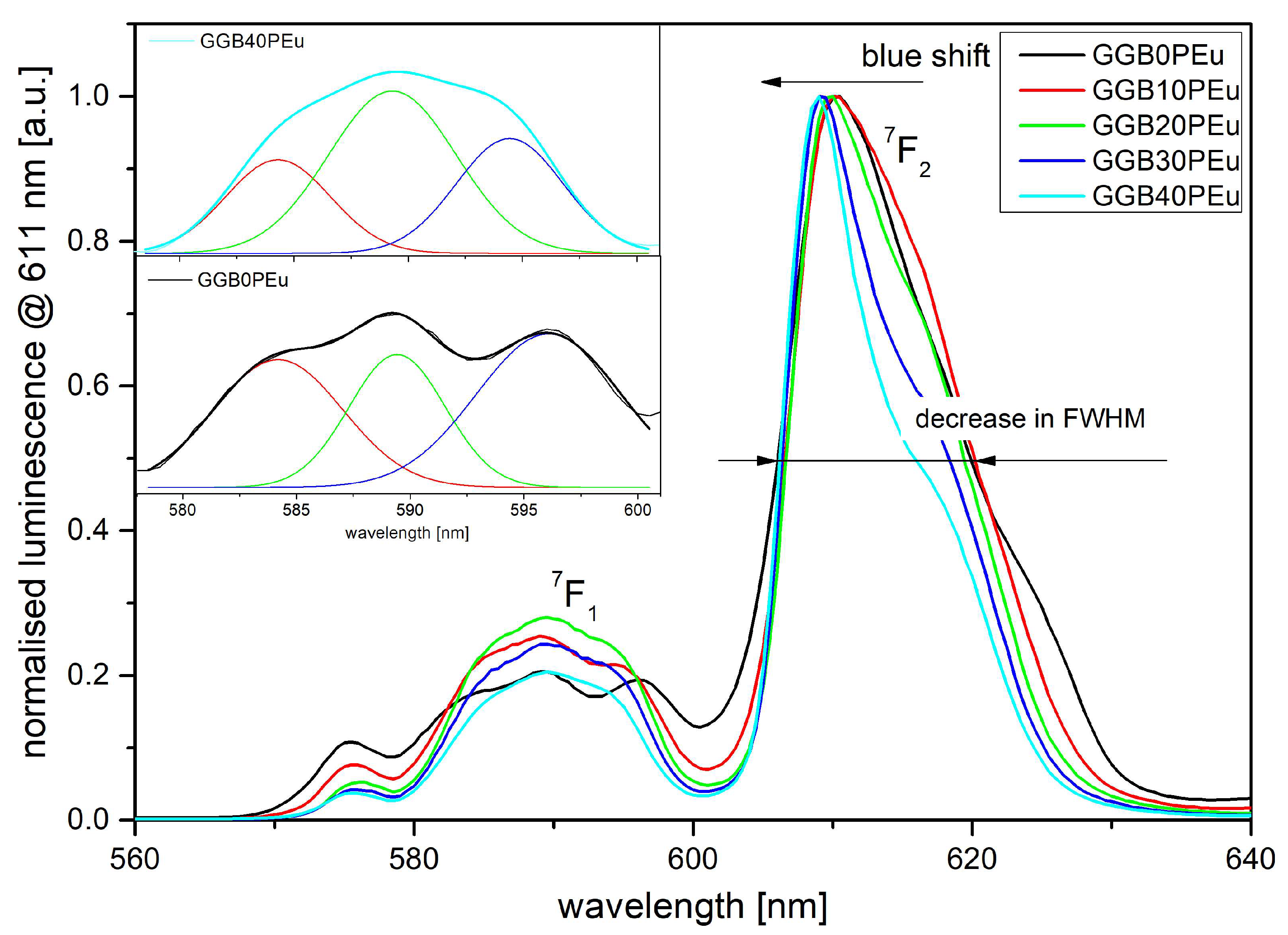
3.2. Photoluminescence Excitation, Emission Spectra, and Luminescence Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gorni, G.; Velázquez, J.J.; Kochanowicz, M.; Dorosz, D.; Balda, R.; Fernández, J.; Durán, A.; Pascual, M.J. Tunable upconversion emission in NaLuF4–glass-ceramic fibers doped with Er3+ and Yb3+. RSC Adv. 2019, 9, 31699–31707. [Google Scholar] [CrossRef] [Green Version]
- Szczodra, A.; Kuusela, L.; Norrbo, I.; Mardoukhi, A.; Hokka, M.; Lastusaari, M.; Petit, L. Successful preparation of fluorine containing glasses with persistent luminescence using the direct doping method. J. Alloys Compd. 2019, 787, 1260–1264. [Google Scholar] [CrossRef]
- Mrázek, J.; Kašík, I.; Procházková, L.; Čuba, V.; Girman, V.; Puchý, V.; Blanc, W.; Peterka, P.; Aubrecht, J.; Cajzl, J.; et al. YAG Ceramic Nanocrystals Implementation into MCVD Technology of Active Optical Fibers. Appl. Sci. 2018, 8, 833. [Google Scholar] [CrossRef] [Green Version]
- Hoon Yeub, J.; Eunsongyi, L.; Soo-Chan, A.; Yeonsoo, L.; Young Chul, J. 3D and 4D printing for optics and metaphotonics. Nanophotonics 2020. [CrossRef]
- Escudero, A.; Becerro, A.I.; Carrillo-Carrión, C.; Núñez, N.O.; Zyuzin, M.V.; Laguna, M.; González-Mancebo, D.; Ocaña, M.; Parak, W.J. Rare earth based nanostructured materials: Synthesis, functionalization, properties and bioimaging and biosensing applications. Nanophotonics 2017, 6, 881. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Sun, J.; Wang, Y.; Liang, Z.; Ma, P.; Zhang, D.; Wang, J.; Niu, J. Synthesis, structure, and luminescent properties of a family of lanthanide-functionalized peroxoniobiophosphates. Sci. Rep. 2017, 7, 10653. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, Z.F.; Tam, H.-Y.; Tao, X. Multifunctional Smart Optical Fibers: Materials, Fabrication, and Sensing Applications. Photonics 2019, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Zmojda, J.; Miluski, P.; Kochanowicz, M. Nanocomposite Antimony-Germanate-Borate Glass Fibers Doped with Eu3+ Ions with Self-Assembling Silver Nanoparticles for Photonic Applications. Appl. Sci. 2018, 8, 790. [Google Scholar] [CrossRef] [Green Version]
- Som, T.; Karmakar, B. Optical properties of Eu3+-doped antimony-oxide-based low phonon disordered matrices. J. Phys. Condens. Matter 2009, 22, 035603. [Google Scholar] [CrossRef]
- Rolli, R.; Camagni, P.; Samoggia, G.; Speghini, A.; Wachtler, M.; Bettinelli, M. Fluorescence line narrowing spectroscopy of a lead germanate glass doped with Eu3+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 2157–2162. [Google Scholar] [CrossRef]
- Żur, L. Structural and luminescence properties of Eu3+, Dy3+ and Tb3+ ions in lead germanate glasses obtained by conventional high-temperature melt-quenching technique. J. Mol. Struct. 2013, 1041, 50–54. [Google Scholar] [CrossRef]
- Sołtys, M.; Pisarska, J.; Leśniak, M.; Sitarz, M.; Pisarski, W.A. Structural and spectroscopic properties of lead phosphate glasses doubly doped with Tb3+ and Eu3+ ions. J. Mol. Struct. 2018, 1163, 418–427. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Żur, L.; Pisarska, J. Optical transitions of Eu3+ and Dy3+ ions in lead phosphate glasses. Opt. Lett. 2011, 36, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Ragin, T.; Baranowska, A.; Kochanowicz, M.; Zmojda, J.; Miluski, P.; Dorosz, D. Study of Mid-Infrared Emission and Structural Properties of Heavy Metal Oxide Glass and Optical Fibre Co-Doped with Ho3+/Yb3+ Ions. Materials 2019, 12, 1238. [Google Scholar] [CrossRef] [Green Version]
- Prakash, M.R.; Neelima, G.; Kummara, V.K.; Ravi, N.; Viswanath, C.S.D.; Rao, T.S.; Jilani, S.M. Holmium doped bismuth-germanate glasses for green lighting applications: A spectroscopic study. Opt. Mater. 2019, 94, 436–443. [Google Scholar] [CrossRef]
- Nalin, M.; Messaddeq, Y.; Ribeiro, S.J.L.; Poulain, M.; Briois, V.; Brunklaus, G.; Rosenhahn, C.; Mosel, B.D.; Eckert, H. Structural organization and thermal properties of the Sb2O3–SbPO4 glass system. J. Mater. Chem. 2004, 14, 3398–3405. [Google Scholar] [CrossRef]
- Moustafa, S.Y.; Sahar, M.R.; Ghoshal, S.K. Spectroscopic attributes of Er3+ ions in antimony phosphate glass incorporated with Ag nanoparticles: Judd-Ofelt analysis. J. Alloy. Compd. 2017, 712, 781–794. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Li, T.; Jiang, Z.H.; Ji, X.H.; Buddhudu, S. 980nm laser-diode-excited intense blue upconversion in Tm3+/Yb3+-codoped gallate–bismuth–lead glasses. Appl. Phys. Lett. 2005, 87, 171911. [Google Scholar] [CrossRef]
- Wen, X.; Tang, G.; Wang, J.; Chen, X.; Qian, Q.; Yang, Z. Tm3+ doped barium gallo-germanate glass single-mode fibers for 2.0 μm laser. Opt. Express 2015, 23, 7722–7731. [Google Scholar] [CrossRef]
- Kochanowicz, M.; Żmojda, J.; Miluski, P.; Baranowska, A.; Ragin, T.; Dorosz, J.; Kuwik, M.; Pisarski, W.A.; Pisarska, J.; Leśniak, M.; et al. 2 μm emission in gallo-germanate glasses and glass fibers co-doped with Yb3+/Ho3+ and Yb3+/Tm3+/Ho3+. J. Lumin. 2019, 211, 341–346. [Google Scholar] [CrossRef]
- Jewell, J.; Busse, L.; Crahan, K.; Harbison, B.; Aggarwal, I. Optical Properties of BaO-Ga2O3-GeO2 Glasses for Fiber and Bulk Optical Applications; SPIE: Bellingham, WA, USA, 1994; Volume 2287. [Google Scholar]
- Jewell, J.M.; Higby, P.L.; Aggarwal, I.D. Properties of BaO–R2O3– Ga2O3–GeO2 (R = Y, Al, La, and Gd) Glasses. J. Am. Ceram. Soc. 1994, 77, 697–700. [Google Scholar] [CrossRef]
- Zur, L.; Janek, J.; Pietrasik, E.; Sołtys, M.; Pisarska, J.; Pisarski, W.A. Influence of MO/MF2 modifiers (M = Ca, Sr, Ba) on spectroscopic properties of Eu3+ ions in germanate and borate glasses. Opt. Mater. 2016, 61, 59–63. [Google Scholar] [CrossRef]
- Jadach, R.; Zmojda, J.; Kochanowicz, M.; Miluski, P.; Lesniak, M.; Sołtys, M.; Pisarska, J.; Pisarski, W.; Lukowiak, A.; Sitarz, M.; et al. Spectroscopic Properties of Rare Earth Doped Germanate Glasses; SPIE: Bellingham, WA, USA, 2018; Volume 10683. [Google Scholar]
- Szal, R.; Zmojda, J.; Kochanowicz, M.; Miluski, P.; Dorosz, J.; Lesniak, M.; Jeleń, P.; Starzyk, B.; Sitarz, M.; Kuwik, M.; et al. Spectroscopic properties of antimony modified germanate glass doped with Eu3+ ions. Ceram. Int. 2019, 45, 24811–24817. [Google Scholar] [CrossRef]
- Nassar, E.; Ciuffi, K.; Calefi, P.; Ávila, L.; Bandeira, L.; Cestari, A.; Marçal, A.L.; Matos, M. Europium III: Different Emission Apectra in Different Matrices, the Same Element; Nova Science Publishers: Hauppauge, NY, USA, 2010. [Google Scholar]
- Jha, K.; Jayasimhadri, M. Structural and emission properties of Eu3+-doped alkaline earth zinc-phosphate glasses for white LED applications. J. Am. Ceram. Soc. 2017, 100, 1402–1411. [Google Scholar] [CrossRef]
- Baranowska, A.; Leśniak, M.; Kochanowicz, M.; Żmojda, J.; Miluski, P.; Dorosz, D. Crystallization Kinetics and Structural Properties of the 45S5 Bioactive Glass and Glass-Ceramic Fiber Doped with Eu3+. Materials 2020, 13, 1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezerskyte, E.; Grigorjevaite, J.; Minderyte, A.; Saitzek, S.; Katelnikovas, A. Temperature-Dependent Luminescence of Red-Emitting Ba2Y5B5O17: Eu3+ Phosphors with Efficiencies Close to Unity for Near-UV LEDs. Materials 2020, 13, 763. [Google Scholar] [CrossRef] [Green Version]
- Bouchouicha, H.; Panczer, G.; de Ligny, D.; Guyot, Y.; Ternane, R. Luminescent properties of Eu-doped calcium aluminosilicate glass-ceramics: A potential tunable luminophore. Opt. Mater. 2018, 85, 41–47. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Q.; Song, J.; Xiao, Z.; Qiang, Y.; Ye, X.; You, W.; Lu, A. A novel Eu3+-doped phosphate glass for reddish orange emission: Preparation, structure and fluorescence properties. J. Lumin. 2020, 221, 117041. [Google Scholar] [CrossRef]
- Rakpanich, S.; Wantana, N.; Kaewkhao, J. Development of bismuth borosilicate glass doped with Eu3+ for reddish orange emission materials application. Mater. Today Proc. 2017, 4, 6389–6396. [Google Scholar] [CrossRef]
- Orozco Hinostroza, I.E.; Desirena, H.; Hernandez, J.; Molina, J.; Moreno, I.; De la Rosa, E. Eu3+-doped glass as a color rendering index enhancer in phosphor-in-glass. J. Am. Ceram. Soc. 2018, 101, 2914–2920. [Google Scholar] [CrossRef]
- Park, J.Y.; Chung, J.W.; Park, S.J.; Yang, H.K. Versatile fluorescent CaGdAlO4:Eu3+ red phosphor for latent fingerprints detection. J. Alloy. Compd. 2020, 824, 153994. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Bu, X.; Fu, Y.; Wang, Z.; Liu, Q. Recent advances in dual-emission ratiometric fluorescence probes for chemo/biosensing and bioimaging of biomarkers. Coord. Chem. Rev. 2019, 383, 82–103. [Google Scholar] [CrossRef]
- Pilch, M.; Ortyl, J.; Chachaj-Brekiesz, A.; Galek, M.; Popielarz, R. Europium-based luminescent sensors for mapping pressure distribution on surfaces. Sens. Actuators B Chem. 2020, 305, 127409. [Google Scholar] [CrossRef]
- Vasilchenko, I.; Carpentiero, A.; Chiappini, A.; Chiasera, A.; Vaccari, A.; Lukowiak, A.; Righini, G.C.; Vereshagin, V.; Ferrari, M. Influence of phosphorous precursors on spectroscopic properties of Er3+-activated SiO2-HfO2-P2O5planar waveguides. J. Phys. Conf. Ser. 2014, 566, 012018. [Google Scholar] [CrossRef] [Green Version]
- Pisarska, J.; Kaczmarczyk, B.; Mazurak, Z.; Żelechower, M.; Goryczka, T.; Pisarski, W.A. Influence of P2O5 concentration on structural, thermal and optical behavior of Pr-activated fluoroindate glass. Phys. B Condens. Matter 2007, 388, 331–336. [Google Scholar] [CrossRef]
- Zmojda, J.; Kochanowicz, M.; Miluski, P.; Baranowska, A.; Pisarski, W.A.; Pisarska, J.; Jadach, R.; Sitarz, M.; Dorosz, D. Optical Characterization of Nano- and Microcrystals of EuPO4 Created by One-Step Synthesis of Antimony-Germanate-Silicate Glass Modified by P2O5. Materials 2017, 10, 1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulshreshtha, S.K.; Jayakumar, O.D.; Vishwanadh, B.; Sudarsan, V. Probing the interface of core shell particles of GaPO4 and AlPO4 by 31P MAS NMR spectroscopy. Solid State Sci. 2011, 13, 484–487. [Google Scholar] [CrossRef]
- Labéguerie, P.; Harb, M.; Baraille, I.; Rérat, M. Structural, electronic, elastic, and piezoelectric properties of α-quartz and M X O 4 (M= Al, Ga, Fe; X= P, As) isomorph compounds: A DFT study. Phys. Rev. B 2010, 81, 045107. [Google Scholar] [CrossRef]
- Jadach, R.; Zmojda, J.; Kochanowicz, M.; Miluski, P.; Pisarska, J.; Pisarski, W.A.; Sołtys, M.; Lesniak, M.; Sitarz, M.; Dorosz, D. Investigation of the aluminum oxide content on structural and optical properties of germanium glasses doped with RE ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 143–152. [Google Scholar] [CrossRef]
- Michel, D.; Colomban, P.; Abolhassani, S.; Voyron, F.; Kahn-Harai, A. Germanium mullite: Structure and vibrational spectra of gels, glasses and ceramics. J. Eur. Ceram. Soc. 1996, 16, 161–168. [Google Scholar] [CrossRef]
- Kochanowicz, M.; Zmojda, J.; Miluski, P.; Jelen, P.; Sitarz, M.; Dorosz, D. Spectroscopic investigations of Yb3+/Ho3+ and Yb3+/Tm3+/Ho3+ co-doped germanate glasses and optical fibers. In Proceedings of the 2016, 18th International Conference on Transparent Optical Networks (ICTON), Trento, Italy, 10–14 July 2016; pp. 1–4. [Google Scholar]
- Zmojda, J.; Kochanowicz, M.; Miluski, P.; Leśniak, M.; Sitarz, M.; Pisarski, W.; Pisarska, J.; Dorosz, D. Effect of GeO2 content on structural and spectroscopic properties of antimony glasses doped with Sm3+ ions. J. Mol. Struct. 2016, 1126, 207–212. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Li, H.; Hu, L.L. Statistical structure analysis of GeO2 modified Yb3+: Phosphate glasses based on Raman and FTIR study. J. Alloy. Compd. 2017, 698, 103–113. [Google Scholar] [CrossRef]
- Stoch, P.; Stoch, A.; Ciecinska, M.; Krakowiak, I.; Sitarz, M. Structure of phosphate and iron-phosphate glasses by DFT calculations and FTIR/Raman spectroscopy. J. Non-Cryst. Solids 2016, 450, 48–60. [Google Scholar] [CrossRef]
- Stefanovsky, S.V.; Stefanovsky, O.I.; Danilov, S.S.; Kadyko, M.I. Phosphate-based glasses and glass ceramics for immobilization of lanthanides and actinides. Ceram. Int. 2019, 45, 9331–9338. [Google Scholar] [CrossRef]
- Jha, P.K.; Pandey, O.P.; Singh, K. FTIR spectral analysis and mechanical properties of sodium phosphate glass–ceramics. J. Mol. Struct. 2015, 1083, 278–285. [Google Scholar] [CrossRef]
- Higby, P.L.; Aggarwal, I.D. Properties of barium gallium germanate glasses. J. Non-Cryst. Solids 1993, 163, 303–308. [Google Scholar] [CrossRef]
- Sadiq, M.; Bensitel, M.; Nohair, K.; Leglise, J.; Lamonier, C. Effect of calcination temperature on the structure of vanadium phosphorus oxide materials and their catalytic activity in the decomposition of 2-propanol. J. Saudi Chem. Soc. 2012, 16, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Wang, Z.; Wu, Q.; Yang, W.; Yang, H.; Xue, X. Evaluation of the role of inherent Ca2+ in phosphorus removal from wastewater system. Water Sci. Technol. 2015, 73, 1644–1651. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium (III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Kindrat, I.I.; Padlyak, B.V. Luminescence properties and quantum efficiency of the Eu-doped borate glasses. Opt. Mater. 2018, 77, 93–103. [Google Scholar] [CrossRef]
- Rice, D.K.; DeShazer, L.G. Spectral Broadening of Europium Ions in Glass. Phys. Rev. 1969, 186, 387–392. [Google Scholar] [CrossRef]
- You, H.; Nogami, M. Optical Properties and Local Structure of Eu3+ Ions in Sol−Gel TiO2−SiO2 Glasses. J. Phys. Chem. B 2004, 108, 12003–12008. [Google Scholar] [CrossRef]
- Wen, H.; Jia, G.; Duan, C.-K.; Tanner, P.A. Understanding Eu3+ emission spectra in glass. Phys. Chem. Chem. Phys. 2010, 12, 9933–9937. [Google Scholar] [CrossRef] [PubMed]


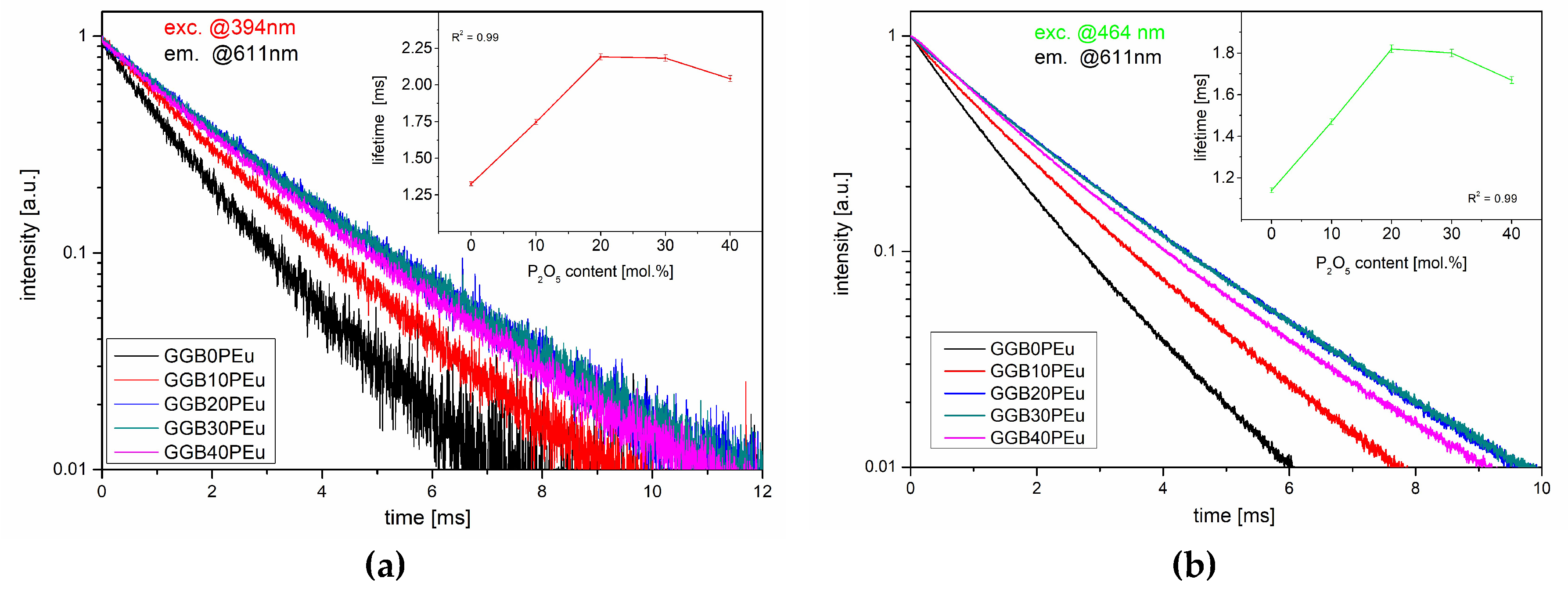
| Glass Sample | ED/MD Ratio | τ394nm [ms] ± 0.01 ms | τ464nm [ms] ± 0.01 ms | |
|---|---|---|---|---|
| exc. @394 nm | exc.@464 nm | |||
| GGB10P | 4.8 | 5.9 | 1.32 | 1.14 |
| GGB10P | 3.9 | 5.1 | 1.74 | 1.47 |
| GGB20P | 3.5 | 4.8 | 2.19 | 1.82 |
| GGB30P | 4 | 5.7 | 2.18 | 1.80 |
| GGB40P | 4.8 | 6.7 | 2.04 | 1.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żmojda, J.; Kochanowicz, M.; Miluski, P.; Golonko, P.; Baranowska, A.; Ragiń, T.; Dorosz, J.; Kuwik, M.; Pisarski, W.; Pisarska, J.; et al. Luminescent Studies on Germanate Glasses Doped with Europium Ions for Photonic Applications. Materials 2020, 13, 2817. https://doi.org/10.3390/ma13122817
Żmojda J, Kochanowicz M, Miluski P, Golonko P, Baranowska A, Ragiń T, Dorosz J, Kuwik M, Pisarski W, Pisarska J, et al. Luminescent Studies on Germanate Glasses Doped with Europium Ions for Photonic Applications. Materials. 2020; 13(12):2817. https://doi.org/10.3390/ma13122817
Chicago/Turabian StyleŻmojda, Jacek, Marcin Kochanowicz, Piotr Miluski, Piotr Golonko, Agata Baranowska, Tomasz Ragiń, Jan Dorosz, Marta Kuwik, Wojciech Pisarski, Joanna Pisarska, and et al. 2020. "Luminescent Studies on Germanate Glasses Doped with Europium Ions for Photonic Applications" Materials 13, no. 12: 2817. https://doi.org/10.3390/ma13122817
APA StyleŻmojda, J., Kochanowicz, M., Miluski, P., Golonko, P., Baranowska, A., Ragiń, T., Dorosz, J., Kuwik, M., Pisarski, W., Pisarska, J., Szal, R., Mach, G., Starzyk, B., Leśniak, M., Sitarz, M., & Dorosz, D. (2020). Luminescent Studies on Germanate Glasses Doped with Europium Ions for Photonic Applications. Materials, 13(12), 2817. https://doi.org/10.3390/ma13122817






