Trace Organic Compound Removal from Wastewater Reverse-Osmosis Concentrate by Advanced Oxidation Processes with UV/O3/H2O2
Abstract
1. Introduction
2. Materials and Methods
2.1. Analyte Selection
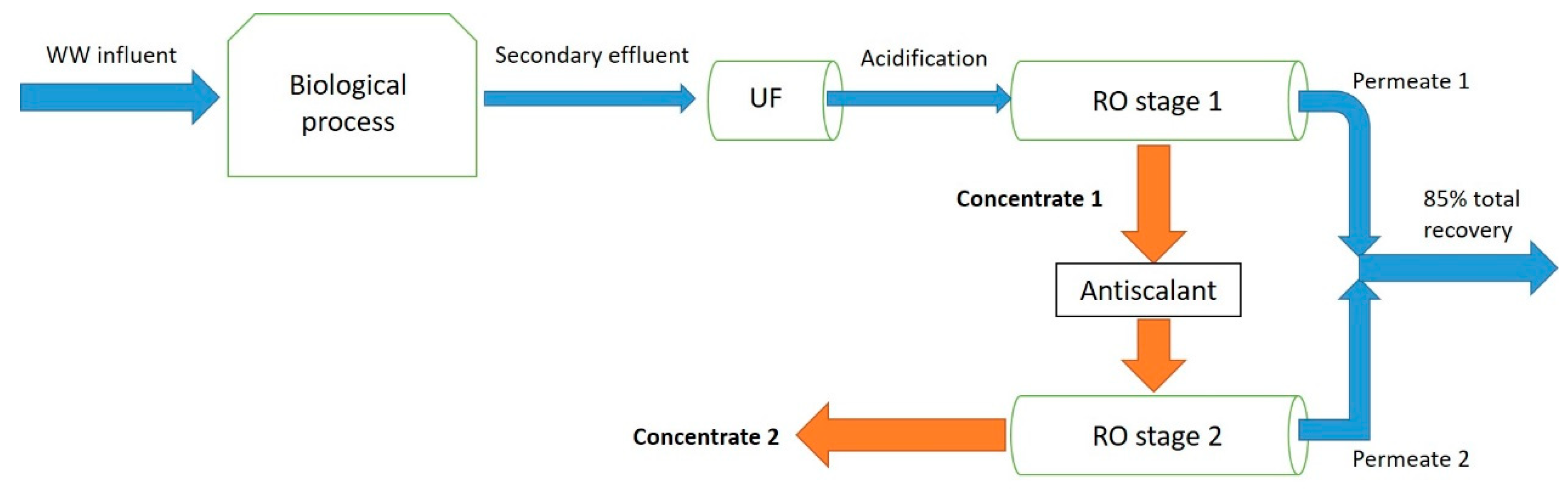
2.2. WWROC Sample Preparation
- Samples adjusted to pH 6,
- Samples adjusted to pH 6 and addition of 60 ppm H2O2–1 ppm H2O2 per 1 ppm initial dissolved organic carbon (DOC),
- Increase in pH to 10.5 with 1N NaOH and removal of the precipitate with a 2.7-µm filter.
2.3. Laboratory-Scale AOP Experiments
2.3.1. Ozone-Based AOP Experiments
2.3.2. UV Photolysis and AOP-Based Experiments
2.4. Analysis
2.4.1. Sample Preparation
2.4.2. Analytical Measurements
3. Results and Discussion
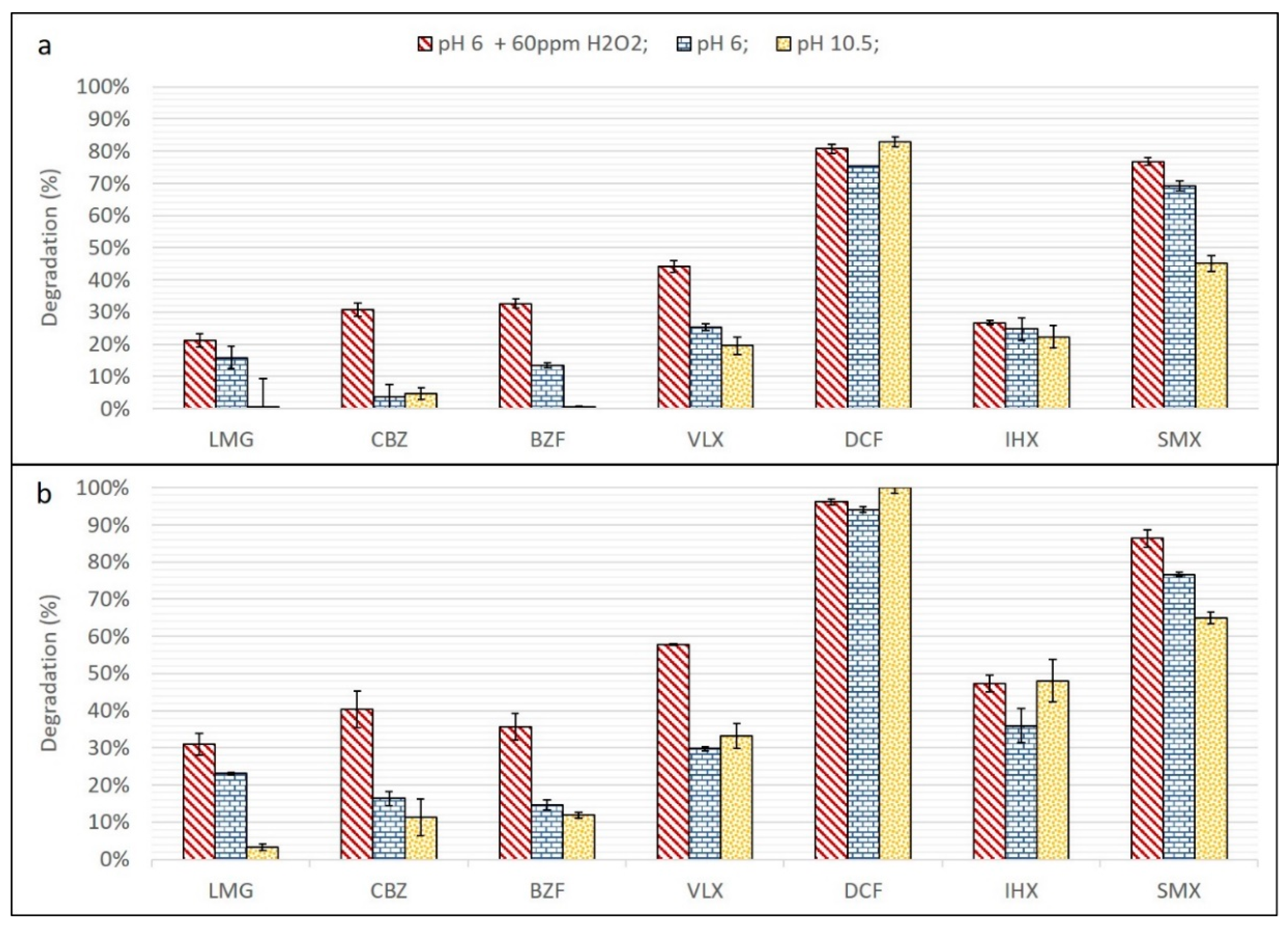
3.1. UV and UV/H2O2
3.2. OH· Scavengers
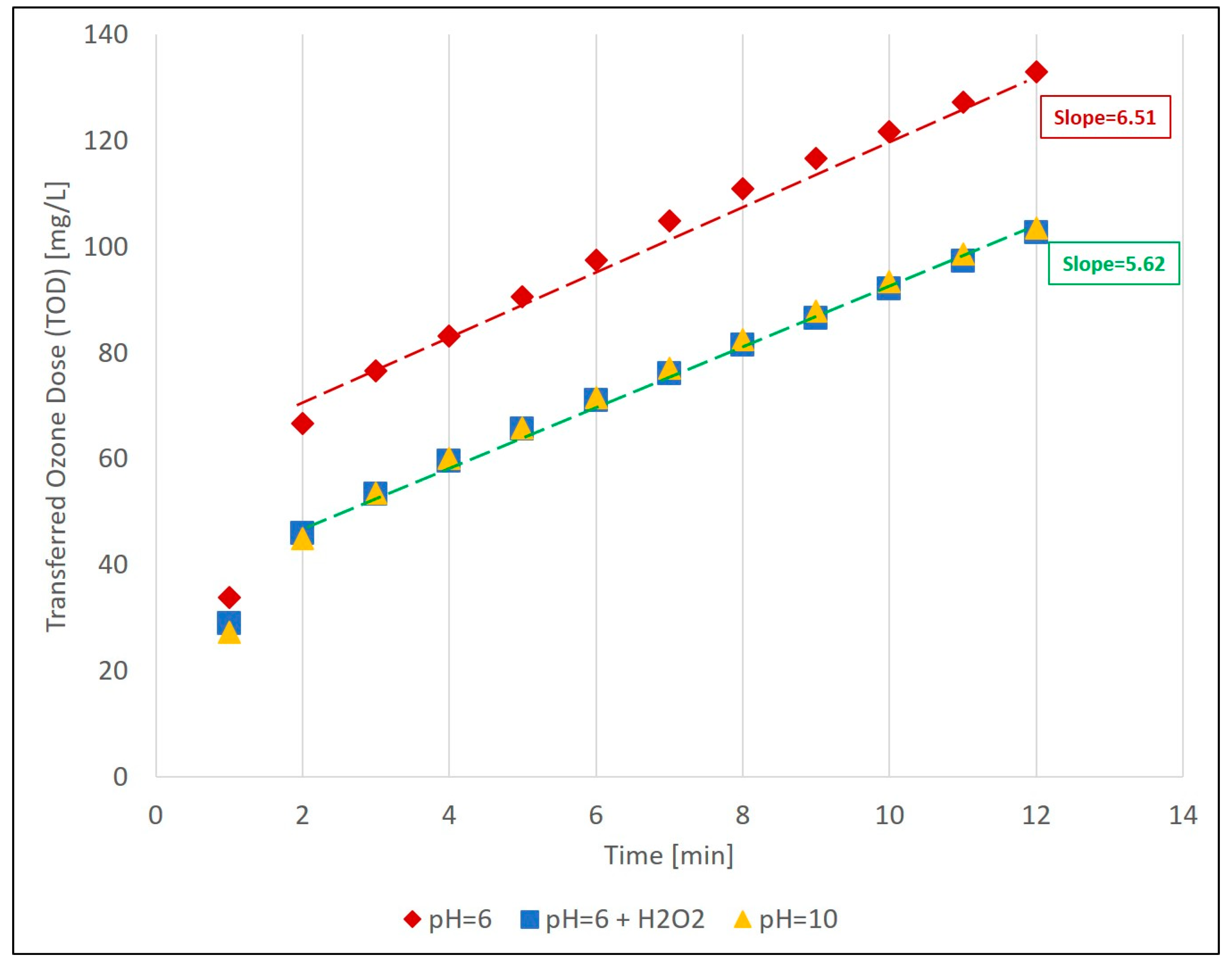
3.3. Ozone and O3/H2O2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNESCO. Leaving No One behind: How Far on the Way to Universal Primary and Secondary Education? UNESCO Digital Library. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000245238 (accessed on 8 January 2020).
- UNPD, Department of Economic and Social Affairs of the United Nations Population Division (UNPD). World Urbanization Prospects: The 2014 Revision, Highlights (ST/ESA/SER.A/352); UNPD: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- WWAP. Wastewater: The Untapped Resource: The United Nations World Water Development Report 2017; UNESCO: Paris, France, 2017. [Google Scholar]
- Wintgens, T.; Melin, T.; Schäfer, A.; Khan, S.; Muston, M.; Bixio, D.; Thoeye, C. The role of membrane processes in municipal wastewater reclamation and reuse. Desalination 2005, 178, 1–11. [Google Scholar] [CrossRef]
- Marron, E.L.; Mitch, W.A.; von Gunten, U.; Sedlak, D.L. A Tale of Two Treatments: The Multiple Barrier Approach to Removing Chemical Contaminants during Potable Water Reuse. Acc. Chem. Res. 2019, 52, 615–622. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M. Evaluation of an MBR-RO system to produce high quality reuse water: Microbial control, DBP formation and nitrate. Water Res. 2005, 39, 3982–3990. [Google Scholar] [CrossRef]
- Mamo, J.; García-Galán, M.J.; Stefani, M.; Rodríguez-Mozaz, S.; Barceló, D.; Monclús, H.; Rodriguez-Roda, I.; Comas, J. Fate of pharmaceuticals and their transformation products in integrated membrane systems for wastewater reclamation. Chem. Eng. J. 2018, 331, 450–461. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Ricart, M.; Köck-Schulmeyer, M.; Guasch, H.; Bonnineau, C.; Proia, L.; de Alda, M.L.; Sabater, S.; Barceló, D. Pharmaceuticals and pesticides in reclaimed water: Efficiency assessment of a microfiltration-reverse osmosis (MF-RO) pilot plant. J. Hazard. Mater. 2015, 282, 165–173. [Google Scholar] [CrossRef]
- Gur-Reznik, S.; Koren-Menashe, I.; Heller-Grossman, L.; Rufel, O.; Dosoretz, C.G. Influence of seasonal and operating conditions on the rejection of pharmaceutical active compounds by RO and NF membranes. Desalination 2011, 277, 250–256. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef]
- Dogan, E.C.; Yasar, A.; Sen, U.; Aydiner, C. Water recovery from treated urban wastewater by ultrafiltration and reverse osmosis for landscape irrigation. Urban Water J. 2016. [CrossRef]
- Joss, A.; Baenninger, C.; Foa, P.; Koepke, S.; Krauss, M.; McArdell, C.S.; Rottermann, K.; Wei, Y.; Zapata, A.; Siegrist, H. Water reuse: >90% water yield in MBR/RO through concentrate recycling and CO2 addition as scaling control. Water Res. 2011, 45, 6141–6151. [Google Scholar] [CrossRef]
- Justo, A.; González, O.; Aceña, J.; Pérez, S.; Barceló, D.; Sans, C.; Esplugas, S. Pharmaceuticals and organic pollution mitigation in reclamation osmosis brines by UV/H2O2 and ozone. J. Hazard. Mater. 2013, 263, 268–274. [Google Scholar] [CrossRef]
- Arola, K.; van der Bruggen, B.; Mänttäri, M.; Kallioinen, M. Treatment options for nanofiltration and reverse osmosis concentrates from municipal wastewater treatment: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1–68. [Google Scholar] [CrossRef]
- Azerrad, S.P.; Isaacs, M.; Dosoretz, C.G. Integrated treatment of reverse osmosis brines coupling electrocoagulation with advanced oxidation processes. Chem. Eng. J. 2019, 356, 771–780. [Google Scholar] [CrossRef]
- Jamil, S.; Loganathan, P.; Kandasamy, J.; Listowski, A.; Khourshed, C.; Naidu, R.; Vigneswaran, S. Removal of dissolved organic matter fractions from reverse osmosis concentrate: Comparing granular activated carbon and ion exchange resin adsorbents. J. Environ. Chem. Eng. 2019, 7, 103126. [Google Scholar] [CrossRef]
- Pérez-González, A.; Urtiaga, A.M.; Ibáñez, R.; Ortiz, I. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012, 46, 267–283. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Choi, Y.; Vigneswaran, S. Membrane distillation for wastewater reverse osmosis concentrate treatment with water reuse potential. J. Memb. Sci. 2017, 524, 565–575. [Google Scholar] [CrossRef]
- Jamil, S.; Loganathan, P.; Listowski, A.; Kandasamy, J.; Khourshed, C.; Vigneswaran, S. Simultaneous removal of natural organic matter and micro-organic pollutants from reverse osmosis concentrate using granular activated carbon. Water Res. 2019, 155, 106–114. [Google Scholar] [CrossRef]
- Fick, J.; Lindberg, R.H.; Tysklind, M.; Larsson, D.G.J. Predicted critical environmental concentrations for 500 pharmaceuticals. Regul. Toxicol. Pharmacol. 2010, 58, 516–523. [Google Scholar] [CrossRef]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef]
- aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment-Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Avisar, D.; Lester, Y.; Mamane, H. pH induced polychromatic UV treatment for the removal of a mixture of SMX, OTC and CIP from water. J. Hazard. Mater. 2010, 175, 1068–1074. [Google Scholar] [CrossRef]
- Klatte, S.; Schaefer, H.-C.; Hempel, M. Pharmaceuticals in the environment – A short review on options to minimize the exposure of humans, animals and ecosystems. Sustain. Chem. Pharm. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Lakretz, A.; Mamane, H.; Cikurel, H.; Avisar, D.; Gelman, E.; Zucker, I. The Role of Soil Aquifer Treatment (SAT) for Effective Removal of Organic Matter, Trace Organic Compounds and Microorganisms from Secondary Effluents Pre-Treated by Ozone. Ozone Sci. Eng. 2017, 39, 385–394. [Google Scholar] [CrossRef]
- Dai, C.; Zhou, X.; Zhang, Y.; Duan, Y.; Qiang, Z.; Zhang, T.C. Comparative study of the degradation of carbamazepine in water by advanced oxidation processes. Environ. Technol. 2012, 33, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Lim, T.T.; Chin, S.S.; Fane, A.G. Treatment of organics in reverse osmosis concentrate from a municipal wastewater reclamation plant: Feasibility test of advanced oxidation processes with/without pretreatment. Chem. Eng. J. 2011, 166, 932–939. [Google Scholar] [CrossRef]
- Luster, E.; Avisar, D.; Horovitz, I.; Lozzi, L.; Baker, M.; Grilli, R.; Mamane, H. N-Doped TiO2-Coated Ceramic Membrane for Carbamazepine Degradation in Different Water Qualities. Nanomaterials 2017, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz, N.; Esquius, L.; Grandjean, D.; Magnet, A.; Tungler, A.; de Alencastro, L.F.; Pulgarín, C. Degradation of emergent contaminants by UV, UV/H2O2 and neutral photo-Fenton at pilot scale in a domestic wastewater treatment plant. Water Res. 2013, 47, 5836–5845. [Google Scholar] [CrossRef]
- Lester, Y.; Avisar, D.; Gozlan, I.; Mamane, H. Removal of pharmaceuticals using combination of UV/H2O2/O3 advanced oxidation process. Water Sci. Technol. 2011, 64, 2230–2238. [Google Scholar] [CrossRef]
- Lester, Y.; Avisar, D.; Mamane, H. Photodegradation of the antibiotic sulphamethoxazole in water with UV/H2O2 advanced oxidation process. Environ. Technol. 2010, 31, 175–183. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.; Gunten, U.R.S.V.O.N. Oxidation of Pharmaceuticals during Ozonation and Advanced Oxidation Processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef]
- Zucker, I.; Mamane, H.; Cikurel, H.; Jekel, M.; Hübner, U.; Avisar, D. A hybrid process of biofiltration of secondary effluent followed by ozonation and short soil aquifer treatment for water reuse. Water Res. 2015, 84, 315–322. [Google Scholar] [CrossRef]
- Keen, O.S.; Ferrer, I.; Thurman, E.M.; Linden, K.G. Degradation pathways of lamotrigine under advanced treatment by direct UV photolysis, hydroxyl radicals, and ozone. Chemosphere 2014, 117, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Lester, Y.; Mamane, H.; Zucker, I.; Avisar, D. Treating wastewater from a pharmaceutical formulation facility by biological process and ozone. Water Res. 2013, 47, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Wols, B.A.; Hofman-Caris, C.H.M.; Harmsen, D.J.H.; Beerendonk, E.F. Degradation of 40 selected pharmaceuticals by UV/H2O2. Water Res. 2013, 47, 5876–5888. [Google Scholar] [CrossRef]
- Akao, P.K.; Cohen-Yaniv, V.; Peretz, R.; Kinel-Tahan, Y.; Yehoshua, Y.; Mamane, H. Effect of ozonation on Spirulina platensis filaments by dynamic imaging particle analysis. Biomass Bioenergy 2019, 127, 105247. [Google Scholar] [CrossRef]
- Kim, I.; Yamashita, N.; Tanaka, H. Performance of UV and UV/H2O2 processes for the removal of pharmaceuticals detected in secondary effluent of a sewage treatment plant in Japan. J. Hazard. Mater. 2009, 166, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Lekkerkerker-Teunissen, K.; Benotti, M.J.; Snyder, S.A.; van Dijk, H.C. Transformation of atrazine, carbamazepine, diclofenac and sulfamethoxazole by low and medium pressure UV and UV/H2O2 treatment. Sep. Purif. Technol. 2012, 96, 33–43. [Google Scholar] [CrossRef]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Effect of matrix components on UV/H2O2 and UV/S2O82−advanced oxidation processes for trace organic degradation in reverse osmosis brines from municipal wastewater reuse facilities. Water Res. 2016, 89, 192–200. [Google Scholar] [CrossRef]
- Rosenfeldt, E.J.; Linden, K.G. The ROH,UV concept to characterize and the model UV/H2O2 process in natural waters. Environ. Sci. Technol. 2007, 41, 2548–2553. [Google Scholar] [CrossRef]
- Dong, M.M.; Mezyk, S.P.; Ortiz, F.R. Reactivity of effluent organic matter (EfOM) with hydroxyl radical as a function of molecular weight. Environ. Sci. Technol. 2010, 44, 5714–5720. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B.; Buxton, G.V.; Greenstock, C.L.; Helman, P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Sharpless, C.M.; Linden, K.G. Experimental and model comparisons of low- and medium-pressure Hg lamps for the direct and H2O2 assisted UV photodegradation of N-nitrosodimethylamine in simulated drinking water. Environ. Sci. Technol. 2003, 37, 1933–1940. [Google Scholar] [CrossRef]
- Zucker, I.; Lester, Y.; Avisar, D.; Hübner, U.; Jekel, M.; Weinberger, Y.; Mamane, H. Influence of wastewater particles on ozone degradation of trace organic contaminants. Environ. Sci. Technol. 2015, 49, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Canonica, S.; von Gunten, U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef] [PubMed]
- Gerrity, D.; Lee, Y.; Gamage, S.; Lee, M.; Pisarenko, A.N.; Trenholm, R.A.; von Guntene, U.; Snyder, S.A. Emerging investigators series: Prediction of trace organic contaminant abatement with UV/H2O2: Development and validation of semi-empirical models for municipal wastewater effluents. Environ. Sci. Water Res. Technol. 2016, 2, 460–473. [Google Scholar] [CrossRef]
- Silva, L.L.S.; Moreira, C.G.; Curzio, B.A.; da Fonseca, F.V. Micropollutant Removal from Water by Membrane and Advanced Oxidation Processes—A Review. J. Water Resour. Prot. 2017, 9, 411–431. [Google Scholar] [CrossRef]
- von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Benner, J.; Salhi, E.; Ternes, T.; von Gunten, U. Ozonation of reverse osmosis concentrate: Kinetics and efficiency of beta blocker oxidation. Water Res. 2008, 42, 3003–3012. [Google Scholar] [CrossRef]
- EPA US; Supply Water, Water Resources Division; Smith, C. 2012 Guidelines for Water Reuse; EPA US: Washington, DC, USA, 2012. [Google Scholar]
- Israeli Ministry of Health, Public Health Regulations, (2010). Available online: https://www.health.gov.il/LegislationLibrary/Briut01.pdf (accessed on 25 August 2019).

| Name | Class | KO3 (M−1s−1) | KOH (109 M−1s−1) | Conc. Found in Secondary Effluent, Shafdan, Israel (µg/L) [25] | Conc. in WWROC, Nir-Ezion, Israel (µg/L) |
|---|---|---|---|---|---|
| Iohexol (IHX) [25] | Contrast media | 1.4 | 3.3 | 21.95–42.03 | Not detected |
| Lamotrigine (LMG) [34] | Antiepileptic | 4 | 2.1 | Not tested | 5.35 ± 0.05 |
| Bezafibrate (BZF) [32] | Lipid regulator | 590 | 7.4 | 0.10–0.15 | 1.41 ± 0.14 |
| Venlafaxine (VLX) [35,36] | Antidepressant | 3.3 × 104 | 8.8 | 0.24–0.29 | 1.97 ± 0.05 |
| Sulfamethoxazole (SMX) [32] | Antibiotic | 2.5 × 106 | 5.5 | 0.21–0.40 | 1.79 ± 0.25 |
| Carbamazepine (CBZ) [32] | Antiepileptic | 3 × 105 | 8.8 | 0.87–1.04 | 11.26 ± 0.11 |
| Diclofenac (DCF) [32] | Anti-inflammatory | 1 × 106 | 7.5 | 0.34–1.00 | 2.40 ± 0.20 |
| Parameter | Result |
|---|---|
| pH | 5.9 + 0.1 |
| Conductivity (mS/cm) | 9.1 + 0.1 |
| UVA254 | 1.49 + 0.1 |
| DOC (mg/L) | 63.8 ± 1.1 |
| COD (mg/L) | 183.5 ± 42.5 |
| Magnesium (mg/L) | 160.6 ± 2.5 |
| Calcium (mg/L) | 614.1 ± 4.0 |
| Potassium (mg/L) | 116.0 ± 7.3 |
| Sodium (mg/L) | >1800 |
| Iron (mg/L) | 0.16 ± 0.001 |
| Treatment Method | WWROC Sample Conditions | Predictable Oxidation Mechanism |
|---|---|---|
| UV | pH = 6pH = 10.5 filtered 2.7 µm | Direct photolysis |
| UV/H2O2 | pH = 6 | By HO·(indirect) |
| O3 | pH = 6pH = 10.5 filtered 2.7 µm | Direct ozone reactions for pH = 6; By HO (indirect) for pH = 10.5 |
| O3/H2O2 | pH = 6 | By HO (indirect) |
| Compound | IHX | LMG | SMX | VLX | CBZ | BZF | DCF |
|---|---|---|---|---|---|---|---|
| [M + H] | 821.884 | 256.017 | 254.059 | 278.209 | 237.102 | 362.117 | 296.023 |
| RT (min) | 3.79 | 8.10 | 8.43 | 9.64 | 12.65 | 14.44 | 17.26 |
| Instrument | Manufacturer | Model | Measurement |
|---|---|---|---|
| UV spectrophotometer | Varian | Cary 100 | UV absorbance |
| pH m | Mettler Toledo | MA 235 | pH |
| Conductivity m | IQ | IQ 170 | Conductivity |
| Total organic carbon analyzer | O.I. | Aurora | DOC |
| Inductively coupled plasma | Spectro | Genesis | Metal ions |
| Photometer | Lovibond | MD 600 | Chemical oxidation demand (COD), Color |
| WWROC | WWROC + O3 + H2O2 | |
|---|---|---|
| Visual | Dark yellow | Clear–pale yellow with light “cloudy” precipitation |
| Color (Pt-Co) | 299 | 32 |
| UVA254 (cm−1) | 1.489 | 0.513 |
| DOC (ppm) | 63.8 ± 1.1 | 55.1 ± 0.8 |
| COD (mgO2/L) | 183.5 ± 42.5 | 101.5 ± 2.5 |
| Copper (ppb) | 540.7 ± 5.7 | 537.5 ± 4.9 |
| Manganese (ppb) | 223.8 ± 4.0 | 215.5 ± 4.0 |
| Nickel (ppb) | 650.2 ± 11.9 | 656.2 ± 10.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, A.; Mamane, H.; Lester, Y.; Avisar, D. Trace Organic Compound Removal from Wastewater Reverse-Osmosis Concentrate by Advanced Oxidation Processes with UV/O3/H2O2. Materials 2020, 13, 2785. https://doi.org/10.3390/ma13122785
Kaplan A, Mamane H, Lester Y, Avisar D. Trace Organic Compound Removal from Wastewater Reverse-Osmosis Concentrate by Advanced Oxidation Processes with UV/O3/H2O2. Materials. 2020; 13(12):2785. https://doi.org/10.3390/ma13122785
Chicago/Turabian StyleKaplan, Aviv, Hadas Mamane, Yaal Lester, and Dror Avisar. 2020. "Trace Organic Compound Removal from Wastewater Reverse-Osmosis Concentrate by Advanced Oxidation Processes with UV/O3/H2O2" Materials 13, no. 12: 2785. https://doi.org/10.3390/ma13122785
APA StyleKaplan, A., Mamane, H., Lester, Y., & Avisar, D. (2020). Trace Organic Compound Removal from Wastewater Reverse-Osmosis Concentrate by Advanced Oxidation Processes with UV/O3/H2O2. Materials, 13(12), 2785. https://doi.org/10.3390/ma13122785






