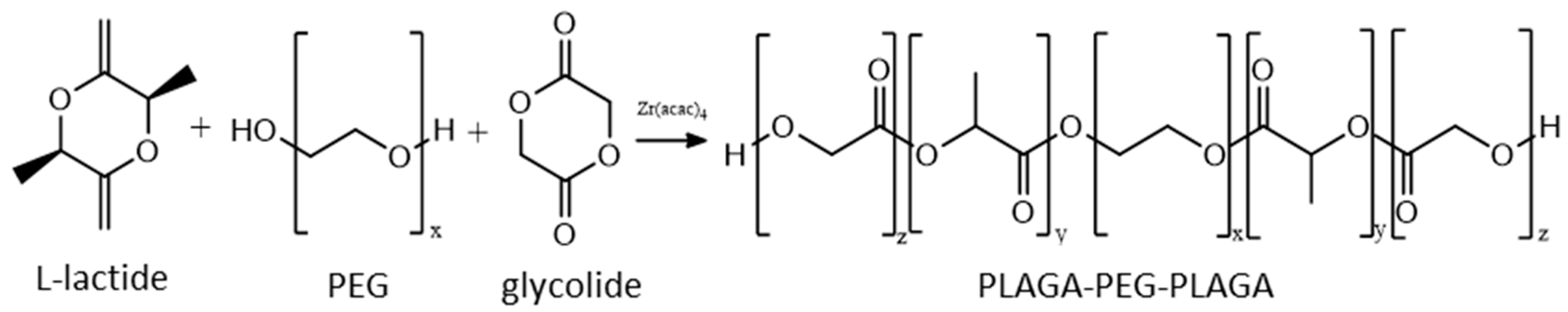
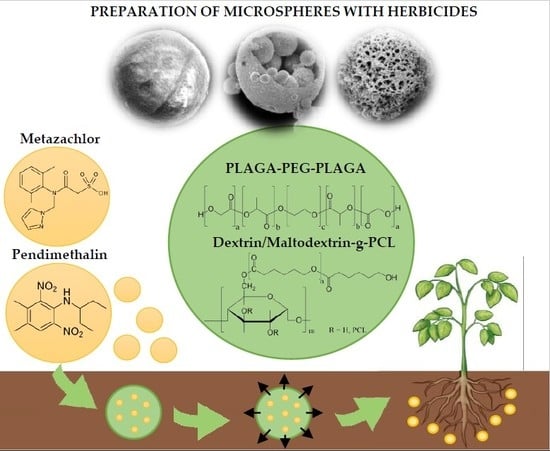
Scheme 1.
The course of terpolymer synthesis.
Scheme 1.
The course of terpolymer synthesis.
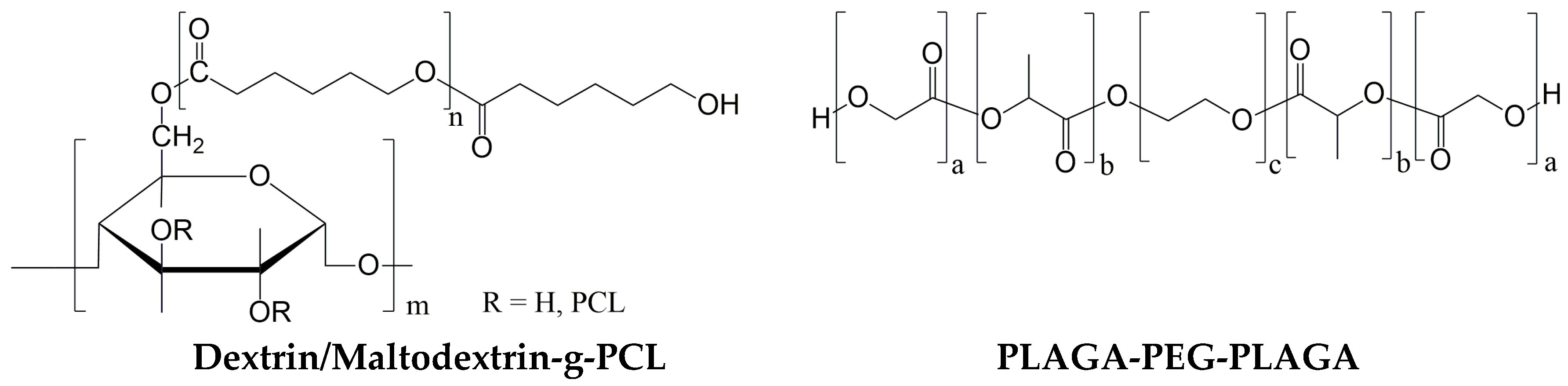
Figure 1.
Schematic structures of synthesized polymers.
Figure 1.
Schematic structures of synthesized polymers.
Figure 2.
Diameters distribution of the microspheres loaded with herbicides (a) for TERPOLYMER and (b) for TER/dextrin and TER/maltodextrin.
Figure 2.
Diameters distribution of the microspheres loaded with herbicides (a) for TERPOLYMER and (b) for TER/dextrin and TER/maltodextrin.
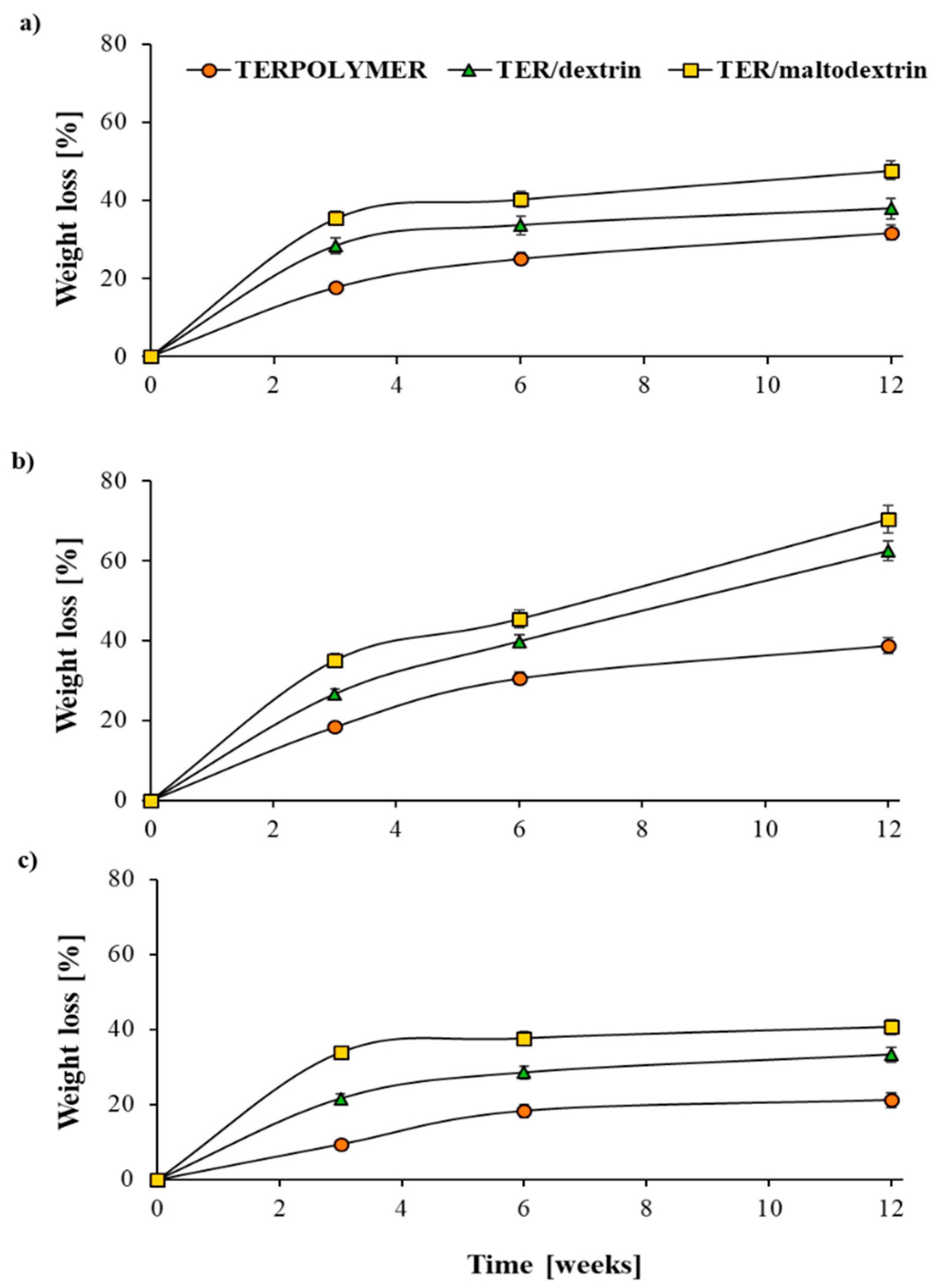
Figure 3.
Percentage weight loss of microspheres of TERPOLYMER; TER/dextrin and TER/maltodextrin, (a)—in water, (b)—in activated sludge, (c)—in soil. The error bars are smaller than the symbols where they are not shown.
Figure 3.
Percentage weight loss of microspheres of TERPOLYMER; TER/dextrin and TER/maltodextrin, (a)—in water, (b)—in activated sludge, (c)—in soil. The error bars are smaller than the symbols where they are not shown.
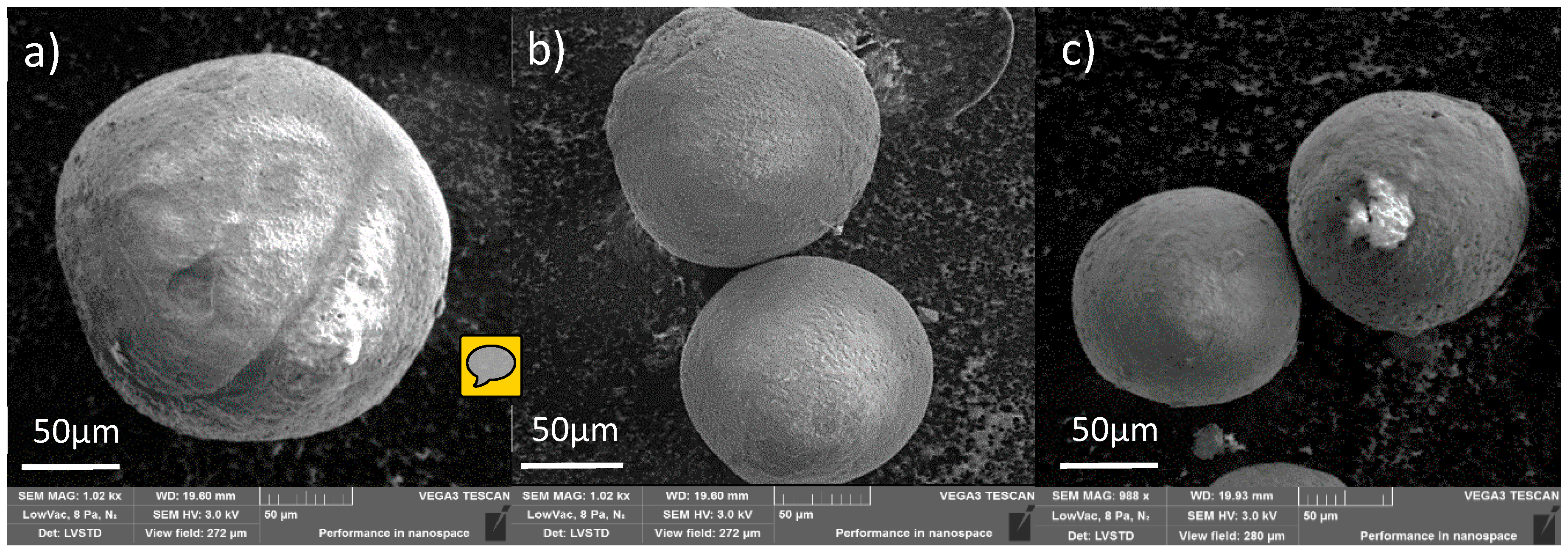
Figure 4.
Scanning electron microscopy (SEM) images of obtained microspheres: (a) terpolymer, (b) TER/dextrin (c) TER/maltodextrin (magnification × 1000).
Figure 4.
Scanning electron microscopy (SEM) images of obtained microspheres: (a) terpolymer, (b) TER/dextrin (c) TER/maltodextrin (magnification × 1000).
Figure 5.
Deterioration of the microspheres surface after each period of exposure to the degradation medium, scanning electron microscopy (SEM) (magnification × 1000).
Figure 5.
Deterioration of the microspheres surface after each period of exposure to the degradation medium, scanning electron microscopy (SEM) (magnification × 1000).
Figure 6.
Cumulative release of pendimethalin (a) and metazachlor (b) from microspheres into water. The error bars are smaller than the symbols where they are not shown.
Figure 6.
Cumulative release of pendimethalin (a) and metazachlor (b) from microspheres into water. The error bars are smaller than the symbols where they are not shown.
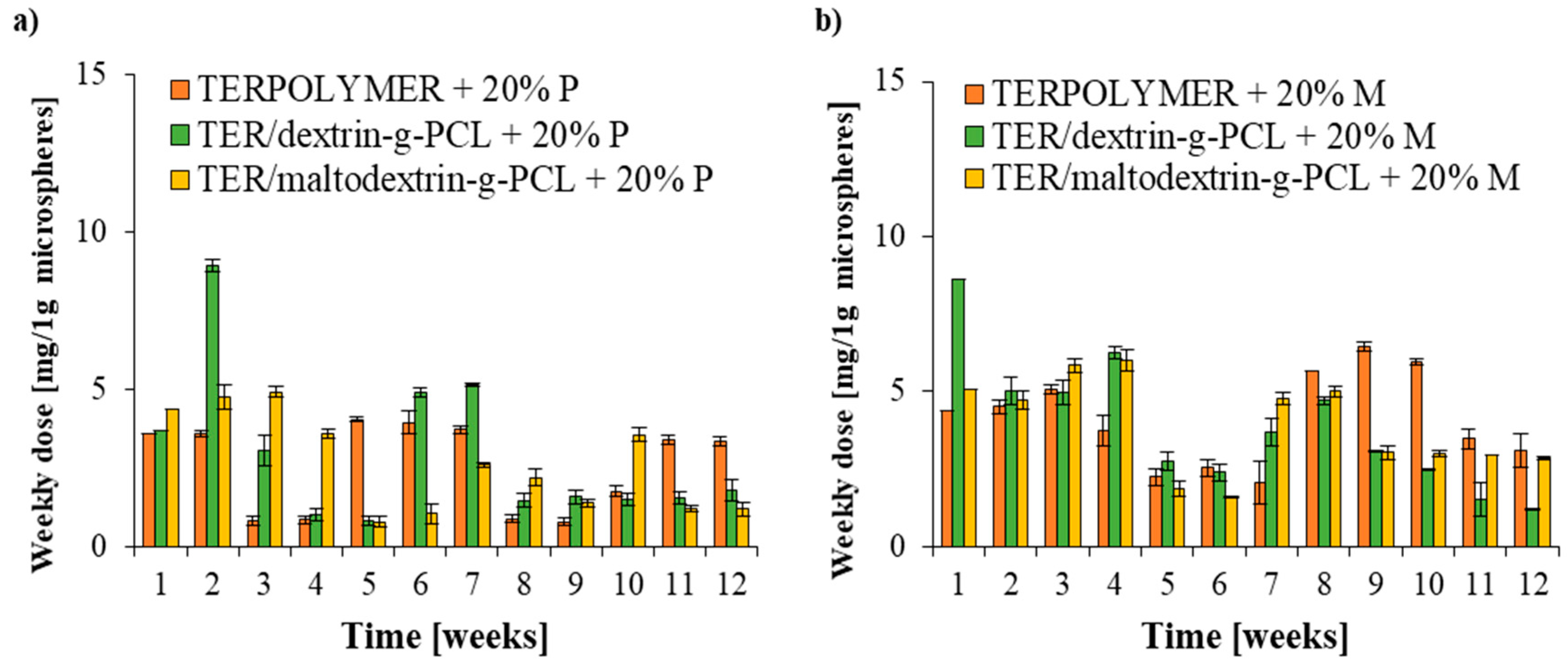
Figure 7.
Weekly dose release of (a) pendimethalin and (b) metazachlor from microspheres into water.
Figure 7.
Weekly dose release of (a) pendimethalin and (b) metazachlor from microspheres into water.
Figure 8.
Herbicidal activity assessment of released metazachlor from microspheres after one month of incubation of microspheres in soil.
Figure 8.
Herbicidal activity assessment of released metazachlor from microspheres after one month of incubation of microspheres in soil.
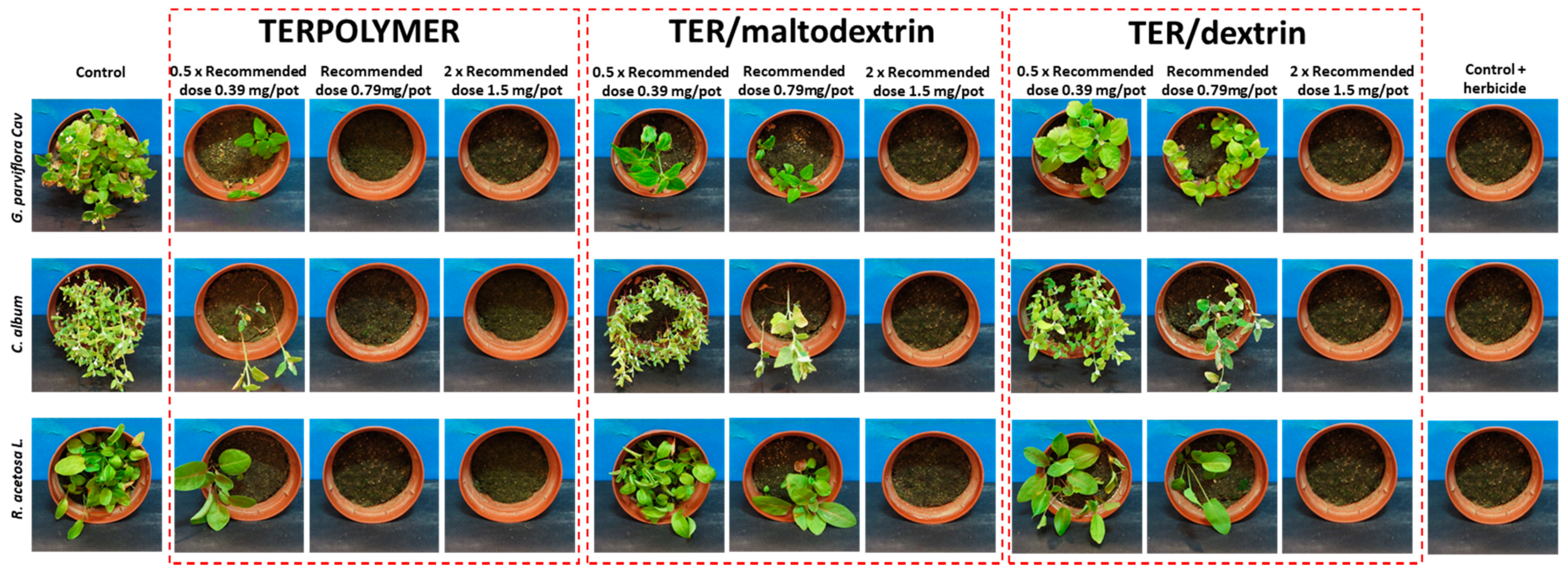
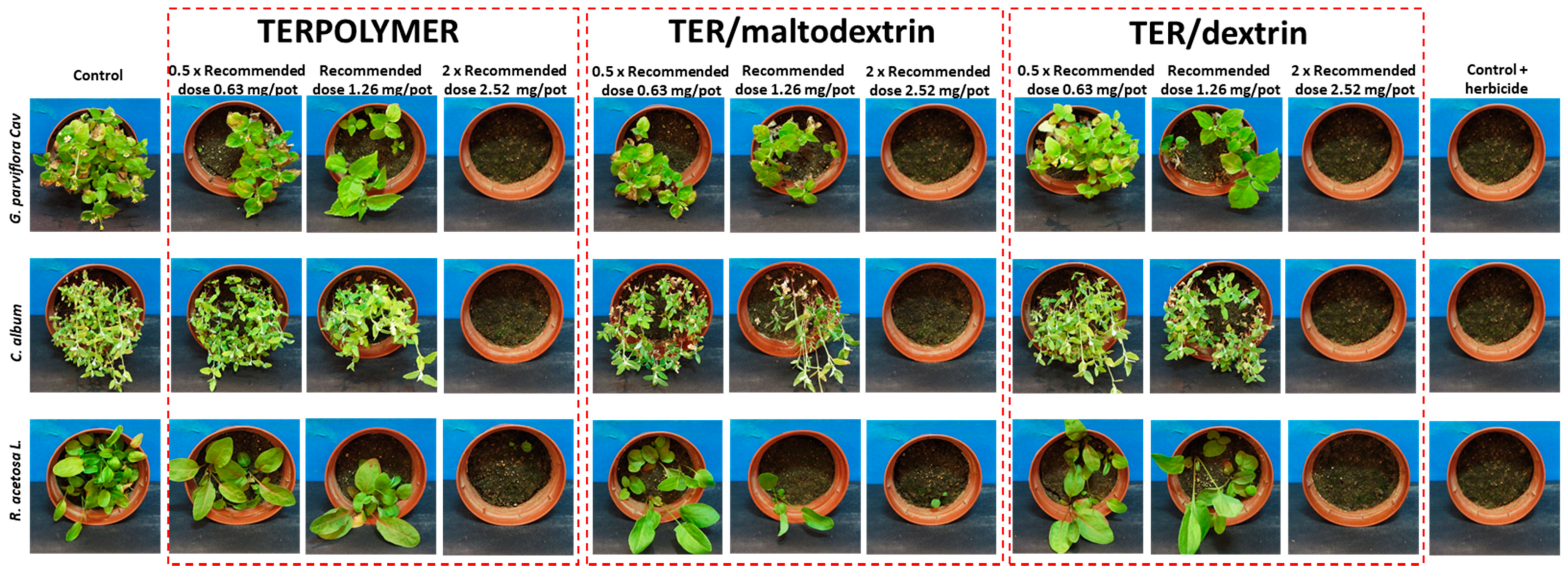
Figure 9.
Herbicidal activity assessment of released pendimethalin from microspheres after one month of incubation of microspheres in soil.
Figure 9.
Herbicidal activity assessment of released pendimethalin from microspheres after one month of incubation of microspheres in soil.
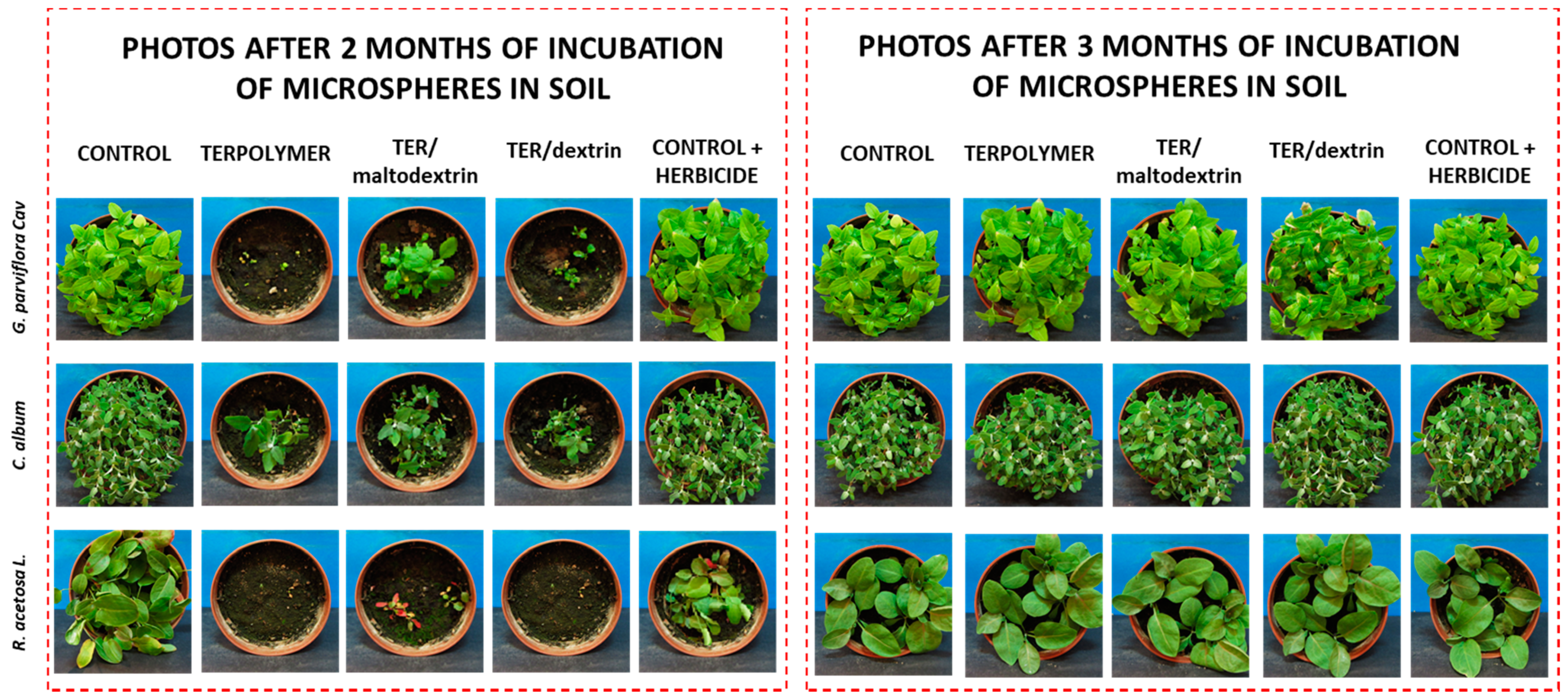
Figure 10.
Herbicidal activity assessment of released metazachlor loaded into microspheres in double recommended dose. Seeds of each plant were sown after two and three months of incubation of microspheres in soil.
Figure 10.
Herbicidal activity assessment of released metazachlor loaded into microspheres in double recommended dose. Seeds of each plant were sown after two and three months of incubation of microspheres in soil.
Figure 11.
Herbicidal activity assessment of released pendimethalin loaded into microspheres in double recommended dose. Seeds of each plant were sown after two and three months of incubation of microspheres in soil.
Figure 11.
Herbicidal activity assessment of released pendimethalin loaded into microspheres in double recommended dose. Seeds of each plant were sown after two and three months of incubation of microspheres in soil.
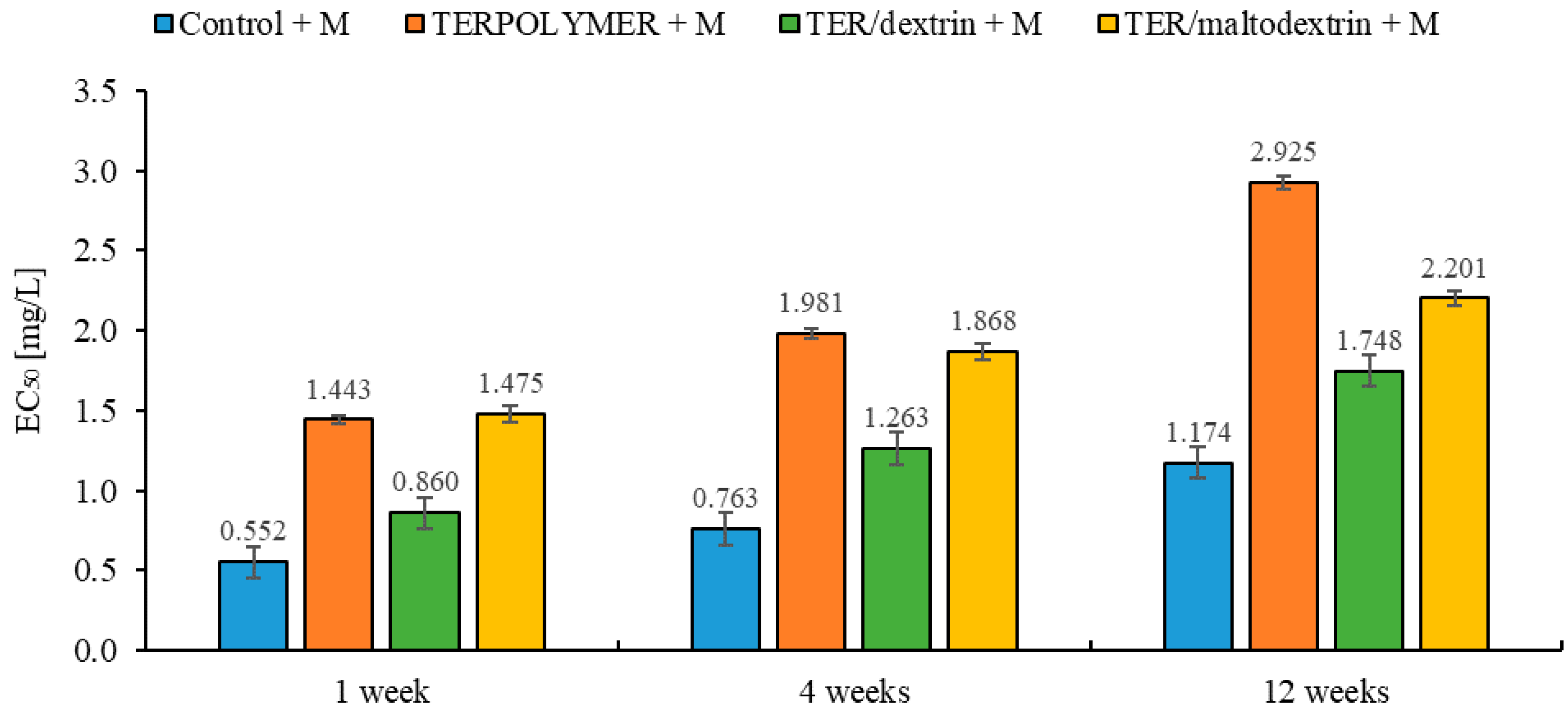
Figure 12.
EC50 values of tested soil with microspheres (TERPOLYMER, TER/dextrin, TER/maltodextrin) loaded with metazachlor measured during exposure to the A. fischeri.
Figure 12.
EC50 values of tested soil with microspheres (TERPOLYMER, TER/dextrin, TER/maltodextrin) loaded with metazachlor measured during exposure to the A. fischeri.
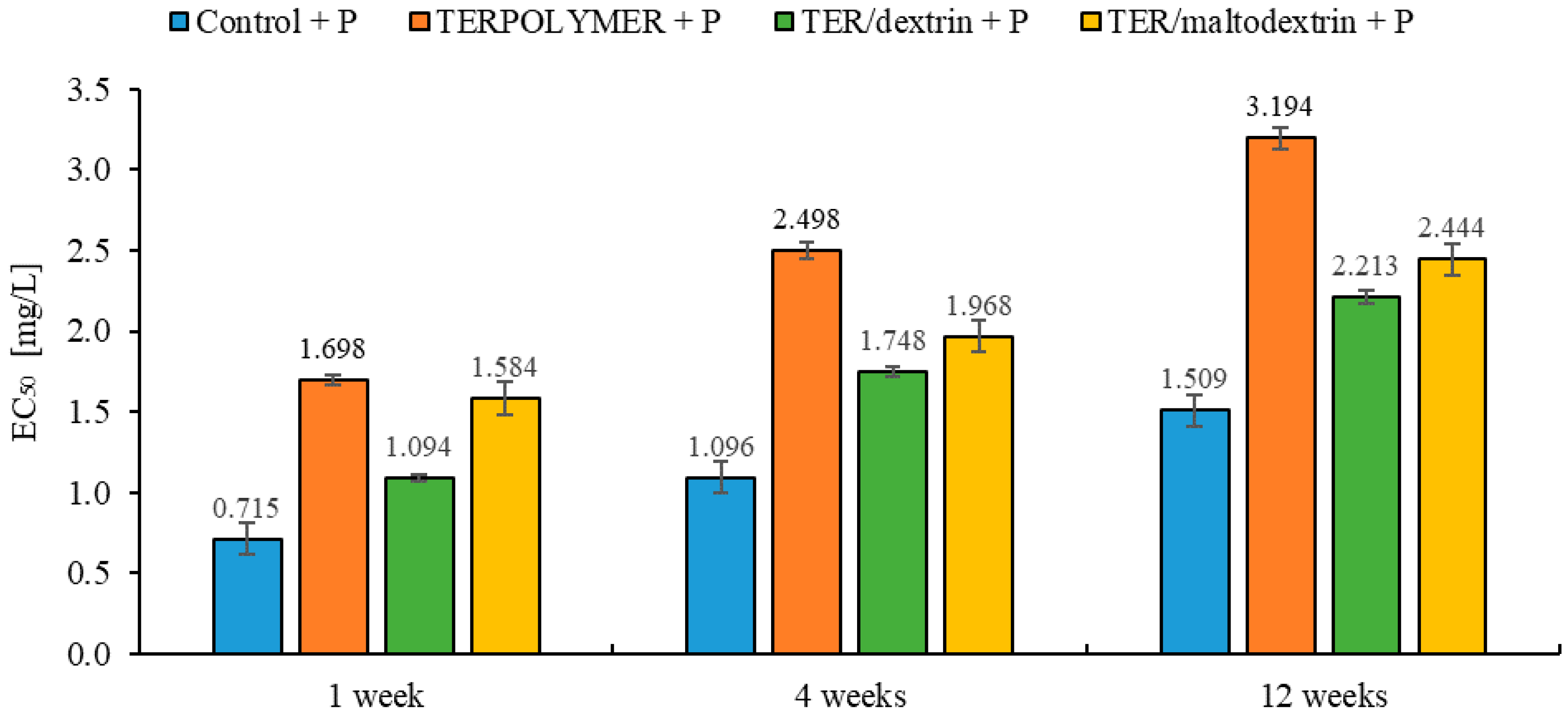
Figure 13.
EC50 values of tested soil with microspheres (TERPOLYMER, TER/dextrin, TER/maltodextrin) loaded with pendimethalin measured during exposure to the A. fischeri.
Figure 13.
EC50 values of tested soil with microspheres (TERPOLYMER, TER/dextrin, TER/maltodextrin) loaded with pendimethalin measured during exposure to the A. fischeri.
Table 1.
Determination of the metazachlor/pendimethalin content in microspheres.
Table 1.
Determination of the metazachlor/pendimethalin content in microspheres.
| Sample | Herbicides | M0H/MP [%] | MeH/MP [%] |
|---|
| TERPOLYMER | Metazachlor | 20 | 16 |
| Pendimethalin | 20 | 15 |
| TER/maltodextrin | Metazachlor | 20 | 17 |
| Pendimethalin | 20 | 17 |
| TER/dextrin | Metazachlor | 20 | 16 |
| Pendimethalin | 20 | 15 |
Table 2.
Herbicidal activity assessment of metazachlor released from microspheres after one month of their incubation in soil (European Weed Research Council (EWRC) scale. Herbicides were loaded in three doses: half of recommended (0.39 mg/pot), recommended (0.79 mg/pot) and double dose (1.5 mg/pot).
Table 2.
Herbicidal activity assessment of metazachlor released from microspheres after one month of their incubation in soil (European Weed Research Council (EWRC) scale. Herbicides were loaded in three doses: half of recommended (0.39 mg/pot), recommended (0.79 mg/pot) and double dose (1.5 mg/pot).
| | TERPOLYMER | TER/Maltodextrin | TER/Dextrin | |
|---|
| Weeds | Control | 0.39 mg/pot | 0.79 mg/pot | 1.5 mg/pot | 0.39 mg/pot | 0.79 mg/pot | 1.5 mg/pot | 0.39 mg/pot | 0.79 mg/pot | 1.5 mg/pot | Control + Herbicide |
| G. parviflora Cav. | − | 3 | 1 | 1 | 5 | 4 | 1 | 8 | 7 | 1 | 1 |
| C. album L. | − | 3 | 1 | 1 | 8 | 3 | 1 | 9 | 6 | 1 | 1 |
| R. acetosa L.
| − | 3 | 1 | 1 | 9 | 6 | 1 | 8 | 5 | 1 | 1 |
Table 3.
Herbicidal activity assessment of pendimethalin released from microspheres after one month of their incubation in soil (EWRC scale). Herbicides were loaded in three doses: half of recommended (0.63 mg/pot), recommended (1.26 mg/pot) and double dose (2.52 mg/pot).
Table 3.
Herbicidal activity assessment of pendimethalin released from microspheres after one month of their incubation in soil (EWRC scale). Herbicides were loaded in three doses: half of recommended (0.63 mg/pot), recommended (1.26 mg/pot) and double dose (2.52 mg/pot).
| | TERPOLYMER | TER/Maltodextrin | TER/Dextrin | |
|---|
| Weeds | Control | 0.63 mg/pot | 1.26 mg/pot | 2.52 mg/pot | 0.63 mg/pot | 1.26 mg/pot | 2.52 mg/pot | 0.63 mg/pot | 1.26 mg/pot | 2.52 mg/pot | Control + Herbicide |
| G. parviflora Cav. | − | 7 | 6 | 1 | 8 | 7 | 1 | 9 | 4 | 1 | 1 |
| C. album L. | − | 9 | 8 | 1 | 9 | 5 | 1 | 9 | 8 | 1 | 1 |
| R. acetosa L.
| − | 7 | 6 | 1 | 8 | 3 | 2 | 8 | 7 | 1 | 1 |
Table 4.
Herbicidal activity assessment of released metazachlor loaded into microspheres in double recommended dose (EWRC scale).
Table 4.
Herbicidal activity assessment of released metazachlor loaded into microspheres in double recommended dose (EWRC scale).
| | | 2 Months after Incubation of Microspheres in Soil | 3 Months after Incubation of Microspheres in Soil |
|---|
| Weeds | Control | TERPOLYMER | TER/maltodextrin | TER/dextrin | Control + Herbicide | TERPOLYMER | TER/maltodextrin | TER/dextrin | Control + Herbicide |
| G. parviflora Cav. | − | 1 | 1 | 1 | 1 | 6 | 6 | 6 | 9 |
| C. album L. | − | 1 | 1 | 1 | 1 | 6 | 6 | 6 | 9 |
| R. acetosa L.
| − | 1 | 1 | 1 | 1 | 8 | 7 | 8 | 9 |
Table 5.
Herbicidal activity assessment of released pendimethalin loaded into microspheres in double recommended dose (EWRC scale).
Table 5.
Herbicidal activity assessment of released pendimethalin loaded into microspheres in double recommended dose (EWRC scale).
| | | 2 Months after Incubation of Microspheres in Soil | 3 Months after Incubation of Microspheres in Soil |
|---|
| Weeds | Control | TERPOLYMER | TER/maltodextrin | TER/dextrin | Control + Herbicide | TERPOLYMER | TER/maltodextrin | TER/dextrin | Control + Herbicide |
| G. parviflora Cav. | − | 3 | 6 | 5 | 9 | 9 | 9 | 9 | 9 |
| C. album L. | − | 4 | 6 | 4 | 9 | 9 | 9 | 9 | 9 |
| R. acetosa L.
| − | 1 | 3 | 1 | 9 | 9 | 9 | 9 | 9 |
Table 6.
Microtox® EC50 values (mg/L) of exposure of the luminescent marine bacteria A. fischeri to tested soil with microspheres (TERPOLYMER, TER/dextrin, TER/maltodextrin) with metazachlor respective to 95% confidence limits (in brackets) obtained in the fit of the data.
Table 6.
Microtox® EC50 values (mg/L) of exposure of the luminescent marine bacteria A. fischeri to tested soil with microspheres (TERPOLYMER, TER/dextrin, TER/maltodextrin) with metazachlor respective to 95% confidence limits (in brackets) obtained in the fit of the data.
| 1 Week | 4 Weeks | 12 Weeks |
|---|
| EC50 (Lower Limit, Upper Limit [mg/L]) | Coefficient of Determination (R2) | EC50 (Lower Limit, Upper Limit [mg/L]) | Coefficient of Determination (R2) | EC50 (Lower Limit, Upper Limit [mg/L]) | Coefficient of Determination (R2) |
|---|
| Control + M |
| 0.552 (0.450; 0.677) | 0.957 | 0.763 (0.647; 0.899) | 0.973 | 1.174 (1.025; 1.344) | 0.978 |
| TERPOLYMER + M |
| 1.443 (1.138; 1.831) | 0.936 | 1.981 (1.572; 2.496) | 0.947 | 2.925 (1.963; 4.357) | 0.845 |
| TER/dextrin + M |
| 0.860 (0.719; 1.029) | 0.965 | 1.263 (1.089; 1.466) | 0.978 | 1.748 (1.529; 1.999) | 0.977 |
| TER/maltodextrin + M |
| 1.475 (1.164; 1.869) | 0.926 | 1.868 (1.315; 2.652) | 0.823 | 2.201 (1.860; 2.605) | 0.958 |
Table 7.
Microtox® EC50 values (mg/L) of exposure of the luminescent marine bacteria A. fischeri to tested soil with microspheres (TERPOLYMER, TER/dextrin, TER/maltodextrin) with pendimethalin respective to 95% confidence limits (in brackets) obtained in the fit of the data.
Table 7.
Microtox® EC50 values (mg/L) of exposure of the luminescent marine bacteria A. fischeri to tested soil with microspheres (TERPOLYMER, TER/dextrin, TER/maltodextrin) with pendimethalin respective to 95% confidence limits (in brackets) obtained in the fit of the data.
| 1 week | 4 weeks | 12 weeks |
|---|
| EC50 (Lower Limit, Upper Limit [mg/L]) | Coefficient of Determination (R2) | EC50 (Lower Limit, Upper Limit [mg/L]) | Coefficient of Determination (R2) | EC50 (Lower Limit, Upper Limit [mg/L]) | Coefficient of Determination (R2) |
|---|
| Control + P |
| 0.715 (0.589; 0.868) | 0.961 | 1.096 (0.971; 1.237) | 0.985 | 1.509 (1.408; 1.618) | 0.995 |
| TERPOLYMER + P |
| 1.698 (1.362; 2.116) | 0.949 | 2.498 (1.926; 3.239) | 0.933 | 3.194 (2.423; 4.211) | 0.906 |
| TER/dextrin + P |
| 1.094 (0.937; 1.278) | 0.973 | 1.748 (1.568; 1.949) | 0.988 | 2.213 (1.950; 2.512) | 0.985 |
| TER/maltodextrin + P |
| 1.584 (1.322; 1.897) | 0.965 | 1.968 (1.648; 2.349) | 0.963 | 2.444 (1.978; 3.019) | 0.939 |