Measuring Heat Production from Burning Al/Zr and Al/Mg/Zr Composite Particles in a Custom Micro-Bomb Calorimeter
Abstract
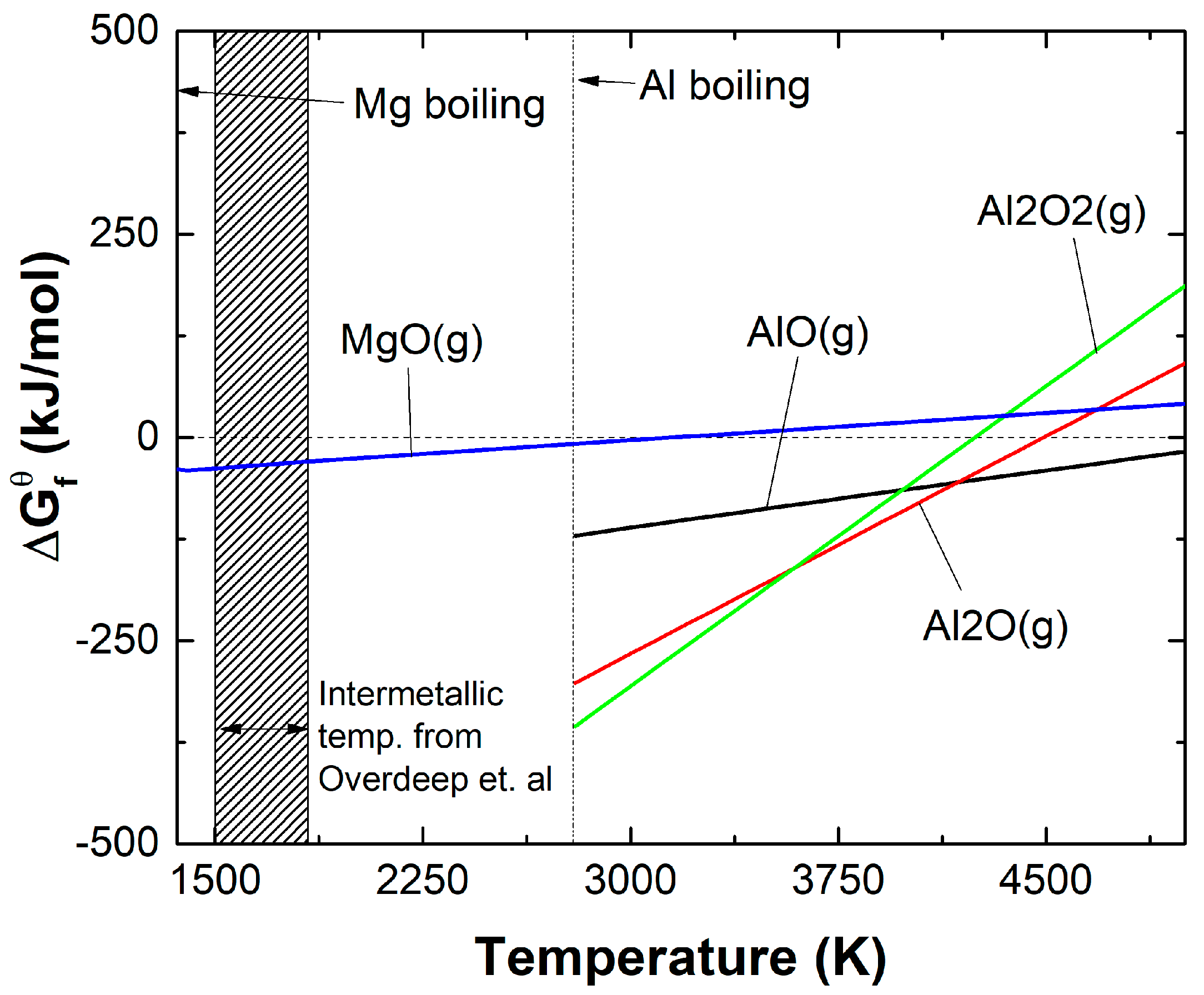
1. Introduction
2. Materials and Methods
2.1. Particle Synthesis
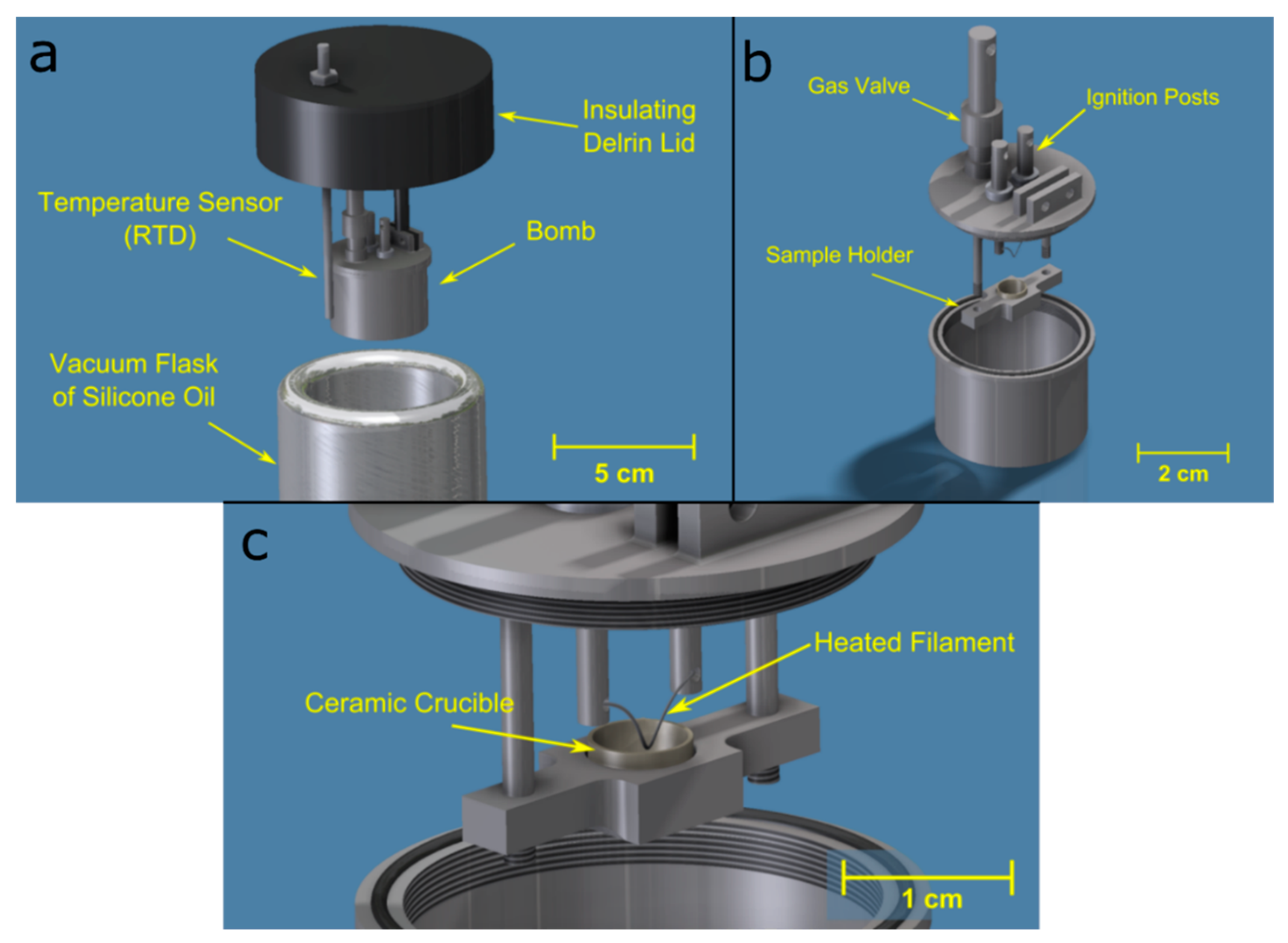
2.2. Custom Particle Bomb Micro-Calorimeter Design and Measurement
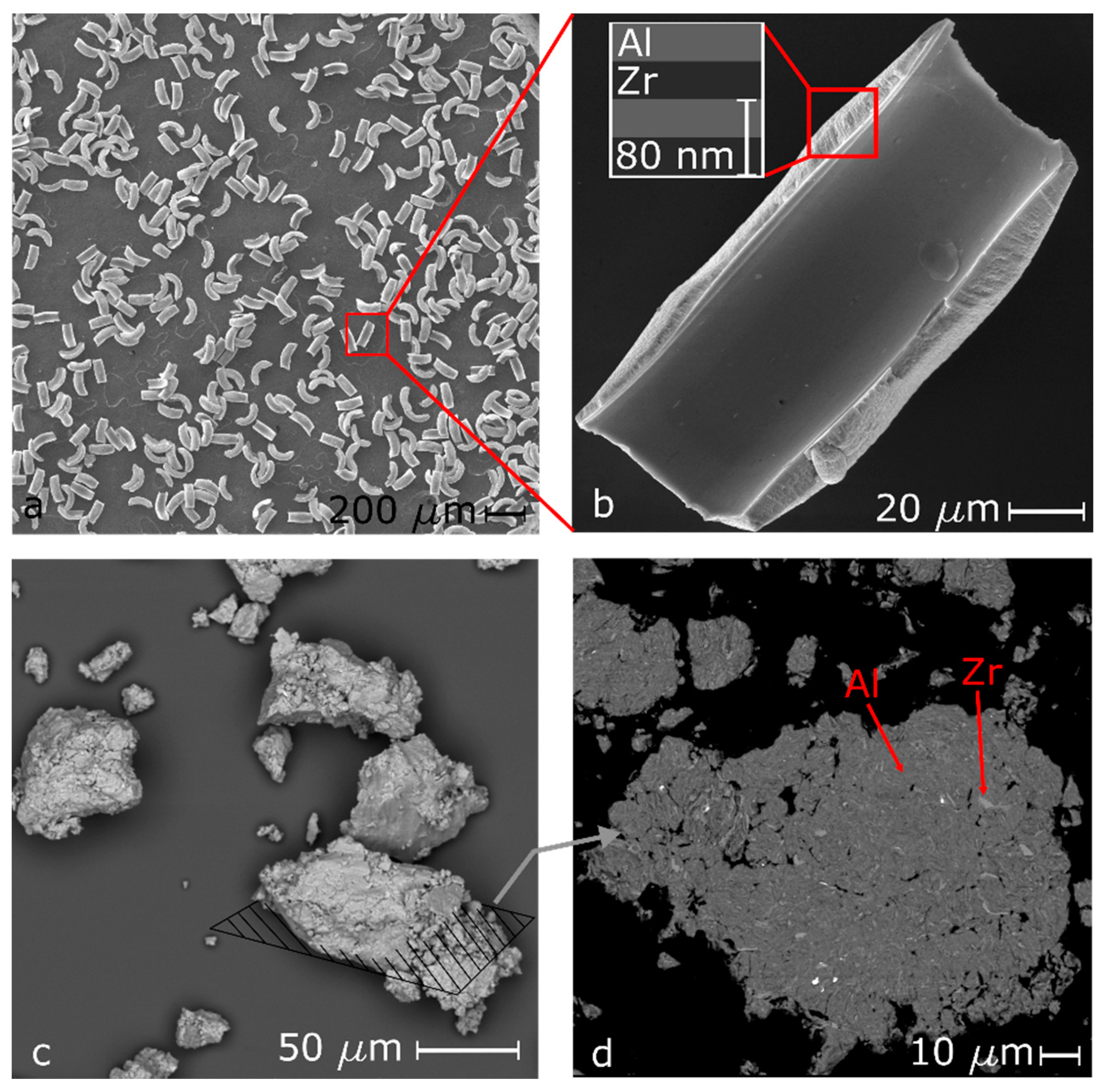
2.3. Particle Characterization
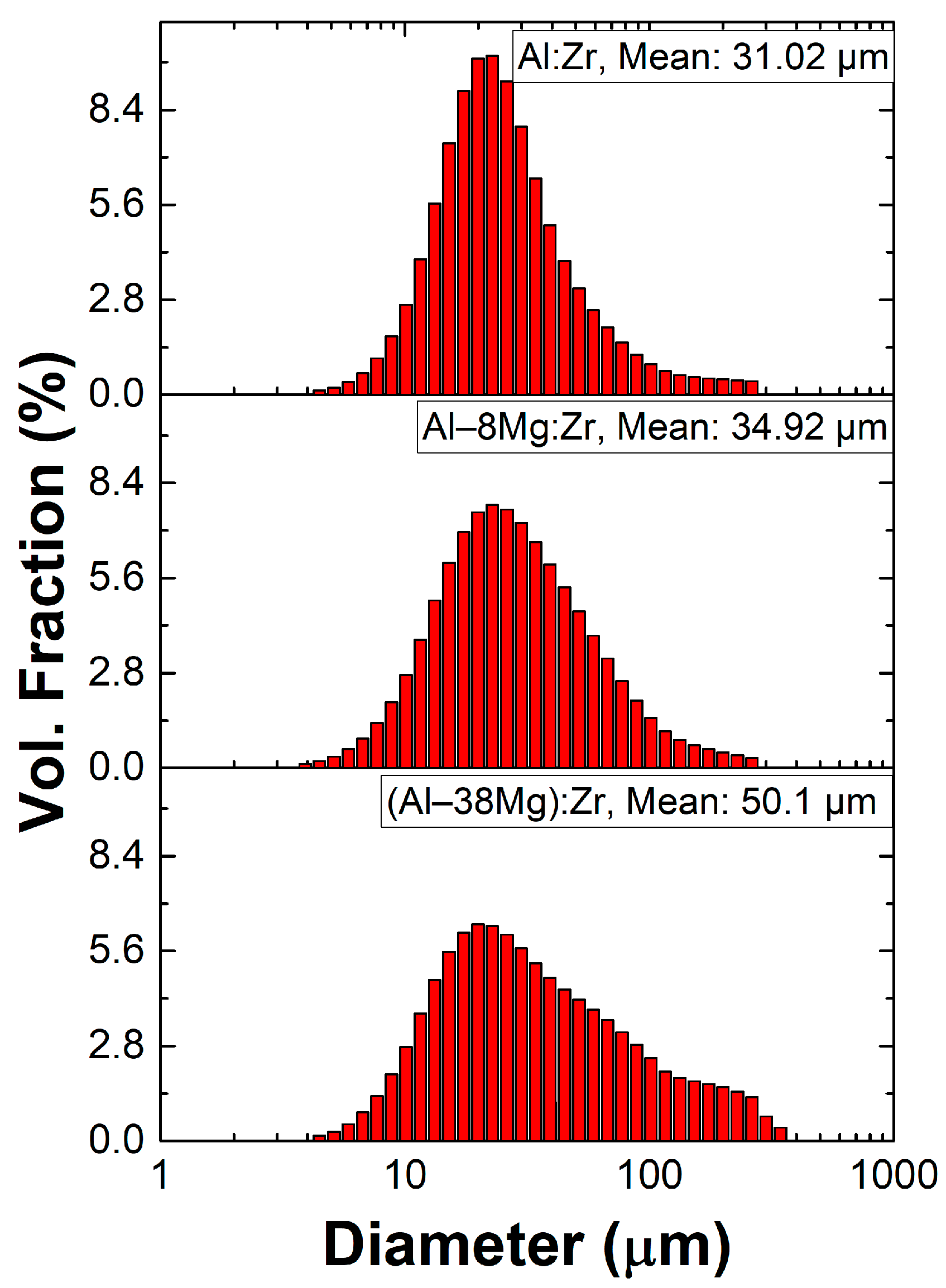
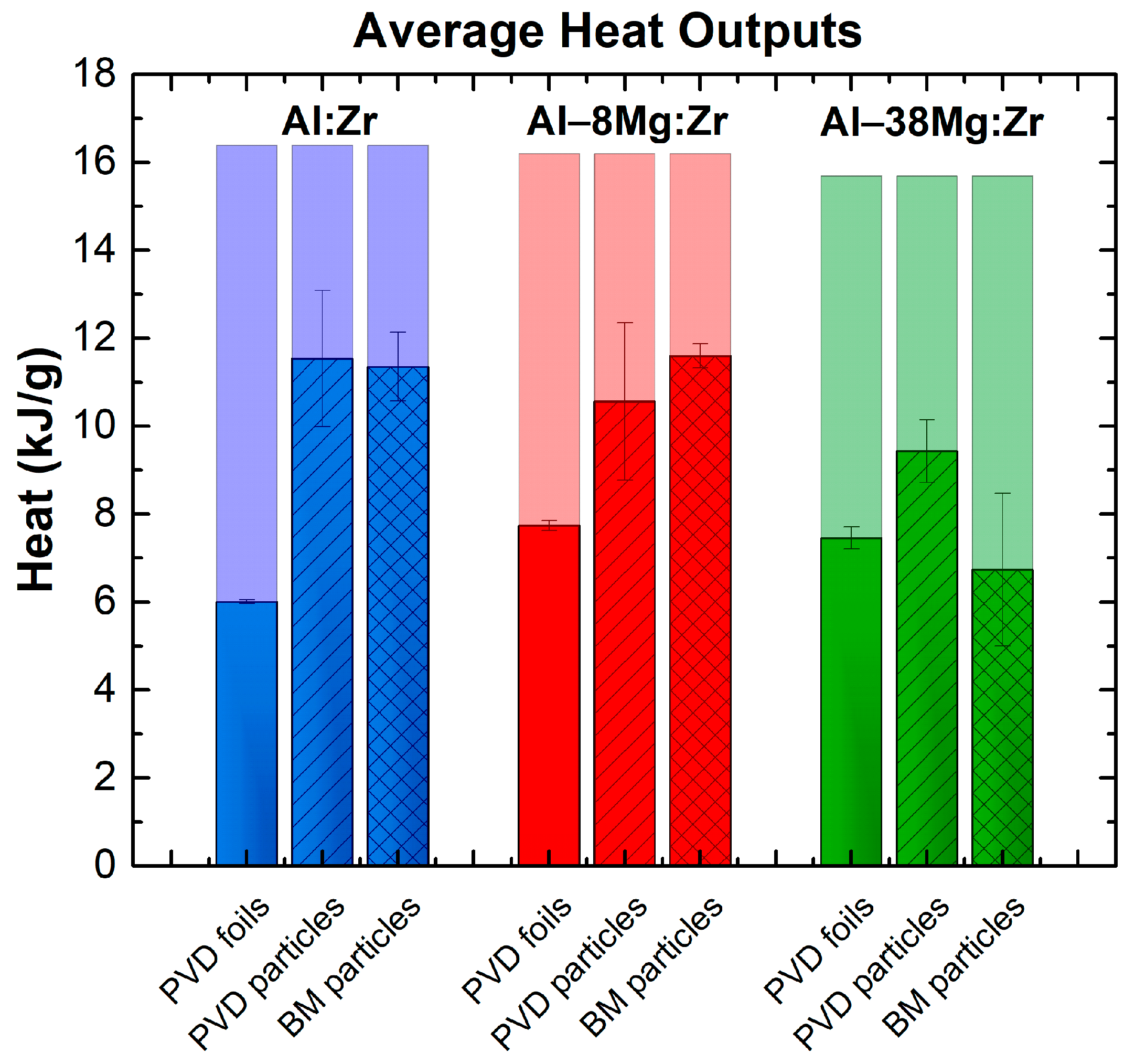
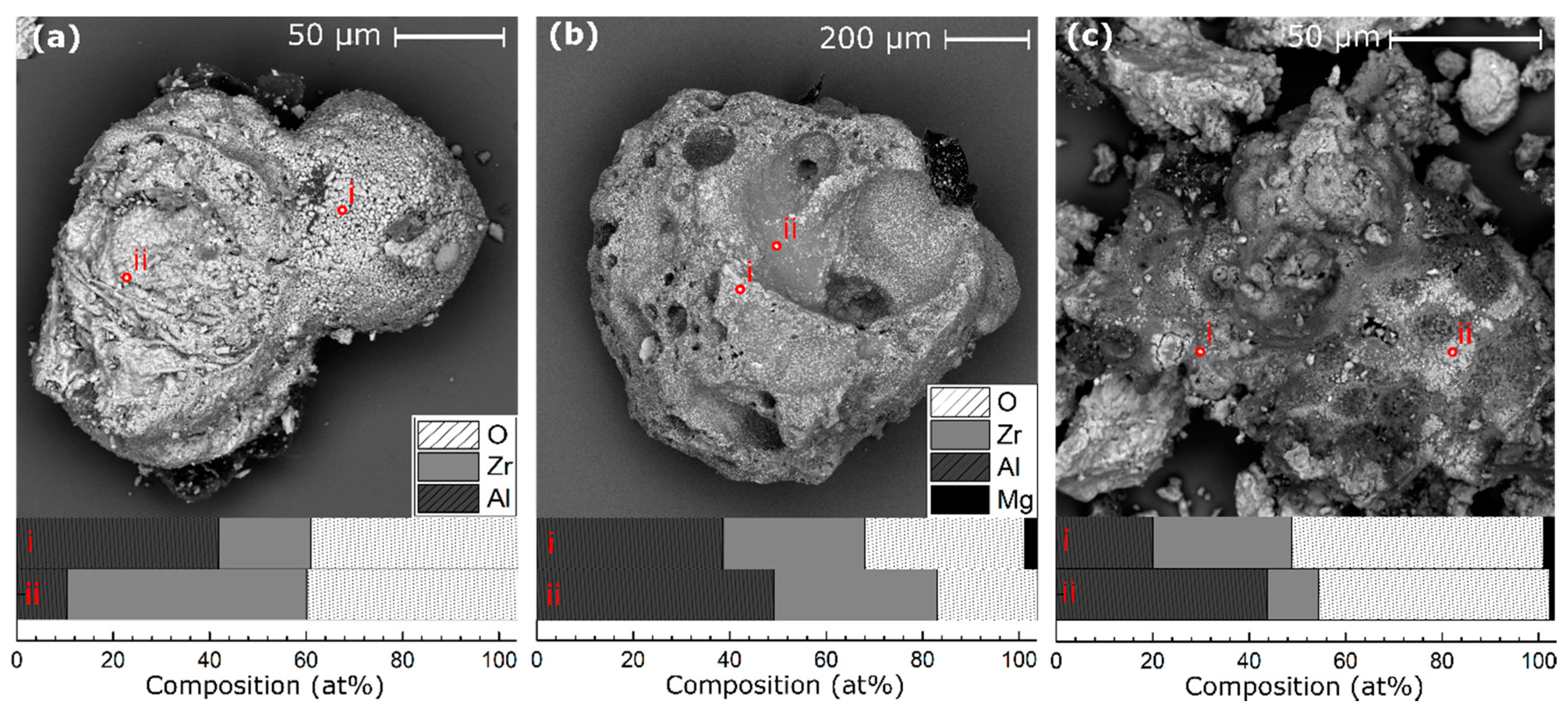
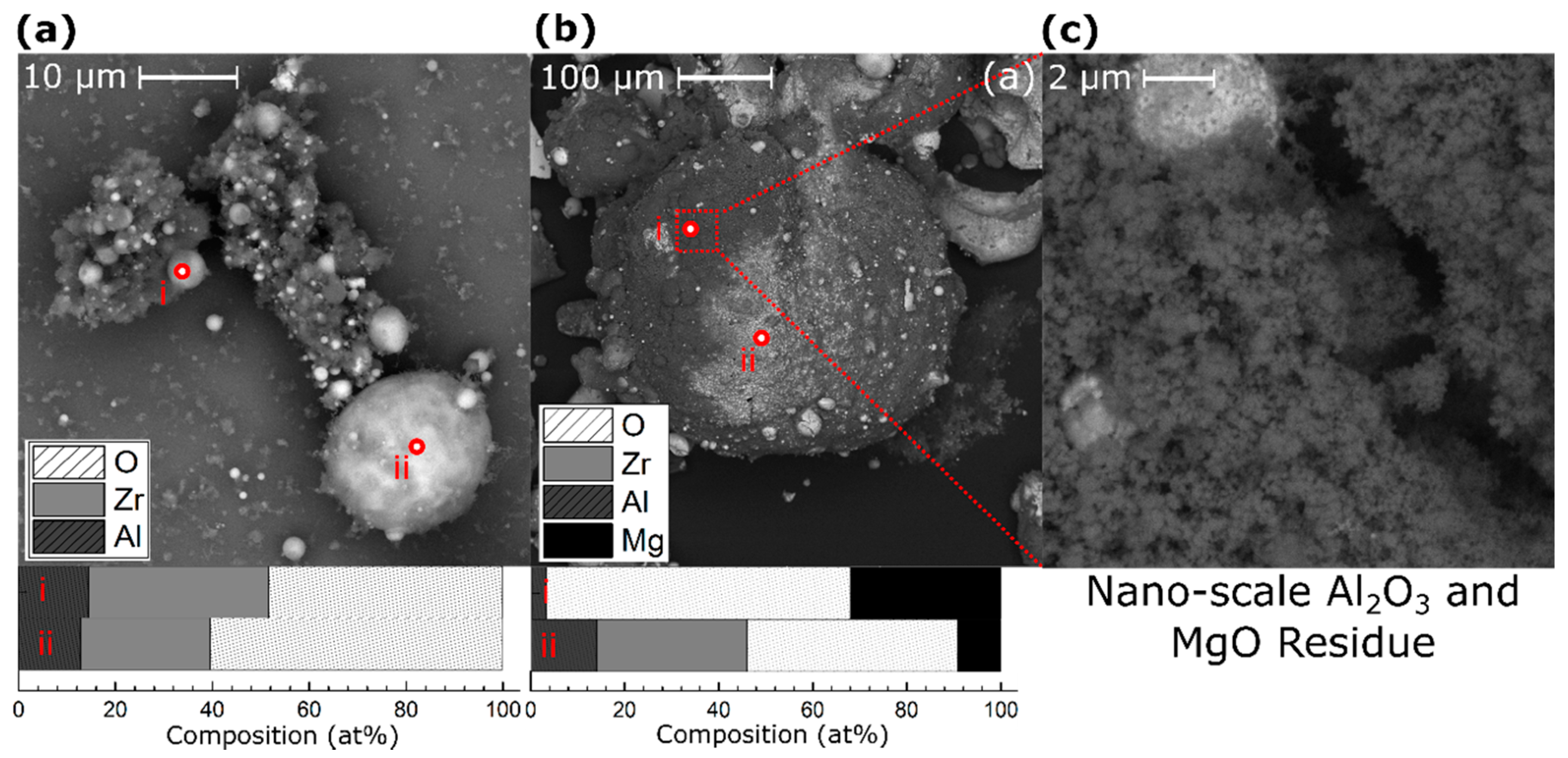
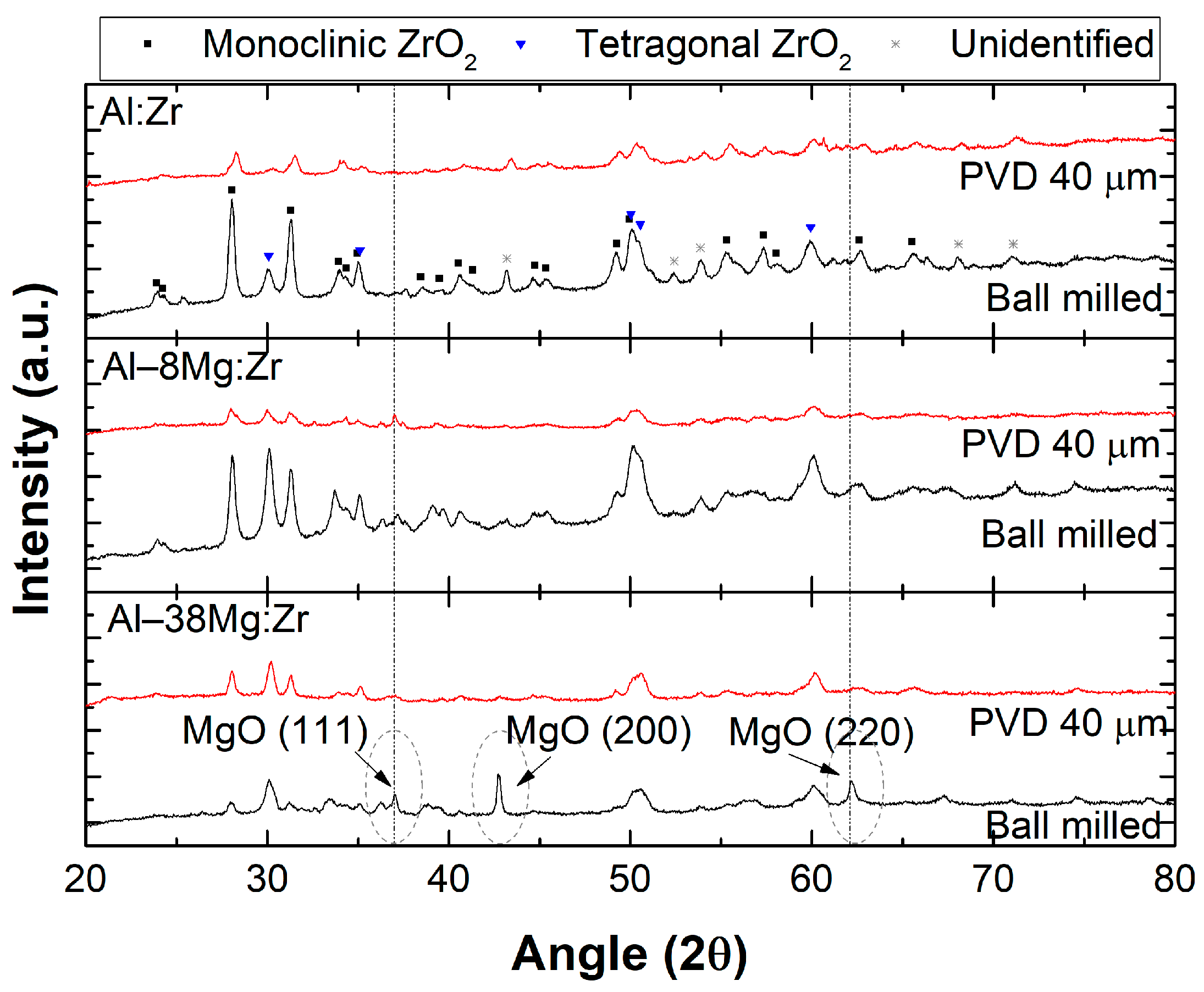
3. Results
4. Discussion
4.1. Particle Geometry Effects
4.2. The Effect of Increasing Mg Content on Heat Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Sundaram, D.; Yang, V.; Yetter, R.A. Metal-based nanoenergetic materials: Synthesis, properties, and applications. Prog. Energy Combust. Sci. 2017, 61, 293–365. [Google Scholar] [CrossRef]
- Yen, N.H.; Wang, L.Y. Reactive metals in explosives. Propellants, Explos. Pyrotech. 2012, 37, 143–155. [Google Scholar] [CrossRef]
- Peiris, S.M.; Bolden-Frazier, N. Applications of Reactive Materials in Munitions. Shock Phenom. Granul. Porous Mater. 2019, 165–191. [Google Scholar] [CrossRef]
- Busta, F.F. Thermal Inactivation Characteristics of Bacterial Spores at Ultrahigh Temperatures. Appl. Microbiol. 1967, 15, 640–645. [Google Scholar] [CrossRef]
- Montville, T.J.; Dengrove, R.; De Siano, T.; Bonnet, M.; Schaffner, D.W. Thermal Resistance of Spores from Virulent Strains of Bacillus anthracis and Potential Surrogates. J. Food Prot. 2005, 68, 2362–2366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gershenfeld, L.; Witlin, B. Iodine Solution as a Sporicidal Agent. J. Am. Pharm. Assoc. (Scientific ed.) 1952, 41, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Setlow, B.; Parish, S.; Zhang, P.; Li, Y.Q.; Neely, W.C.; Setlow, P. Mechanism of killing of spores of Bacillus anthracis in a high-temperature gas environment, and analysis of DNA damage generated by various decontamination treatments of spores of Bacillus anthracis, Bacillus subtilis and Bacillus thuringiensis. J. Appl. Microbiol. 2014, 116, 805–814. [Google Scholar] [CrossRef]
- Camilleri, E.; Korza, G.; del Carmen Huesca-Espita, L.; Setlow, B.; Stamatis, D.; Setlow, P. Mechanisms of killing of Bacillus thuringiensis Al Hakam spores in a blast environment with and without iodic acid. J. Appl. Microbiol. 2020, 1–12. [Google Scholar] [CrossRef]
- Grinshpun, S.A.; Yermakov, M.; Indugula, R.; Abraham, A.; Schoenitz, M.; Dreizin, E.L. Aluminum-based materials for inactivation of aerosolized spores of Bacillus anthracis surrogates. Aerosol Sci. Technol. 2017, 51, 224–234. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Schoenitz, M.; Grinshpun, S.A.; Yermakov, M.; Dreizin, E.L. Biocidal effectiveness of combustion products of iodine-bearing reactive materials against aerosolized bacterial spores. J. Aerosol Sci. 2018, 116, 106–115. [Google Scholar] [CrossRef]
- Ermoline, A.; Yildiz, D.; Dreizin, E.L. Model of heterogeneous combustion of small particles. Combust. Flame 2013, 160, 2982–2989. [Google Scholar] [CrossRef]
- Sundaram, D.S.; Yang, V.; Zarko, V.E. Combustion of nano aluminum particles (Review). Combust. Explos. Shock Waves 2015, 51, 173–196. [Google Scholar] [CrossRef]
- Mohan, S.; Trunov, M.A.; Dreizin, E.L. On possibility of vapor-phase combustion for fine aluminum particles. Combust. Flame 2009, 156, 2213–2216. [Google Scholar] [CrossRef]
- Glassman, I.; Yetter, R.A.; Glumac, N.G. Combustion; Academic Press: Cambridge, MA, USA, 2014; ISBN 0124115551. [Google Scholar]
- Vummidi Lakshman, S.; Gibbins, J.D.; Wainwright, E.R.; Weihs, T.P. The effect of chemical composition and milling conditions on composite microstructure and ignition thresholds of Al–Zr ball milled powders. Powder Technol. 2019, 343, 87–94. [Google Scholar] [CrossRef]
- Joress, H.; Barron, S.C.; Livi, K.J.T.; Aronhime, N.; Weihs, T.P. Self-sustaining oxidation initiated by rapid formation reactions in multilayer foils Self-sustaining oxidation initiated by rapid formation reactions in multilayer foils. Appl. Phys. Lett. 2012, 101, 111908. [Google Scholar] [CrossRef]
- Overdeep, K.R.; Joress, H.; Zhou, L.; Livi, K.J.T.; Barron, S.C.; Grapes, M.D.; Shanks, K.S.; Dale, D.S.; Tate, M.W.; Philipp, H.T.; et al. Mechanisms of oxide growth during the combustion of Al:Zr nanolaminate foils. Combust. Flame 2018, 191, 442–452. [Google Scholar] [CrossRef]
- Fritz, G.M.; Joress, H.; Weihs, T.P. Enabling and controlling slow reaction velocities in low-density compacts of multilayer reactive particles. Combust. Flame 2011, 158, 1084–1088. [Google Scholar] [CrossRef]
- Wainwright, E.R.; Dean, S.W.; Vummidi Lakshman, S.; Weihs, T.P.; Gottfried, J.L. Evaluating Compositional Effects on the Laser-induced Combustion and Shock Velocities of Al/Zr-based Composite Fuels. Combust. Flame 2020, 213, 357–368. [Google Scholar] [CrossRef]
- Stamatis, D.; Wainwright, E.R.; Vummidi Lakshman, S.; Kessler, M.S.; Weihs, T.P. Combustion of explosively dispersed Al-Mg-Zr composite particles. Combust. Flame 2020, 217, 93–102. [Google Scholar] [CrossRef]
- Aly, Y.; Schoenitz, M.; Dreizin, E.L. Aluminum-Metal Reactive Composites. Combust. Sci. Technol. 2011, 183, 1107–1132. [Google Scholar] [CrossRef]
- Michaelsen, C.; Barmak, K.; Weihs, T.P. Investigating the thermodynamics and kinetics of thin film reactions by differential scanning calorimetry. J. Phys. D. Appl. Phys. 1997, 30, 3167–3186. [Google Scholar] [CrossRef]
- Rogachev, A.S. Exothermic reaction waves in multilayer nanofilms. Russ. Chem. Rev. 2008, 77, 21–37. [Google Scholar] [CrossRef]
- Fogagnolo, J.; Velasco, F.; Robert, M.; Torralba, J. Effect of mechanical alloying on the morphology, microstructure and properties of aluminium matrix composite powders. Mater. Sci. Eng. A 2003, 342, 131–143. [Google Scholar] [CrossRef]
- Stover, A.K.; Krywopusk, N.M.; Fritz, G.M.; Barron, S.C.; Gibbins, J.D.; Weihs, T.P. An analysis of the microstructure and properties of cold-rolled Ni:Al laminate foils. J. Mater. Sci. 2013, 48, 5917–5929. [Google Scholar] [CrossRef]
- Stover, A.K.; Krywopusk, N.M.; Gibbins, J.D.; Weihs, T.P. Mechanical fabrication of reactive metal laminate powders. J. Mater. Sci. 2014, 49, 5821–5830. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Hadjiafxenti, a.; Gunduz, I.E.; Doumanidis, C.C.; Rebholz, C. Spark ignitable ball milled powders of Al and Ni at NiAl composition. Vacuum 2014, 101, 275–278. [Google Scholar] [CrossRef]
- Adams, D.P. Reactive multilayers fabricated by vapor deposition: A critical review. Thin Solid Films 2015, 576, 98–128. [Google Scholar] [CrossRef]
- Knepper, R.; Snyder, M.R.; Fritz, G.; Fisher, K.; Knio, O.M.; Weihs, T.P. Effect of varying bilayer spacing distribution on reaction heat and velocity in reactive Al/Ni multilayers. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- DesJardin, P.E.; Felske, J.D.; Carrara, M.D. Mechanistic Model for Aluminum Particle Ignition and Combustion in Air. J. Propuls. Power 2005, 21, 478–485. [Google Scholar] [CrossRef]
- Barron, S.C.; Knepper, R.; Walker, N.; Weihsa, T.P. Characterization of self-propagating formation reactions in Ni/Zr multilayered foils using reaction heats, velocities, and temperature-time profiles. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Fisher, K.; Barron, S.C.; Bonds, M.A.; Knepper, R.; Livi, K.J.T.; Campbell, G.H.; Browning, N.D.; Weihs, T.P. Phase transformations, heat evolution, and atomic diffusion during slow heating of Al-rich Al/Zr multilayered foils. J. Appl. Phys. 2013, 114. [Google Scholar] [CrossRef]
- Swaminathan, P.; Grapes, M.D.; Woll, K.; Barron, S.C.; Lavan, D.A.; Weihs, T.P. Studying exothermic reactions in the Ni-Al system at rapid heating rates using a nanocalorimeter. J. Appl. Phys. 2013, 113. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Vadchenko, S.G.; Baras, F.; Politano, O.; Rouvimov, S.; Sachkova, N.V.; Grapes, M.D.; Weihs, T.P.; Mukasyan, A.S. Combustion in reactive multilayer Ni/Al nanofoils: Experiments and molecular dynamic simulation. Combust. Flame 2016, 166, 158–169. [Google Scholar] [CrossRef]
- Weihs, T.P. Fabrication and characterization of reactive multilayer films and foils. In Metallic Films for Electronic, Optical and Magnetic Applications; Barmak, K., Coffey, K., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 160–243. ISBN 978-0-85709-057-7. [Google Scholar]
- Levashov, E.A.; Mukasyan, A.S.; Rogachev, A.S.; Shtansky, D.V. Self-propagating high-temperature synthesis of advanced materials and coatings. Int. Mater. Rev. 2016, 62, 1–37. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Mukasyan, A.S. Combustion of heterogeneous nanostructural systems (review). Combust. Explos. Shock Waves 2010, 46, 243–266. [Google Scholar] [CrossRef]
- Dreizin, E.L.; Schoenitz, M. Mechanochemically prepared reactive and energetic materials: A review. J. Mater. Sci. 2017, 52, 11789–11809. [Google Scholar] [CrossRef]
- Kohga, M.; Hagihara, Y. Rheology of Concentrated AP/HTPB Suspensions Prepared at the Upper Limit of AP Content. Propellants Explos. Pyrotech. 2000, 25, 199–202. [Google Scholar] [CrossRef]
- Maggi, F. Curing viscosity of HTPB-based binder embedding microand nano-aluminum particles. Propellants Explos. Pyrotech. 2014, 39, 755–760. [Google Scholar] [CrossRef]
- Stamatis, D.; Zhu, X.; Schoenitz, M.; Dreizin, E.L.; Redner, P. Consolidation and mechanical properties of reactive nanocomposite powders. Powder Technol. 2011, 208, 637–642. [Google Scholar] [CrossRef]
- Wainwright, E.R.; Lakshman, S.V.; Leong, A.F.T.; Kinsey, A.H.; Gibbins, J.D.; Arlington, S.Q.; Sun, T.; Fezzaa, K.; Hufnagel, T.C.; Weihs, T.P. Viewing internal bubbling and microexplosions in combusting metal particles via x-ray phase contrast imaging. Combust. Flame 2019, 199, 194–203. [Google Scholar] [CrossRef]
- Wainwright, E.R.; Schmauss, T.A.; Vummidi Lakshman, S.; Overdeep, K.R.; Weihs, T.P. Observations during Al:Zr composite particle combustion in varied gas environments. Combust. Flame 2018, 196, 487–499. [Google Scholar] [CrossRef]
- Overdeep, K.R.; Livi, K.J.T.; Allen, D.J.; Glumac, N.G.; Weihs, T.P. Using magnesium to maximize heat generated by reactive Al/Zr nanolaminates. Combust. Flame 2015, 162, 2855–2864. [Google Scholar] [CrossRef]
- Aly, Y.; Dreizin, E.L. Ignition and combustion of Al·Mg alloy powders prepared by different techniques. Combust. Flame 2015, 162, 1440–1447. [Google Scholar] [CrossRef]
- Aly, Y.; Schoenitz, M.; Dreizin, E.L. Ignition and combustion of mechanically alloyed Al-Mg powders with customized particle sizes. Combust. Flame 2013, 160, 835–842. [Google Scholar] [CrossRef]
- Corcoran, A.L.; Wang, S.; Aly, Y.; Dreizin, E.L. Combustion of Mechanically Alloyed Al·Mg Powders in Products of a Hydrocarbon Flame. Combust. Sci. Technol. 2014, 187, 807–825. [Google Scholar] [CrossRef]
- Aly, Y.; Hoffman, V.K.; Schoenitz, M.; Dreizin, E.L. Reactive, Mechanically Alloyed Al·Mg Powders with Customized Particle Sizes and Compositions. J. Propuls. Power 2014, 30, 96–104. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, L.; Xia, Z.; Huang, L.; Yang, D. Ignition and combustion characteristics of single gas-atomized Al–Mg alloy particles in oxidizing gas flow. Energy 2020, 196, 117036. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal Oxide Nanoparticles as Bactericidal Agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Wagner, G.W.; Bartram, P.W.; Koper, O.; Klabunde, K.J. Reactions of VX, GD, and HD with Nanosize MgO. J. Phys. Chem. B 1999, 103, 3225–3228. [Google Scholar] [CrossRef]
- Overdeep, K.R.; Weihs, T.P. Design and functionality of a high-sensitivity bomb calorimeter specialized for reactive metallic foils. J. Therm. Anal. Calorim. 2015. [Google Scholar] [CrossRef]
- Jacob, R.J.; Ortiz-Montalvo, D.L.; Overdeep, K.R.; Weihs, T.P.; Zachariah, M.R. Incomplete reactions in nanothermite composites. J. Appl. Phys. 2017, 121. [Google Scholar] [CrossRef]
- Wagner, W.; Pruß, A. The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef]
- Santos, L.M.N.B.F.; Silva, M.T.; Schröder, B.; Gomes, L. Labtermo: Methodologies for the calculation of the corrected temperature rise in isoperibol calorimetry. J. Therm. Anal. Calorim. 2007, 89, 175–180. [Google Scholar] [CrossRef]
- Park, J. Bioceramics: Properties, Characterizations, and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; Volume 741. [Google Scholar]
- Gill, R.J.; Mohan, S.; Dreizin, E.L. Sizing and burn time measurements of micron-sized metal powders. Rev. Sci. Instrum. 2009, 80, 064101. [Google Scholar] [CrossRef]
- Beckstead, M.W. Correlating Aluminum Burning Times. Combust. Explos. Shock Waves 2005, 41, 533–546. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Piekiel, N.W.; Wu, C.; Chowdhury, S.; Kelly, S.T.; Hufnagel, T.C.; Fezzaa, K.; Zachariah, M.R. Reactive sintering: An important component in the combustion of nanocomposite thermites. Combust. Flame 2012, 159, 2–15. [Google Scholar] [CrossRef]
- Egan, G.C.; Lagrange, T.; Zachariah, M.R. Time-resolved nanosecond imaging of nanoscale condensed phase reaction. J. Phys. Chem. C 2015, 119, 2792–2797. [Google Scholar] [CrossRef]
- Chakraborty, P.; Zachariah, M.R. Do nanoenergetic particles remain nano-sized during combustion? Combust. Flame 2014, 161, 1408–1416. [Google Scholar] [CrossRef]
- Trunov, M.A.; Schoenitz, M.; Dreizin, E.L. Ignition of aluminum powders under different experimental conditions. Propellants Explos. Pyrotech. 2005, 30, 36–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Evans, J.R.G.; Yang, S. Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. J. Chem. Eng. Data 2011, 56, 328–337. [Google Scholar] [CrossRef]
- Chase, M.W.; Davies, C.A.; Downey, J.R.; Frurip, D.J.; McDonald, R.A.; Syverud, A.N. NIST-JANAF Thermochemical Tables. NIST Stand. Ref. Database 1985. [Google Scholar] [CrossRef]
- Taiebat, M.; Mutabaruka, P.; Pellenq, R.; Radjai, F. Effect of particle size distribution on 3D packings of spherical particles. EPJ Web Conf. 2017, 140, 02030. [Google Scholar] [CrossRef]
- Desmond, K.W.; Weeks, E.R. Influence of particle size distribution on random close packing of spheres. Phys. Rev. E 2014, 90, 022204. [Google Scholar] [CrossRef]


| Composition | Mass (mg) |
|---|---|
| Al:Zr | 44.76 |
| Al–8Mg:Zr | 45.2 |
| Al–38Mg:Zr | 46.95 |
| Particle Size | Avg. Particle Width (µm) | Avg. Particle Length (µm) | Avg. Particle Thickness (µm) | Avg. SA/V | Heat (kJ/g) |
|---|---|---|---|---|---|
| “Small” | 40 ± 6 | 145 ± 5 | 10 ± 6 | 0.083 | 10.2 ± 1.3 |
| “Standard” | 68 ± 5 | 141 ± 7 | 44 ± 5 | 0.048 | 11.5 ± 1.6 |
| “Large” | 84 ± 6 | 204 ± 9 | 52 ± 6 | 0.038 | 11.7 ± 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wainwright, E.R.; Mueller, M.A.; Overdeep, K.R.; Vummidi Lakshman, S.; Weihs, T.P. Measuring Heat Production from Burning Al/Zr and Al/Mg/Zr Composite Particles in a Custom Micro-Bomb Calorimeter. Materials 2020, 13, 2745. https://doi.org/10.3390/ma13122745
Wainwright ER, Mueller MA, Overdeep KR, Vummidi Lakshman S, Weihs TP. Measuring Heat Production from Burning Al/Zr and Al/Mg/Zr Composite Particles in a Custom Micro-Bomb Calorimeter. Materials. 2020; 13(12):2745. https://doi.org/10.3390/ma13122745
Chicago/Turabian StyleWainwright, Elliot R., Madeline A. Mueller, Kyle R. Overdeep, Shashank Vummidi Lakshman, and Timothy P. Weihs. 2020. "Measuring Heat Production from Burning Al/Zr and Al/Mg/Zr Composite Particles in a Custom Micro-Bomb Calorimeter" Materials 13, no. 12: 2745. https://doi.org/10.3390/ma13122745
APA StyleWainwright, E. R., Mueller, M. A., Overdeep, K. R., Vummidi Lakshman, S., & Weihs, T. P. (2020). Measuring Heat Production from Burning Al/Zr and Al/Mg/Zr Composite Particles in a Custom Micro-Bomb Calorimeter. Materials, 13(12), 2745. https://doi.org/10.3390/ma13122745





