Effects of Different Ions and Temperature on Corrosion Behavior of Pure Iron in Anoxic Simulated Groundwater
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Simulated Groundwater Solution
2.3. Electrochemical Measurement
2.4. Immersion Test
2.5. Surface Characterization
3. Result and Discussion
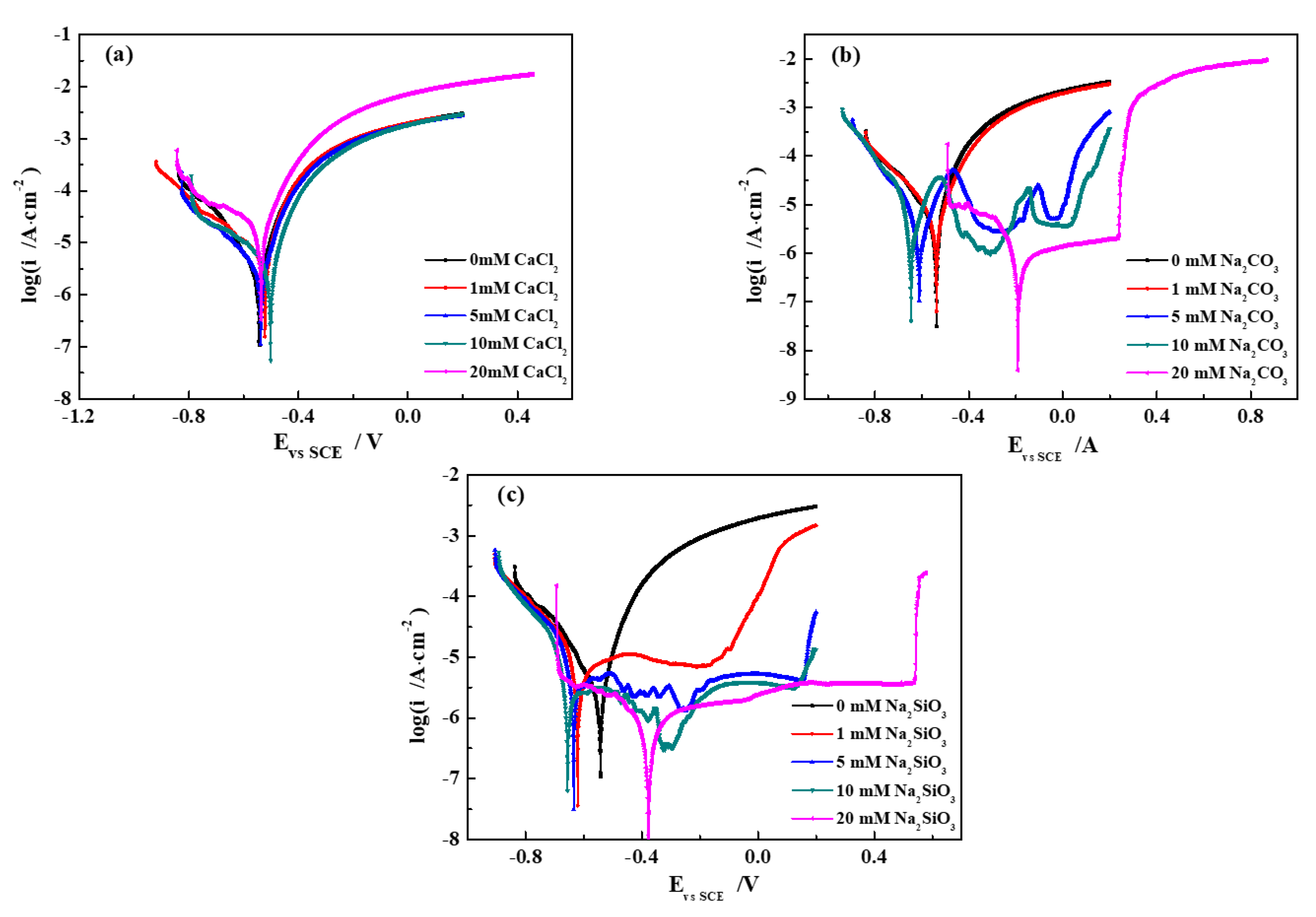
3.1. Potentiodynamic Polarization Curves
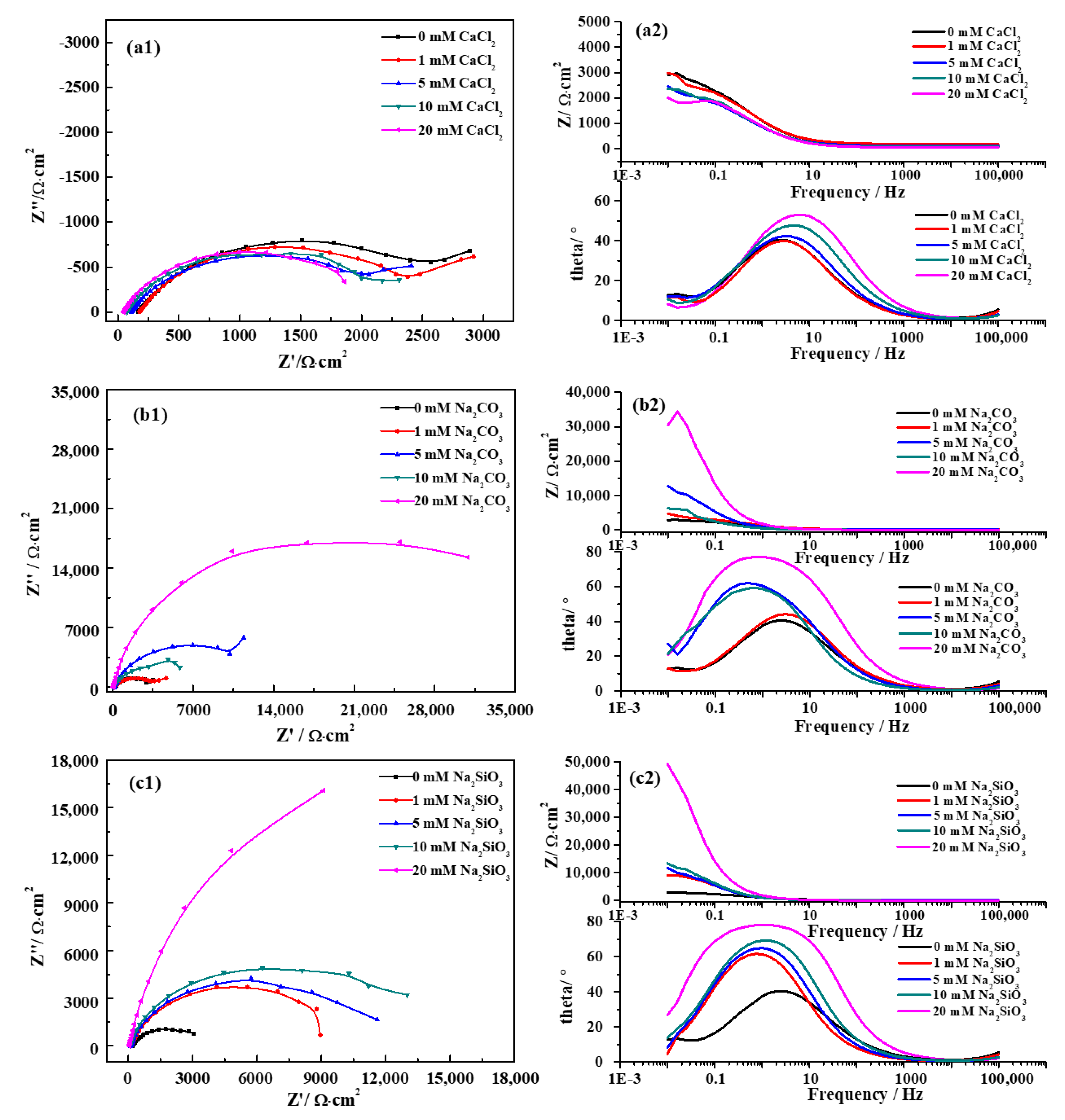
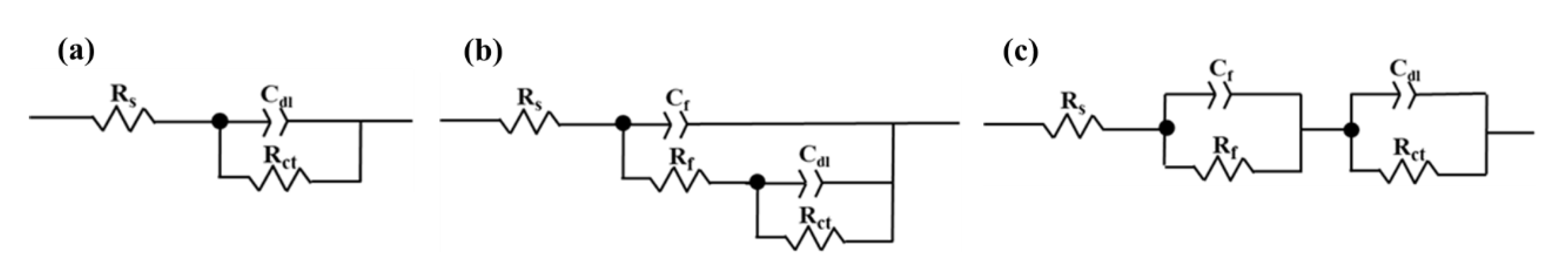
3.2. Electrochemical Impedance Spectroscopy
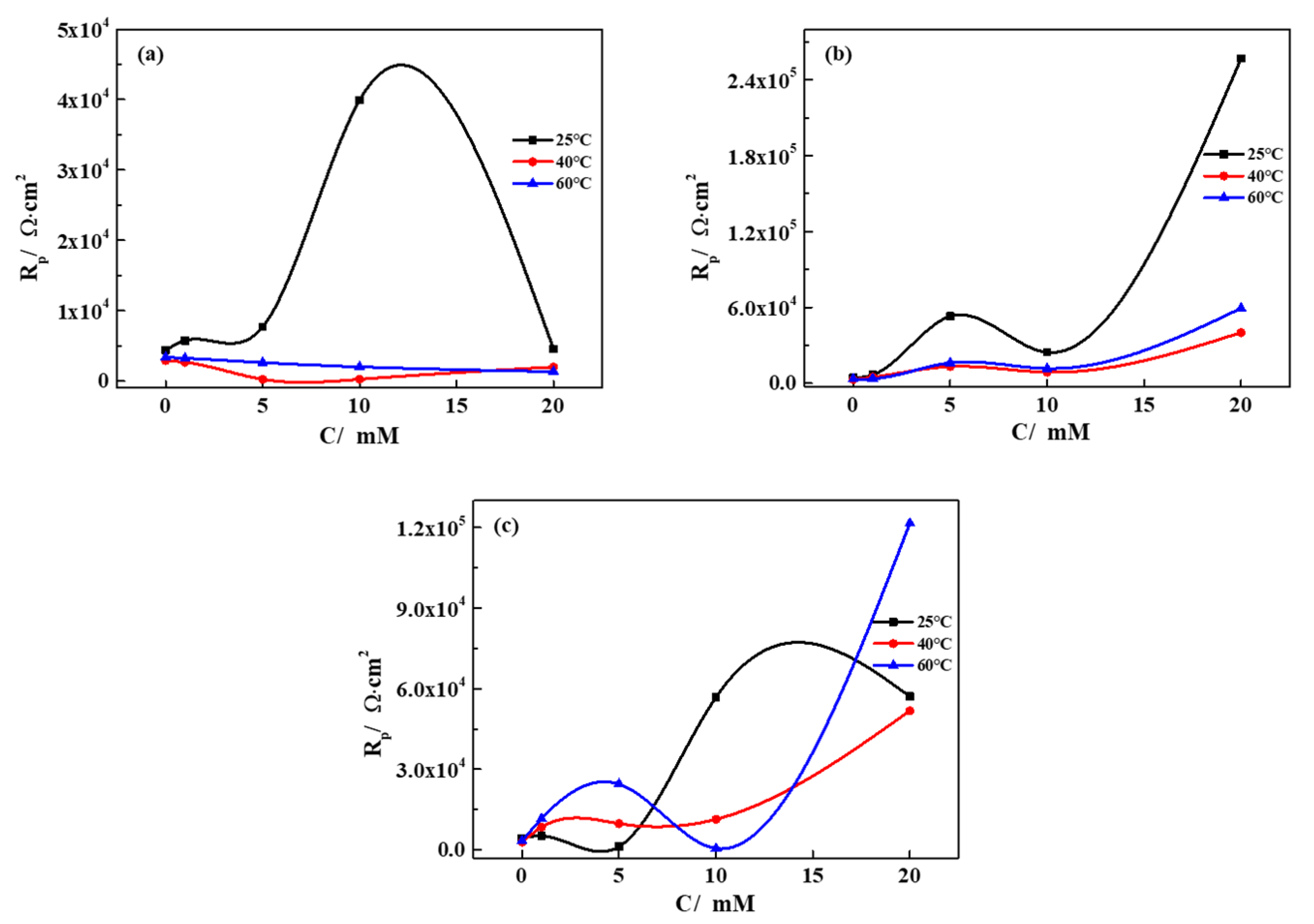
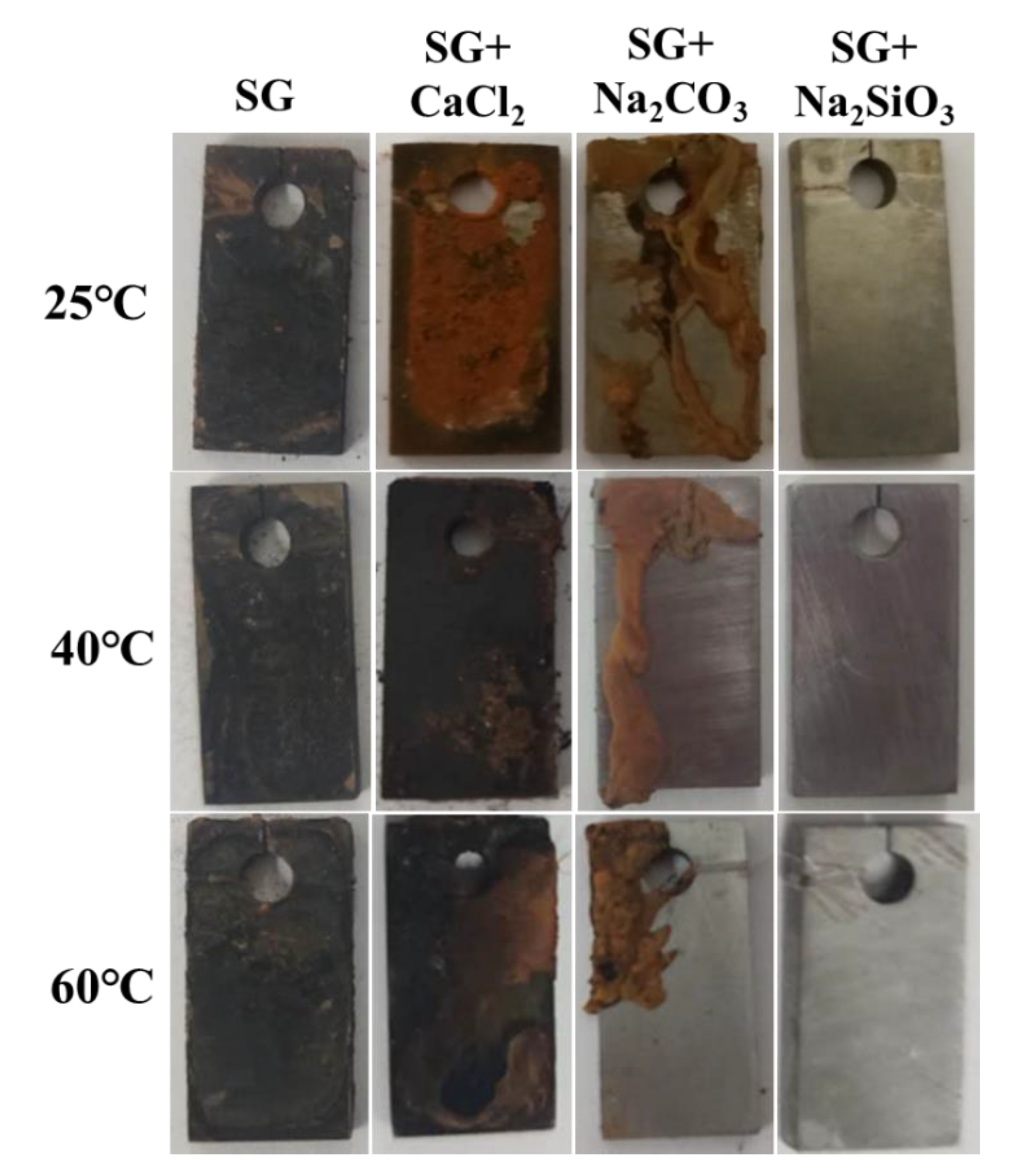
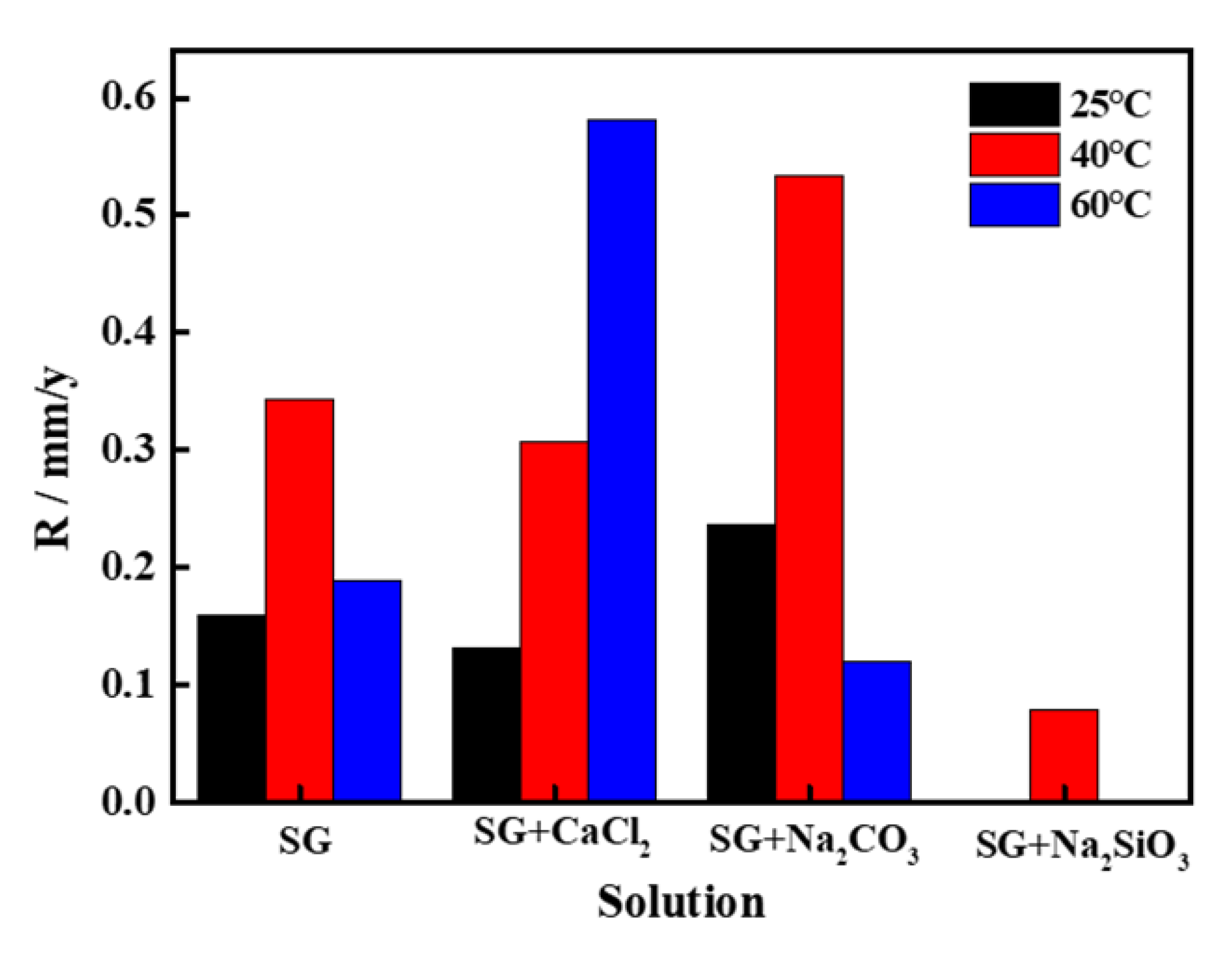
3.3. Corrosion Rate Obtained from Immersion Tests
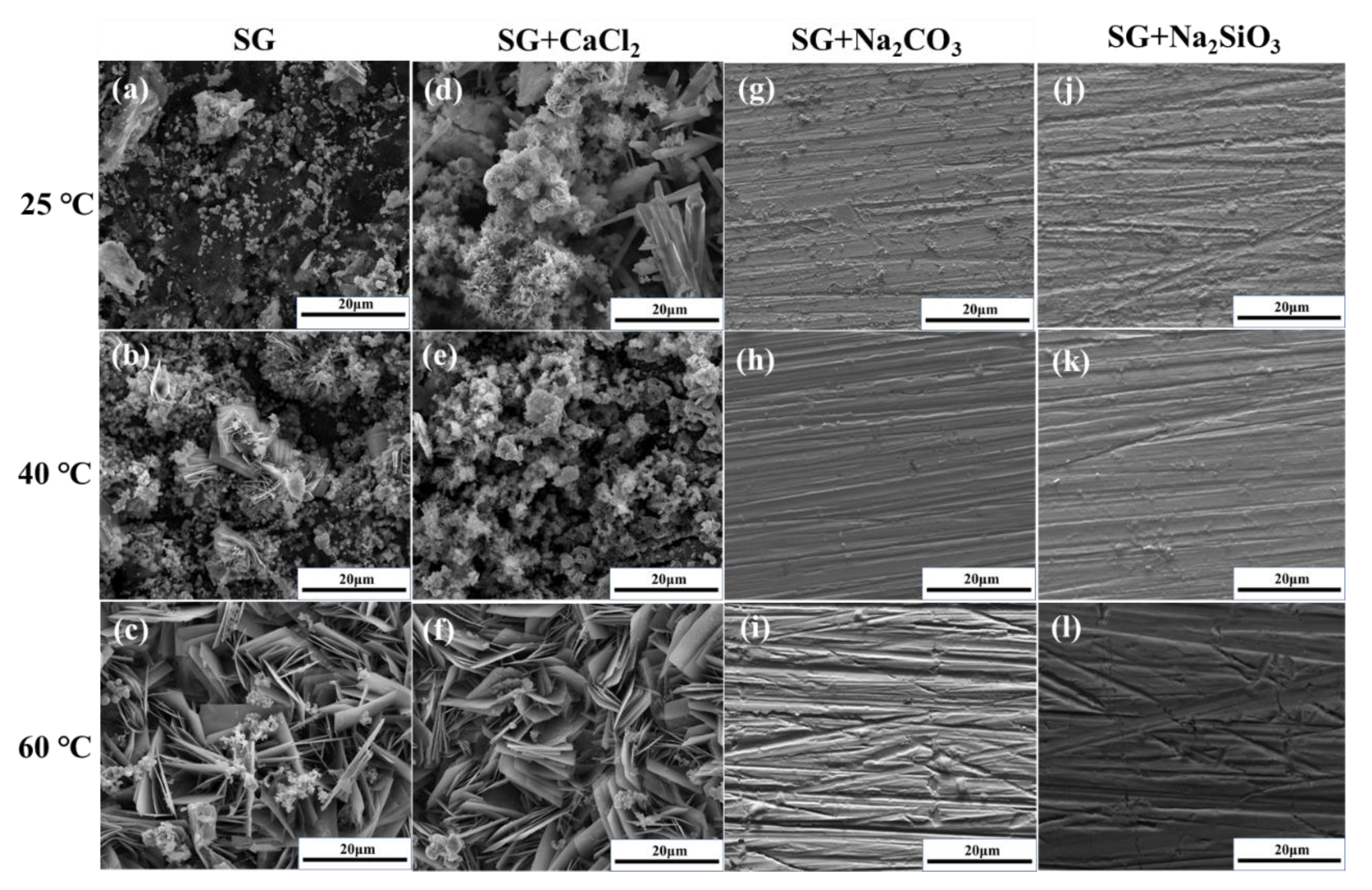
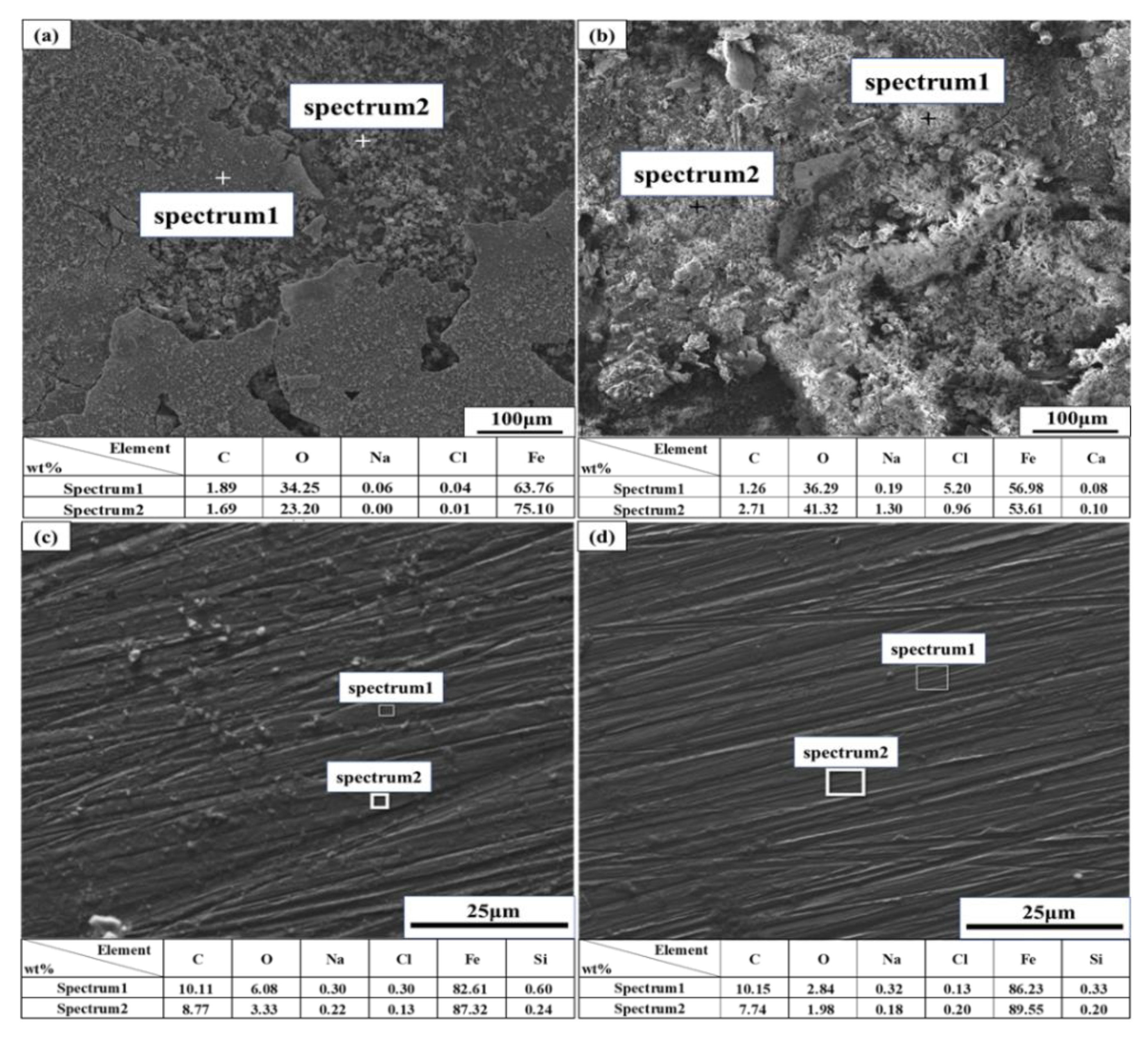
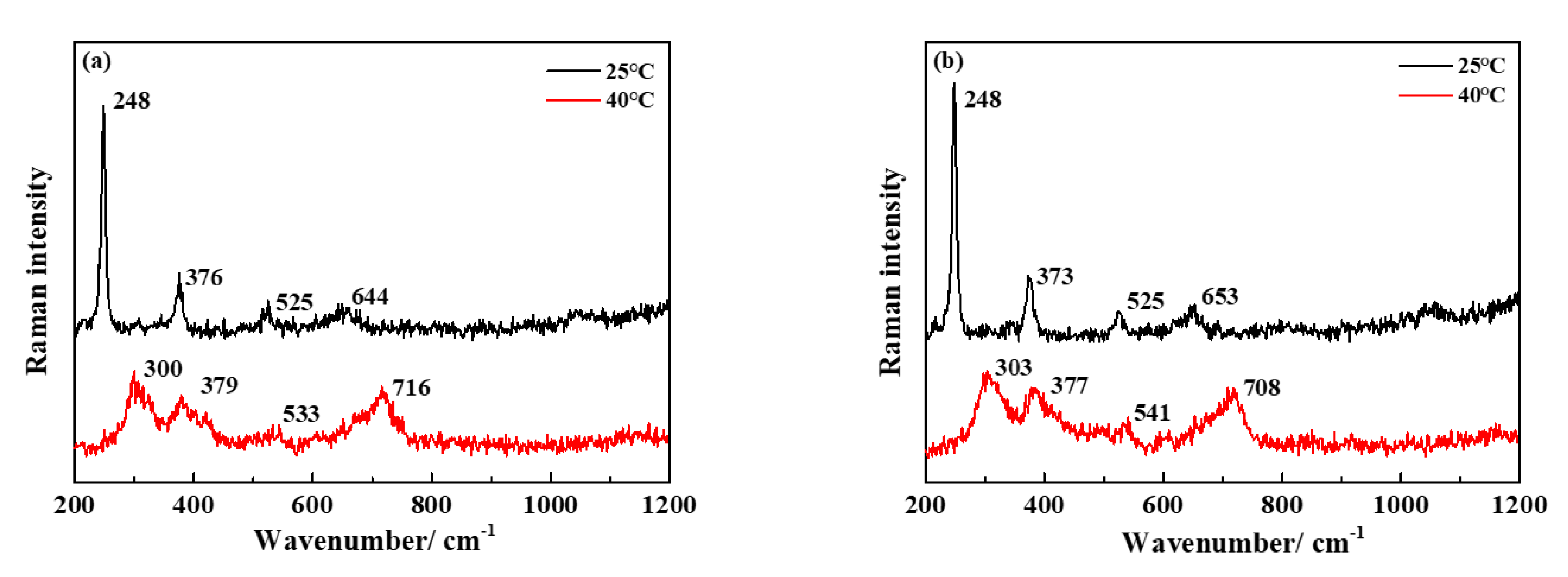
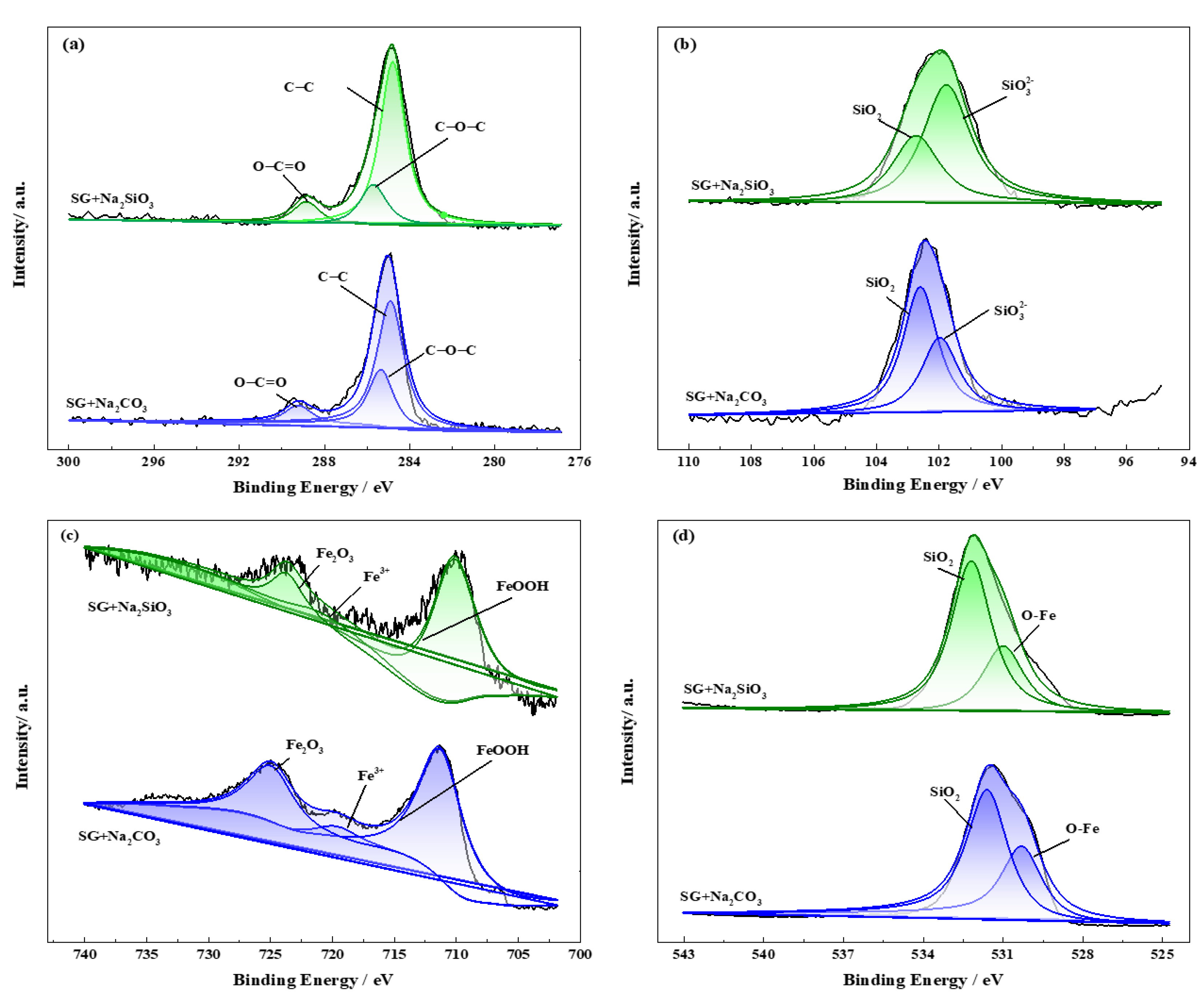
3.4. Characterization of Corrosion Products and Passivation Film
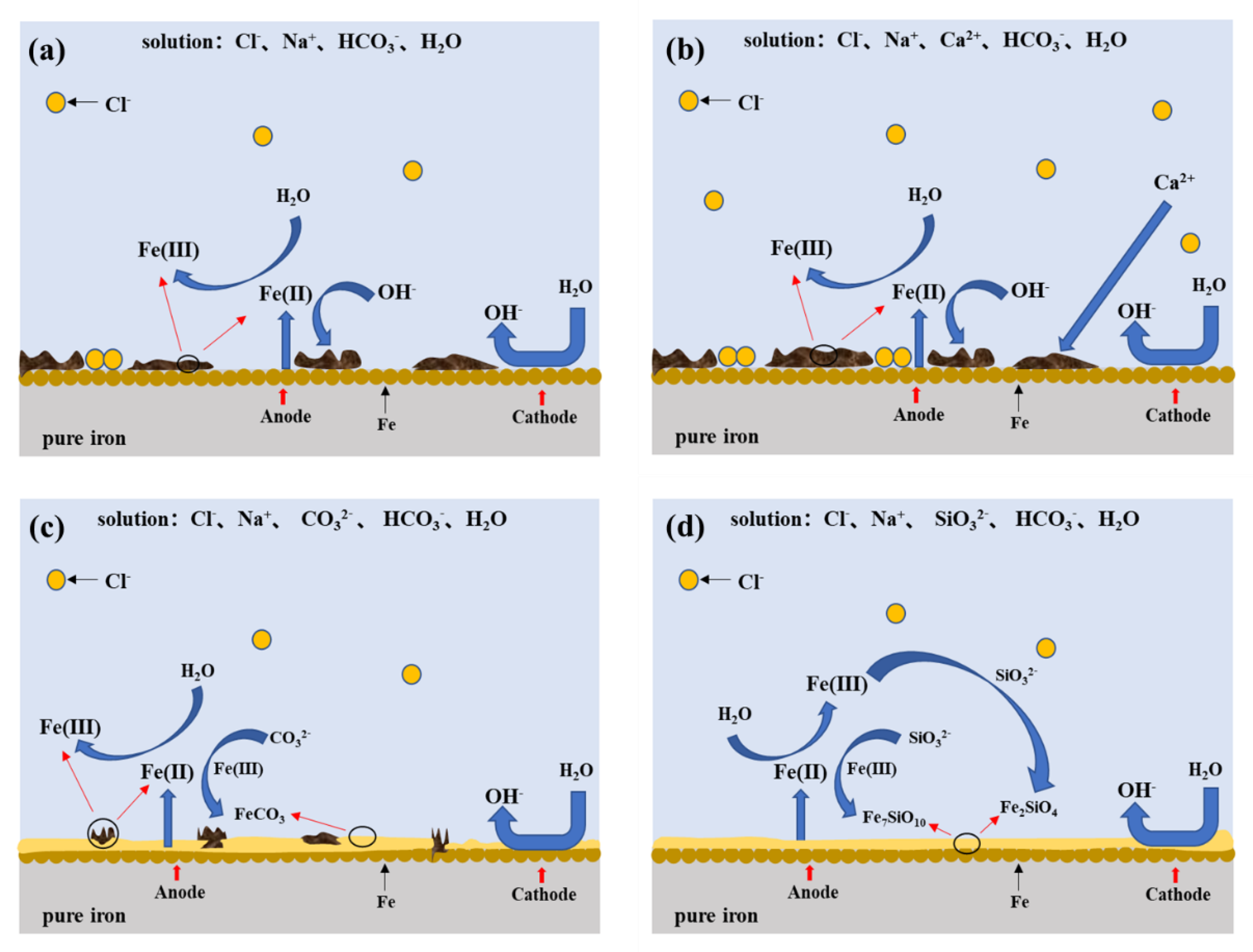
3.5. Mechanism of Corrosion Behavior of Iron in Simulated Groundwater
4. Conclusions
- The corrosion rate of pure iron in the SG + CaCl2 solution increases with the increasing concentration and temperature. The effects of Ca2− on the corrosion behavior of iron is negligible, however, Cl− plays important roles in the corrosion processes. The increased concentration and temperature accelerate the activity of Cl− and promote the dissolution of corrosion products, leading to more severe corrosion behavior. The formation of the corrosion products is mainly consisted of γ-FeOOH, α-FeOOH and γ-Fe2O3 in the SG and SG + CaCl2 solutions;
- The corrosion resistance of iron is largely improved by adding CO32− and SiO32− in the SG + Na2CO3/Na2SiO3 solution. A uniform passivation film gradually forms with the increased concentration, playing important roles in increasing the barrier effects of the iron surface and decreasing the formation of corrosion products. The passivation film is mainly composed of SiO2, ferric carbonate and silicate;
- The effects of temperature on the corrosion behavior of iron in the SG + Na2CO3/Na2SiO3 solution are not consistent. Cl− is dominant in enhancing the corrosion activity in the low-concentration solution, so the corrosion rates increase with the increased temperature. In the high-concentration solution, the synergistic effects of CO32−/SiO32− and Cl− contribute to the synergistic effects in the formation of the passivation film, leading to the cycles of passivation–dissolution processes. Hence, the corrosion rates are fluctuant with the varying temperature;
- The effects of CO32− and SiO32− are beneficial to iron in simulated groundwater. The formation of ferric silicate is dominant with the addition of SiO32−, resulting in a more uniform and denser passivation film than the film formed with the addition of CO32−. The penetration of Cl− is effectively prevented and the corrosion resistance of iron is largely improved.
Author Contributions
Funding
Conflicts of Interest
References
- Cui, D.; Spahiu, K. The reduction of U(VI) on corroded iron under anoxic conditions. Radiochim. Acta 2002, 90, 623–628. [Google Scholar] [CrossRef]
- Johnson, L.; Snellman, N.M.; Pastina, B. Safety assessment for a KBS3H spent nuclear fuel repository at Olkiluoto. Atlas Clin. Urol. 2007, 358, 127–140. [Google Scholar]
- Keech, P.G.; Vo, P.; Ramamurthy, S.; Chen, J.; Jacklin, R.; Shoesmith, D.W. Design and development of copper coatings for long term storage of used nuclear fuel. Corros. Eng. Sci. Technol. 2014, 49, 425–430. [Google Scholar] [CrossRef]
- Ibrahim, B.; Zagidulin, D.; Behazin, M.; Ramamurthy, S.; Wren, J.C.; Shoesmith, D.W. The corrosion of copper in irradiated and unirradiated humid air. Corros. Sci. 2018, 141, 53–62. [Google Scholar] [CrossRef]
- Guo, X.; Gin, S.; Lei, P.; Yao, T.; Liu, H.; Schreiber, D.K.; Ngo, D.; Viswanathan, G.; Li, T.; Kim, S.H.; et al. Self-accelerated corrosion of nuclear waste forms at material interfaces. Nat. Mater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, A.; Gaboreau, S.; Tinseau, E.; Bartier, D.; Petit, S.; Grauby, O.; Foct, F.; Beaufort, D. Mineralogical reactions in the Tournemire argillite after in-situ interaction with steels. Appl. Clay Sci. 2009, 43, 196–207. [Google Scholar] [CrossRef]
- Cui, D.; Low, J.; Rondinella, V.V.; Spahiu, K. Hydrogen catalytic effects of nanostructured alloy particles in spent fuel on radionuclide immobilization. Appl. Catal. B Environ. 2010, 94, 173–178. [Google Scholar] [CrossRef]
- Yang, H.; Cui, D.; Grolimund, D.; Rondinella, V.V.; Brütsch, R.; Amme, M.; Kutahyali, C.; Wiss, A.T.; Puranen, A.; Spahiu, K. Reductive precipitation of neptunium on iron surfaces under anaerobic conditions. J. Nucl. Mater. 2017, 496, 109–116. [Google Scholar] [CrossRef]
- Odorowski, M.; Jegou, C.; De Windt, L.; Broudic, V.; Jouan, G.; Peuget, S.; Martin, C. Effect of metallic iron on the oxidative dissolution of UO2 doped with a radioactive alpha emitter in synthetic Callovian-Oxfordian groundwater. Geochim. Cosmochim. Acta 2017, 219, 1–21. [Google Scholar] [CrossRef]
- He, J.; Ma, B.; Kang, M.; Wang, C.; Nie, Z.; Liu, C. Migration of 75Se(IV) in crushed Beishan granite: Effects of the iron content. J. Hazard. Mater. 2017, 324, 564–572. [Google Scholar] [CrossRef]
- Cui, D.; Rondinella, V.V.; Fortner, J.A.; Kropf, A.J.; Eriksson, L.; Wronkiewicz, D.J.; Spahiu, K. Characterization of alloy particles extracted from spent nuclear fuel. J. Nucl. Mater. 2012, 420, 328–333. [Google Scholar] [CrossRef]
- Björkbacka, Å.; Hosseinpour, S.; Johnson, M.; Leygraf, C.; Jonsson, M. Radiation induced corrosion of copper for spent nuclear fuel storage. Radiat. Phys. Chem. 2013, 92, 80–86. [Google Scholar] [CrossRef]
- Cui, D.; Ranebo, Y.; Low, J.; Rondinella, V.V.; Pan, J.; Spahiu, K. Immobilization of radionuclides on iron canister material at simulated near-field conditions. In Proceedings of the Materials Research Society Symposium, Boston, MA, USA, 2–4 December 2009; Volume 1124, pp. 111–116. [Google Scholar]
- Rosborg, B.; Werme, L. The Swedish nuclear waste program and the long-term corrosion behaviour of copper. J. Nucl. Mater. 2008, 379, 142–153. [Google Scholar] [CrossRef]
- Smart, N.R.; Blackwood, D.J.; Werme, L. Anaerobic corrosion of carbon steel and cast iron in artificial groundwaters: Part 2-Gas generation. Corrosion 2002, 58, 627–637. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Zhang, Z.; Han, E.H. Studies on corrosion behaviour of low carbon steel canister with and without γ-irradiation in China’s HLW disposal repository. Corros. Eng. Sci. Technol. 2017, 52, 136–140. [Google Scholar] [CrossRef]
- Martin, F.A.; Perrin, S.; Bataillon, C. Evaluating the corrosion rate of low alloyed steel in Callovo-Oxfordian clay: Towards a complementary EIS, gravimetric and structural study. In Proceedings of the Materials Research Society Symposium, Boston, MA, USA, 25–30 November 2012. [Google Scholar]
- Standish, T.E.; Braithwaite, L.J.; Shoesmith, D.W.; Noël, J.J. Influence of area ratio and chloride concentration on the galvanic coupling of copper and carbon steel. J. Electrochem. Soc. 2019. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, M.; Huang, Y.; Kunte, H.J.; Wang, X.; Liu, Y.; Zheng, C. Long term corrosion estimation of carbon steel, titanium and its alloy in backfill material of compacted bentonite for nuclear waste repository. Sci. Rep. 2019, 9, 3195. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, M.C.; Caporuscio, F.A.; Jové Colón, C.F.; Norskog, K.E. Fe-saponite growth on low-carbon and stainless steel in hydrothermal-bentonite experiments. J. Nucl. Mater. 2018. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, J.; Ke, W. Effects of Cl− ions on the corrosion behaviour of low alloy steel in deaerated bicarbonate solutions. J. Mater. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Zhang, Z.; Han, E.-H.; Liu, W.; Liang, D.; Yang, Z. Characterization of corrosion behavior of irradiated X65 low carbon steel in aerobic and unsaturated gaomiaozi bentonite. Acta Metall. Sin. Engl. Lett. 2019, 032, 506–516. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Tuček, J.; Prucek, R.; Kolařík, J.; Zoppellaro, G.; Petr, M.; Filip, J.; Sharma, V.K.; Zbořil, R. Zero-valent iron nanoparticles reduce arsenites and arsenates to As(0) firmly embedded in core-shell superstructure: Challenging strategy of arsenic treatment under anoxic conditions. ACS Sustain. Chem. Eng. 2017, 5, 3027–3038. [Google Scholar] [CrossRef]
- Das, S.; Lindsay, M.B.; Hendry, M.J. Selenate removal by zero-valent iron under anoxic conditions effects. Environ. Earth Sci. 2019, 78, 528. [Google Scholar] [CrossRef]
- Ni, Q.; Xia, X.; Zhang, J.; Dai, N.; Fan, Y. Electrochemical and SVET studies on the typical polarity reversal of Cu–304 stainless steel galvanic couple in Cl−-containing solution with different pH. Electrochim. Acta 2017, 247, 207–215. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Wang, Z.; Zhang, S.; Wu, X.; Huang, Y.; Li, X. The effect of nanosized NbC precipitates on electrochemical corrosion behavior of high-strength low-alloy steel in 3.5%NaCl solution. Int. J. Hydrogen Energy 2017, 42, 22175–22184. [Google Scholar] [CrossRef]
- Kazum, O.; Mathan, B.; Beladi, H.; Timokhina, I.; Hodgson, P.; Khoddam, S. Aqueous corrosion performance of nanostructured bainitic steel. Mater. Des. 2014, 54, 67–71. [Google Scholar] [CrossRef]
- Marques, A.G.; Simões, A.M. EIS and SVET assessment of corrosion resistance of thin Zn-55% Al-rich primers: Effect of immersion and of controlled deformation. Electrochim. Acta 2014, 148, 153–163. [Google Scholar] [CrossRef]
- Moreto, J.A.; Marino, C.E.B.; Filho, W.W.B.; Rocha, L.A.; Fernandes, J.C.S. SVET, SKP and EIS study of the corrosion behaviour of high strength Al and Al–Li alloys used in aircraft fabrication. Corros. Sci. 2014, 84, 30–41. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, K.; Lv, W.; Pang, X. Effects of alloyed Cr and Cu on the corrosion behavior of low-alloy steel in a simulated groundwater solution. Corros. Sci. 2016, 102, 114–124. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, S.; Smith, K.; Yu, K.; Hu, H.; Jiang, W.; Li, Y. Characterization of corrosion scale formed on stainless steel delivery pipe for reclaimed water treatment. Water Res. 2016, 88, 816–825. [Google Scholar] [CrossRef]
- Thompson, S.P.; Day, S.J.; Parker, J.E.; Evans, A.; Tang, C.C. Fine-grained amorphous calcium silicate CaSiO 3 from vacuum dried sol-gel—Production, characterisation and thermal behaviour. J. Non Cryst. Solids 2012, 358, 885–892. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Villar, S.E.J.; Jehlicka, J.; Munshi, T. FT-Raman spectroscopic study of calcium-rich and magnesium-rich carbonate minerals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Liu, Z.; Zhou, C.; Guo, Y.; Feng, Y.; Yang, M. Degradation mechanism of methylene blue by H2O2 and synthesized carbon nanodots/graphitic carbon nitride/Fe(II) composite. J. Phys. Chem. C 2019, 123, 26921–26931. [Google Scholar] [CrossRef]
- De Oliveira, L.A.; Correa, O.V.; Dos Santos, D.J.; Páez, A.A.Z.; De Oliveira, M.C.L.; Antunes, R.A. Effect of silicate-based films on the corrosion behavior of the API 5L X80 pipeline steel. Corros. Sci. 2018, 139, 21–34. [Google Scholar] [CrossRef]
- Lopez-Garrity, O.; Frankel, G.S. Corrosion inhibition of aa2024-t3 by sodium silicate. Electrochim. Acta 2014, 130, 9–21. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, Q.; Su, Y.; Li, J.; Wang, A.; Xing, Z. Preparation of MgAlFe-LDHs as a deicer corrosion inhibitor to reduce corrosion of chloride ions in deicing salts. Ecotoxicol. Environ. Saf. 2019, 174, 164–174. [Google Scholar] [CrossRef]
- Gibson, B.D.; Blowes, D.W.; Lindsay, M.B.J.; Ptacek, C.J. Mechanistic investigations of Se(VI) treatment in anoxic groundwater using granular iron and organic carbon: An EXAFS study. J. Hazard. Mater. 2012, 241–242, 92–100. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.; Hu, B.; Liu, Z.; Wang, C.; Han, J.; Su, M.; Li, Y.; Li, C. Modified chitosan-oligosaccharide and sodium silicate as efficient sustainable inhibitor for carbon steel against chloride-induced corrosion. J. Clean. Prod. 2019, 238, 117823. [Google Scholar] [CrossRef]

| Material | Composition (wt.%) | |||||
|---|---|---|---|---|---|---|
| C | S | P | Si | Mn | Fe | |
| Pure iron | 0.003 | 0.002 | 0.002 | 0.002 | 0.001 | balance |
| Solution | Chemicals Concentration (mM) | ||||
|---|---|---|---|---|---|
| NaCl | NaHCO3 | CaCl2 | Na2CO3 | Na2SiO3 | |
| SG | 10 | 2 | 0 | 0 | 0 |
| SG + CaCl2 | 10 | 2 | 1 | 0 | 0 |
| 10 | 2 | 5 | 0 | 0 | |
| 10 | 2 | 10 | 0 | 0 | |
| 10 | 2 | 20 | 0 | 0 | |
| SG + Na2CO3 | 10 | 2 | 0 | 1 | 0 |
| 10 | 2 | 0 | 5 | 0 | |
| 10 | 2 | 0 | 10 | 0 | |
| 10 | 2 | 0 | 20 | 0 | |
| SG + Na2SiO3 | 10 | 2 | 0 | 0 | 1 |
| 10 | 2 | 0 | 0 | 5 | |
| 10 | 2 | 0 | 0 | 10 | |
| 10 | 2 | 0 | 0 | 20 | |
| 40 °C | C/mM | 0 | 1 | 5 | 10 | 20 | |
|---|---|---|---|---|---|---|---|
| i0/E0 | |||||||
| CaCl2 | i0/A·cm−2 | 4.40 × 10−6 | 8.25 × 10−6 | 2.33 × 10−5 | 3.09 × 10−5 | 3.76 × 10−5 | |
| E0/V | −0.543 | −0.542 | −0.542 | −0.540 | −0.536 | ||
| Na2CO3 | i0/A·cm−2 | 4.40 × 10−6 | 7.19 × 10−6 | 7.91 × 10−6 | 1.33 × 10−6 | 3.27 × 10−6 | |
| E0/V | −0.543 | −0.537 | −0.610 | −0.642 | −0.161 | ||
| Na2SiO3 | i0/A·cm−2 | 4.40 × 10−6 | 1.56 × 10−5 | 4.19 × 10−6 | 5.40 × 10−6 | 3.93 × 10−6 | |
| E0/V | −0.543 | −0.621 | −0.634 | −0.655 | −0.377 | ||
| Solution | C/mM | Rs/Ω | Rct/Ω | Rf/Ω | Rp/Ω | Cr/S·secn | Cdl/S·secn |
|---|---|---|---|---|---|---|---|
| CaCl2 | 0 mM | 169.0 | 2889.0 | − | 2889.0 | − | 0.00024 |
| 1 mM | 176.8 | 2653.0 | − | 2653.0 | − | 0.000023 | |
| 5 mM | 108.6 | 226.4 | − | 226.4 | − | 0.00030 | |
| 10 mM | 72.3 | 227.1 | − | 227.1 | − | 0.00026 | |
| 20 mM | 44.9 | 1977.0 | − | 1977.0 | − | 0.00022 | |
| Na2CO3 | 1 mM | 174.8 | 3535.0 | 1277.0 | 4812 | 0.00017 | 0.0085 |
| 5 mM | 115.9 | 12.7 | 13,280.0 | 13,292.7 | 0.00021 | 0.000031 | |
| 10 mM | 68.9 | 13.5 | 8780.0 | 8793.5 | 0.000052 | 0.00044 | |
| 20 mM | 54.9 | 89.6 | 40,060.0 | 40,149.6 | 0.000063 | 0.000043 | |
| Na2SiO3 | 1 mM | 168.7 | 8164.0 | 275.1 | 8439.1 | 0.00020 | 0.0020 |
| 5 mM | 275.1 | 9494.0 | 289.1 | 9783.1 | 0.00021 | 0.021 | |
| 10 mM | 85.1 | 0.3 | 11,400.0 | 11,400.3 | 0.00023 | 0.00019 | |
| 20 mM | 51.6 | 28.3 | 51,770.0 | 51,798.3 | 0.00016 | 0.00010 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Huang, G.; Feng, Y.; Yang, M.; Wang, L.; Cui, D.; Zhang, X. Effects of Different Ions and Temperature on Corrosion Behavior of Pure Iron in Anoxic Simulated Groundwater. Materials 2020, 13, 2713. https://doi.org/10.3390/ma13122713
Li T, Huang G, Feng Y, Yang M, Wang L, Cui D, Zhang X. Effects of Different Ions and Temperature on Corrosion Behavior of Pure Iron in Anoxic Simulated Groundwater. Materials. 2020; 13(12):2713. https://doi.org/10.3390/ma13122713
Chicago/Turabian StyleLi, Teng, Guokai Huang, Yanpeng Feng, Miao Yang, Lingyu Wang, Daqing Cui, and Xian Zhang. 2020. "Effects of Different Ions and Temperature on Corrosion Behavior of Pure Iron in Anoxic Simulated Groundwater" Materials 13, no. 12: 2713. https://doi.org/10.3390/ma13122713
APA StyleLi, T., Huang, G., Feng, Y., Yang, M., Wang, L., Cui, D., & Zhang, X. (2020). Effects of Different Ions and Temperature on Corrosion Behavior of Pure Iron in Anoxic Simulated Groundwater. Materials, 13(12), 2713. https://doi.org/10.3390/ma13122713






