Rivet-Inspired Modification of Aramid Fiber by Decorating with Silica Particles to Enhance the Interfacial Interaction and Mechanical Properties of Rubber Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Grafting PAA onto AF
2.3. Silanization of SiO2
2.4. Preparation of SiO2@AF
2.5. Preparation of the Natural Rubber Composites
2.6. Characterization
3. Results and Discussions
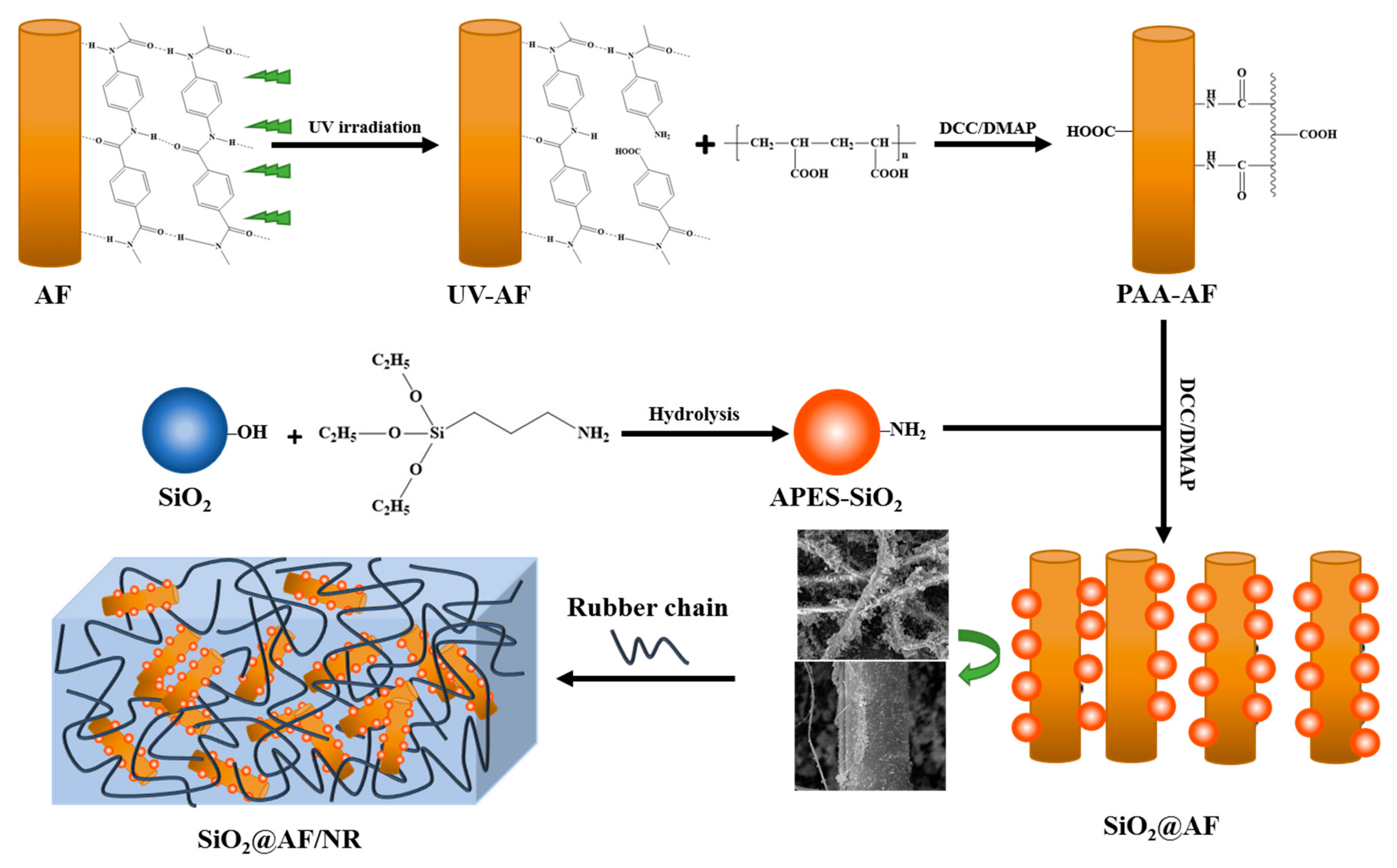
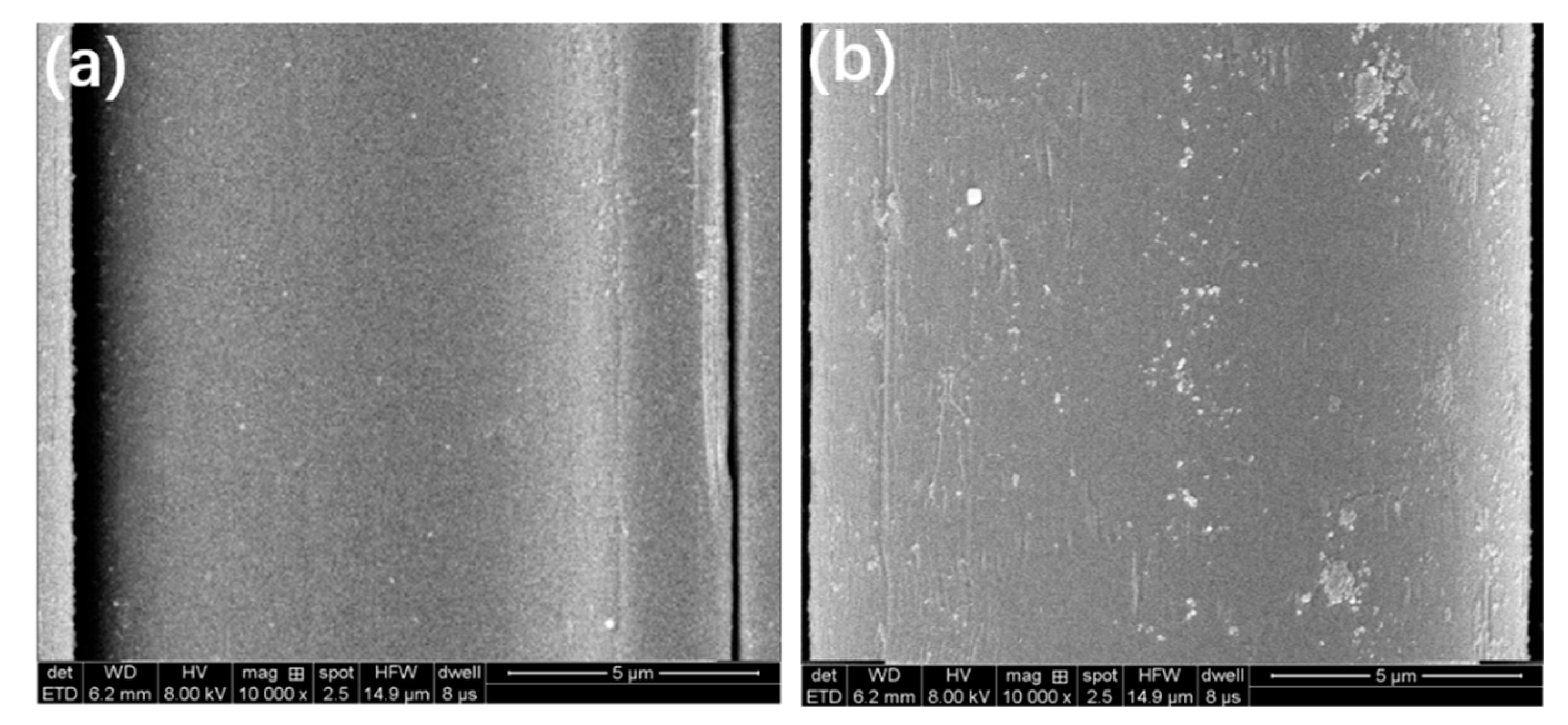
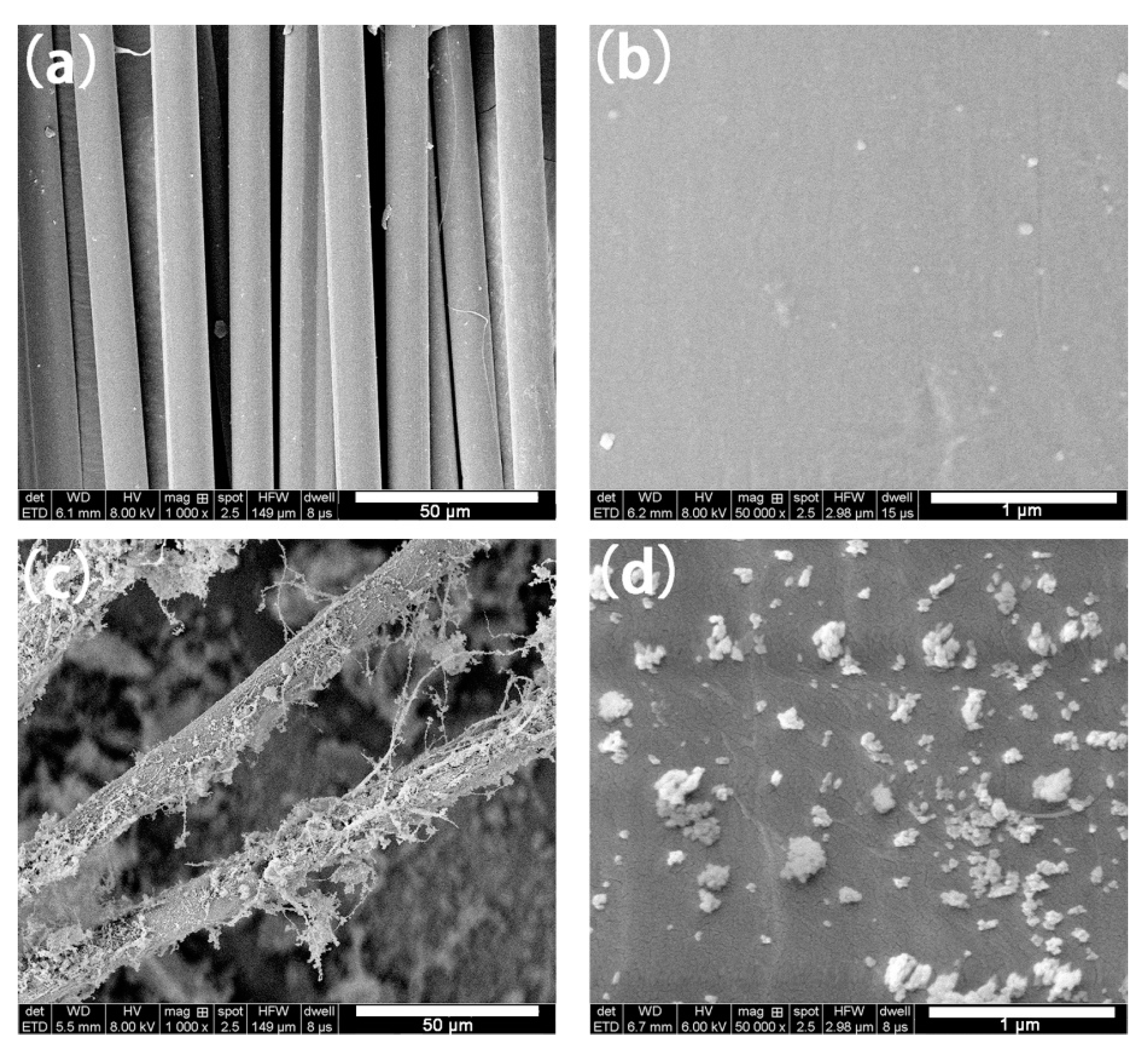
3.1. Characterizations of SiO2@AF
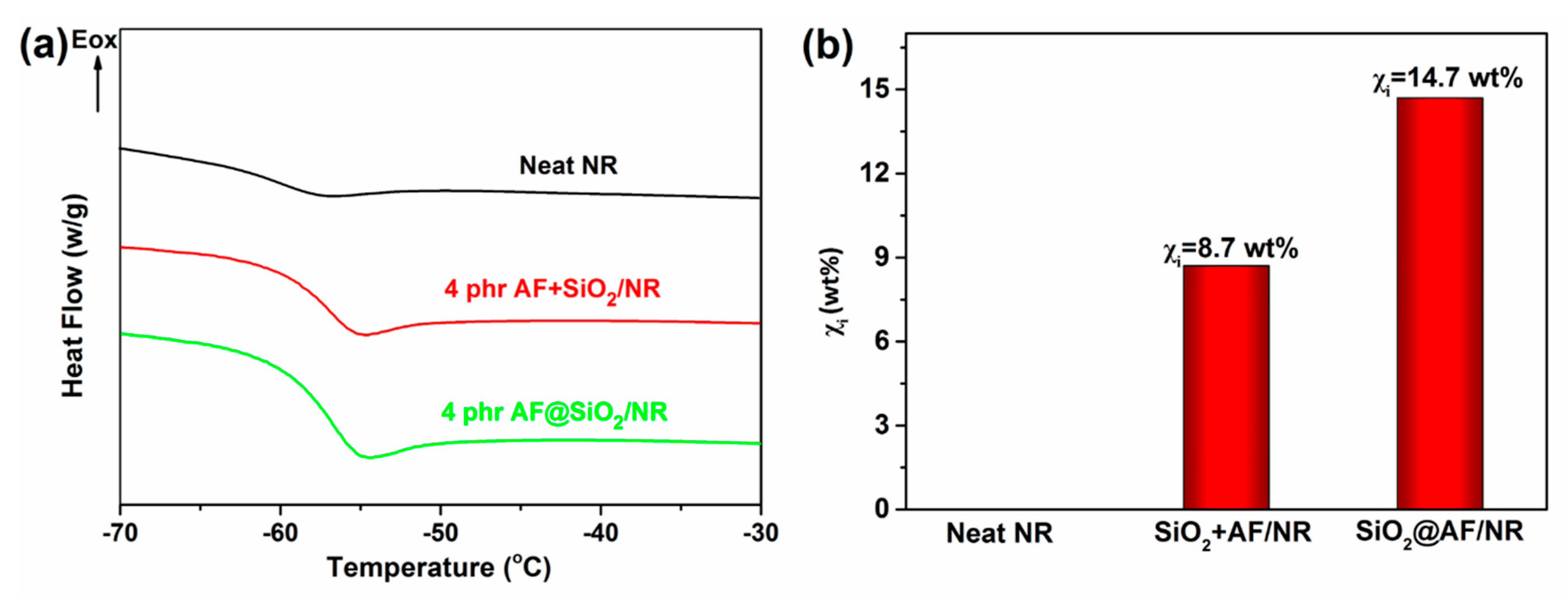
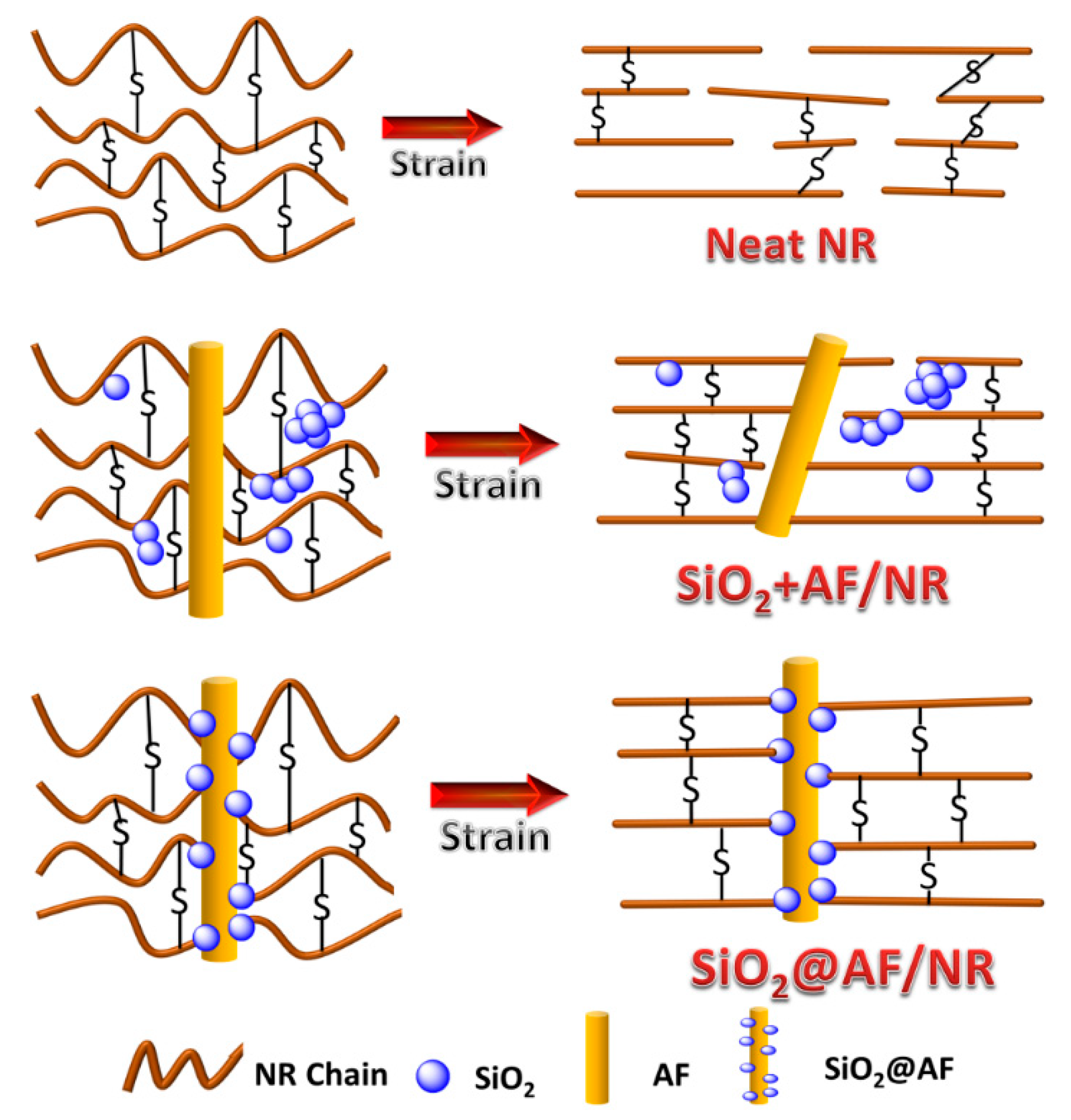
3.2. Analysis of Immobilized Rubber Approaching the Filler Surface
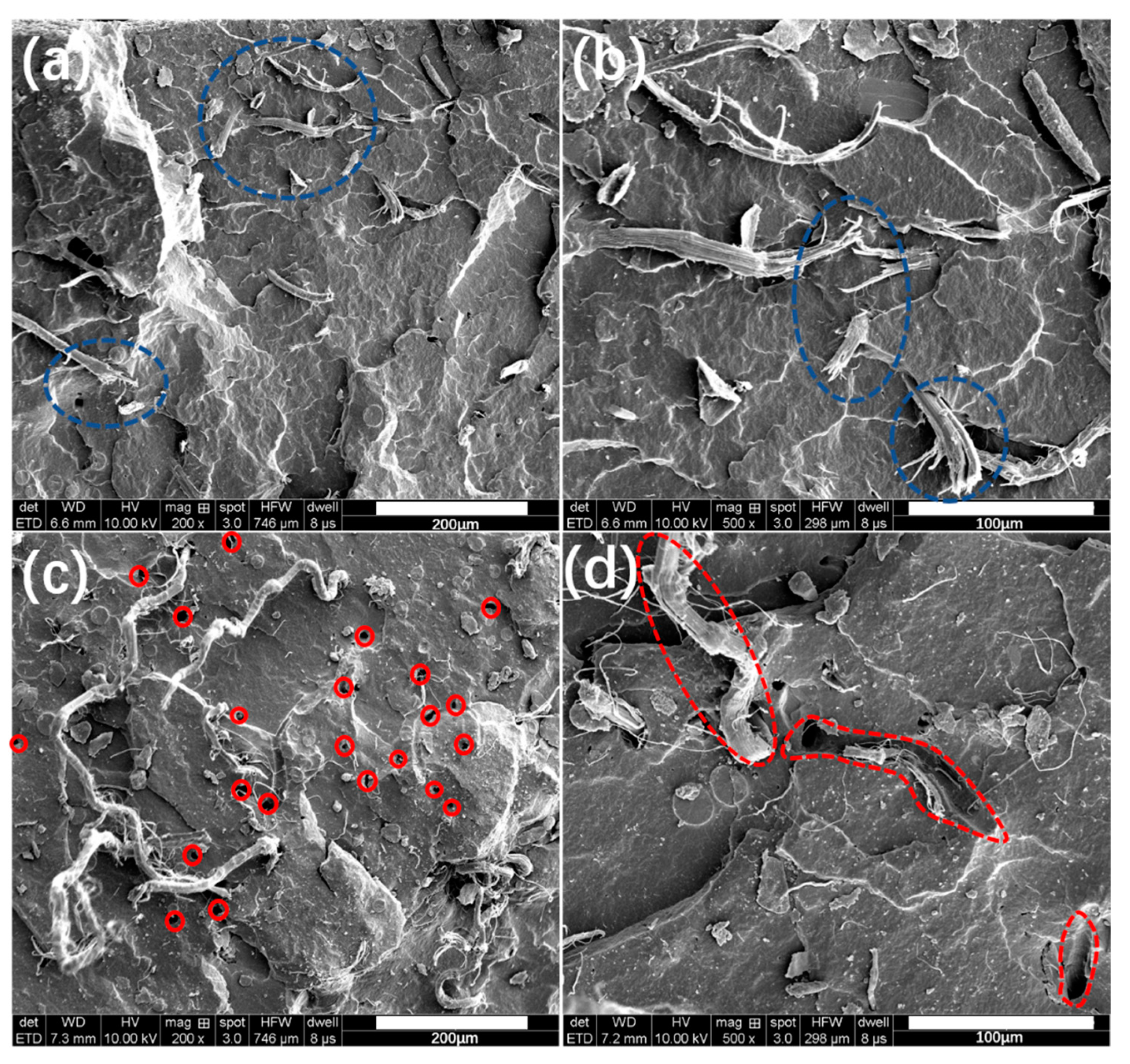
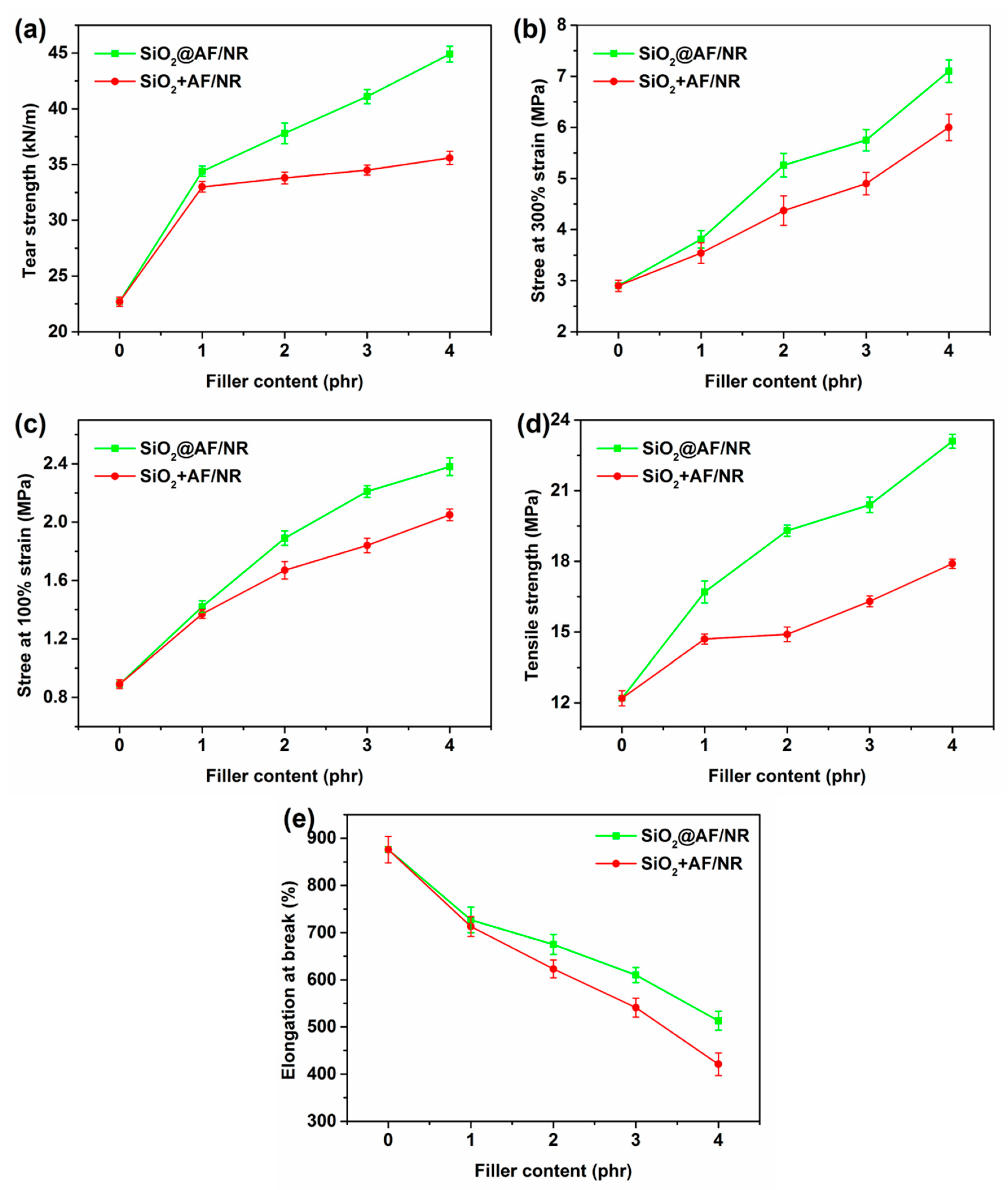
3.3. Mechanical Properties
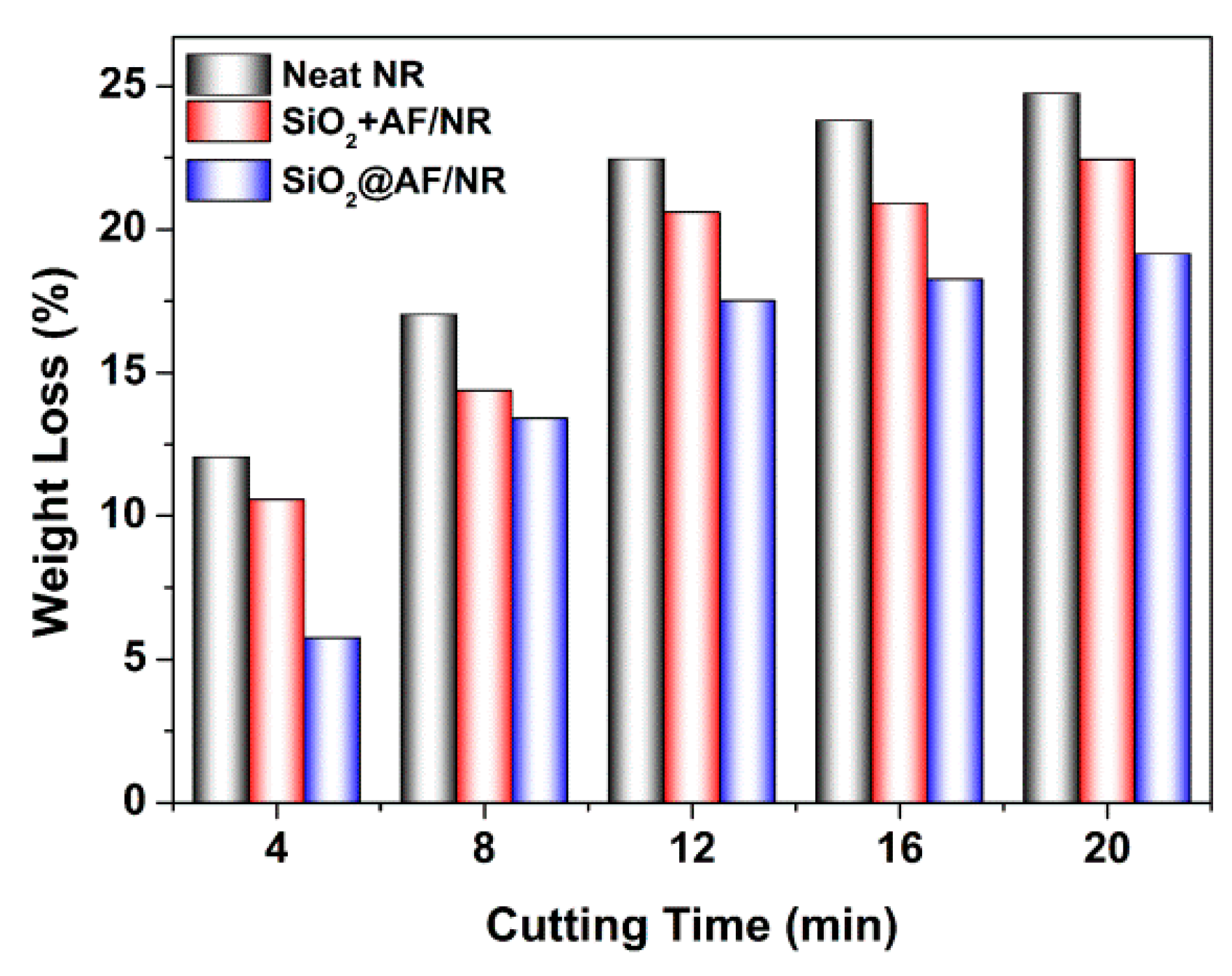
3.4. Cutting Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhan, Y.; Wang, J.; Zhang, K.; Li, Y.; Meng, Y.; Yan, N.; Wei, W.; Peng, F.; Xia, H. Fabrication of a flexible electromagnetic interference shielding Fe3O4@reduced graphene oxide/natural rubber composite with segregated network. Chem. Eng. J. 2018, 344, 184–193. [Google Scholar] [CrossRef]
- Das, A.; Stockelhuber, K.W.; Jurk, R.; Saphiannikova, M.; Fritzsche, J.; Lorenz, H.; Kluppel, M.; Heinrich, G. Modified and unmodified multiwalled carbon nanotubes in high performance solution-styrene–butadiene and butadiene rubber blends. Polymer 2008, 49, 5276–5283. [Google Scholar] [CrossRef]
- Rajan, V.V.; Dierkes, W.K.; Joseph, R.; Noordermeer, J.W.M. Science and technology of rubber reclamation with special attention to NR-based waste latex products. Prog. Polym. Sci. 2006, 31, 811–834. [Google Scholar] [CrossRef]
- Wu, J.; Huang, G.; Li, H.; Wu, S.; Liu, Y.; Zheng, J. Enhanced mechanical and gas barrier properties of rubber nanocomposites with surface functionalized graphene oxide at low content. Polymer 2013, 54, 1930–1937. [Google Scholar] [CrossRef]
- Jiang, M.; Xiong, Y.; Xue, B.; Zhang, Q.; Wan, Q.; Zhao, H. Multi-layer graphene oxide synergistically modified by two coupling agents and its application in reinforced natural rubber composites. RSC Adv. 2018, 8, 29847–29854. [Google Scholar] [CrossRef]
- Raji Vijay, V.; Anitha, A.M.; Ravindranatha Menon, A.R. Studies on blends of natural rubber and butadiene rubber containing silica-organomodified kaolin hybrid filler systems. Polymer 2016, 89, 135–142. [Google Scholar] [CrossRef]
- Luo, H.; Klüppel, M.; Schneider, H. Study of filled SBR elastomers using NMR and mechanical measurements. Macromolecules 2004, 37, 8000–8009. [Google Scholar] [CrossRef]
- Gerspacher, M.; O"Farrell, C.P.; Nikiel, L.; Yang, H.; Méhauté, F. High Frequency Viscoelasticity of Carbon Black Filled Compounds. Rubber Chem. Technol. 1996, 69, 786–800. [Google Scholar] [CrossRef]
- Kulik, V.M.; Boiko, A.; Bardakhanov, S.; Chun, H.; Lee, I. Viscoelastic properties of silicone rubber with admixture of SiO2 nanoparticles. Mat. Sci. Eng. A-Struct. 2011, 528, 5729–5732. [Google Scholar] [CrossRef]
- Meier, J.G.; Fritzsche, J.; Guy, L.; Bomal, Y.; Klüppel, M. Relaxation dynamics of hydration water at activated silica interfaces in high-performance elastomer composites. Macromolecules 2009, 42, 2127–2134. [Google Scholar] [CrossRef]
- Chen, L.; Jia, Z.; Guo, X.; Zhong, B.; Chen, Y.; Luo, Y.; Jia, D. Functionalized HNTs nanocluster vulcanized natural rubber with high filler-rubber interaction. Chem. Eng. J. 2018, 336, 748–756. [Google Scholar] [CrossRef]
- Zhu, J.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. A novel strategy of fabricating high performance UV-resistant aramid fibers with simultaneously improved surface activity, thermal and mechanical properties through building polydopamine and graphene oxide bi-layer coatings. Chem. Eng. J. 2017, 310, 134–147. [Google Scholar] [CrossRef]
- Pittayavinai, P.; Thanawan, S.; Amornsakchai, T. Comparative study of natural rubber and acrylonitrile rubber reinforced with aligned short aramid fiber. Polym. Test. 2017, 64, 109–116. [Google Scholar] [CrossRef]
- Yin, L.; Zhou, Z.; Luo, Z. Reinforcing effect of aramid fibers on fatigue behavior of SBR/aramid fiber composites. Polym. Test. 2019, 80, 106092. [Google Scholar] [CrossRef]
- Surajarusarn, B.; Hajjar-Garreau, S.; Schrodj, G.; Mougin, K.; Amornsakchai, T. Comparative study of pineapple leaf microfiber and aramid fiber reinforced natural rubbers using dynamic mechanical analysis. Polym. Test. 2020, 82, 106289. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Chen, S.; Wang, W.; Tian, M.; Ning, N.; Zhang, L. Highly efficient mussel-like inspired modification of aramid fibers by UV-accelerated catechol/polyamine deposition followed chemical grafting for high-performance polymer composites. Chem. Eng. J. 2017, 314, 583–593. [Google Scholar] [CrossRef]
- Zhou, L.; Yuan, L.; Guan, Q.; Gu, A.; Liang, G. Building unique surface structure on aramid fibers through a green layer-by-layer self-assembly technique to develop new high performance fibers with greatly improved surface activity, thermal resistance, mechanical properties and UV resistance. Appl. Surf. Sci. 2017, 411, 34–45. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, B.; Huang, J.; Chen, T.; Liu, Y.; Wang, X.; Liu, X. Covalent modification of Aramid fibers’ surface via direct fluorination to enhance composite interfacial properties. Mater. Design 2016, 106, 216–225. [Google Scholar] [CrossRef]
- Wang, C.X.; Du, M.; Lv, J.C.; Zhou, Q.Q.; Ren, Y.; Liu, C.L.; Gao, D.W.; Jin, L.M. Surface modification of aramid fiber by plasma induced vapor phase graft polymerization of acrylic acid. I. Influence of plasma conditions. Appl. Surf. Sci. 2015, 349, 333–342. [Google Scholar] [CrossRef]
- Xing, L.X.; Li, L.; Huang, Y.D.; Jiang, D.W.; Jiang, B.; He, J.M. Enhanced interfacial properties of domestic aramid fiber-12 via high energy gamma ray irradiation. Compos. Part B-Eng. 2015, 69, 50–57. [Google Scholar] [CrossRef]
- Vilay, V.; Mariatti, M.; Mat Taib, R.; Todo, M. Effect of fiber surface treatment and fiber loading on the properties of bagasse fiber–reinforced unsaturated polyester composites. Compos. Sci. Technol. 2008, 68, 631–638. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, L.; Jiang, C.; Dai, Y.; Meng, C.; Luo, L.; Liu, X. Aramid fiber with excellent interfacial properties suitable for resin composite in a wide polarity range. Chem. Eng. J. 2018, 347, 483–492. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, P.; Li, B.; Chen, T.; Liu, Y.; Ren, M.; Wang, Z.; Lai, W.; Wang, X.; Liu, X. Surface chain cleavage behavior of PBIA fiber induced by direct fluorination. Appl. Surf. Sci. 2016, 384, 480–486. [Google Scholar] [CrossRef]
- Li, Z.M.; Liu, B.H.; Kong, H.J.; Yu, M.H.; Qin, M.L.; Teng, C.Q. Layer-by-Layer Self-Assembly Strategy for Surface Modification of Aramid Fibers to Enhance Interfacial Adhesion to Epoxy Resin. Polymers. 2018, 10, 820. [Google Scholar] [CrossRef]
- Wei, B.; Cao, H.L.; Song, S.H. Surface modification and characterization of basalt fibers with hybrid sizing. Compos. Part A 2011, 42, 22–29. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Y.; Ni, Q.; Fu, Y.; Fu, X. Surface modification and characterization of aramid fibers with hybrid coating. Appl. Surf. Sci. 2014, 321, 103–108. [Google Scholar] [CrossRef]
- Li, S.; Gu, A.; Xue, J.; Liang, G.; Yuan, L. The influence of the short-term ultraviolet radiation on the structure and properties of poly(p-phenylene terephthalaramide) fibers. Appl. Surf. Sci. 2013, 265, 519–526. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, M.; Nie, J.; Liu, G.; Tan, J.; Yang, B.; Song, S.; Zhao, J. A deep insight into the structure and performance evolution of aramid nanofiber films induced by UV irradiation. Polym. Degrad. Stab. 2019, 167, 170–178. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Chen, J.; Hao, X.; Wang, S.; Feng, X.; Guo, Y. Effects of solar UV irradiation on the tensile properties and structure of PPTA fiber. Polym. Degrad. Stab. 2006, 91, 2761–2767. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, Z.; Guo, B.; Zhang, L. Significantly improved rubber-silica interface via subtly controlling surface chemistry of silica. Compos. Sci. Technol. 2018, 156, 70–77. [Google Scholar] [CrossRef]
- Dai, J.; Xiong, Y.; Cui, L.; Li, X.; Wang, B.; Wu, S. Study on modification of aramid fiber by UV irradiation. J. Synth. Cryst. 2016, 45, 2705–2710. [Google Scholar]
- Xu, Y.; Li, Y.; Hua, W.; Zhang, A.; Bao, J. Light-weight silver plating foam and carbon nanotube hybridized epoxy composite foams with exceptional conductivity and electromagnetic shielding property. ACS Appl. Mater. Inter. 2016, 8, 24131–24142. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.; Teng, C. Covalent functionalization of aramid fibers with zinc oxide nano-interphase for improved UV resistance and interfacial strength in composites. Compos. Sci. Technol. 2020, 188, 107996. [Google Scholar] [CrossRef]
- Morent, R.; Geyter, N.D.; Trentesaux, M.; Gengembre, L.; Dubruel, P.; Leys, C.; Payen, E. Stability study of polyacrylic acid films plasma-polymerized on polypropylene substrates at medium pressure. Appl. Surf. Sci. 2010, 257, 372–380. [Google Scholar] [CrossRef]
- Dai, Y.; Meng, C.; Tang, S.; Qin, J.; Liu, X. Construction of dendritic structure by nano-SiO2 derivate grafted with hyperbranched polyamide in aramid fiber to simultaneously improve its mechanical and compressive properties. Eur. Polym. J. 2019, 119, 367–375. [Google Scholar] [CrossRef]
- Mitic, Z.; Cakic, M.; Nikolic, G.M.; Nikolic, R.; Nikolic, G.S.; Pavlovic, R.; Santaniello, E. Synthesis, physicochemical and spectroscopic characterization of copper (II)-polysaccharide pullulan complexes by UV–vis, ATR-FTIR, and EPR. Carbohydr. Res. 2011, 346, 434–441. [Google Scholar] [CrossRef]
- Xue, B.; Ji, L.; Deng, J.; Zhang, J. In situ FTIR spectroscopy study on the rapid dissolution process of modified poly(vinyl alcohol). J. Polym. Res. 2016, 23, 209. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, Z.; Schwieger, W. Vibrational spectroscopic studies of layered silicates. Chem. Mater. 1999, 11, 1210–1217. [Google Scholar] [CrossRef]
- Xue, B.; Bao, J.; Zhang, J. Ultrafine fly ash as a reinforcing filler in poly(lactic acid) matrix. J. Appl. Polym. Sci. 2016, 133, 43716. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, H.; Wirth, M. Chemical modification of the surface of poly(dimethylsiloxane) by atom-transfer radical polymerization of acrylamide. Langmuir 2002, 18, 9971–9976. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, S.; Peng, J.; Liu, L. The filler–rubber interface and reinforcement in styrene butadiene rubber composites with graphene/silica hybrids: A quantitative correlation with the constrained region. Compos. Part A 2016, 86, 19–30. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, C.; Li, H.; Du, Z. Fabrication of silica nanoparticles on the surface of functionalized multi-walled carbon nanotubes. Carbon 2011, 49, 126–132. [Google Scholar] [CrossRef]
- Kripotou, S.; Pissis, P.; Savelyev, Y.V.; Robota, L.P.; Travinskaya, T.V. Polymer dynamics in polyurethane/clay nanocomposites studied by dielectric and thermal techniques. J. Macromol. Sci. Part B Phys. 2010, 49, 86–110. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Pissis, P.; Bokobza, L. Glass transition and molecular dynamics in poly (dimethylsiloxane)/silica nanocomposites. Polymer 2005, 46, 6001–6008. [Google Scholar] [CrossRef]
- Ruggerone, R.; Geiser, V.; Vacche, S.D.; Leterrier, Y.; Manson, J. Immobilized polymer fraction in hyperbranched polymer/silica nanocomposites suspensions. Macromolecules 2010, 43, 10490–10497. [Google Scholar] [CrossRef]
- Tadiello, L.; D’Arienzo, M.; Di Credico, B.; Hanel, T.; Matejka, L.; Mauri, M. Filler-rubber interface in styrene butadiene nanocomposites with anisotropic silica particles: Morphology and dynamic properties. Soft Matter 2015, 11, 4022–4033. [Google Scholar] [CrossRef]
- Hao, M.Y.; Wu, H.W.; Qiu, F.; Wang, X.W. Interface Bond Improvement of Sisal Fibre Reinforced Polylactide Composites with Added Epoxy Oligomer. Materials 2018, 11, 398. [Google Scholar] [CrossRef]
- Xu, T.; Jia, Z.; Li, J.; Luo, Y.; Jia, D.; Peng, Z. Study on the dispersion of carbon black/silica in SBR/BR composites and its properties by adding epoxidized natural rubber as a compatilizer. Polym. Compos. 2018, 39, 377–385. [Google Scholar] [CrossRef]


| Ingredient | Phr * |
|---|---|
| NR | 100 |
| SiO2@AF or SiO2+AF | 1–4 |
| Zinc oxide (ZnO) | 5 |
| Stearic acid (SA) | 4 |
| Accelerator diphenylhydrazine (D) | 0.5 |
| Tetramethylthiuramdisulfide (TMTD) | 0.32 |
| 2-Mercaptobenzothiazole (M) | 2.21 |
| 2,2′-dibenzothiazoledisulfde (DM) | 1.96 |
| Antioxidant N-isopropyl-N′-phenyl-4-phenylenediamin (4010NA) | 1.5 |
| Sulphur | 1.71 |
| Sample | ϕ (wt%) | Tg (K) | ΔCp (J/gK) | ΔCpn (J/gK) |
|---|---|---|---|---|
| Neat NR | 0 | 207.61 | 0.503 | 0.503 |
| AF+SiO2/NR | 3.300 | 216.34 | 0.444 | 0.459 |
| SiO2@AF/NR | 3.300 | 218.00 | 0.409 | 0.429 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xiong, Y.; Zhang, Q. Rivet-Inspired Modification of Aramid Fiber by Decorating with Silica Particles to Enhance the Interfacial Interaction and Mechanical Properties of Rubber Composites. Materials 2020, 13, 2665. https://doi.org/10.3390/ma13112665
Li Y, Xiong Y, Zhang Q. Rivet-Inspired Modification of Aramid Fiber by Decorating with Silica Particles to Enhance the Interfacial Interaction and Mechanical Properties of Rubber Composites. Materials. 2020; 13(11):2665. https://doi.org/10.3390/ma13112665
Chicago/Turabian StyleLi, Yihang, Yuzhu Xiong, and Qingpo Zhang. 2020. "Rivet-Inspired Modification of Aramid Fiber by Decorating with Silica Particles to Enhance the Interfacial Interaction and Mechanical Properties of Rubber Composites" Materials 13, no. 11: 2665. https://doi.org/10.3390/ma13112665
APA StyleLi, Y., Xiong, Y., & Zhang, Q. (2020). Rivet-Inspired Modification of Aramid Fiber by Decorating with Silica Particles to Enhance the Interfacial Interaction and Mechanical Properties of Rubber Composites. Materials, 13(11), 2665. https://doi.org/10.3390/ma13112665





