Magnetic Behaviour of Mn12-Stearate Single-Molecule Magnets Immobilized on the Surface of 300 nm Spherical Silica Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
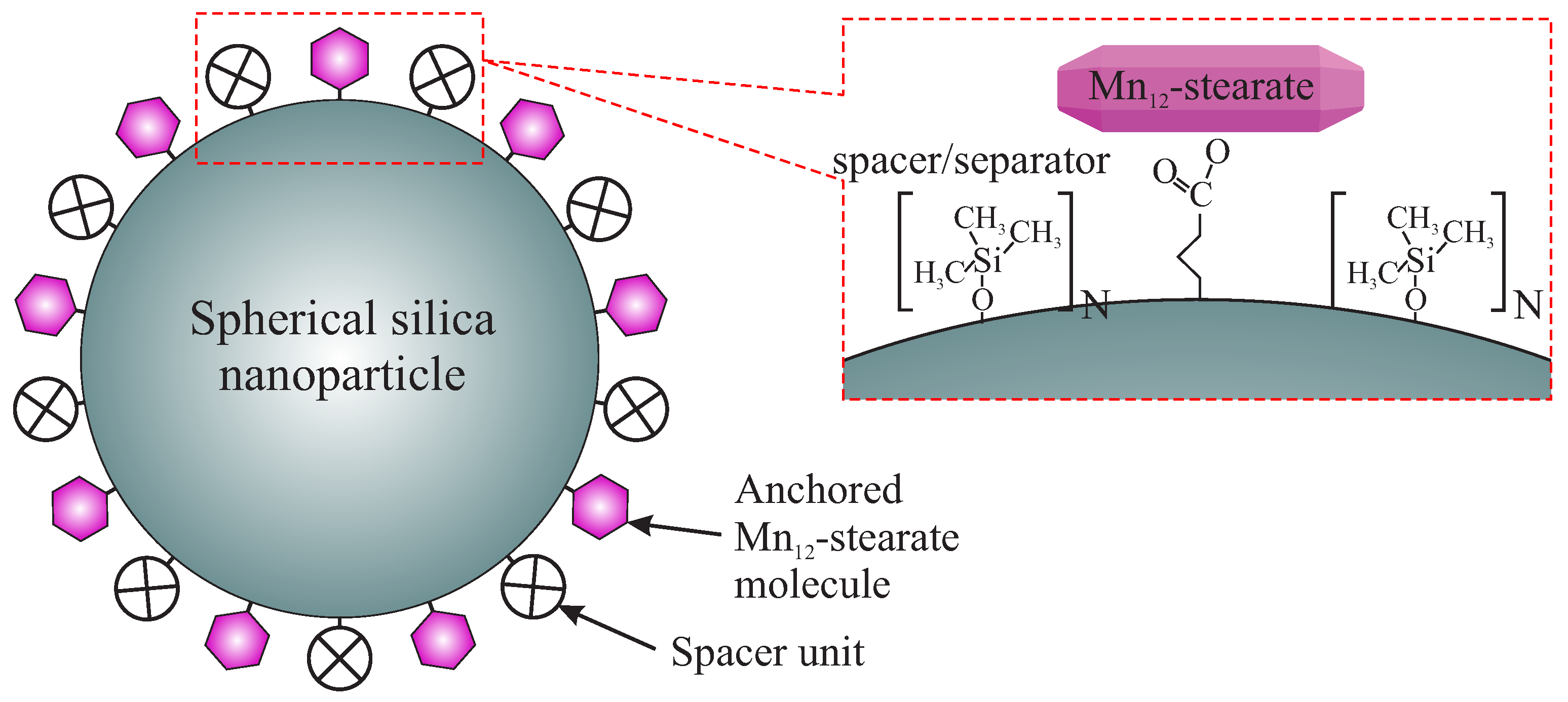
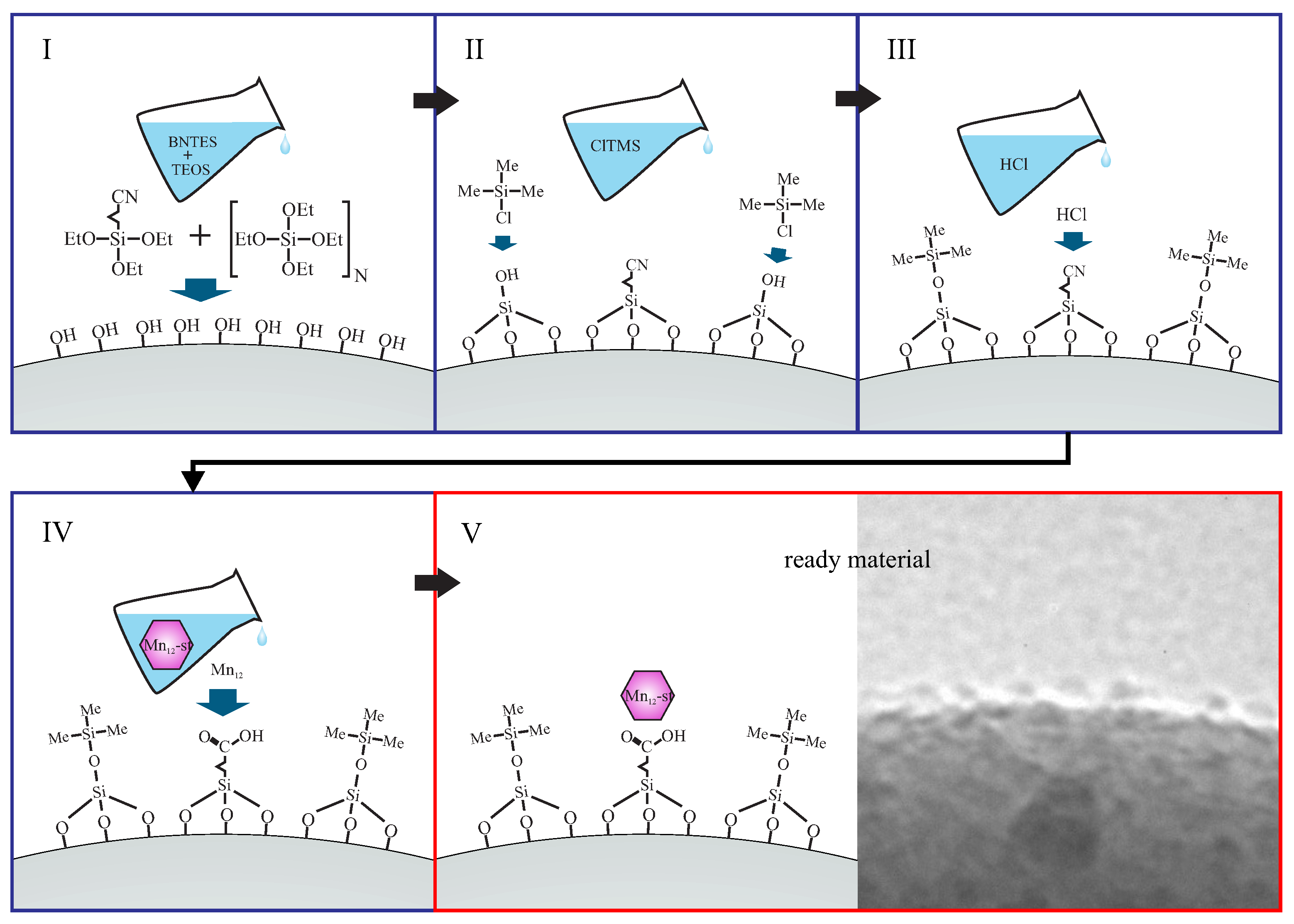
2.1. Samples Synthesis
- SilS-MnN1: sample with a highest possible concentration of Mn-st at the surface and the most rigidly anchored molecules (multiple bonds between silica surface and the SMMs);
- SilS-MnN3: sample with a highest possible concentration of Mn-st at the surface, rigidly anchored molecules but lower number of bonds between silica surface and the SMMs, than for the previously listed sample;
- SilS-MnN6: sample with a highest possible concentration of Mn-st at the surface bonded via single bonds—free-floating molecules of Mn;
- SilS-MnN9: sample with a significantly lower concentration of Mn-st at the surface bonded via single bonds—free-floating molecules of Mn.
2.2. Characterization Methods
3. Results and Discussion
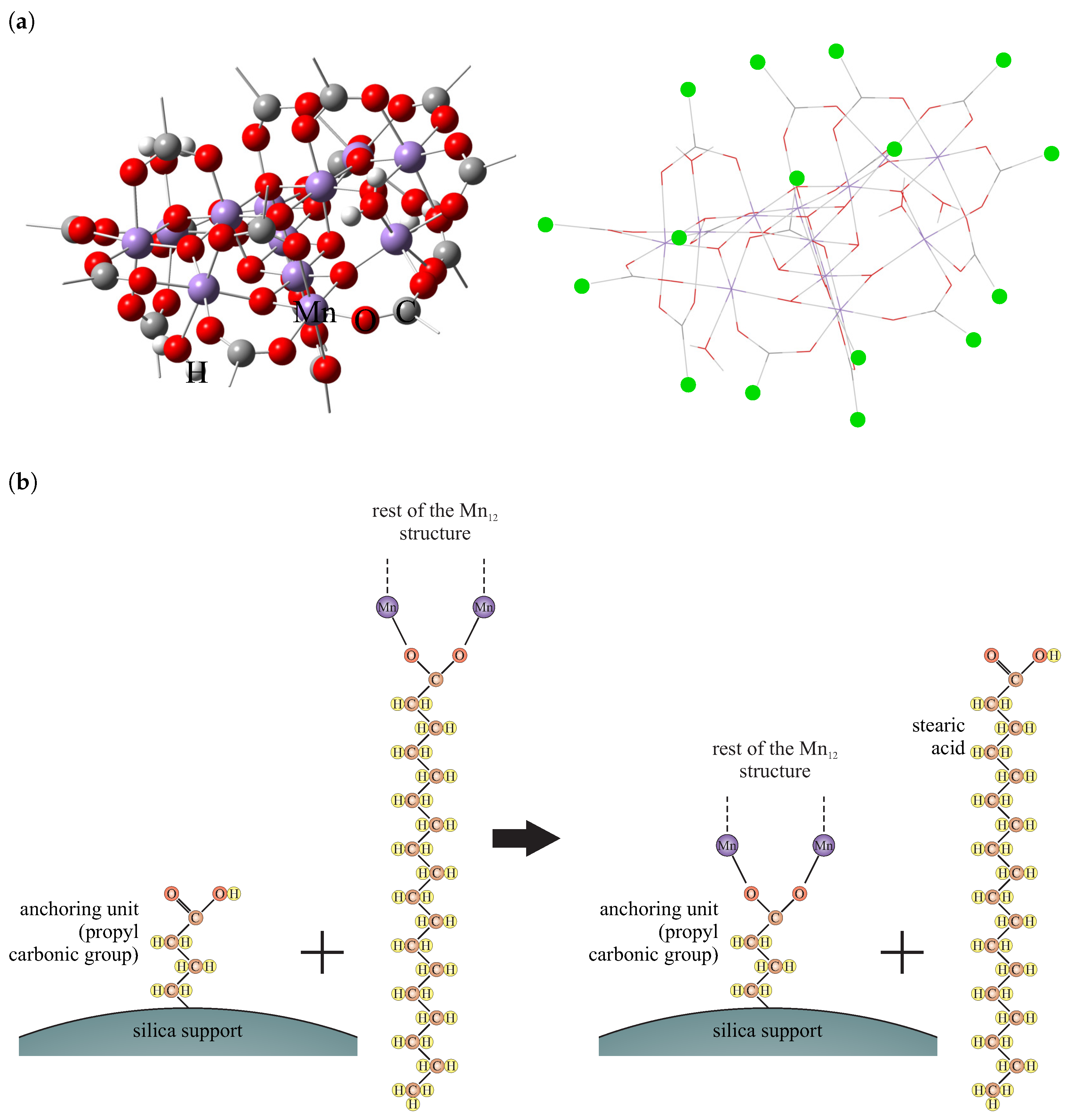
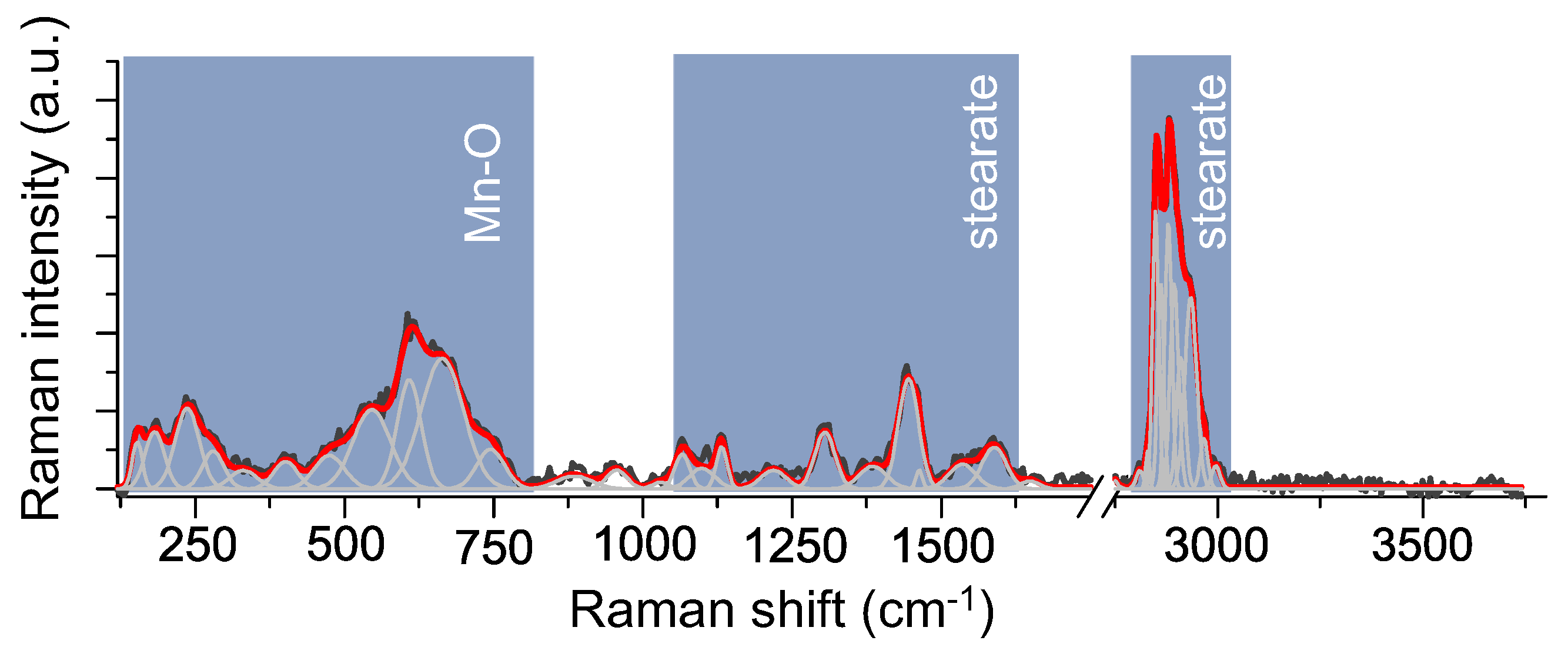
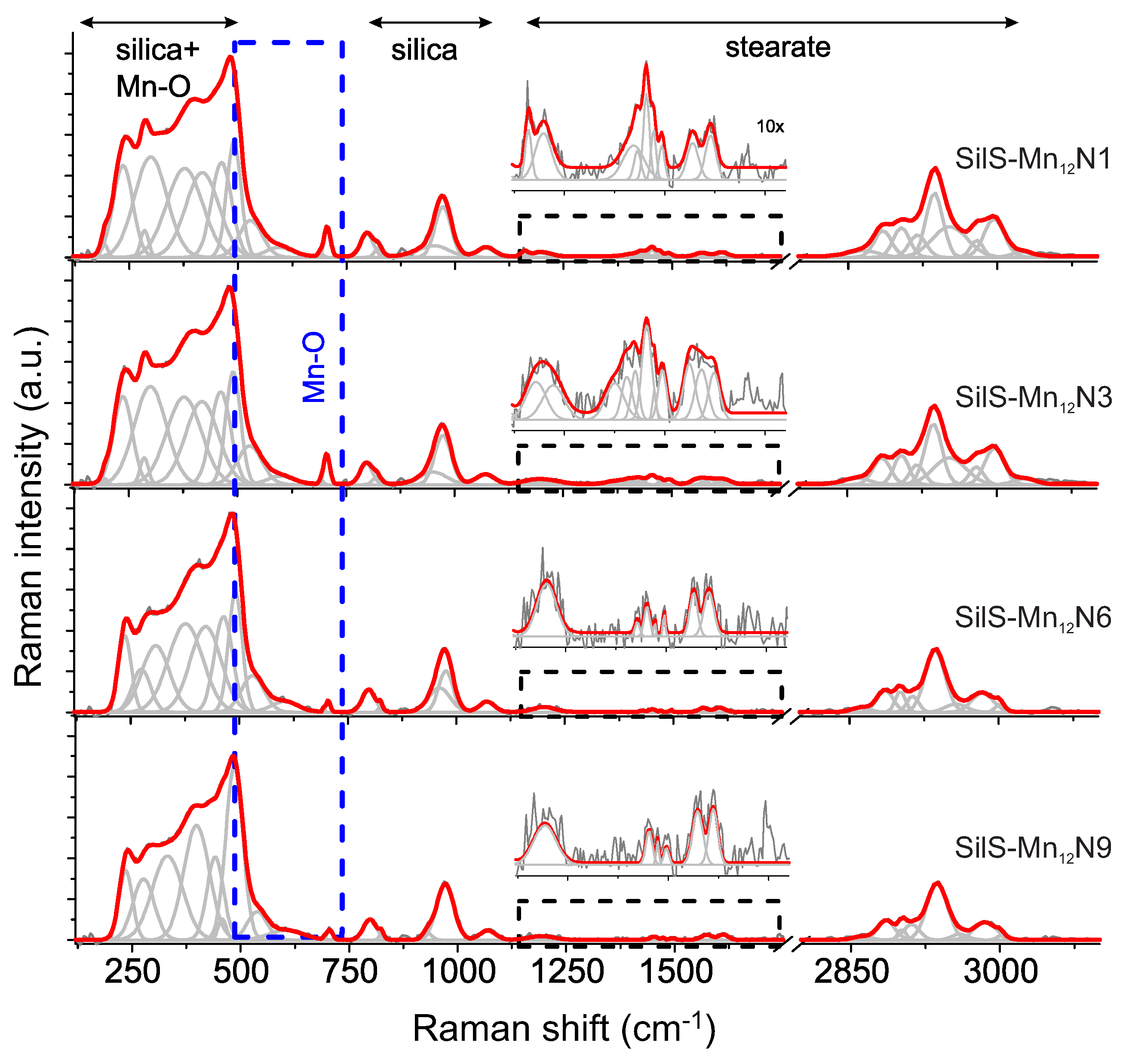
3.1. Structural Investigations
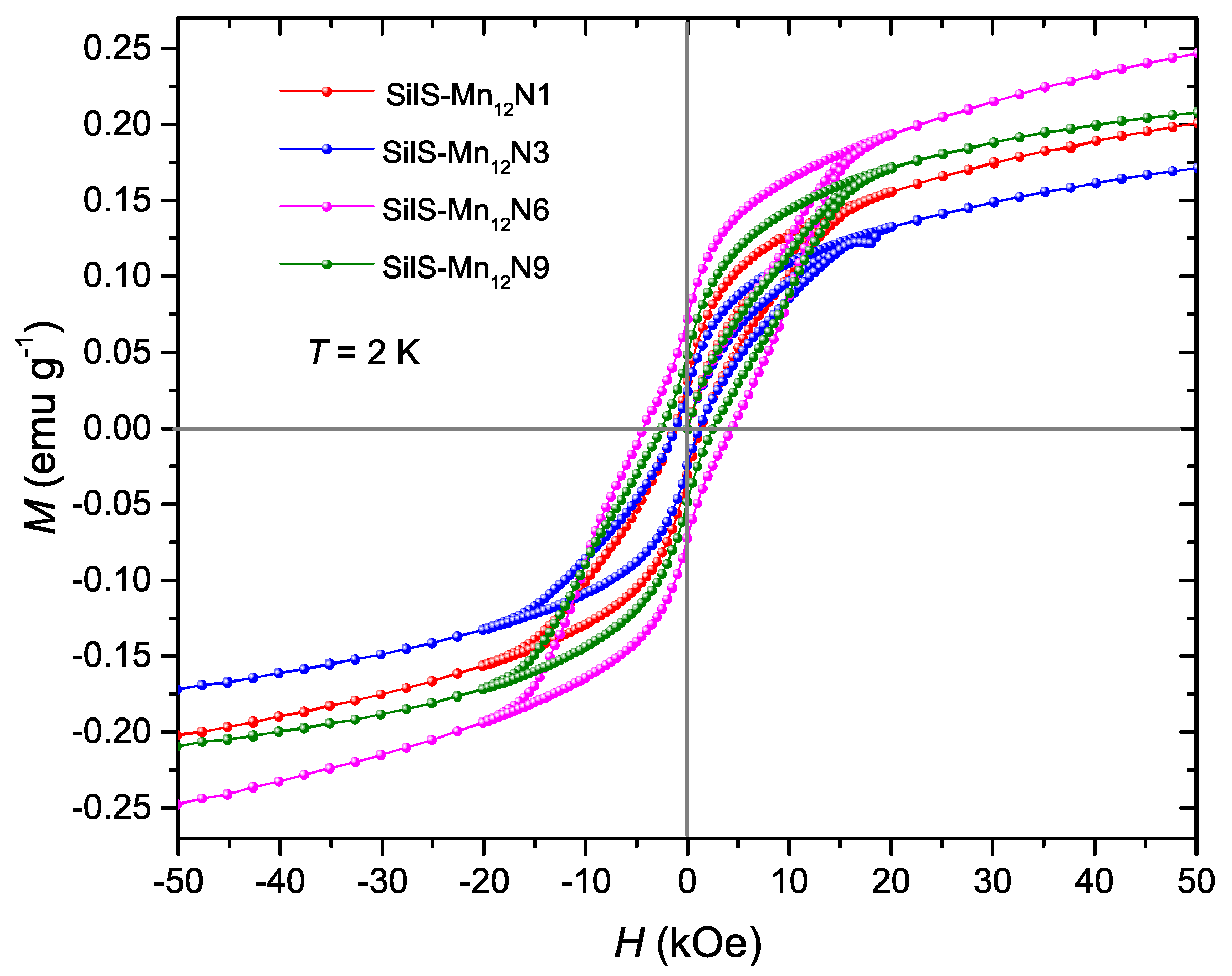
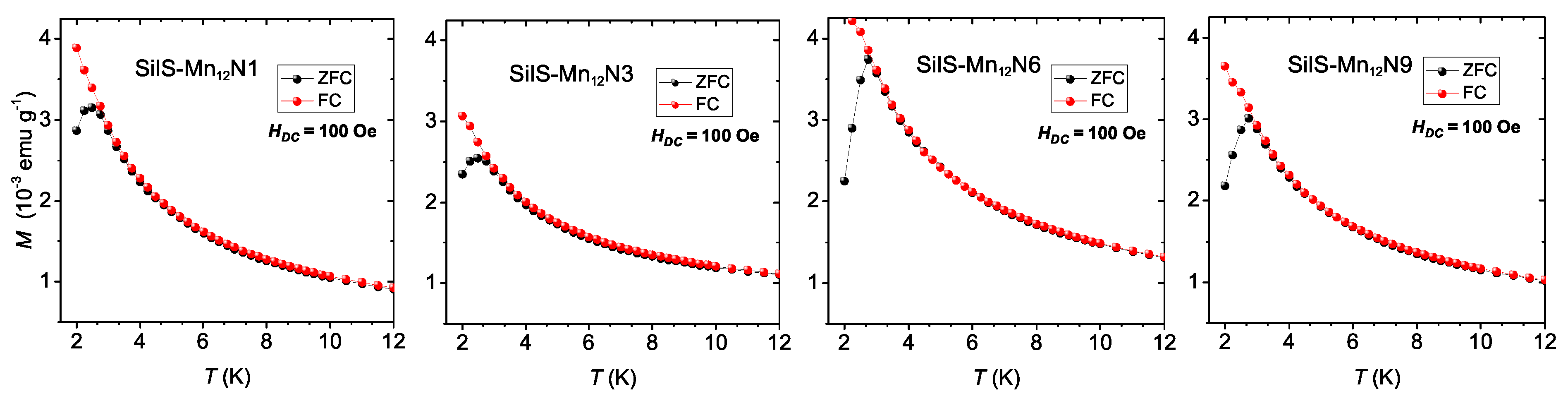
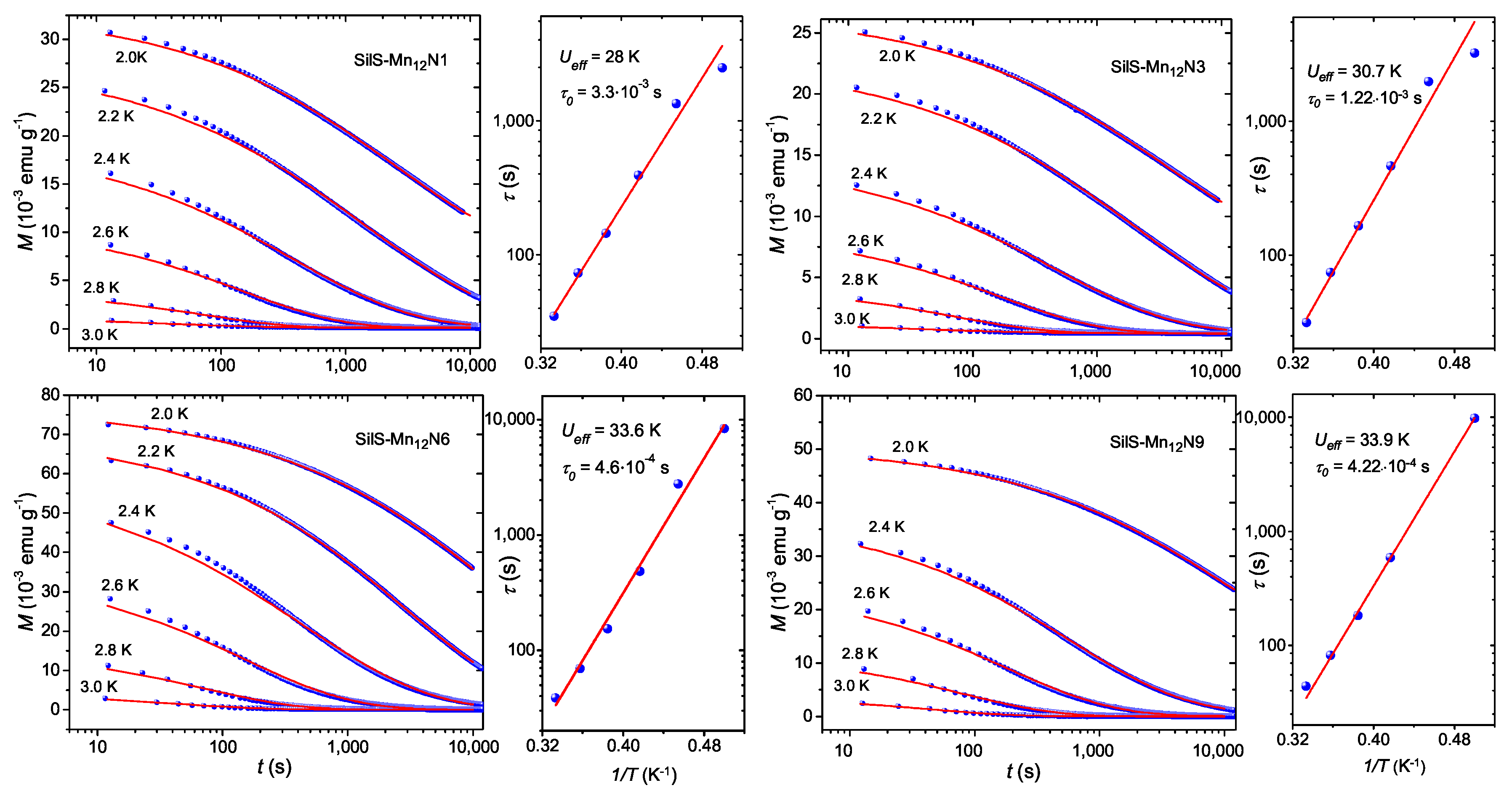
3.2. Magnetic Studies
3.3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Mnac | |
| Mn-st | derivative of Mn containing strearic acid ligands: [ |
| TEOS | tetraethyl orthosilicate |
| BNTES | butyronitriletriethoxysilane |
| ClTMS | chlorotrimethylsilane |
| Me | methyl groups |
| Et | ethyl units |
| QTM | quantum tunnelling of magnetization |
References
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Barra, A.L.; Brunel, L.C.; Guillot, M. Alternating current susceptibility, high field magnetization, and millimeter band EPR evidence for a ground S = 10 state in [Mn12O12 (CH3COO) 16 (H2O) 4] 2CH3COOH·4H2O. J. Am. Chem. Soc. 1991, 113, 5873–5874. [Google Scholar] [CrossRef]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Lis, T. Preparation, structure, and magnetic properties of a dodecanuclear mixed-valence manganese carboxylate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 2042–2046. [Google Scholar] [CrossRef]
- Joachim, C.; Gimzewski, J.; Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 2000, 408, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Mannini, M.; Bonacchi, D.; Zobbi, L.; Piras, F.M.; Speets, E.A.; Caneschi, A.; Cornia, A.; Magnani, A.; Ravoo, B.J.; Reinhoudt, D.N.; et al. Advances in single-molecule magnet surface patterning through microcontact printing. Nano Lett. 2005, 5, 1435–1438. [Google Scholar] [CrossRef]
- Mannini, M.; Messina, P.; Sorace, L.; Gorini, L.; Fabrizioli, M.; Caneschi, A.; Manassen, Y.; Sigalotti, P.; Pittana, P.; Gatteschi, D. Addressing individual paramagnetic molecules through ESN-STM. Inorg. Chim. Acta 2007, 360, 3837–3842. [Google Scholar] [CrossRef]
- Cornia, A.; Fabretti, A.C.; Pacchioni, M.; Zobbi, L.; Bonacchi, D.; Caneschi, A.; Gatteschi, D.; Biagi, R.; Del Pennino, U.; De Renzi, V.; et al. Direct observation of single-molecule magnets organized on gold surfaces. Angew. Chem. Int. Ed. 2003, 42, 1645–1648. [Google Scholar] [CrossRef]
- Rigamonti, L.; Piccioli, M.; Nava, A.; Malavolti, L.; Cortigiani, B.; Sessoli, R.; Cornia, A. Structure, magnetic properties and thermal sublimation of fluorinated Fe4 Single-Molecule Magnets. Polyhedron 2017, 128, 9–17. [Google Scholar] [CrossRef]
- Mannini, M.; Pineider, F.; Sainctavit, P.; Danieli, C.; Otero, E.; Sciancalepore, C.; Talarico, A.M.; Arrio, M.A.; Cornia, A.; Gatteschi, D.; et al. Magnetic memory of a single-molecule quantum magnet wired to a gold surface. Nat. Mater. 2009, 8, 194–197. [Google Scholar] [CrossRef]
- Barra, A.L.; Bianchi, F.; Caneschi, A.; Cornia, A.; Gatteschi, D.; Gorini, L.; Gregoli, L.; Maffini, M.; Parenti, F.; Sessoli, R.; et al. New Single-Molecule Magnets by Site-Specific Substitution: Incorporation of “Alligator Clips” into Fe4 Complexes. Eur. J. Inorg. Chem. 2007, 2007, 4145–4152. [Google Scholar] [CrossRef]
- Park, C.D.; Jeong, D.Y. Soluble Single-Molecule Magnet: Mn12-stearate. Bull. Korean Chem. Soc. 2001, 22, 611–615. [Google Scholar]
- Laskowski, L.; Kityk, I.; Konieczny, P.; Pastukh, O.; Schabikowski, M.; Laskowska, M. The Separation of the Mn12 Single-Molecule Magnets onto Spherical Silica Nanoparticles. Nanomaterials 2019, 9, 764. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, M.; Pastukh, O.; Kuźma, D.; Laskowski, Ł. How to Control the Distribution of Anchored, Mn12–Stearate, Single-Molecule Magnets. Nanomaterials 2019, 9, 1730. [Google Scholar] [CrossRef]
- Laskowska, M.; Oyama, M.; Kityk, I.; Marszalek, M.; Dulski, M.; Laskowski, L. Surface functionalization by silver-containing molecules with controlled distribution of functionalities. Appl. Surf. Sci. 2019, 481, 433–436. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Verma, S.; Verma, A.; Srivastava, A.K.; Gupta, A.; Singh, S.P.; Singh, P. Structural and magnetic properties of Mn12-Stearate nanomagnets. Mater. Chem. Phys. 2016, 177, 140–146. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and R aman Spectra of Inorganic and Coordination Compounds. In Handbook of Vibrational Spectroscopy; Wiley Online Library: Hoboken, NJ, USA, 2006. [Google Scholar]
- Laskowska, M.; Bałanda, M.; Fitta, M.; Dulski, M.; Zubko, M.; Pawlik, P.; Laskowski, Ł. Magnetic behaviour of Mn12-stearate single-molecule magnets immobilized inside SBA-15 mesoporous silica matrix. J. Magn. Magn. Mater. 2019, 478, 20–27. [Google Scholar] [CrossRef]
- Verma, A.; Verma, S.; Singh, P.; Gupta, A. Ageing effects on the magnetic properties of Mn12-based Acetate and Stearate SMMs. J. Magn. Magn. Mater. 2017, 439, 76–81. [Google Scholar] [CrossRef]
- Pointillart, F.; Bernot, K.; Golhen, S.; Le Guennic, B.; Guizouarn, T.; Ouahab, L.; Cador, O. Magnetic memory in an isotopically enriched and magnetically isolated mononuclear dysprosium complex. Angew. Chem. Int. Ed. 2015, 54, 1504–1507. [Google Scholar] [CrossRef]
- Mitcov, D.; Pedersen, A.H.; Ceccato, M.; Gelardi, R.M.; Hassenkam, T.; Konstantatos, A.; Reinholdt, A.; Sørensen, M.A.; Thulstrup, P.W.; Vinum, M.G.; et al. Molecular multifunctionality preservation upon surface deposition for a chiral single-molecule magnet. Chem. Sci. 2019, 10, 3065–3073. [Google Scholar] [CrossRef]
- Garanin, D.; Chudnovsky, E. Dislocation-induced spin tunneling in Mn 12 acetate. Phys. Rev. B 2002, 65, 094423. [Google Scholar] [CrossRef]
- Cornia, A.; Mannini, M.; Sainctavit, P.; Sessoli, R. Chemical strategies and characterization tools for the organization of single molecule magnets on surfaces. Chem. Soc. Rev. 2011, 40, 3076–3091. [Google Scholar] [CrossRef]
- Sun, H.; Li, W.; Wollenberg, L.; Li, B.; Wu, L.; Li, F.; Xu, L. Self-organized honeycomb structures of Mn12 single-molecule magnets. J. Phys. Chem. B 2009, 113, 14674–14680. [Google Scholar] [CrossRef]
- Thomas, L.; Caneschi, A.; Barbara, B. Nonexponential Dynamic Scaling of the Magnetization Relaxation in Mn 12 Acetate. Phys. Rev. Lett. 1999, 83, 2398. [Google Scholar] [CrossRef]
- Heu, M.; Suh, B.; Yoon, S.; Jeon, W.; Kim, Y.; Jung, D. Magnetic Relaxation in a Single-Molecule Magnet Mn~1~2-Chlorobutylate. J. Korean Phys. Soc. 2003, 43, 544–547. [Google Scholar]
- Yoon, S.; Heu, M.; Jeon, W.; Jung, D.Y.; Suh, B.; Yoon, S. Quantum tunneling and magnetic relaxation in Mn 12 chloropropionate. Phys. Rev. B 2003, 67, 052402. [Google Scholar] [CrossRef]
- Aubin, S.M.; Sun, Z.; Eppley, H.J.; Rumberger, E.M.; Guzei, I.A.; Folting, K.; Gantzel, P.K.; Rheingold, A.L.; Christou, G.; Hendrickson, D.N. Single-molecule magnets: Jahn- Teller isomerism and the origin of two magnetization relaxation processes in Mn12 complexes. Inorg. Chem. 2001, 40, 2127–2146. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Coronado, E.; Forment-Aliaga, A.; Gaita-Arino, A.; Giménez-Saiz, C.; Romero, F.M.; Wernsdorfer, W.; Biagi, R.; Corradini, V. Electronic and Magnetic Study of Polycationic Mn12 Single-Molecule Magnets with a Ground Spin State S= 11. Inorg. Chem. 2010, 49, 386–396. [Google Scholar] [CrossRef]
- Parois, P.; Moggach, S.A.; Sanchez-Benitez, J.; Kamenev, K.V.; Lennie, A.R.; Warren, J.E.; Brechin, E.K.; Parsons, S.; Murrie, M. Pressure-induced Jahn–Teller switching in a Mn12 nanomagnet. Chem. Commun. 2010, 46, 1881–1883. [Google Scholar] [CrossRef]
- Sessoli, R. Large Magnetic Anisotropy in High Spin Clusters; a Route to Magnetic Hysteresis at the Molecular Level. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1995, 274, 145–157. [Google Scholar] [CrossRef]
- Cheesman, M.R.; Oganesyan, V.S.; Sessoli, R.; Gatteschi, D.; Thomson, A.J. Magnetically induced optical bi-stability of the molecular nanomagnet Mn12O12 (OOCMe) 16 (H2O) 4 in an organic glass. Chem. Commun. 1997, 1677–1678. [Google Scholar] [CrossRef]
- McInnes, E.J.; Pidcock, E.; Oganesyan, V.S.; Cheesman, M.R.; Powell, A.K.; Thomson, A.J. Optical detection of spin polarization in single-molecule magnets [Mn12O12 (O2CR) 16 (H2O) 4]. J. Am. Chem. Soc. 2002, 124, 9219–9228. [Google Scholar] [CrossRef] [PubMed]
- Domingo, N.; Williamson, B.; Gómez-Segura, J.; Gerbier, P.; Ruiz-Molina, D.; Amabilino, D.B.; Veciana, J.; Tejada, J. Magnetism of isolated Mn 12 single-molecule magnets detected by magnetic circular dichroism: Observation of spin tunneling with a magneto-optical technique. Phys. Rev. B 2004, 69, 052405. [Google Scholar] [CrossRef]

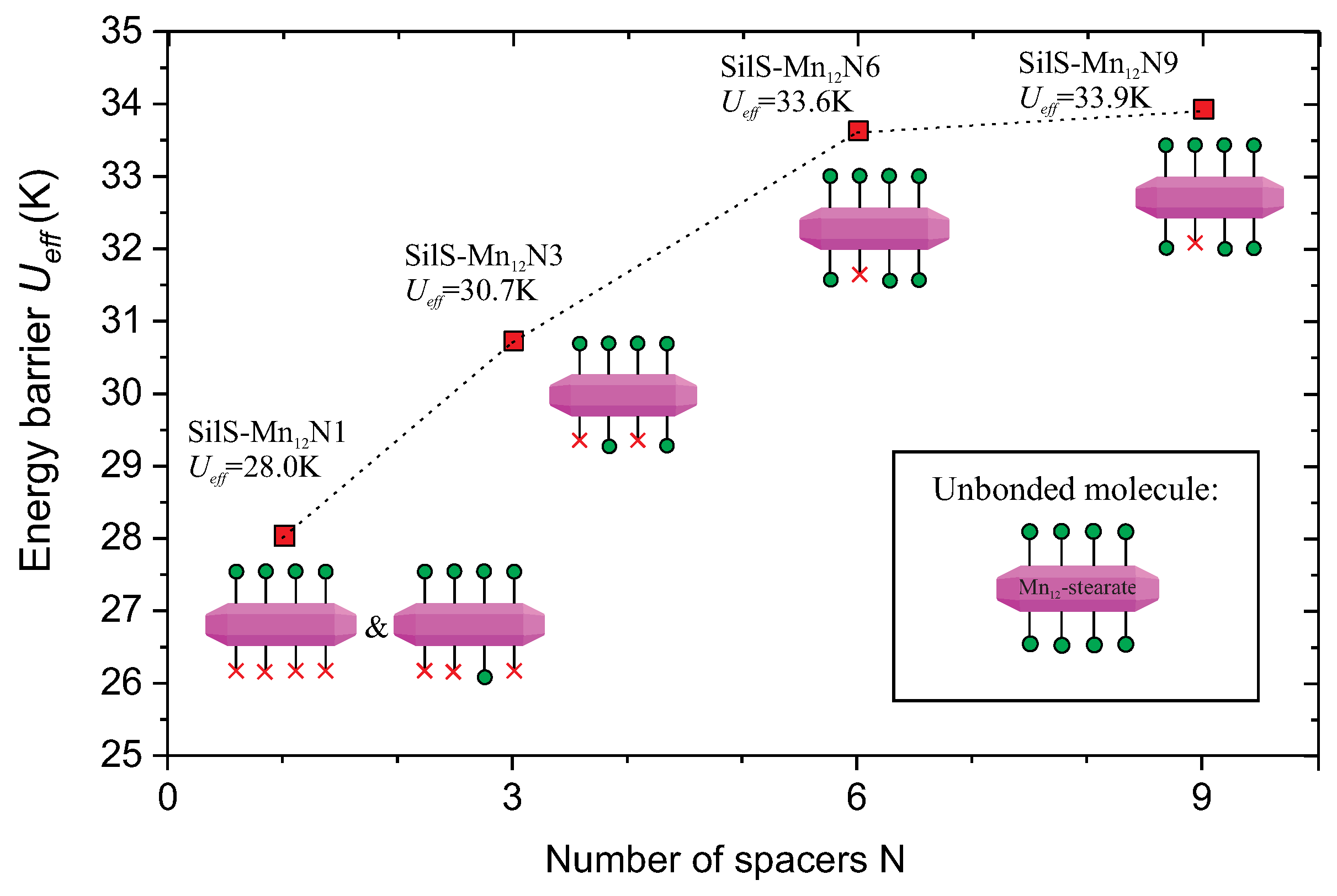
| Sample | (kOe) | (emu g) | (K) | (K) | (s) |
|---|---|---|---|---|---|
| SilS-MnN1 | 1.32 | 0.029 | 2.65 | 28 ± 1.5 | 3.3 × 10 |
| SilS-MnN3 | 1.16 | 0.023 | 2.65 | 31 ± 2 | 1.1 × 10 |
| SilS-MnN6 | 4.51 | 0.072 | 2.74 | 33.6 ± 1.7 | 4.6 × 10 |
| SilS-MnN9 | 2.55 | 0.047 | 2.74 | 33.9 ± 1.6 | 4.2 × 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskowska, M.; Pastukh, O.; Konieczny, P.; Dulski, M.; Zalsiński, M.; Laskowski, L. Magnetic Behaviour of Mn12-Stearate Single-Molecule Magnets Immobilized on the Surface of 300 nm Spherical Silica Nanoparticles. Materials 2020, 13, 2624. https://doi.org/10.3390/ma13112624
Laskowska M, Pastukh O, Konieczny P, Dulski M, Zalsiński M, Laskowski L. Magnetic Behaviour of Mn12-Stearate Single-Molecule Magnets Immobilized on the Surface of 300 nm Spherical Silica Nanoparticles. Materials. 2020; 13(11):2624. https://doi.org/10.3390/ma13112624
Chicago/Turabian StyleLaskowska, Magdalena, Oleksandr Pastukh, Piotr Konieczny, Mateusz Dulski, Marcin Zalsiński, and Lukasz Laskowski. 2020. "Magnetic Behaviour of Mn12-Stearate Single-Molecule Magnets Immobilized on the Surface of 300 nm Spherical Silica Nanoparticles" Materials 13, no. 11: 2624. https://doi.org/10.3390/ma13112624
APA StyleLaskowska, M., Pastukh, O., Konieczny, P., Dulski, M., Zalsiński, M., & Laskowski, L. (2020). Magnetic Behaviour of Mn12-Stearate Single-Molecule Magnets Immobilized on the Surface of 300 nm Spherical Silica Nanoparticles. Materials, 13(11), 2624. https://doi.org/10.3390/ma13112624








