Effect of Laser Parameters on Processing of Biodegradable Magnesium Alloy WE43 via Selective Laser Melting Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Production
2.2. Analysis of Shape and Continuity
2.3. Analysis of Surface Quality
2.4. Measurement of Microhardness and Local Chemical Composition
3. Results
3.1. Analysis of Shape and Continuity
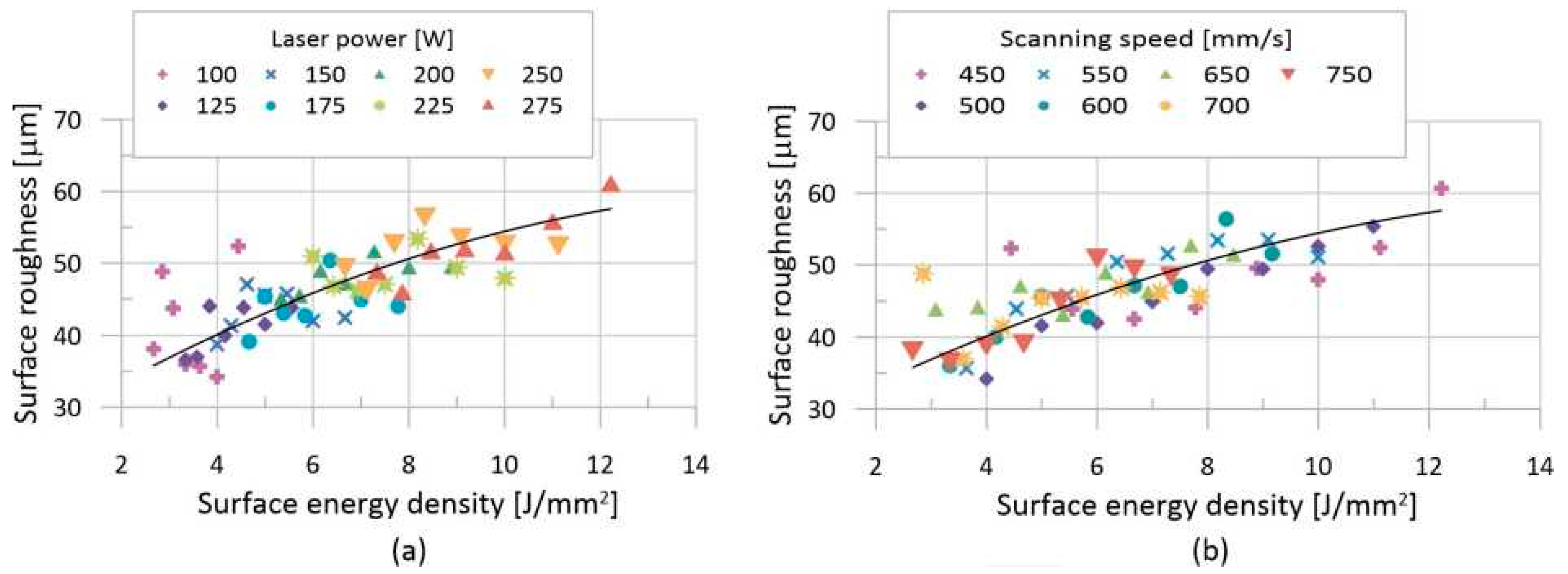
3.2. Analysis of Surface Quality
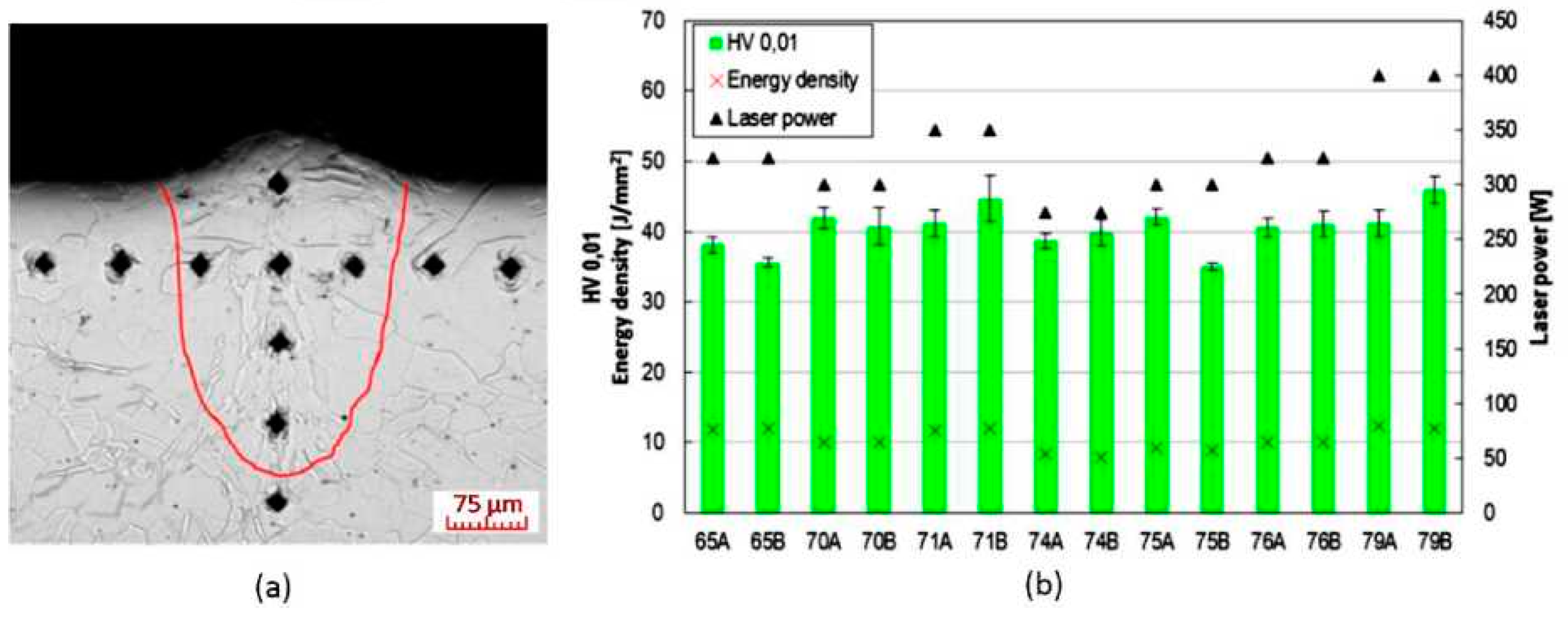
3.3. Evaluation of Microhardness
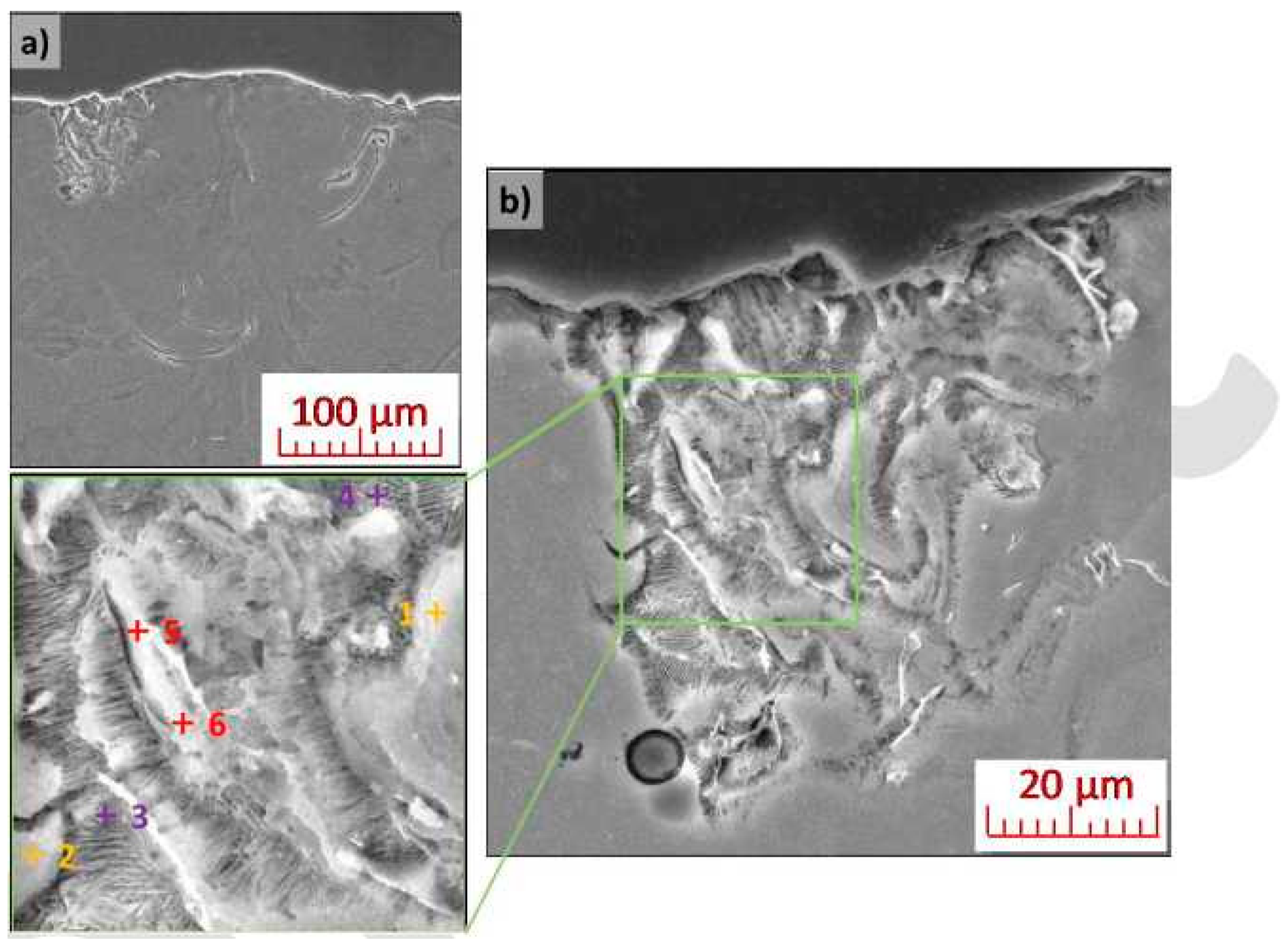
3.4. Analysis of Local Chemical Composition
4. Discussion
4.1. Sample Shape and Continuity
4.2. Analysis of Surface Quality
4.3. Microhardness
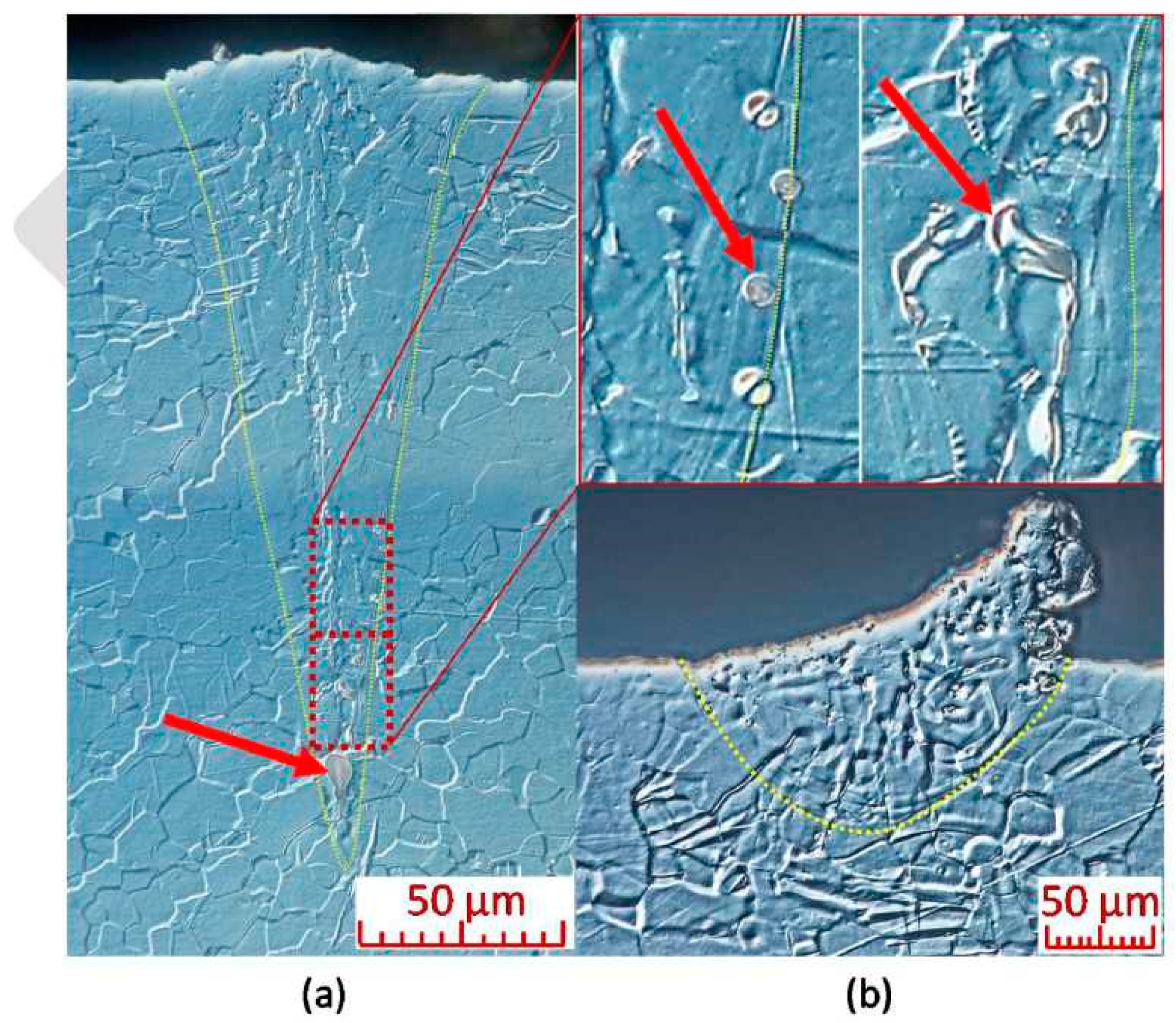
4.4. Microstructural Analysis and Local Microanalysis of Chemical Composition
5. Conclusions
- -
- Stable and continuous weld depositions can be achieved within an energy density range of 5.5–12 J/mm2.
- -
- The layering of weld deposits on thin walls led to an average increase of 15–17% in the width of the melt pool.
- -
- The layering of the weld deposits led to reducing the required energy density for a stable weld track to 4.5 J/mm2.
- -
- An equation for estimating surface roughness based on process parameters has been created.
Author Contributions
Funding
Conflicts of Interest
References
- Cohen, J. Current concepts review. Corrosion of metal orthopaedic implants. J. Bone Jt. Surg. Am. 1998, 80, 1554. [Google Scholar] [CrossRef] [PubMed]
- Milošev, I.; Pišot, V.; Campbell, P. Serum levels of cobalt and chromium in patients with Sikomet metal-metal total hip replacements. J. Orthop. Res. 2005, 23, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Niki, Y.; Matsumoto, H.; Suda, Y.; Otani, T.; Fujikawa, K.; Toyama, Y.; Hisamori, N.; Nozue, A. Metal ions induce bone-resorbing cytokine production through the redox pathway in synoviocytes and bone marrow macrophages. Biomaterials 2003, 24, 1447–1457. [Google Scholar] [CrossRef]
- Granchi, D.; Ciapetti, G.; Stea, S.; Savarino, L.; Filippini, F.; Sudanese, A.; Zinghi, G.; Montanaro, L. Cytokine release in mononuclear cells of patients with Co-Cr hip prosthesis. Biomaterials 1999, 20, 1079–1086. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wicklund, B.H.; Gustilo, R.B.; Tsukayama, D.T. Titanium, chromium and cobalt ions modulate the release of bone-associated cytokines by human monocytes/macrophages in vitro. Biomaterials 1996, 17, 2233–2240. [Google Scholar] [CrossRef]
- Nagels, J.; Stokdijk, M.; Rozing, P.M. Stress shielding and bone resorption in shoulder arthroplasty. J. Shoulder Elb. Surg. 2003, 12, 35–39. [Google Scholar] [CrossRef]
- Saris, N.E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Okuma, T. Magnesium and bone strength. Nutrition 2001, 17, 679–680. [Google Scholar] [CrossRef]
- Hartwig, A. Role of magnesium in genomic stability. Micronutr. Genom. STable 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Revell, P.A.; Damien, E.; Zhang, X.S.; Evans, P.; Howlett, C.R. The Effect of Magnesium Ions on Bone Bonding to Hydroxyapatite Coating on Titanium Alloy Implants. Key Eng. Mater. 2004, 254–256, 447–450. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, Y.; Yoshida, Y.; Okazaki, M.; Shimazu, A.; Kubo, T.; Akagawa, Y.; Uchida, T. Action of FGMgCO3Ap-collagen composite in promoting bone formation. Biomaterials 2003, 24, 4913–4920. [Google Scholar] [CrossRef]
- Levorova, J.; Duskova, J.; Drahos, M.; Vrbova, R.; Vojtech, D.; Kubasek, J.; Bartos, M.; Dugova, L.; Ulmann, D.; Foltan, R. In vivo study on biodegradable magnesium alloys: Bone healing around WE43 screws. J. Biomater. Appl. 2018, 32, 886–895. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Li, N.; Guo, C.; Wu, Y.H.; Zheng, Y.F.; Ruan, L.Q. Comparative study on corrosion behaviour of pure Mg and WE43 alloy in static, stirring and flowing Hank’s solution. Corros. Eng. Sci. Technol. 2012, 47, 346–351. [Google Scholar] [CrossRef]
- Ge, S.; Wang, Y.; Tian, J.; Lei, D.; Yu, Q.; Wang, G. An in vitro study on the biocompatibility of WE magnesium alloys. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 482–487. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D.; Jablonská, E.; Pospíšilová, I.; Lipov, J.; Ruml, T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn-Mg alloys. Mater. Sci. Eng. C 2016, 58, 24–35. [Google Scholar] [CrossRef]
- Riza, S.H.; Masood, S.H.; Wen, C. Laser-Assisted Additive Manufacturing for Metallic Biomedical Scaffolds. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 285–301. ISBN 9780080965338. [Google Scholar]
- Gu, D. Laser Additive Manufacturing of High-Performance Materials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–311. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2. [Google Scholar] [CrossRef]
- Ng, C.C.; Savalani, M.M.; Man, H.C.; Gibson, I. Layer manufacturing of magnesium and its alloy structures for future applications. Virtual Phys. Prototyp. 2010, 5, 13–19. [Google Scholar] [CrossRef]
- Chung Ng, C.; Savalani, M.; Chung Man, H. Fabrication of magnesium using selective laser melting technique. Rapid Prototyp. J. 2011, 17, 479–490. [Google Scholar] [CrossRef]
- Ng, C.C.; Savalani, M.M.; Lau, M.L.; Man, H.C. Microstructure and mechanical properties of selective laser melted magnesium. Appl. Surf. Sci. 2011, 257, 7447–7454. [Google Scholar] [CrossRef]
- Wei, K.; Gao, M.; Wang, Z.; Zeng, X. Effect of energy input on formability, microstructure and mechanical properties of selective laser melted AZ91D magnesium alloy. Mater. Sci. Eng. A 2014, 611, 212–222. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Y.; Zhang, D.; Hao, L.; Jiang, J.; Li, Z.; Chen, Y. Experimental Investigation on Selective Laser Melting of Bulk Net-Shape Pure Magnesium. Mater. Manuf. Process. 2015, 30, 1298–1304. [Google Scholar] [CrossRef]
- Taltavull, C.; Torres, B.; López, A.J.; Rodrigo, P.; Otero, E.; Rams, J. Selective laser surface melting of a magnesium-aluminium alloy. Mater. Lett. 2012, 85, 98–101. [Google Scholar] [CrossRef]
- Schmid, D.; Renza, J.; Zaeh, M.F.; Glasschroeder, J. Process influences on laser-beam melting of the magnesium alloy AZ91. Phys. Procedia 2016, 83, 927–936. [Google Scholar] [CrossRef]
- Pawlak, A.; Rosienkiewicz, M.; Chlebus, E. Design of experiments approach in AZ31 powder selective laser melting process optimization. Arch. Civ. Mech. Eng. 2017, 17, 9–18. [Google Scholar] [CrossRef]
- Tandon, R.; Palmer, T.; Gieseke, M.; Noelke, C. Additive Manufacturing of Magnesium Alloy Powders: Investigations Into Process Development Using Elektron®MAP+43 Via Laser Powder Bed Fusion and Directed Energy Deposition. Euro PM2016 2016, 91, 4–9. [Google Scholar]
- Gangireddy, S.; Gwalani, B.; Liu, K.; Faierson, E.J.; Mishra, R.S. Microstructure and mechanical behavior of an additive manufactured (AM) WE43-Mg alloy. Addit. Manuf. 2019, 26, 53–64. [Google Scholar] [CrossRef]
- Zumdick, N.A.; Jauer, L.; Kersting, L.C.; Kutz, T.N.; Schleifenbaum, J.H.; Zander, D. Additive manufactured WE43 magnesium: A comparative study of the microstructure and mechanical properties with those of powder extruded and as-cast WE43. Mater. Charact. 2019, 147, 384–397. [Google Scholar] [CrossRef]
- Jauer, L.; Jülich, B.; Voshage, M.; Meiners, W. Selective Laser Melting of magnesium alloys. 2015, 30, 824682. [Google Scholar]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Tümer, N.; Schröder, K.U.; Mol, J.M.C.; Weinans, H.; et al. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Jauer, L.; Meiners, W.; Vervoort, S.; Gayer, C.; Zumdick, N.A.; Zander, D. Selective laser melting of magnesium alloys. In Proceedings of the World PM 2016 Congress and Exhibition, Hamburg, Germany, 9–13 October 2016; European Powder Metallurgy Association (EPMA): Shrewsbury, UK, 2016. [Google Scholar]
- Hyer, H.; Zhou, L.; Benson, G.; McWilliams, B.; Cho, K.; Sohn, Y. Additive Manufacturing of Dense WE43 Mg Alloy by Laser Powder Bed Fusion. Addit. Manuf. 2020, 33, 101123. [Google Scholar] [CrossRef]
- Zhang, W.N.; Wang, L.Z.; Feng, Z.X.; Chen, Y.M. Research progress on selective laser melting (SLM) of magnesium alloys: A review. Optik (Stuttg) 2020, 207, 163842. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Zhang, X.Y.; Leeflang, M.A.; Li, W.; Pouran, B.; Tichelaar, F.D.; Weinans, H.; Zhou, J.; Zadpoor, A.A. Biodegradation-affected fatigue behavior of additively manufactured porous magnesium. Addit. Manuf. 2019, 28, 299–311. [Google Scholar] [CrossRef]
- Król, M.; Taski, T. Surface quality research for selective laser melting of TI-6AL-4V alloy. Arch. Metall. Mater. 2016, 61, 945–950. [Google Scholar] [CrossRef]
- Balc, N.; Cosma, S.C.; Kessler, J.; Mager, V. Research on Improving the Outer Surface Quality of the Parts Made by SLM. Appl. Mech. Mater. 2015, 808, 199–204. [Google Scholar] [CrossRef]
- Majeed, A.; Lv, J.; Zhang, Y.; Muzamil, M.; Waqas, A.; Shamim, K.; Qureshi, M.E.; Zafar, F. An investigation into the influence of processing parameters on the surface quality of AlSi10Mg parts by SLM process. In Proceedings of the 2019 16th International Bhurban Conference on Applied Sciences and Technology IBCAST 2019, Islamabad, Pakistan, 8–12 January 2019; pp. 143–147. [Google Scholar] [CrossRef]
- Savalani, M.M.; Pizarro, J.M. Effect of preheat and layer thickness on selective laser melting (SLM) of magnesium. Rapid Prototyp. J. 2016, 22, 115–122. [Google Scholar] [CrossRef]
- Guo, Y.; Jia, L.; Kong, B.; Wang, N.; Zhang, H. Single track and single layer formation in selective laser melting of niobium solid solution alloy. Chin. J. Aeronaut. 2018, 31, 860–866. [Google Scholar] [CrossRef]
- Kempen, K.; Thijs, L.; Yasa, E.; Badrossamay, M.; Verheecke, W.; Kruth, J.-P. Process Optimization and Microstructural Analysis for Selective Laser Melting of AlSi10Mg. In Proceedings of the 22nd Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Sarasota, FL, USA, 7–17 April 2011; pp. 484–495. [Google Scholar]
- Li, R.; Liu, J.; Shi, Y.; Wang, L.; Jiang, W. Balling behavior of stainless steel and nickel powder during selective laser melting process. Int. J. Adv. Manuf. Technol. 2012, 59, 1025–1035. [Google Scholar] [CrossRef]
- Czerwinski, F. Controlling the ignition and flammability of magnesium for aerospace applications. Corros. Sci. 2014, 86, 1–16. [Google Scholar] [CrossRef]
- Tekumalla, S.; Gupta, M. An insight into ignition factors and mechanisms of magnesium based materials: A review. Mater. Des. 2017, 113, 84–98. [Google Scholar] [CrossRef]
- Zhan, X.; Chen, J.; Liu, J.; Wei, Y.; Zhou, J.; Meng, Y. Microstructure and magnesium burning loss behavior of AA6061 electron beam welding joints. Mater. Des. 2016, 99, 449–458. [Google Scholar] [CrossRef]
- Sercombe, T.B.; Li, X.; Sercombe, T.B.; Li, X. Selective laser melting of aluminium and aluminium metal matrix composites: Review. Mater. Technol. 2016, 7857. [Google Scholar] [CrossRef]
- Zhang, B.; Liao, H.; Coddet, C. Effects of processing parameters on properties of selective laser melting Mg–9%Al powder mixture. Mater. Des. 2012, 34, 753–758. [Google Scholar] [CrossRef]
- Wei, K.; Wang, Z.; Zeng, X. Influence of element vaporization on formability, composition, microstructure, and mechanical performance of the selective laser melted Mg-Zn-Zr components. Mater. Lett. 2015, 156, 187–190. [Google Scholar] [CrossRef]
- Kruth, J.; Badrossamay, M.; Yasa, E.; Deckers, J.; Thijs, L.; Humbeeck, J. Van Part and material properties in selective laser melting of metals. In Proceedings of the 16th International Symposium on Electromachining, Shanghai, China, 19–23 April 2010; pp. 1–12. [Google Scholar]
- Pinkerton, A.J. Laser direct metal deposition: Theory and applications in manufacturing and maintenance. Adv. Laser Mater. Process. 2010, 461–491. [Google Scholar] [CrossRef]
- Krauss, H.; Zaeh, M.F. Investigations on manufacturability and process reliability of selective laser melting. Phys. Procedia 2013, 41, 815–822. [Google Scholar] [CrossRef]
- Louvis, E.; Fox, P.; Sutcliffe, C.J. Selective laser melting of aluminium components. J. Mater. Process. Technol. 2011, 211, 275–284. [Google Scholar] [CrossRef]
- Aboulkhair, N.T.; Maskery, I.; Tuck, C.; Ashcroft, I.; Everitt, N.M. On the formation of AlSi10Mg single tracks and layers in selective laser melting: Microstructure and nano-mechanical properties. J. Mater. Process. Technol. 2016, 230, 88–98. [Google Scholar] [CrossRef]
- Zong, F.; Meng, C.; Guo, Z.; Ji, F.; Xiao, H.; Zhang, X.; Ma, J.; Ma, H. Synthesis and characterization of magnesium nitride powder formed by Mg direct reaction with N2. J. Alloys Compd. 2010, 508, 172–176. [Google Scholar] [CrossRef]
- Salehi, M.; Maleksaeedi, S.; Farnoush, H.; Nai, M.L.S.; Meenashisundaram, G.K.; Gupta, M. An investigation into interaction between magnesium powder and Ar gas: Implications for selective laser melting of magnesium. Powder Technol. 2018, 333, 252–261. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Liang, J.; Zhou, J.; Wang, L. Microstructure and corrosion behaviour of laser surface melting treated WE43 magnesium alloy. RSC Adv. 2016, 6, 30642–30651. [Google Scholar] [CrossRef]
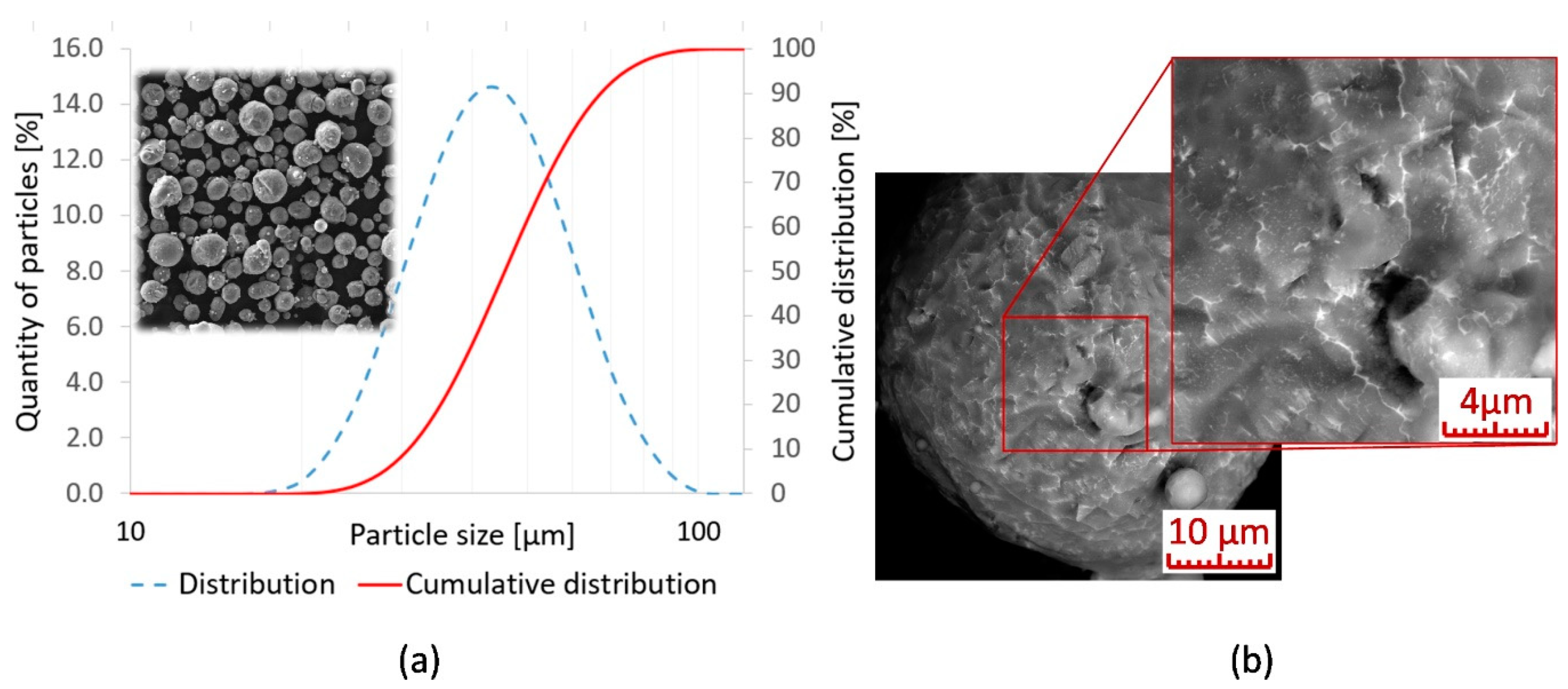
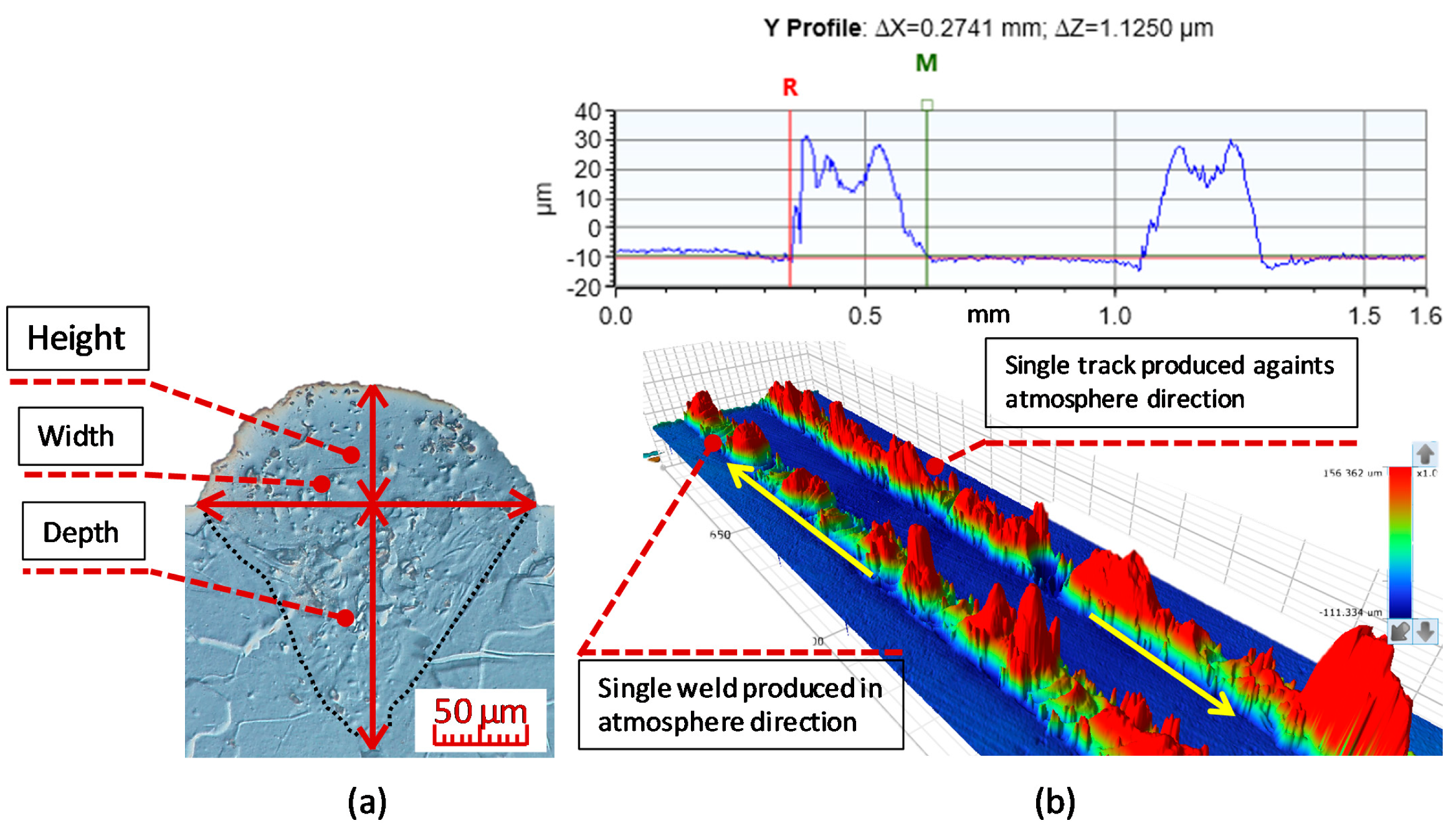
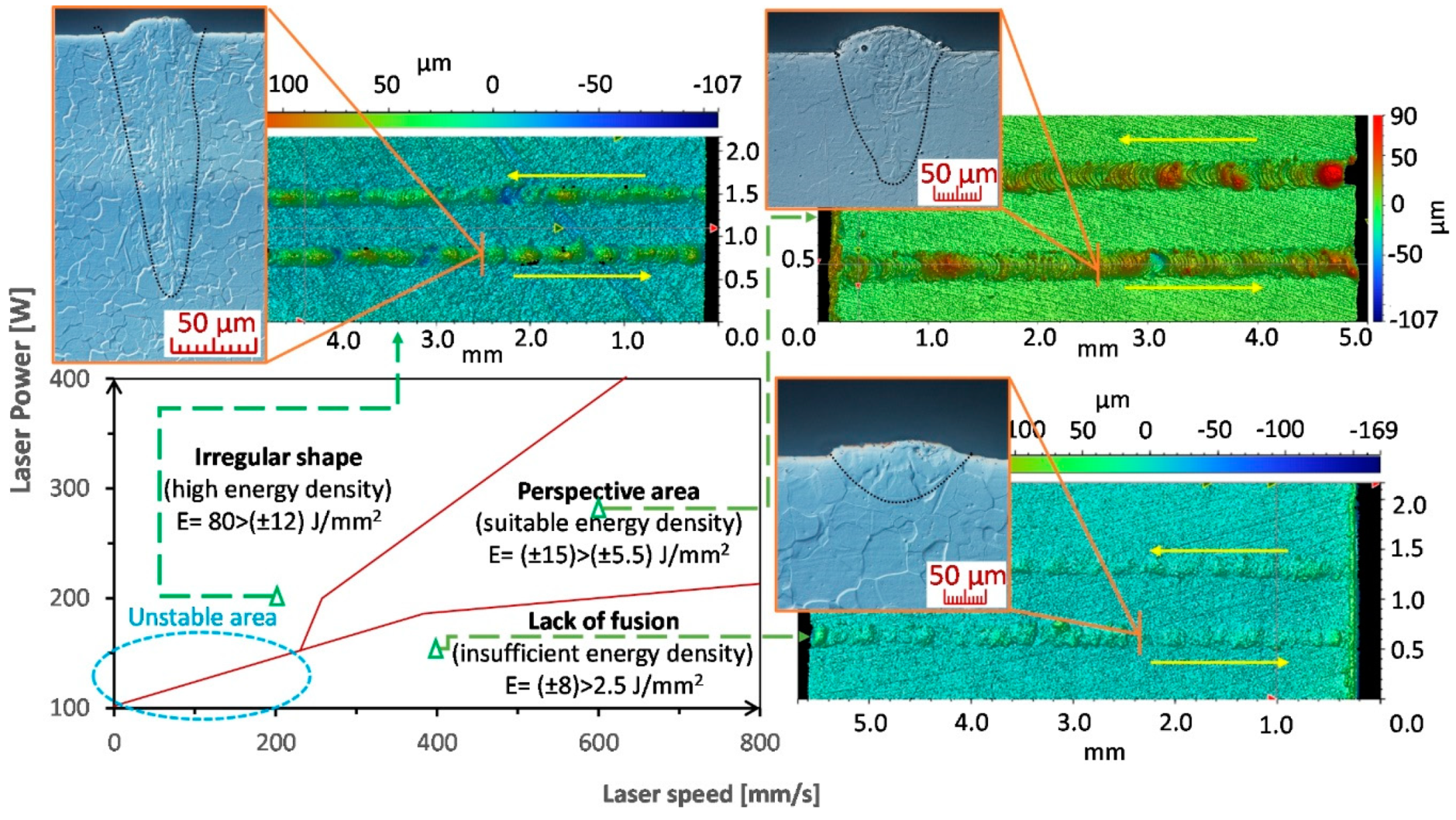
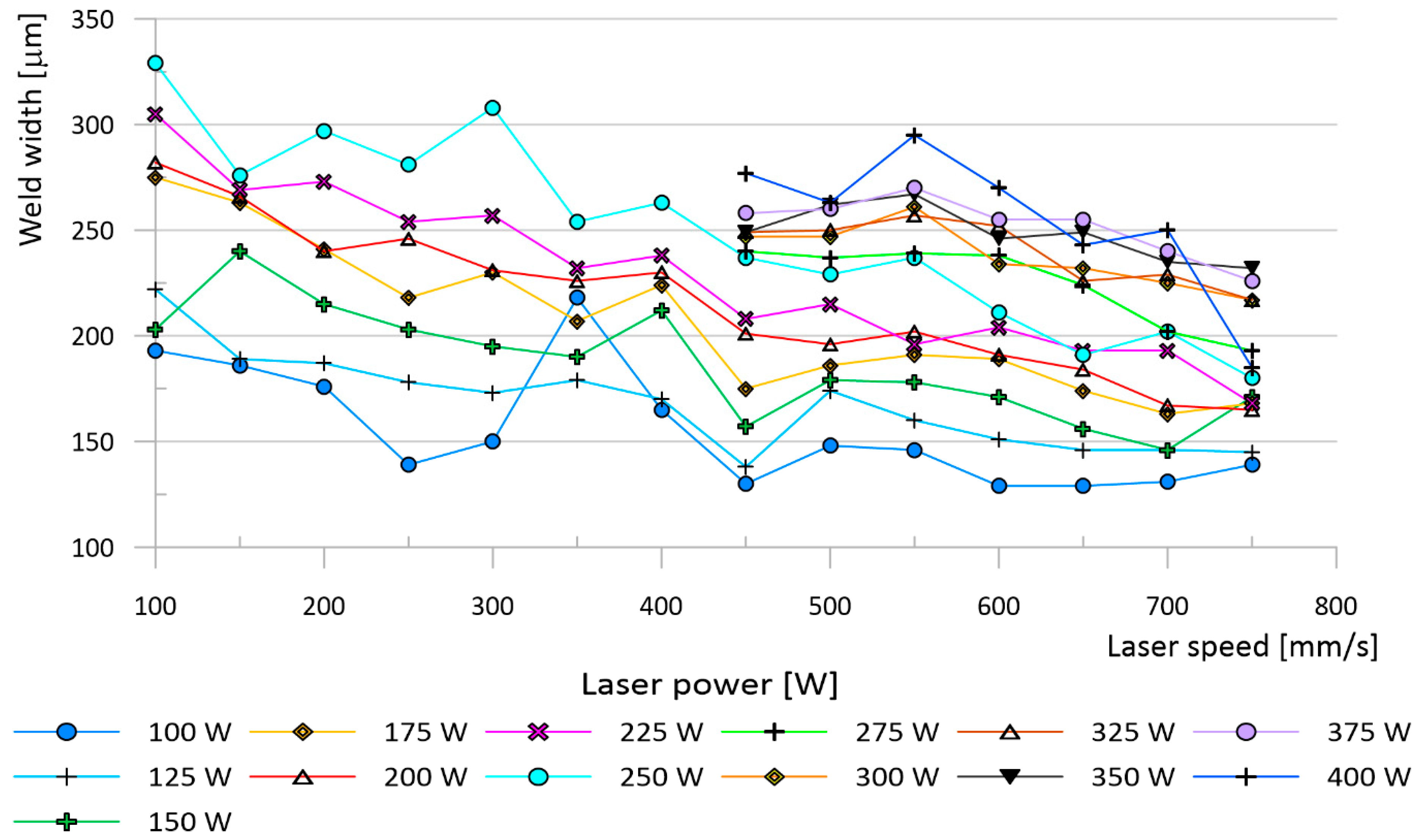


| The Mark of a Sample | Y | Zr | Nd | Si | Cu |
|---|---|---|---|---|---|
| ASTM B93/B93M-06 | 3.7–4.3 | 0.3–1.0 | 2.0–2.5 | Max 0.01 | Max 0.01 |
| WE43 powder | 3.96 | 0.56 | 2.30 | <0.01 | <0.01 |
| Area | Mg | O | Si | Y | Nd |
|---|---|---|---|---|---|
| 1 | 97.3 | 2.4 | ND | 0.2 | 0.1 |
| 2 | 96.3 | 3.0 | 0.4 | 0.2 | 0.1 |
| 3 | 97.9 | 0.4 | ND | 0.4 | 1.3 |
| 4 | 94.3 | 5.0 | ND | 0.4 | 0.3 |
| 5 | 93.6 | 5.1 | 0.7 | 0.5 | 0.1 |
| 6 | 96.1 | 2.5 | 0.6 | 0.7 | 0.1 |
| Area | Mg | O | Y | Nd | Zr |
|---|---|---|---|---|---|
| 1 | 92.2 | 6.5 | 0.8 | 0.4 | 0.1 |
| 2 | 93.2 | 6.0 | 0.4 | 0.3 | 0.1 |
| 3 | 90.9 | 8.2 | 0.5 | 0.3 | 0.1 |
| 4 | 96.4 | 3.1 | 0.3 | 0.2 | ND |
| 5 | 97.4 | 2.4 | 0.1 | 0.1 | ND |
| 6 | 96.4 | 3.4 | 0.1 | 0.1 | ND |
| 7 | 97.2 | 2.8 | ND | ND | ND |
| 8 | 97.2 | 2.8 | ND | ND | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchy, J.; Horynová, M.; Klakurková, L.; Palousek, D.; Koutny, D.; Celko, L. Effect of Laser Parameters on Processing of Biodegradable Magnesium Alloy WE43 via Selective Laser Melting Method. Materials 2020, 13, 2623. https://doi.org/10.3390/ma13112623
Suchy J, Horynová M, Klakurková L, Palousek D, Koutny D, Celko L. Effect of Laser Parameters on Processing of Biodegradable Magnesium Alloy WE43 via Selective Laser Melting Method. Materials. 2020; 13(11):2623. https://doi.org/10.3390/ma13112623
Chicago/Turabian StyleSuchy, Jan, Miroslava Horynová, Lenka Klakurková, David Palousek, Daniel Koutny, and Ladislav Celko. 2020. "Effect of Laser Parameters on Processing of Biodegradable Magnesium Alloy WE43 via Selective Laser Melting Method" Materials 13, no. 11: 2623. https://doi.org/10.3390/ma13112623
APA StyleSuchy, J., Horynová, M., Klakurková, L., Palousek, D., Koutny, D., & Celko, L. (2020). Effect of Laser Parameters on Processing of Biodegradable Magnesium Alloy WE43 via Selective Laser Melting Method. Materials, 13(11), 2623. https://doi.org/10.3390/ma13112623






