Sintering Temperature Effect on Structural and Optical Properties of Heat Treated Coconut Husk Ash Derived SiO2 Mixed with ZnO Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.; Ullah, S.; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Mittal, J.; Batra, A.; Singh, A.; Sharma, M.M. Phytofabrication of nanoparticles through plant as nanofactories. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043002. [Google Scholar] [CrossRef]
- Aisyah, S.; Wahab, A.; Amin, K.; Hj, S.; Aziz, A.; Zakiah, A.; Azman, K.; Emilia, R.; Khaidir, M.; Zulhasif, M.; et al. Synthesis of cobalt oxide Co3O4 doped zinc silicate based glass- ceramic derived for LED applications. Optik 2019, 179, 919–926. [Google Scholar]
- Khaidir, R.E.M.; Fen, Y.W.; Mohd Zaid, M.H.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Abdul Wahab, S.A.; Khirel Azman, A.Z. Addition of ZnO nanoparticles on waste rice husk as potential host material for red-emitting phosphor. Mater. Sci. Semicond. Process. 2020, 106, 104774. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A.; Aziz, S.H.A.; Alassan, Z.N.; Samsudin, N.F. Development and characterization studies of Eu3+-doped Zn2SiO4 phosphors with waste silicate sources. Procedia Chem. 2016, 19, 21–29. [Google Scholar] [CrossRef]
- Faizul, C.P.; Chik, A.; Bari, M.F. Palm Ash as an alternative source for silica production. MATEC Web Conf. 2016, 78, 01062. [Google Scholar]
- Norsuraya, S.; Fazlena, H.; Norhasyimi, R. Sugarcane bagasse as a renewable source of silica to synthesize Santa Barbara Amorphous-15 (SBA-15). Procedia Eng. 2016, 148, 839–846. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Matori, K.A.; Mohd, M.H. The physical and optical studies of crystalline silica derived from green synthesis of coconut husk ash. Appl. Sci. 2020, 10, 2128. [Google Scholar] [CrossRef]
- Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Khaidir, R.E.M. Synthesis and structural properties of coconut husk as potential silica source. Results Phys. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Kundu, T.K.; Karak, N.; Barik, P.; Saha, S. Optical properties of ZnO nanoparticles prepared by chemical method using poly vinyl alcohol (PVA) as capping agent. Int. J. Soft Comput. Eng. 2011, 1, 19–24. [Google Scholar]
- Yang, Y.; Yang, B.; Fu, Z.; Yan, H.; Zhen, W.; Dong, W.; Xia, L.; Liu, W.; Jian, Z.; Li, F. Enhanced yellow-green photoluminescence from ZnO-SiO2 composite opal. J. Phys. Condens. Matter 2004, 16, 7277–7286. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, N.; Tu, J.; Li, X.; Wang, R.; Zhang, T.; Shao, C. Preparation and humidity sensitive property of mesoporous ZnO-SiO 2 composite. Sens. Actuators B Chem. 2010, 149, 413–419. [Google Scholar] [CrossRef]
- Meyer, M.; Damonte, L.C. Study of Co and Fe-doped ZnO milled nanopowders. Powder Technol. 2015, 286, 371–377. [Google Scholar] [CrossRef]
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 10. [Google Scholar] [CrossRef]
- Pearton, S.J.; Norton, D.P.; Ip, K.; Heo, Y.W.; Steiner, T. Recent progress in processing and properties of ZnO. Prog. Mater. Sci. 2005, 50, 293–340. [Google Scholar] [CrossRef]
- Tani, T.; Mädler, L.; Pratsinis, S.E. Synthesis of zinc oxide/silica composite nanoparticles by flame spray pyrolysis. J. Mater. Sci. 2002, 37, 4627–4632. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Xin, S.; Wang, Q.; Li, Y.; Wu, Q.; Wang, C.; Wang, X.; Ding, X.; Geng, W. Recent development in rare earth doped phosphors for white light emitting diodes. J. Rare Earths 2015, 33, 1–12. [Google Scholar] [CrossRef]
- Takesue, M.; Hayashi, H.; Smith, R.L. Thermal and chemical methods for producing zinc silicate (willemite): A review. Prog. Cryst. Growth Charact. Mater. 2009, 55, 98–124. [Google Scholar] [CrossRef]
- Engku Ali, E.A.G.; Matori, K.A.; Saion, E.; Aziz, S.H.A.; Zaid, M.H.M.; Alibe, I.M. Structural and Optical Properties of Heat Treated Zn2SiO4 Composite Prepared by Impregnation of ZnO on SiO2 Amorphous Nanoparticles. ASM Sci. J. Spec. Issue 2018, 1, 75–85. [Google Scholar]
- Zhang, Q.Y.; Pita, K.; Kam, C.H. Sol-gel derived zinc silicate phosphor films for full-color display applications. J. Phys. Chem. Solids 2003, 64, 333–338. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Chen, Z.; Zhao, X. Hydrothermal synthesis and optical properties of zinc silicate hierarchical superstructures. Mater. Lett. 2011, 65, 3030–3033. [Google Scholar] [CrossRef]
- Rasdi, N.M.; Fen, Y.W.; Azis, R.S.; Omar, N.A.S. Photoluminescence studies of cobalt (II) doped zinc silicate nanophosphors prepared via sol-gel method. Optik 2017, 149, 409–415. [Google Scholar] [CrossRef]
- Ouyang, X.; Kitai, A.H.; Xiao, T. Electroluminescence of the oxide thin film phosphors Zn2SiO4 and Y2SiO5. J. Appl. Phys. 1996, 79, 3229–3234. [Google Scholar] [CrossRef]
- Li, Q.H.; Komarneni, S.; Roy, R. Control of morphology of Zn2SiO4 by hydrothermal preparation. J. Mater. Sci. 1995, 30, 2358–2363. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Zou, Y. Photoluminescence property of ZnO-SiO2 composites synthesized by sol-gel method. J. Phys. D. Appl. Phys. 2003, 36, 2972–2975. [Google Scholar] [CrossRef]
- Syamimi, N.F.; Matori, K.A.; Lim, W.F.; Aziz, S.A.; Hafiz, M.; Zaid, M. Effect of sintering temperature on structural and morphological properties of europium (III oxide doped willemite. J. Spectrosc. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Effendy, N.; Abdul, Z.; Mohamed, H.; Amin, K.; Hj, S.; Aziz, A.; Hafiz, M.; Zaid, M. Structural and optical properties of Er 3 + -doped willemite glass-ceramics from waste materials. Opt. Int. J. Light Electron Opt. 2016, 127, 11698–11705. [Google Scholar] [CrossRef]
- Rasdi, N.M.; Fen, Y.W.; Omar, N.A.S.; Azis, R.S.; Zaid, M.H.M. Effects of cobalt doping on structural, morphological, and optical properties of Zn2SiO4 nanophosphors prepared by sol-gel method. Results Phys. 2017, 7, 3820–3825. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A.; Zaid, M.H.M.; Norhafizah, M.R.; Nurzilla, M.; Zamratul, M.I.M. Synthesis and optical properties of europium doped zinc silicate prepared using low cost solid state reaction method. J. Mater. Sci. Mater. Electron. 2016, 27, 1092–1099. [Google Scholar] [CrossRef]
- Tarafder, A.; Molla, A.R.; Dey, C.; Karmakar, B. Thermal, structural, and enhanced photoluminescence properties of Eu3+-doped transparent willemite glass-ceramic nanocomposites. J. Am. Ceram. Soc. 2013, 96, 2424–2431. [Google Scholar] [CrossRef]
- Azman, A.Z.K.; Matori, K.A.; Ab Aziz, S.H.; Zaid, M.H.M.; Wahab, S.A.A.; Khaidir, R.E.M. Comprehensive study on structural and optical properties of Tm2O3 doped zinc silicate based glass–ceramics. J. Mater. Sci. Mater. Electron. 2018, 29, 19861–19866. [Google Scholar] [CrossRef]
- Sanaeishoar, H.; Sabbaghan, M.; Mohave, F. Synthesis and characterization of micro-mesoporous MCM-41 using various ionic liquids as co-templates. Microporous Mesoporous Mater. 2015, 217, 219–224. [Google Scholar] [CrossRef]
- Babu, B.C.; Rao, B.V.; Ravi, M.; Babu, S. Structural, microstructural, optical, and dielectric properties of Mn2+: Willemite Zn2SiO4 nanocomposites obtained by a sol-gel method. J. Mol. Struct. 2017, 1127, 6–14. [Google Scholar] [CrossRef]
- Sarrigani, G.V.; Quah, H.J.; Lim, W.F.; Matori, K.A.; Mohd Razali, N.S.; Kharazmi, A.; Hashim, M.; Bahari, H.R. Characterization of waste material derived willemite-based glass-ceramics doped with erbium. Adv. Mater. Sci. Eng. 2015, 201, 1–7. [Google Scholar] [CrossRef]
- Rashid, S.S.A.; Aziz, S.H.A.; Matori, K.A.; Zaid, M.H.M.; Mohamed, N. Comprehensive study on effect of sintering temperature on the physical, structural and optical properties of Er 3+ doped ZnO-GSLS glasses. Results Phys. 2017, 7, 2224–2231. [Google Scholar] [CrossRef]
- Naceur, H.; Megriche, A.; El Maaoui, M. Effect of sintering temperature on microstructure and electrical properties of Sr 1−x (Na 0.5 Bi 0.5) x Bi 2 Nb 2 O 9 solid solutions. J. Adv. Ceram. 2014, 3, 17–30. [Google Scholar] [CrossRef][Green Version]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–636. [Google Scholar] [CrossRef]
- Khaidir, R.E.M.; Fen, Y.W.; Zaid, M.H.M.; Matori, K.A.; Omar, N.A.S.; Anuar, M.F.; Wahab, S.A.A.; Azman, A.Z.K. Optical band gap and photoluminescence studies of Eu 3+ -doped zinc silicate derived from waste rice husks. Optik 2019, 182, 486–495. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Matori, K.A.; Zaid, M.H.M.; Samsudin, N.F. Structural and optical properties of Eu3+activated low cost zinc soda lime silica glasses. Results Phys. 2016, 6, 640–644. [Google Scholar] [CrossRef]
- Nazrin, S.N.; Halimah, M.K.; Muhammad, F.D.; Yip, J.S.; Hasnimulyati, L.; Faznny, M.F.; Hazlin, M.A.; Zaitizila, I. The effect of erbium oxide in physical and structural properties of zinc tellurite glass system. J. Non. Cryst. Solids 2018, 490, 35–43. [Google Scholar] [CrossRef]
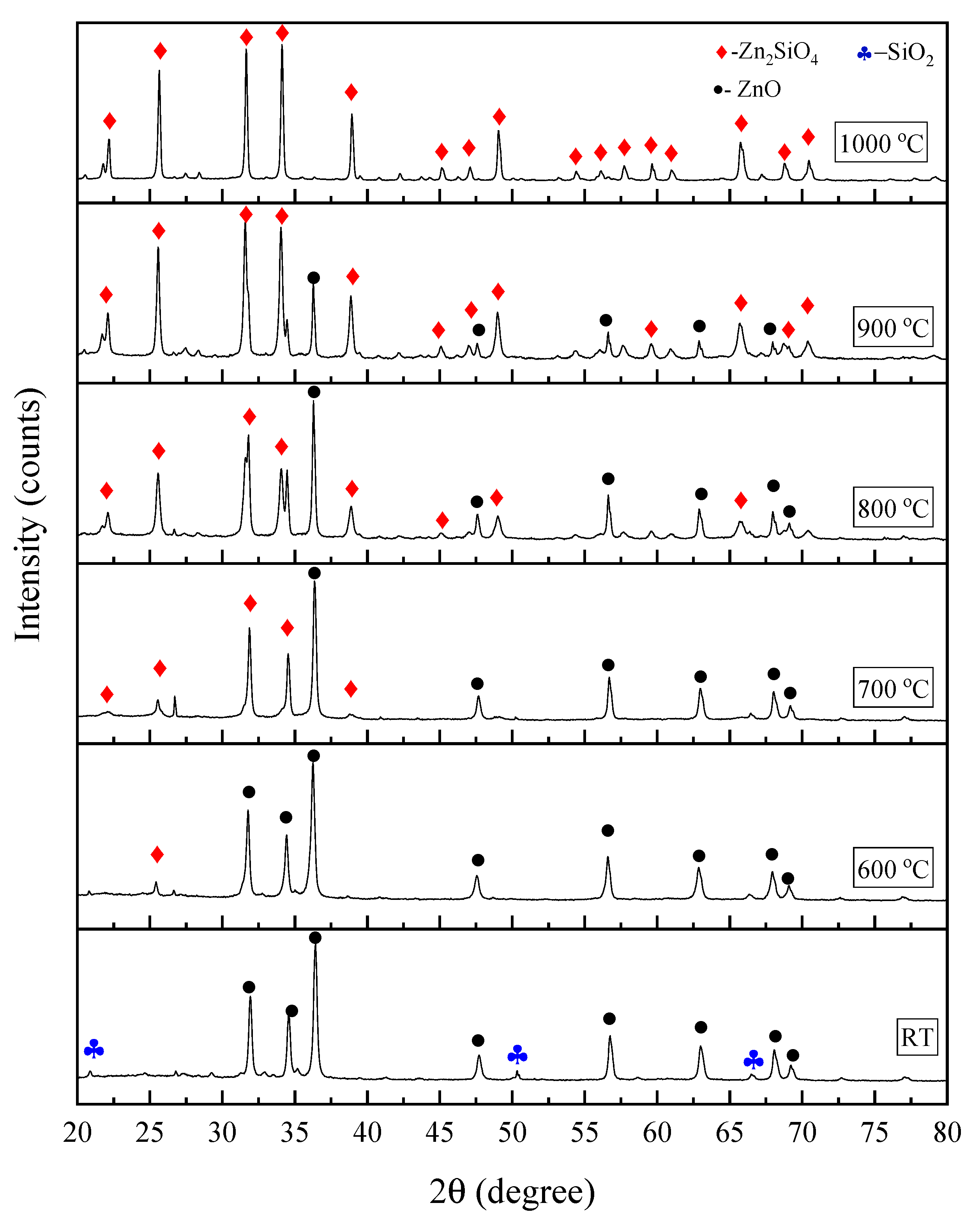
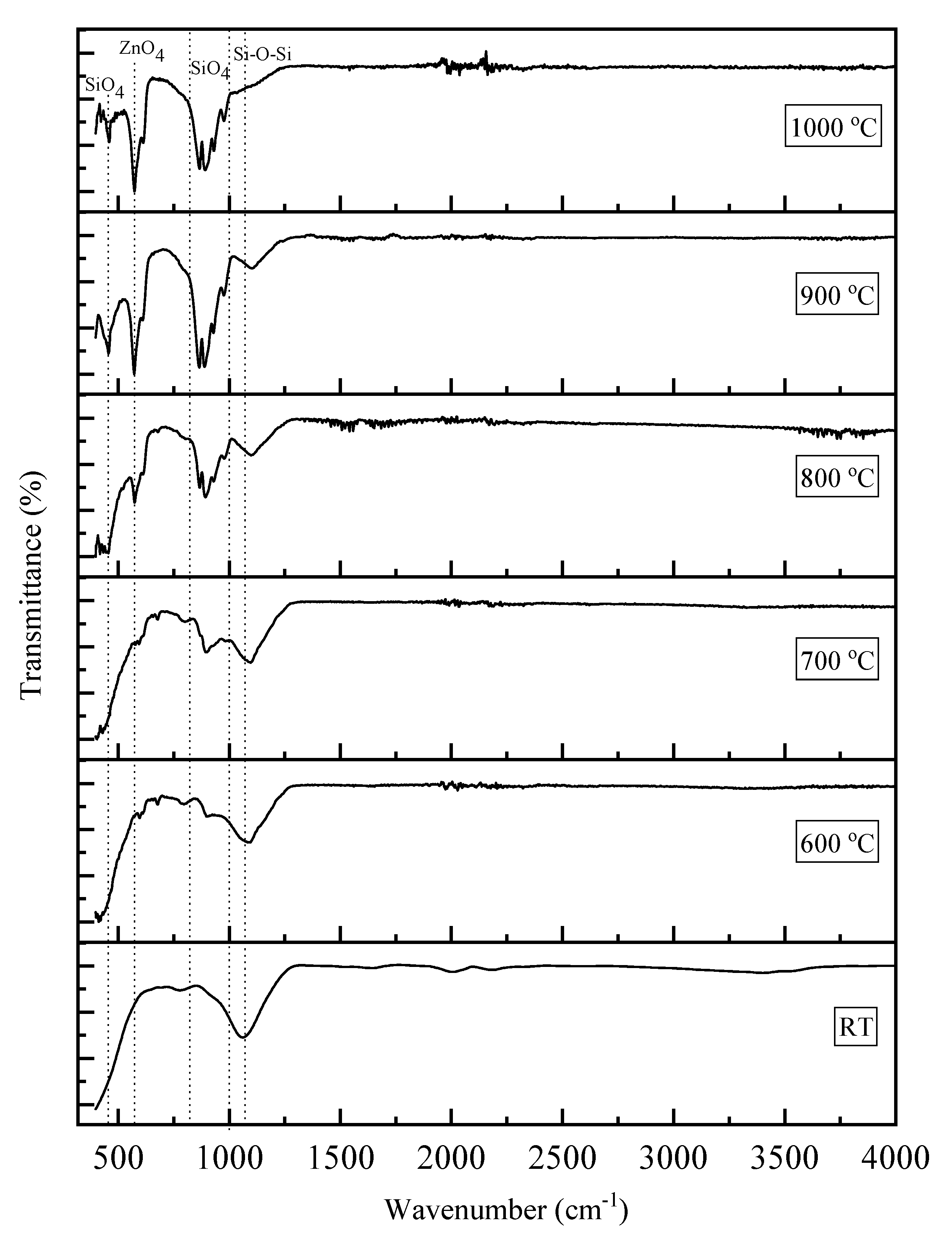

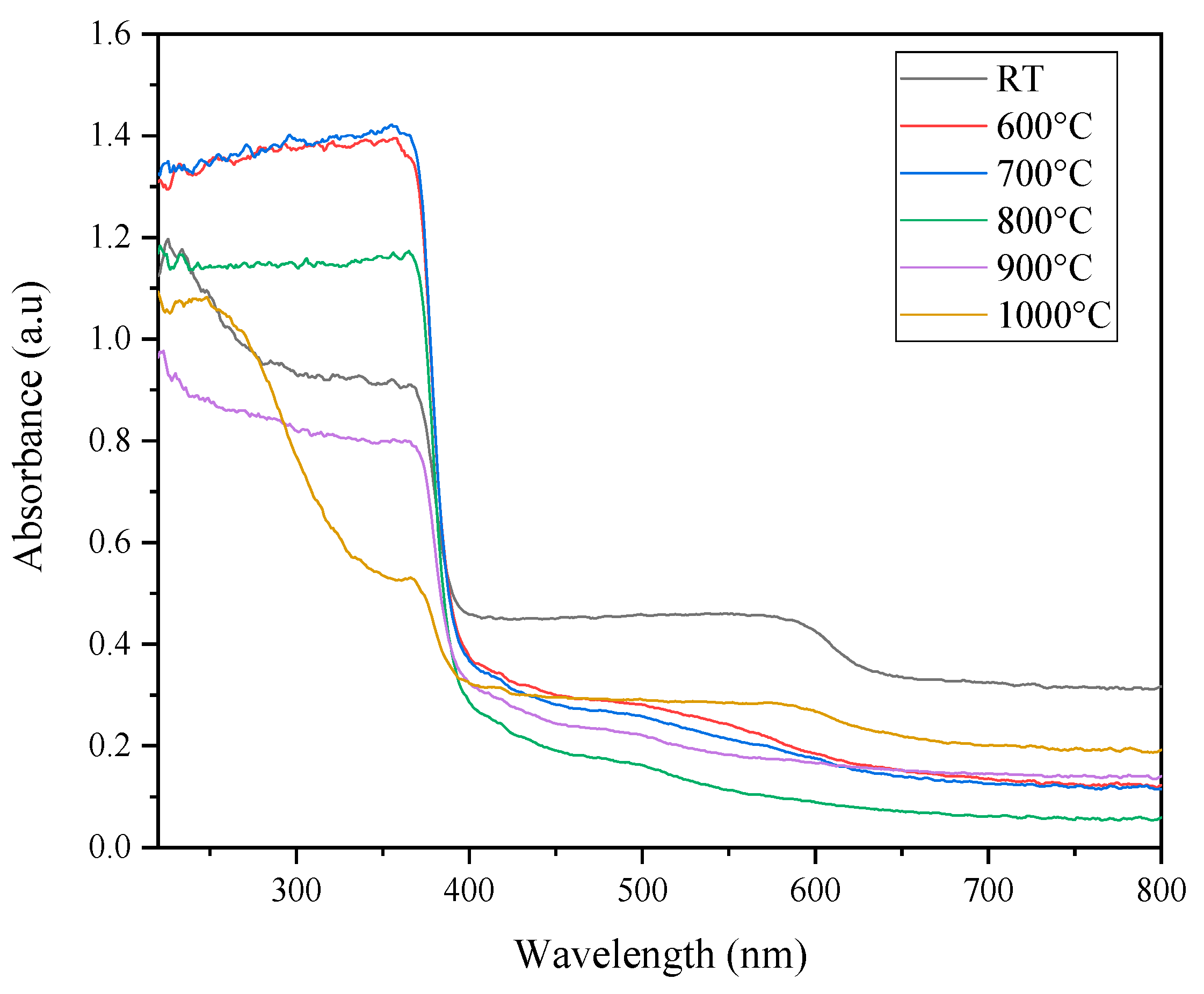
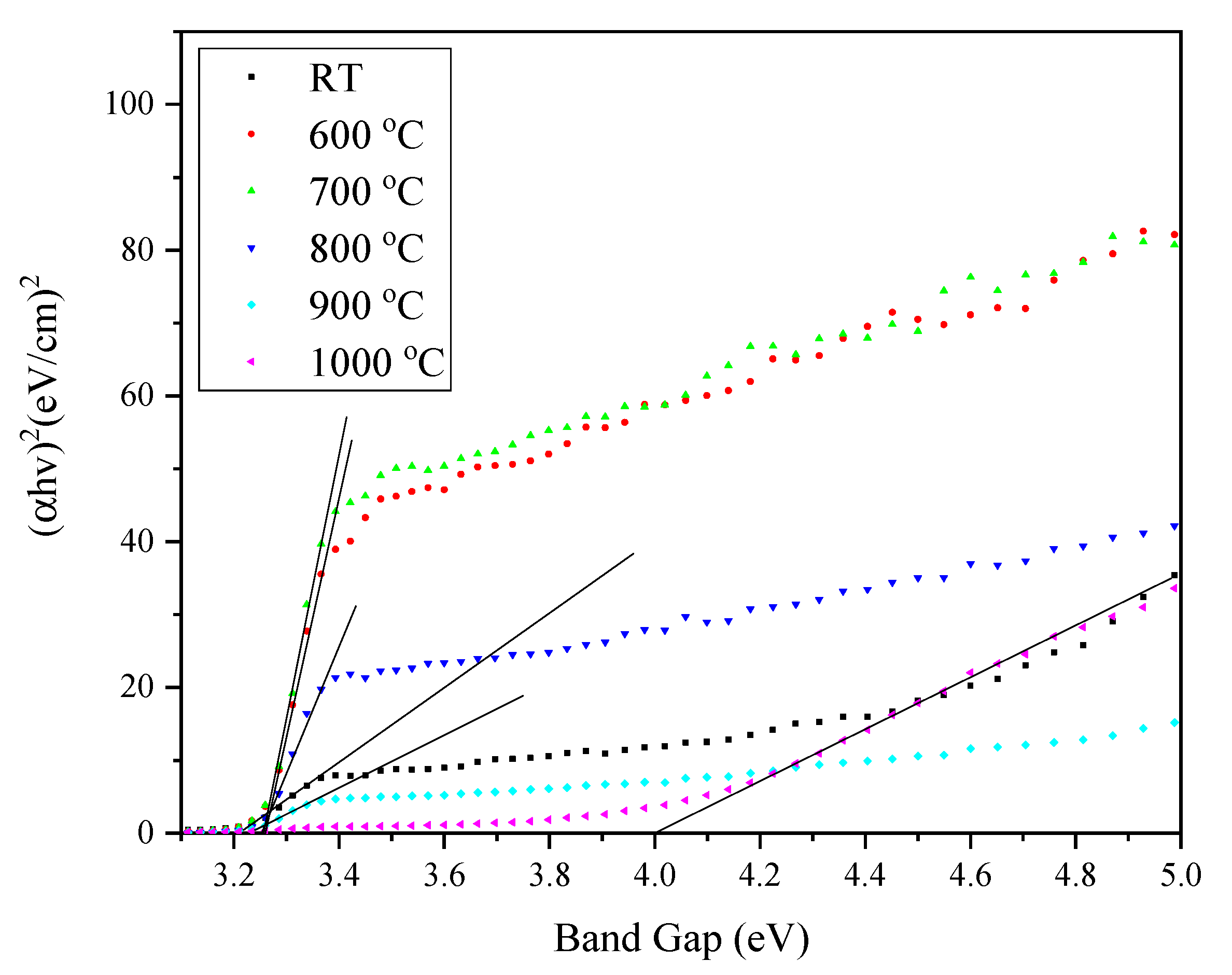
| Sintering Temperature (°C) | Average Grain Sizes (nm) |
|---|---|
| RT | 72.23 |
| 600 | 94.23 |
| 700 | 121.37 |
| 800 | 299.37 |
| 900 | 326.83 |
| 1000 | 515.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anuar, M.F.; Fen, Y.W.; Zaid, M.H.M.; Omar, N.A.S.; Khaidir, R.E.M. Sintering Temperature Effect on Structural and Optical Properties of Heat Treated Coconut Husk Ash Derived SiO2 Mixed with ZnO Nanoparticles. Materials 2020, 13, 2555. https://doi.org/10.3390/ma13112555
Anuar MF, Fen YW, Zaid MHM, Omar NAS, Khaidir REM. Sintering Temperature Effect on Structural and Optical Properties of Heat Treated Coconut Husk Ash Derived SiO2 Mixed with ZnO Nanoparticles. Materials. 2020; 13(11):2555. https://doi.org/10.3390/ma13112555
Chicago/Turabian StyleAnuar, Muhammad Fahmi, Yap Wing Fen, Mohd Hafiz Mohd Zaid, Nur Alia Sheh Omar, and Rahayu Emilia Mohamed Khaidir. 2020. "Sintering Temperature Effect on Structural and Optical Properties of Heat Treated Coconut Husk Ash Derived SiO2 Mixed with ZnO Nanoparticles" Materials 13, no. 11: 2555. https://doi.org/10.3390/ma13112555
APA StyleAnuar, M. F., Fen, Y. W., Zaid, M. H. M., Omar, N. A. S., & Khaidir, R. E. M. (2020). Sintering Temperature Effect on Structural and Optical Properties of Heat Treated Coconut Husk Ash Derived SiO2 Mixed with ZnO Nanoparticles. Materials, 13(11), 2555. https://doi.org/10.3390/ma13112555






