Systemic and Local Biocompatibility Assessment of Graphene Composite Dental Materials in Experimental Mandibular Bone Defect
Abstract
1. Introduction
2. Materials and Methods
2.1. Composite Dental Materials
Cell Culture Tests of Materials
- Extract Preparation
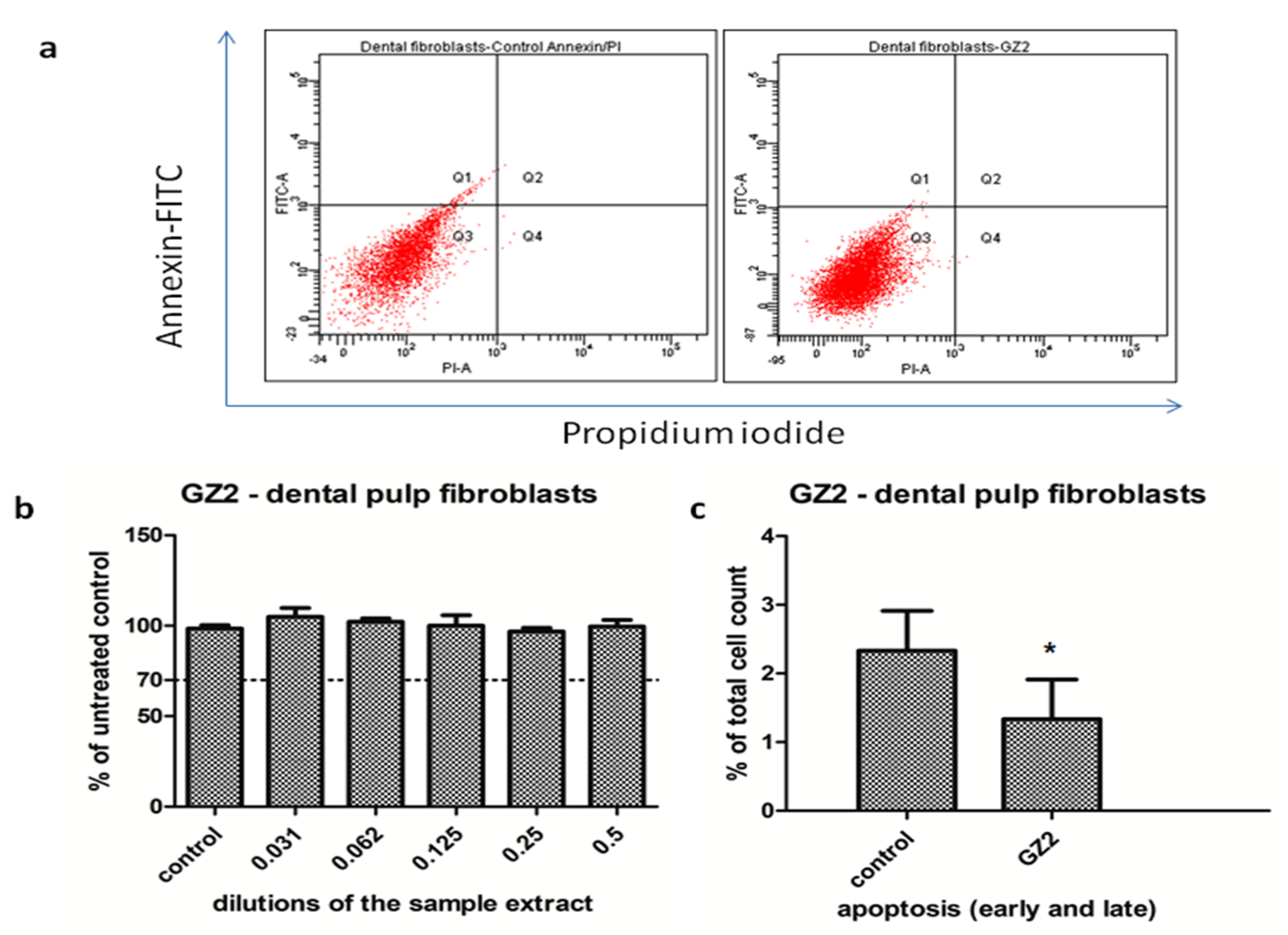
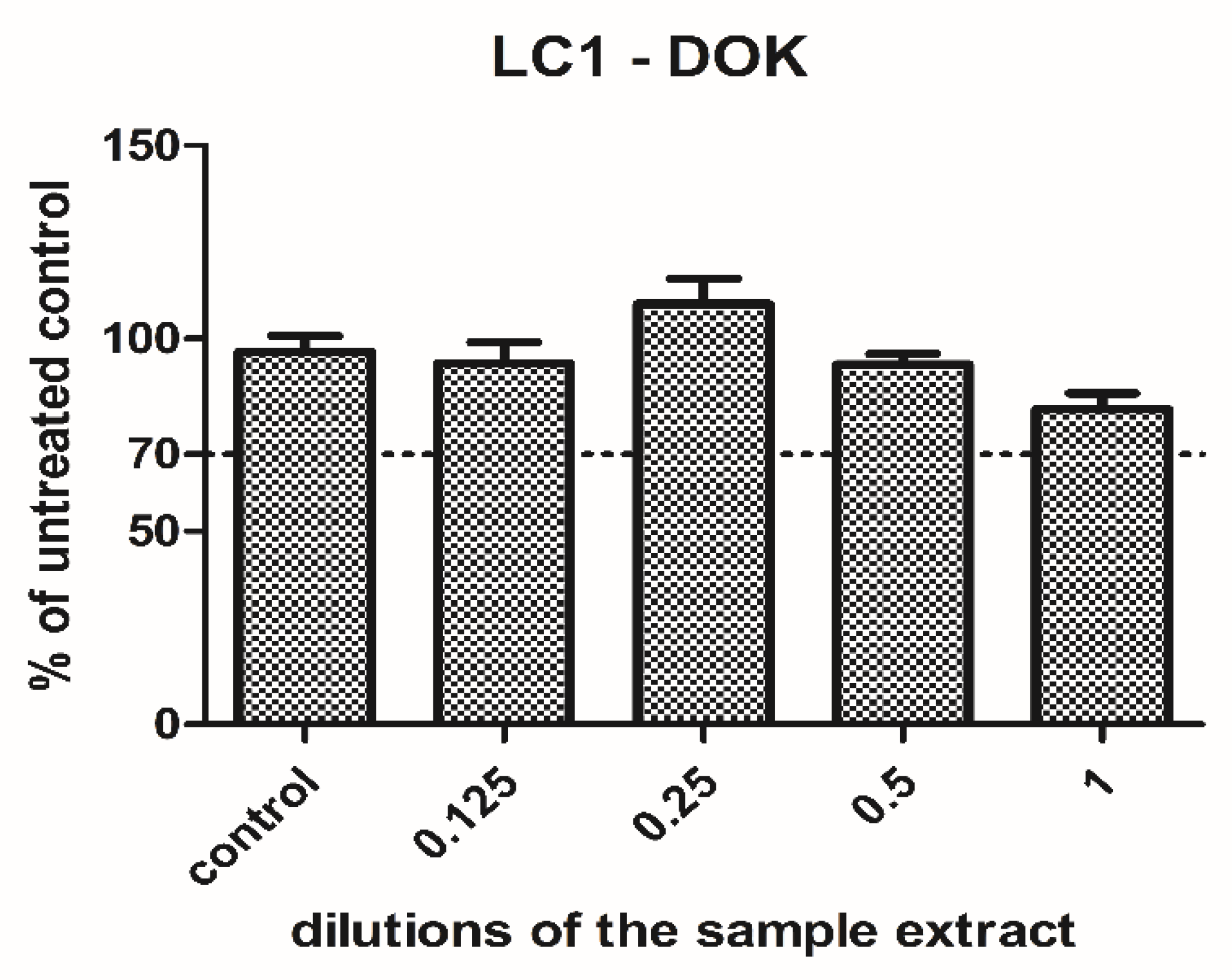
- Viability assay
- Flow Cytometry Assay
2.2. Animals
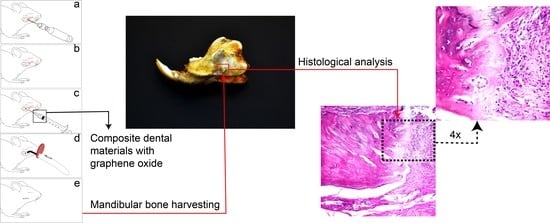
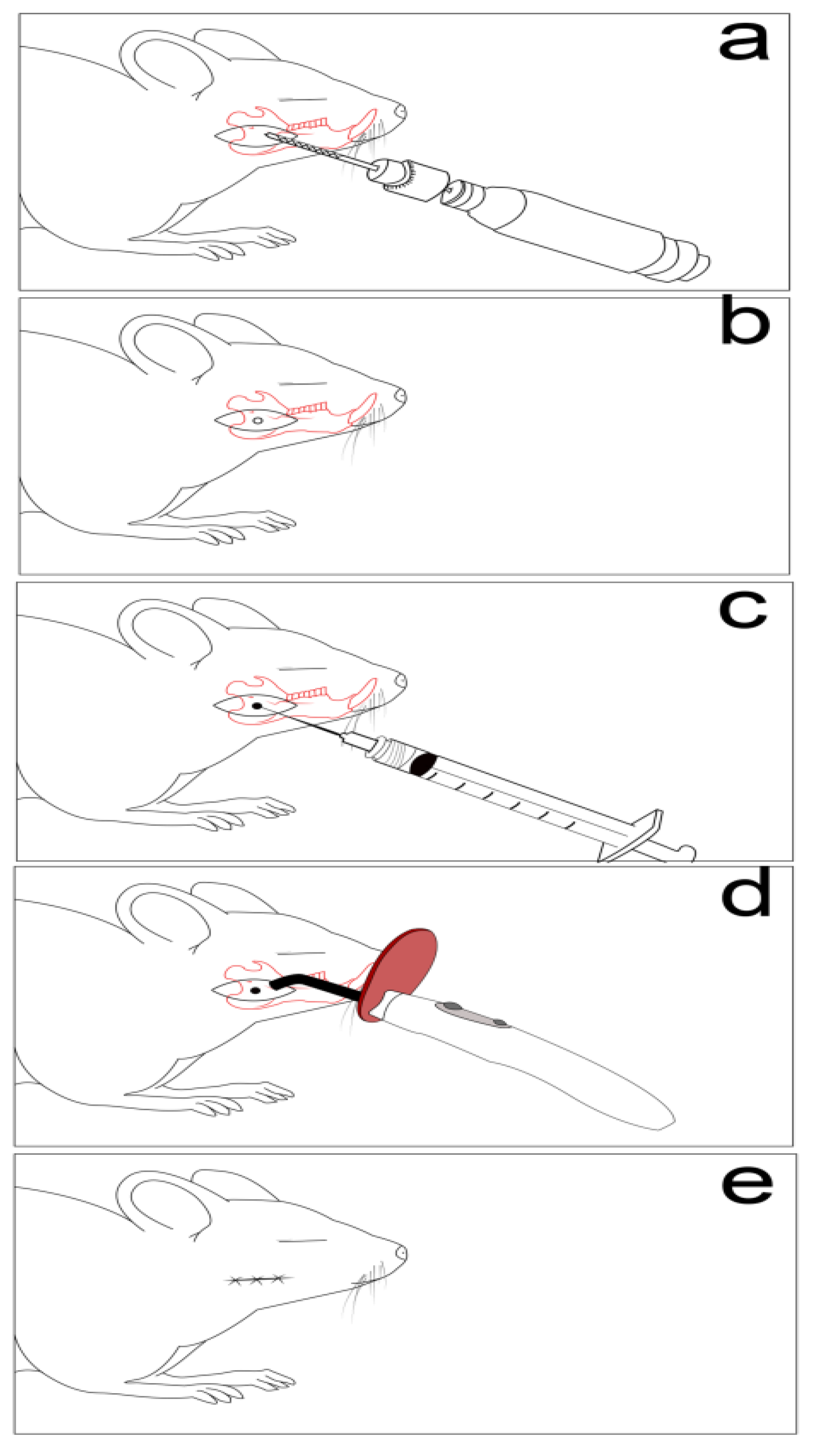
2.3. Experimental Protocols
2.4. Biochemistry
2.5. Oxidative Stress Analysis
2.6. Histological Analysis
2.7. Statistical Analysis
3. Results
3.1. Cell Culture Tests
3.2. Clinical Assessment
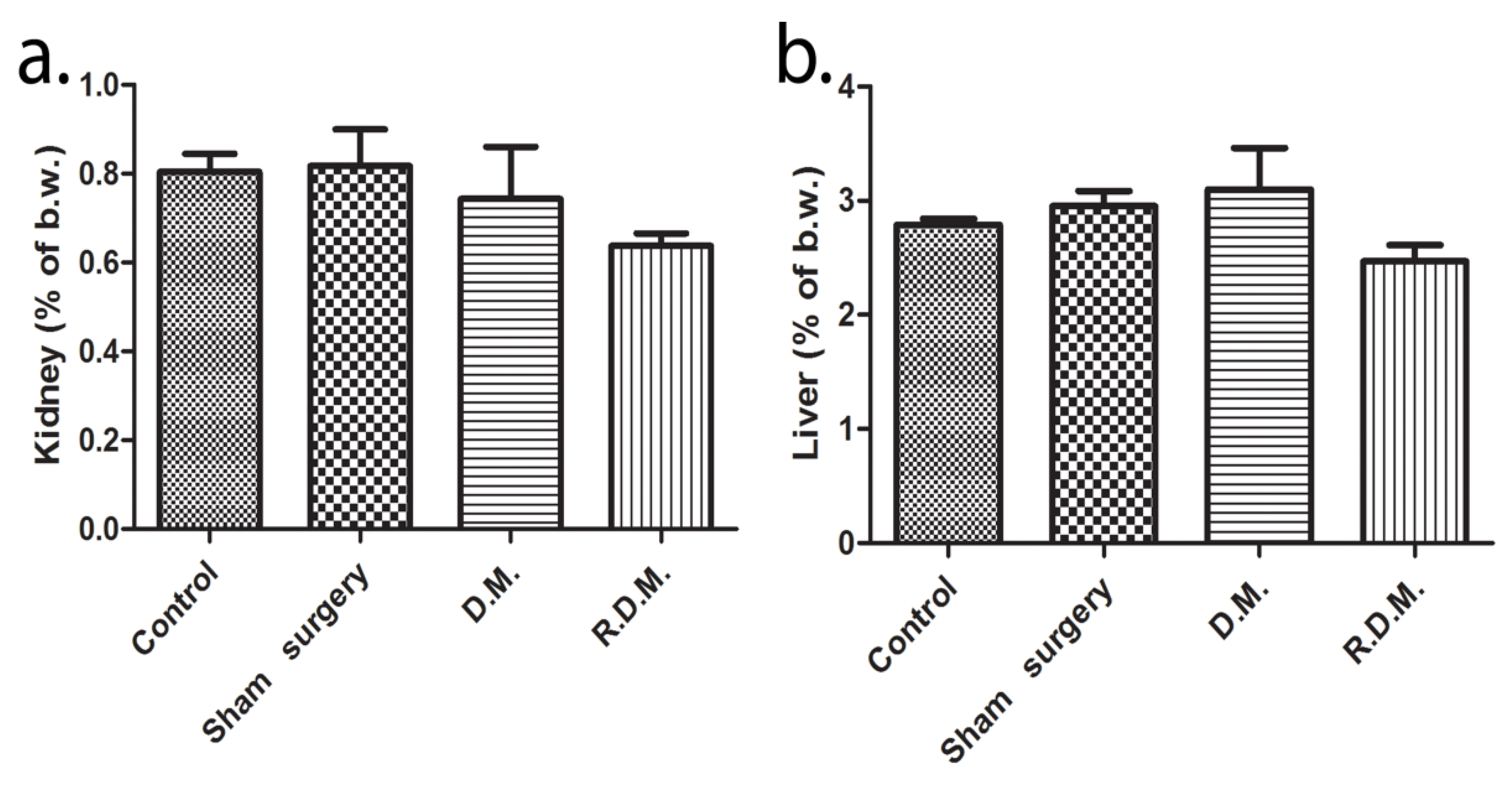
3.3. Oxidative Stress, Biochemical Analysis, and Organ Weight Analysis
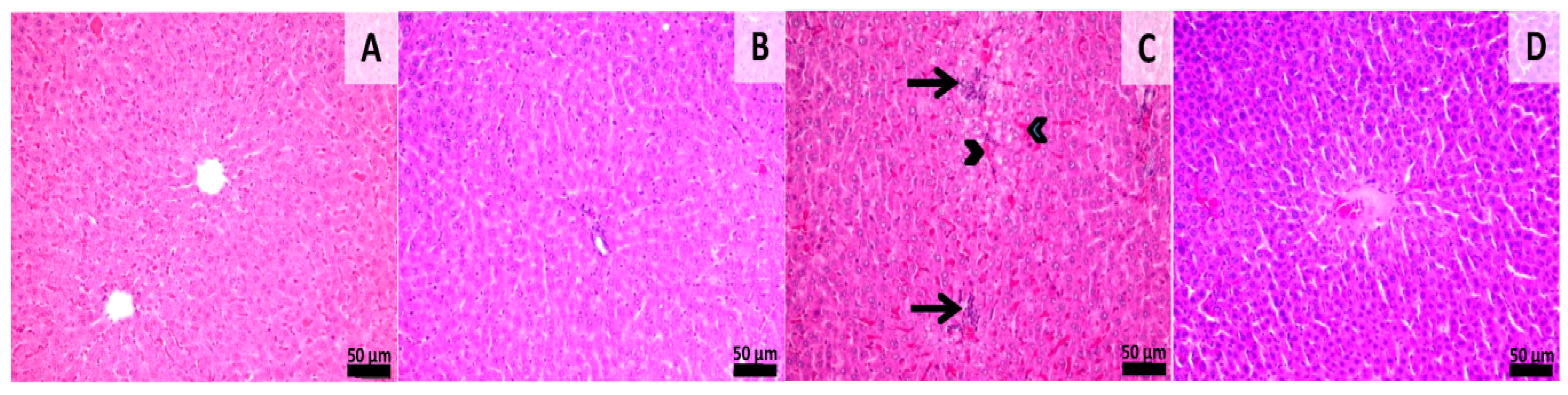
3.4. Histological Assessment
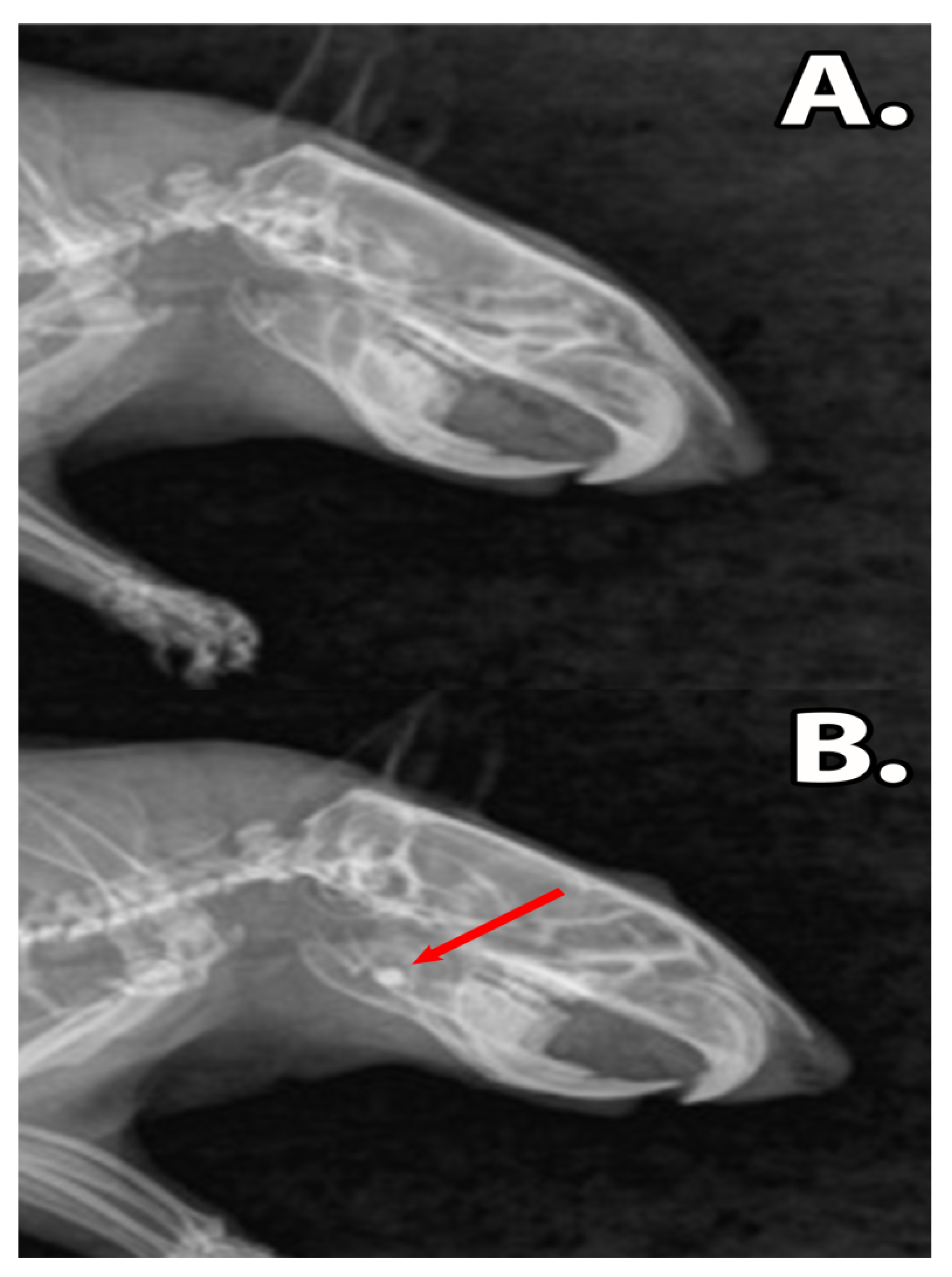
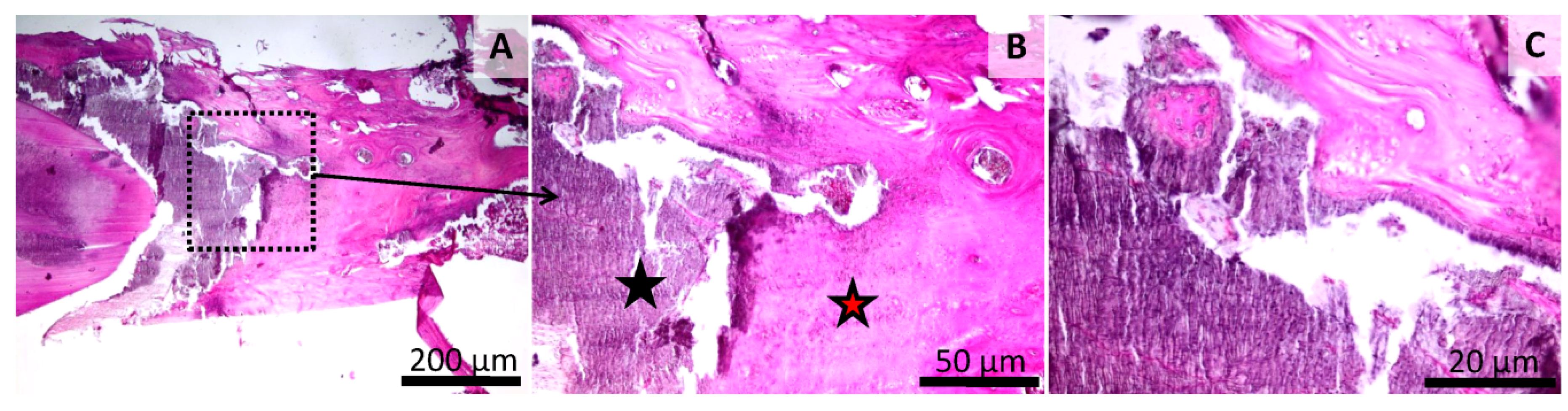
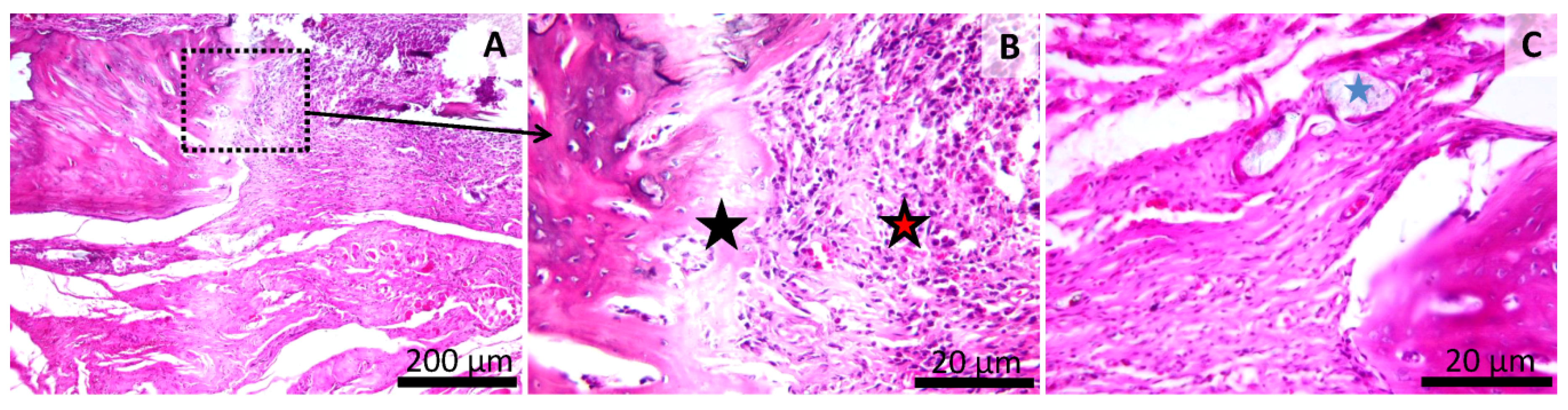
3.5. Histology of the Bone Defect
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bullock, C.J.; Buss, C. Biocompatibility considerations in the design of graphene biomedical materials. Adv. Mater. Interfaces 2019, 6, 1900229. [Google Scholar] [CrossRef]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Graphene: A versatile nanoplatform for biomedical applications. Nanoscale 2012, 4, 3833–3842. [Google Scholar] [CrossRef] [PubMed]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-based nanomaterials for tissue engineering in the dental field. Nanomater 2018, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Mehta, K.K. Top-down versus bottom-up nanoengineering routes to design advanced oropharmacological products. Curr. Pharm. Des. 2016, 22, 1534–1545. [Google Scholar] [CrossRef]
- Xie, H.; Cao, T.; Rodriguez-Lozano, F.J.; Luong-Van, E.K.; Rosa, V. Graphene for the development of the next generation of biocomposites for dental and medical application. Dent. Mater. 2017, 33, 765–774. [Google Scholar] [CrossRef]
- Dubey, N.; Bentini, R.; Islam, I.; Cao, T.; Castro Neto, A.H.; Rosa, V. Graphene: A versatile carbon-based material for bone tissue engineering. Stem Cells Int. 2015, 2015, 804213. [Google Scholar] [CrossRef]
- Menaa, F.; Abdelghani, A.; Menaa, B. Graphene Nano-materials as biocompatible and conductive scaffolds for stem cells: Impact for tissue engineering and regenerative medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1321–1338. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachack, A.; Hurt, R.H.; Kane, A.B. Biological interactions of grapheme—Family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2011, 25, 15–34. [Google Scholar] [CrossRef]
- Seabea, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Duran, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Sydlik, S.A.; Jhunjhunwala, S.; Webber, W.J.; Anderson, D.G.; Langer, R. In vivo compatibility of graphene oxide with differing oxidation states. ACS Nano 2015, 9, 3866–3874. [Google Scholar] [CrossRef]
- Rosu, M.C.; Socaci, C.; Avram, F.V.; Borodi, G.; Pogacean, F.; Coros, M.; Magerusan, L.; Pruneanu, S. Photocatalytic performance of graphene/TiO2-Ag composites on amaranth dye degradation. Mater. Chem. Phys. 2016, 179, 232–241. [Google Scholar] [CrossRef]
- Olteanu, D.; Filip, A.; Socaci, C.; Biris, A.R.; Filip, X.; Coros, M.; Pruneanu, S. Cytotoxicity assessment of grapheme-based nanomaterials on humandental follicle stem cells. Colloids Surf. B Biointerfaces 2015, 136, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Roșu, M.C.; Pall, E.; Socaci, C.; Magerus, L.; Pogacean, F.; Coros, M.; Turza, A.; Pruneanu, S. Cytotoxicity of mehylcellulose-based films containing graphenes and curcumin on human lung fibloblasts. Process. Biochem. 2016, 52, 243–249. [Google Scholar] [CrossRef]
- Gociu, M.; Patroi, D.; Prejmerean, C.; Pastrav, O.; Boboia, S.; Prodan, D.; Moldovan, M. Biology and cytotoxicity of dental materials: An in vitro study. Rom. J. Morphol. Embryol. 2013, 54, 261–265. [Google Scholar]
- Patroi, D.; Gociu, M.; Prejmerean, C.; Colceriu, L.; Silaghi Dumitrescu, L.; Moldovan, M. Assessing the biocompatibility of a dental composite product. Rom. J. Morphol. Embryol. 2013, 54, 321–326. [Google Scholar]
- Swetha, B.; Mathew, S.; Murthy, B.V.S.; Shruthi, N.; Bhandi, S.H. Determination of biocompatibility: A review. Int. Dent. Med. J. Adv. Res. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Hauman, C.H.J.; Love, R.M. Biocompatibility of dental materials used in contemporary endodontic therapy: A review. Part Intracanal drugs and substances. Int. Endontic. J. 2002, 36, 75–85. [Google Scholar] [CrossRef]
- Herford, A.S.; Stoffella, E.; Tandon, R. Reconstruction of mandibular defects using bone morphogenic protein: Can growth factors replace the need for autologous bone grafts? A systematic review of the Literature. Plast Surg. Int. 2011, 2011, 165824. [Google Scholar] [CrossRef]
- Shah, S.R.; Young, S.; Goldman, J.L.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. A composite critical-size rabbit mandibular defect for evaluation of craniofacial tissue regeneration. Nat. Protoc. 2016, 11, 1989–2009. [Google Scholar] [CrossRef]
- Prejmerean, C.; Vezsenyi, M.; Moldovan, M.; Grecu, R.; Muşat, O.; Brie, M. The synnthesis of Bis-GMA oligomers and their effects upon the properties of some experimental dental composites. Rev. Roum. Chim. 2000, 45, 567–577. [Google Scholar]
- Baldea, I.; Olteanu, D.E.; Bolfa, P.; Ion, R.M.; Decea, N.; Cenariu, M.; Banciu, M.; Sesarman, A.V.; Filip, A.G. Efficiency of photodynamic therapy on WM35 melanoma with synthetic porphyrins: Role of chemical structure, intracellular targeting and antioxidant defense. J. Photoch. Photobio. B 2015, 151, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Srouji, S.; Rachmiel, A.; Blumenfeld, I.; Livne, E. Mandibular defect repair by TGF-β and IGF-1 released from a biodegradable osteoconductive hydrogel. J. Maxillofac. Surg. 2005, 33, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Ren, J.; Jia, X.; Pan, K. The bone formation in vitro and mandibular defect repair using PLGA porous scaffolds. J. Biomater. Res. A 2005, 74, 562–569. [Google Scholar]
- Hedenqvist, P. Anaesthesia and Analgesia for Surgery in Rabbits and Rats: A Comparison of the Effects of Diffrent Compounds. Ph.D. Thesis, Karolinska Institutet, Stockholm, Sweden, 2008. [Google Scholar]
- Briebly, G.I.; Tredinnick, S.; Lynham, A.; Woodruff, M.A. Critical sized mandibular defect regeneration in preclinical in vivo models. Curr. Mol. Bio. Rep. 2016, 2, 83–89. [Google Scholar] [CrossRef][Green Version]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Harma, M.; Harma, M.; Erel, O. Increased oxidative stress in pacients with hydatiform mole. Swiss Med. Wkly. 2003, 133, 563–566. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Mitev, D.; Gradeva, H.; Stoyanova, Z.; Petrova, N.; Karova, N.; Dimov, D.; Iliev, V.; Koychev, A.; Prakova, G.; Vlaykova, T. Evaluation of thiol compounds and lipid peroxidative products in plasma of patients with COPD. Trakia J. Sci. 2010, 8, 306–314. [Google Scholar]
- Rodrigues, F.A.P.; Prata, M.M.G.; Oliveira, I.C.M.; Alvez, N.T.Q.; Freitas, R.E.M.; Monteiro, H.S.A.; Silva, J.A.; Vieira, P.C.; Viana, D.A.; Liborio, A.B.; et al. Gingerol fraction from Zingiber officinale protects against gentamicin-induced nephrotoxicity. Antimicrob. Agents Chemother. 2014, 58, 1872–1878. [Google Scholar] [CrossRef]
- Thoolen, B.; Maronpot, R.R.; Harada, T.; Nyska, A.; Rousseaux, C.; Nolte, T.; Malarkey, D.E.; Kaufmann, W.; Kuttler, K.; Deschl, U.; et al. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol. Pathol. 2010, 38, 5s–81s. [Google Scholar] [CrossRef] [PubMed]
- Wallig, M.A.; Janovitz, E.B. Morphologic Manifestations of Toxic Cell Injury. In Fundamentals of Toxicologic Pathology, 3rd ed.; Wallig, M.A., Rousseaux, C.G., Haschek, W.M., Bolon, B., Eds.; Academic Pres: Urbana, IL, USA; Elsevier Inc.: Urbana, IL, USA, 2018; pp. 59–81. [Google Scholar]
- Sava, S.; Moldovan, M.; Sarosi, C.; Mesaros, A.; Dudea, D.; Alb, C. Effects of graphene addition on the mechanical properties of composites for dental restoration. Mater. Plast 2015, 52, 90–92. [Google Scholar]
- Sava, S.; Sarosi, C.; Boboia, S.; Tonea, A.; Alb, C.; Dudea, D. The study of new composites with graphene used in dentistry. Stud. Univ. Babes-Bol. Chem. 2015, 2, 71–80. [Google Scholar]
- Sarosi, C.; Biris, A.R.; Antoniac, A.; Boboia, S.; Alb, C.; Antoniac, I.; Moldovan, M. The nanofiller effect on properties of experimental graphene dental nanocomposites. J. Adhes. Sci. Technol. 2016, 30, 1779–1794. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Cui, D. Biocompatibility of grapheneoxide. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar]
- Fujioka-Kobayashi, M.; Miron, R.J.; Lussi, A.; Gruber, R.; Ilie, N.; Price, R.B.; Schmalz, D. Effect of the degree of conversion of resin-based composites on cytotoxicity, cell attachment, and gene expression. Dent. Mater. 2019, 35, 1173–1193. [Google Scholar] [CrossRef]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxideand graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Costa, C.A.; Oliveira, M.F.; Giro, E.M.; Hebling, J. Biocompatibility of resin-based materials as pulp-capping agents. Int. Endod. J. 2003, 36, 831–839. [Google Scholar] [CrossRef]
- Cao, T.; Saw, T.Y.; Heng, B.C.; Liu, H.; Yap, A.U.; Mah-Lee Ng, M. Comparison of different test models for assessment of cytotoxicity of composite resins. J. Appl. Toxicol. 2005, 25, 101–108. [Google Scholar] [CrossRef]
- Demirci, M.; Hiller, K.A.; Bosl, C.; Galler, K.; Schmalz, G.; Schweikl, H. The induction of oxidative stress, citotoxicity, and genotoxicity by dental adhesives. Dent. Mater. 2008, 24, 362–371. [Google Scholar] [CrossRef]
- Dentistry IQ. Available online: http://www.dentistryiq.com/articles/2013/09/oxidative-stress-and-the-use-of-antioxidants-in-dentistry-part-i.html (accessed on 20 February 2018).
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the degree of conversion, residual monomers and mechanical properties of some light-cured dental resin composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, H.; Zhang, G.; Wu, X.; Zou, H.; Feng, L.; Wang, D.; Li, M.; Zhao, J.; Du, J.; et al. Restoration of a critical mandibular bone defect using human alveolar bone-derived stem cells and porous nano-HA/collagen/PLA scaffold. Stem Cells Int. 2016, 2016, 8741641. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The influence of graphene in improvement of physico-mechanical properties in PMMA denture base resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.; Prodan, D.; Sarosi, C.; Carpa, R.; Socaci, C.; Rosu, M.C.; Pruneanu, S. Synthesis, morpho-structural properties and antibacterial effect of silicate based composites containing graphene oxide/hydroxyapatite. Mater. Chem. Phys. 2018, 217, 48–53. [Google Scholar] [CrossRef]
- Ren, J.; Ren, T.; Zhao, P.; Huang, Y.; Pan, K. Repair of mandibular defects using MSCs-seeded biodegradable polyester porous scaffolds. J. Biomat. Sci. 2012, 18, 505–517. [Google Scholar] [CrossRef]
- Gupta, S.K.; Saxena, P.; Pant, V.A.; Pant, A.B. Release and toxicity of dental resin composite. Toxicol. Int. 2012, 19, 225–234. [Google Scholar]
- Bain, P.J. Liver. Urinary system. In Duncan and Prasse’s Veterinary Laboratory Medicine, Clinical Pathology, 5th ed.; Latimer, K.S., Ed.; Wiley- Blackwel Inc.: West Sussex, UK, 2011; pp. 211–283. [Google Scholar]
- Singh, V.; Kashyap, S.; Yadav, U.; Srivastava, A.; Singh, A.V.; Singh, R.K.; Singh, S.K.; Saxena, P.S. Nitrogen doped carbon quantum dots demonstrate no toxicity under in vitro conditions in a cervical cell line and in vivo in Swiss albino mice. Toxicol. Res. 2019, 8, 395–406. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.T.; Liu, Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of pegylated graphene in mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef]
- Cuozzo, R.C.; Sartoretto, S.C.; Resende, R.F.; Alves, A.T.N.; Mavropoulos, E.; Prado da Silva, M.H.; Calasans-Maia, M.D. Biological evaluation of zinc-containing calcium alginate-hydroxyapatite composite microspheres for bone regeneration. J. Biomed. Mater. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, M.K.; Nayak, M.K. Thrombus inducing property of atomically thin graphene oxide sheets. ACS Nano 2011, 5, 4987–4996. [Google Scholar] [CrossRef]
- Wu, C.; Xia, L.; Han, P.; Xu, M.; Fang, B.; Wang, J.; Chang, J.; Xiao, Y. Graphene-oxide-modified beta-tricalcium phosphate bioceramics stimulate in vitro and in vivo osteogenesis. Carbon 2015, 93, 116–129. [Google Scholar] [CrossRef]
- Roudsari, J.M.; Mahjoub, S. Quantification and comparison of bone-specific alkaline phosphatase with two methods in normal and page’s specimens. Casp. J. Intern. Med. 2012, 3, 478–483. [Google Scholar]
- Baker, S.B.; Worthley, L.I. The essentials of calcium, magnesium and phosphate metabolism: Part Physiology. Crit. Care. Resusc. 2002, 4, 301–306. [Google Scholar] [PubMed]
- Mousabvinasab, S.M. Biocompatibility of composite resins. Dent. Res. G 2011, 8, S21–S29. [Google Scholar]
- Tassery, H.; Remusat, M.; Koubi, G.; Pertot, W.J. Comparison of the intraosseous biocompatibility of Vitremer and super EBA by implantation into the mandible of rabbits. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 1997, 83, 602–608. [Google Scholar] [CrossRef]
- Tecu, C.; Antoniac, A.; Goller, G.; Gok, M.G.; Manole, M.; Mohan, A.; Moldovan, H.; Earar, K. The sintering behaviour and mechanical properties of hydroxyapatite - based composites for bone tissue regeneration. Rev. Chim. 2018, 69, 1272–1275. [Google Scholar] [CrossRef]


| Materials | Type of Materials | Composition | Manufacturer |
|---|---|---|---|
| GZ2 | light-curing hybrid restorative composite | Organic matrix: Bis-GMA, UDMA, TEGDMA. Fillers: 0.3% rGO/ZrO2; HA-ZrO2 (particle size 0.01–60 μm and 5–8 nm), silica, BaO glass (particle size 0.01–0.035 μm and 2–6 nm), quartz. 80% wt | UBB-ICCRR, Cluj-Napoca, Romania |
| LC1 | light-curing hybrid cement | Organic matrix: Bis-GMA, UDMA, TEGDMA. Fillers: 0.3% TiO2-Ag-GO, silica, HA-F, BaF2 glass (particle size 2–6 nm). 70% wt | UBB-ICCRR, Cluj-Napoca, Romania |
| Weeks | Control Group | Sham Surgery | LC1 Group | GZ2 Group |
|---|---|---|---|---|
| 1 | 253 ± 18.45 | 239.8 ± 23.95 | 242.8 ± 10.32 | 244.6 ± 28.58 |
| 2 | 275.4 ± 25.46 | 246.6 ± 25.61 | 248.8 ± 20.22 | 255.4 ± 26.21 |
| 3 | 283.8 ± 23.08 | 256.2 ± 27.9 | 270.2 ± 19.32 | 267 ± 17.94 |
| 4 | 297.6 ± 32.94 | 279.8 ± 31.09 | 284.3 ± 20.37 | 263.2 ± 23.79 |
| 5 | 308.4 ± 30.16 | 281.2 ± 32.61 | 292.8 ± 19.39 | 274.4 ± 15.54 |
| 6 | 314.8 ± 26.25 | 295 ± 32.82 | 297.8 ± 26.41 | 282.2 ± 4.32 |
| 7 | 326.2 ± 21.12 | 301 ± 30.74 | 313 ± 21.96 | 308.8 ± 10.77 |
| Control Group | Sham Surgery | LC1 Group | GZ2 Group | |
|---|---|---|---|---|
| TAC | 1.08 ± 0 | 1.08 ± 0 | 1.08 ± 0 | 1.08 ± 0 |
| TOS | 19.5 ± 1.91 | 24.4 ± 1.40 * | 22.55 ± 1.17 | 22.46 ± 1.77 |
| OSI | 17.98 ± 1.75 | 22.68 ± 1.31 *** | 20.98 ± 1.37 * | 20.67 ± 1.63 |
| NO | 40.59 ± 2.74 | 70.27 ± 3.29 *** | 49.50 ± 7.14 *** | 53.23 ± 2.37 *** |
| MDA | 3.05 ± 0.25 | 4.35 ± 0.95 * | 3.42 ± 0.13 | 3.71 ± 0.92 |
| SH | 0.76 ± 0.07 | 0.43 ± 0.06 *** | 0.36 ± 0.06 *** | 0.27 ± 0.01 *** |
| Parameters | Control Group | Sham Surgery | LC1 Group | GZ2 Group |
|---|---|---|---|---|
| Creatinine | 0.46 ± 0.10 | 0.39 ± 0.03 | 0.41 ± 0.18 | 0.49 ± 0.19 |
| Urea | 18.96 ± 2.51 | 19.84 ± 3.93 | 19.68 ± 3.32 | 18.98 ± 4.20 |
| ALT | 43.14 ± 3.13 | 45.62 ± 3.17 | 40.24 ± 10.01 | 43.7 ± 9.46 |
| AST | 173.56 ± 28.73 | 153.92 ± 32.80 | 110.38 ± 28.48 ** | 129.7 ± 33.51 |
| ALP | 133.46 ± 35.3 | 357.08 ± 132.9 ** | 302.3 ± 41.09 ** | 303.96 ± 58.26 ** |
| CK | 227.8 ± 50.9 | 287.12 ± 134.1 | 208.78 ± 54.63 | 190.24 ± 41.28 |
| Ca | 10.42 ± 0.51 | 13.22 ± 2.19 | 15.52 ± 0.46 *** | 14.18 ± 2.36 * |
| P | 7.16 ± 0.58 | 6.72 ± 0.80 | 6 ± 0.29 * | 5.74 ± 0.35 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreanca, A.; Sarosi, C.; Parvu, A.E.; Blidaru, M.; Enacrachi, G.; Purdoiu, R.; Nagy, A.; Sevastre, B.; Oros, N.A.; Marcus, I.; et al. Systemic and Local Biocompatibility Assessment of Graphene Composite Dental Materials in Experimental Mandibular Bone Defect. Materials 2020, 13, 2511. https://doi.org/10.3390/ma13112511
Dreanca A, Sarosi C, Parvu AE, Blidaru M, Enacrachi G, Purdoiu R, Nagy A, Sevastre B, Oros NA, Marcus I, et al. Systemic and Local Biocompatibility Assessment of Graphene Composite Dental Materials in Experimental Mandibular Bone Defect. Materials. 2020; 13(11):2511. https://doi.org/10.3390/ma13112511
Chicago/Turabian StyleDreanca, Alexandra, Codruta Sarosi, Alina Elena Parvu, Mihai Blidaru, George Enacrachi, Robert Purdoiu, Andras Nagy, Bogdan Sevastre, Nechita Adrian Oros, Ioan Marcus, and et al. 2020. "Systemic and Local Biocompatibility Assessment of Graphene Composite Dental Materials in Experimental Mandibular Bone Defect" Materials 13, no. 11: 2511. https://doi.org/10.3390/ma13112511
APA StyleDreanca, A., Sarosi, C., Parvu, A. E., Blidaru, M., Enacrachi, G., Purdoiu, R., Nagy, A., Sevastre, B., Oros, N. A., Marcus, I., & Moldovan, M. (2020). Systemic and Local Biocompatibility Assessment of Graphene Composite Dental Materials in Experimental Mandibular Bone Defect. Materials, 13(11), 2511. https://doi.org/10.3390/ma13112511