Engineering of 2D Ti3C2 MXene Surface Charge and its Influence on Biological Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the MAX Phase and 2D MXene
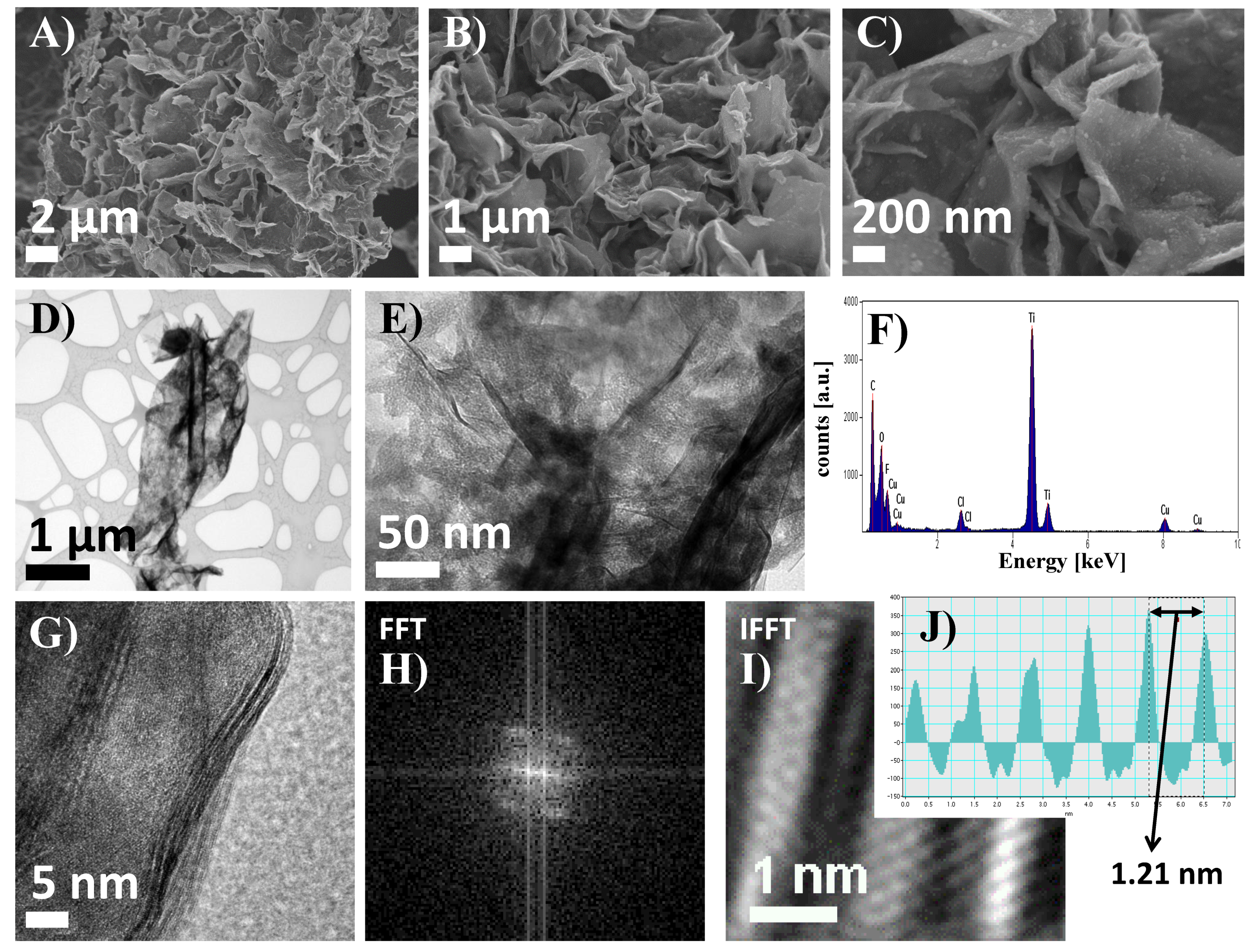
2.2. Studies on Morphology and Structure
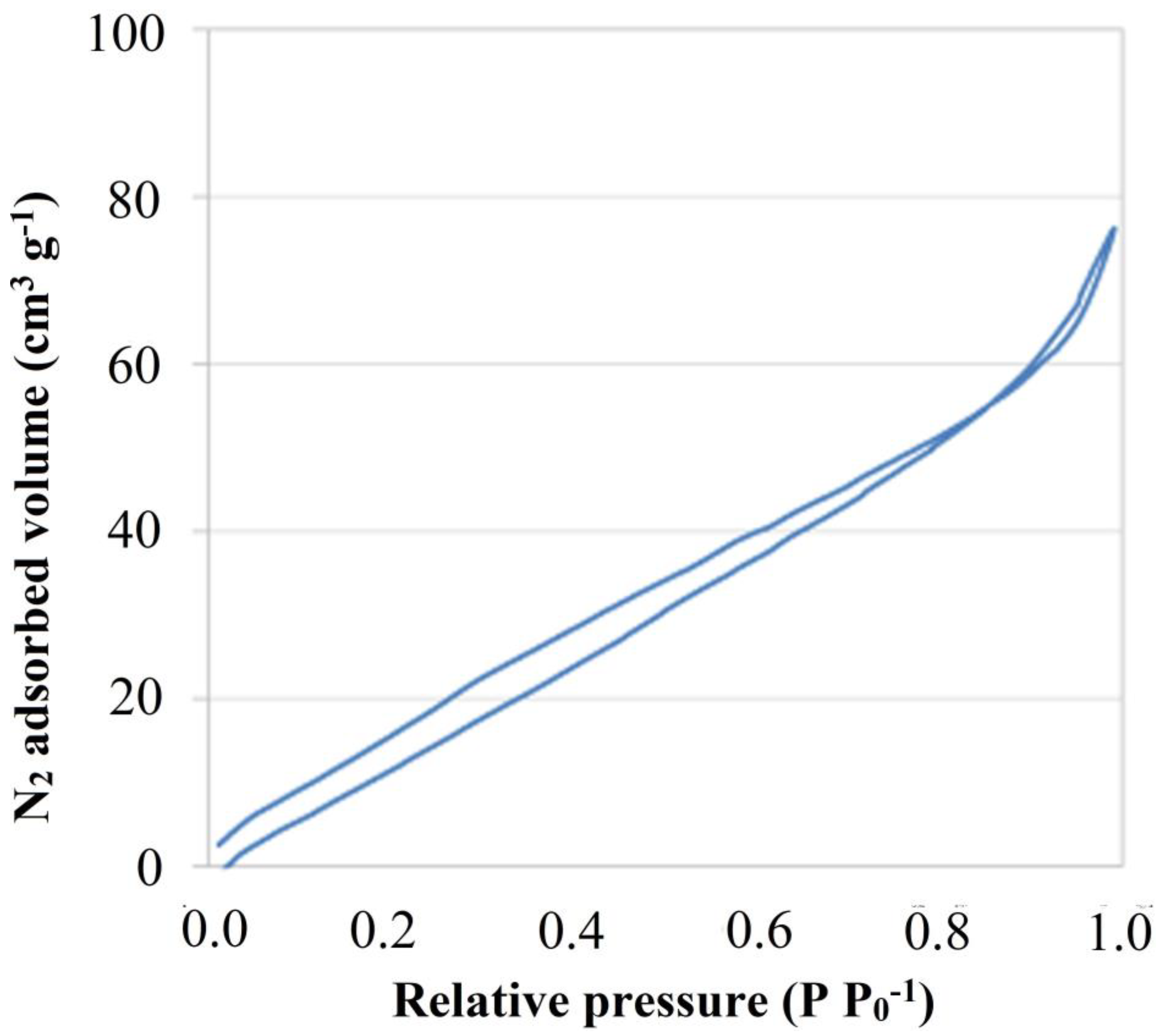
2.3. Studies on Physical Properties
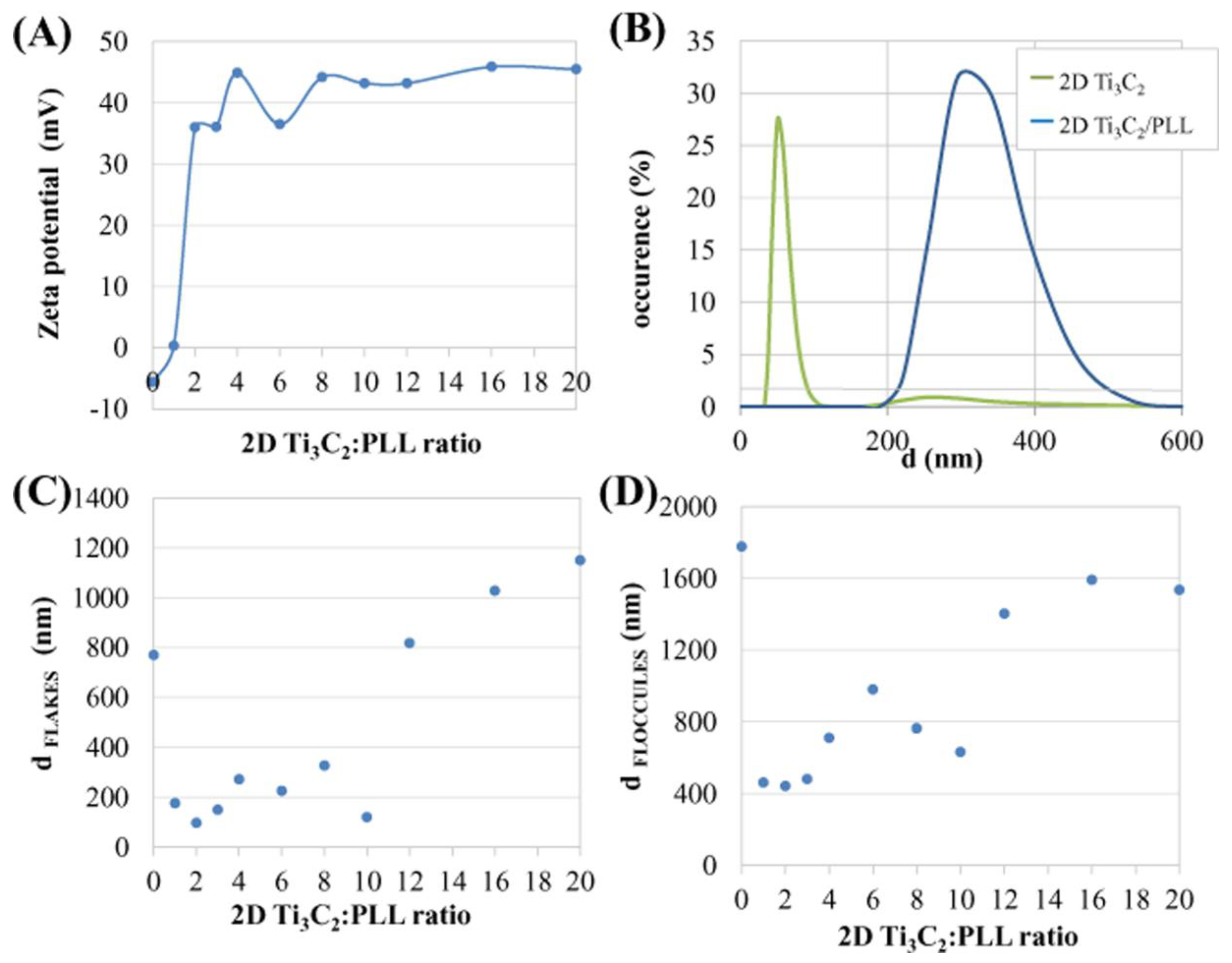
2.4. Studies on Colloidal Properties and PLL Adsorption
2.5. FTIR Measurements
2.6. Antibacterial Properties Tests
2.7. Analysis of Cytotoxicity In Vitro
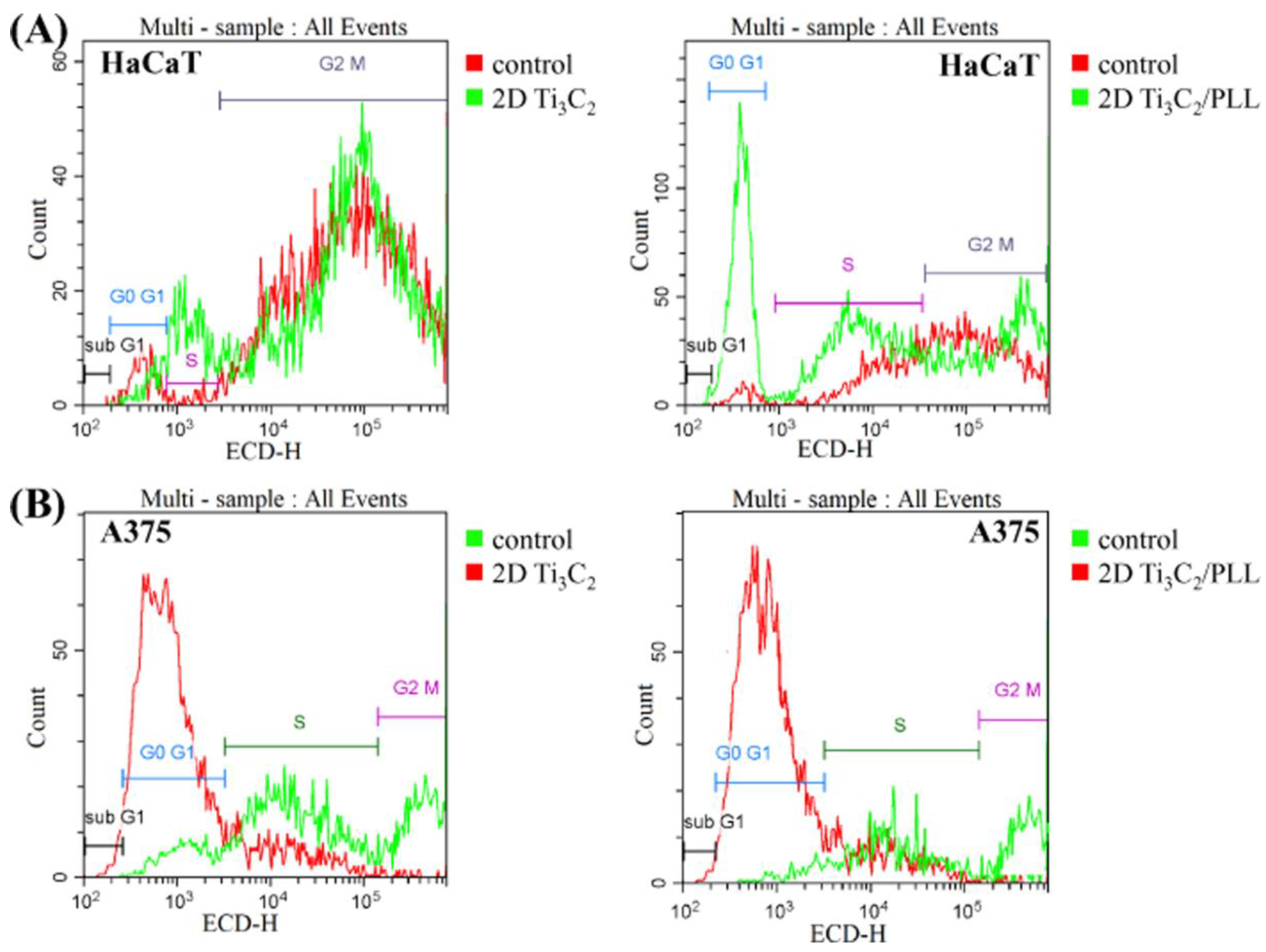
2.8. Cell Cycle Analysis
2.9. Statistical Manipulation
3. Results
3.1. Materials Characterization
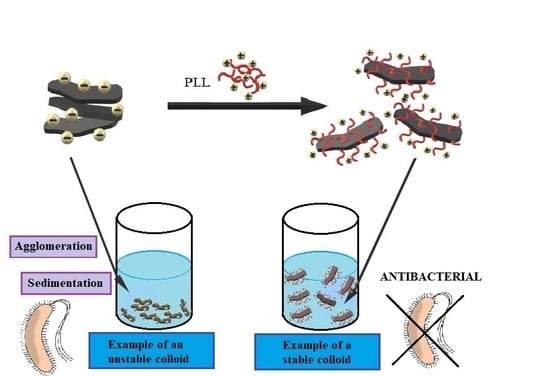
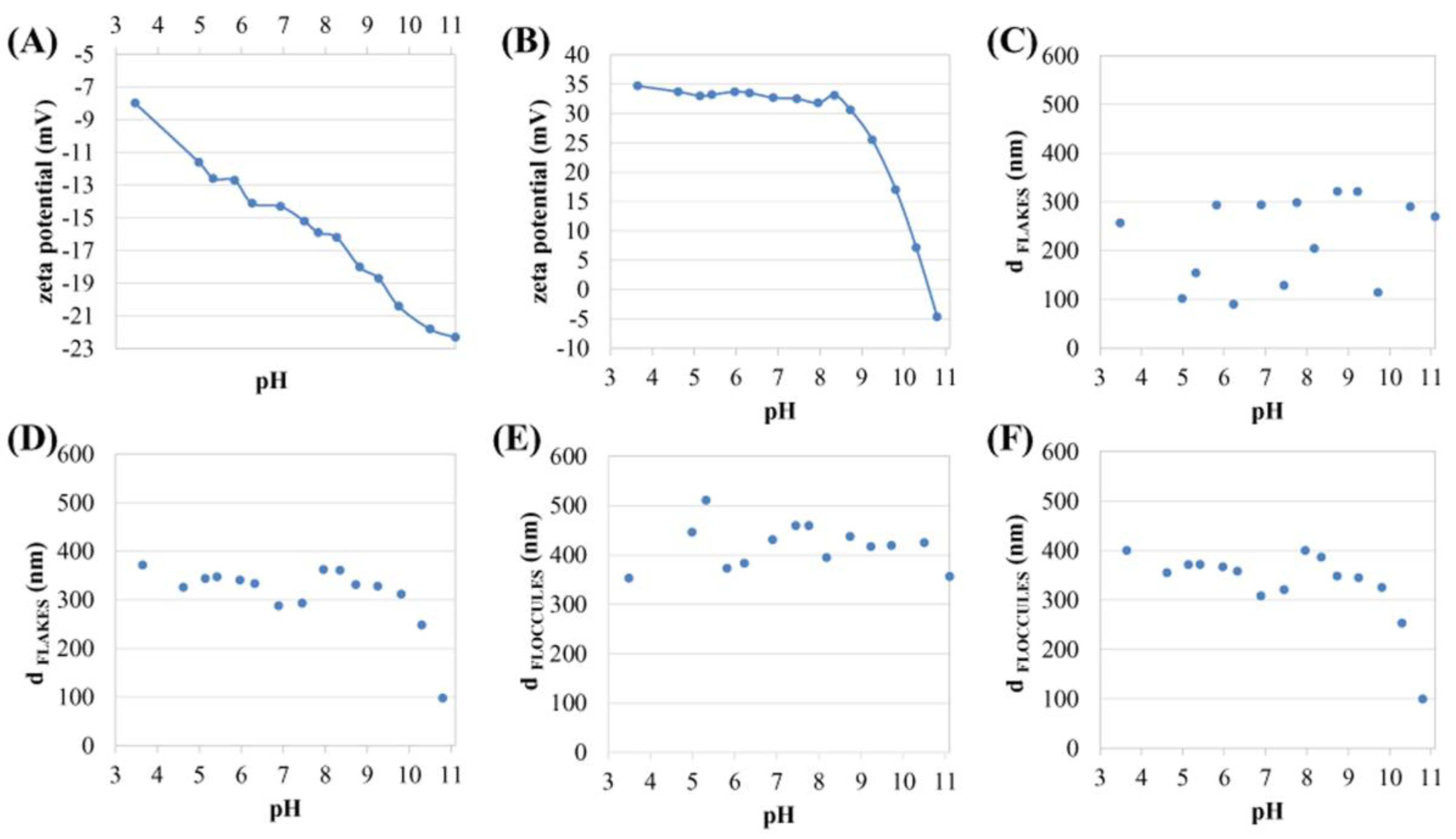
3.2. Poly L-Lysine Adsorption Studies and Colloidal Stabilities
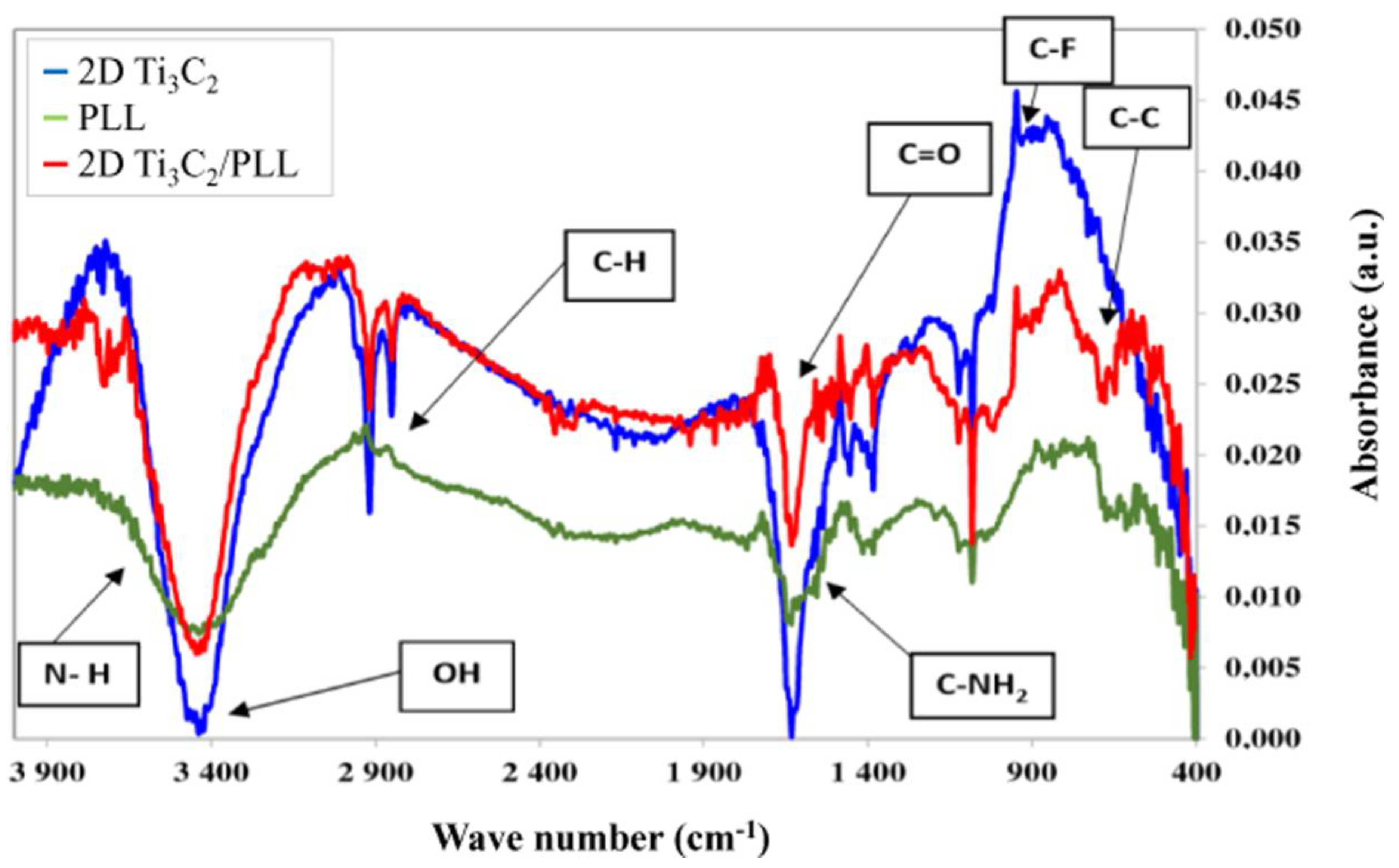
3.3. FTIR Results
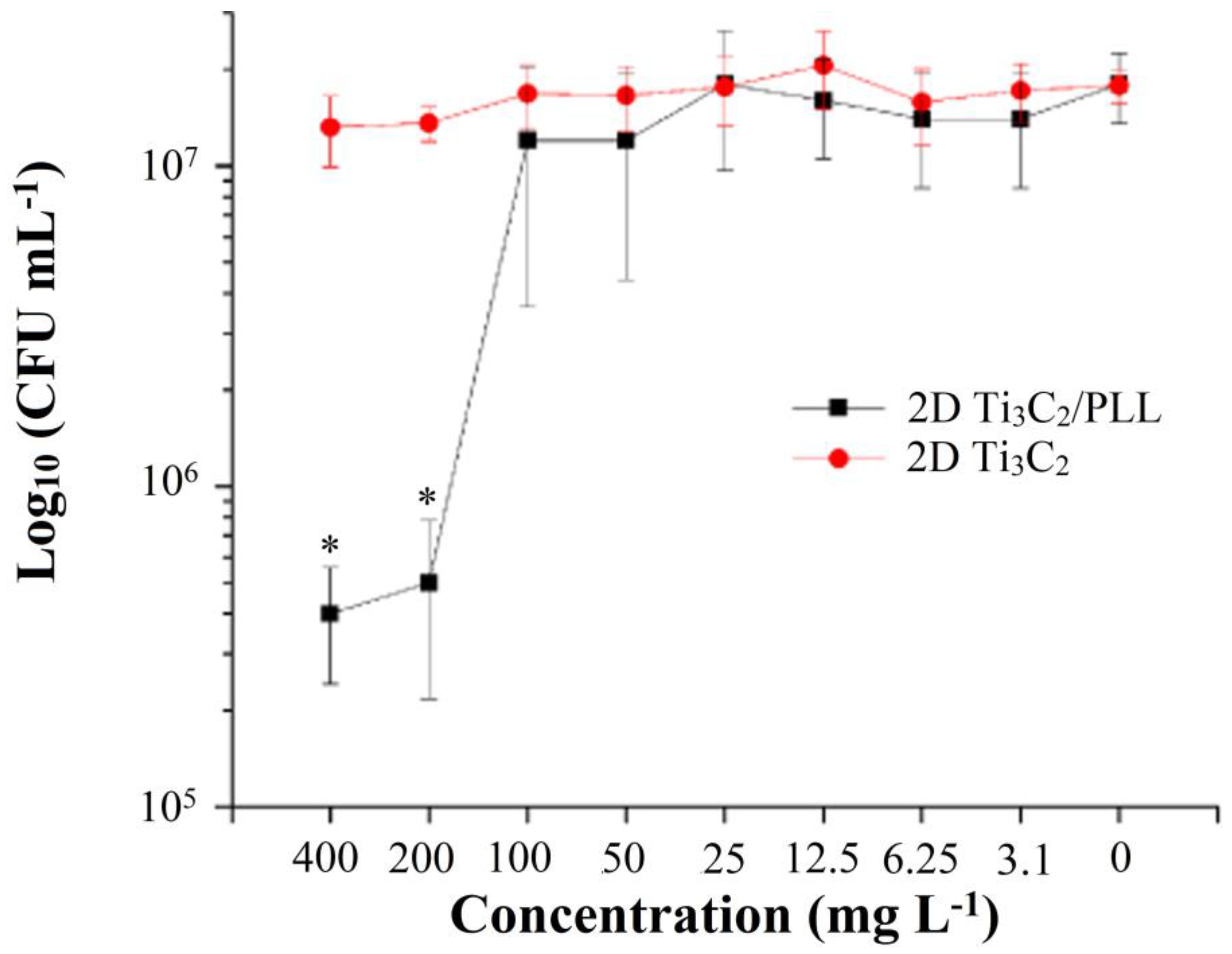
3.4. Analysis of Antibacterial Properties
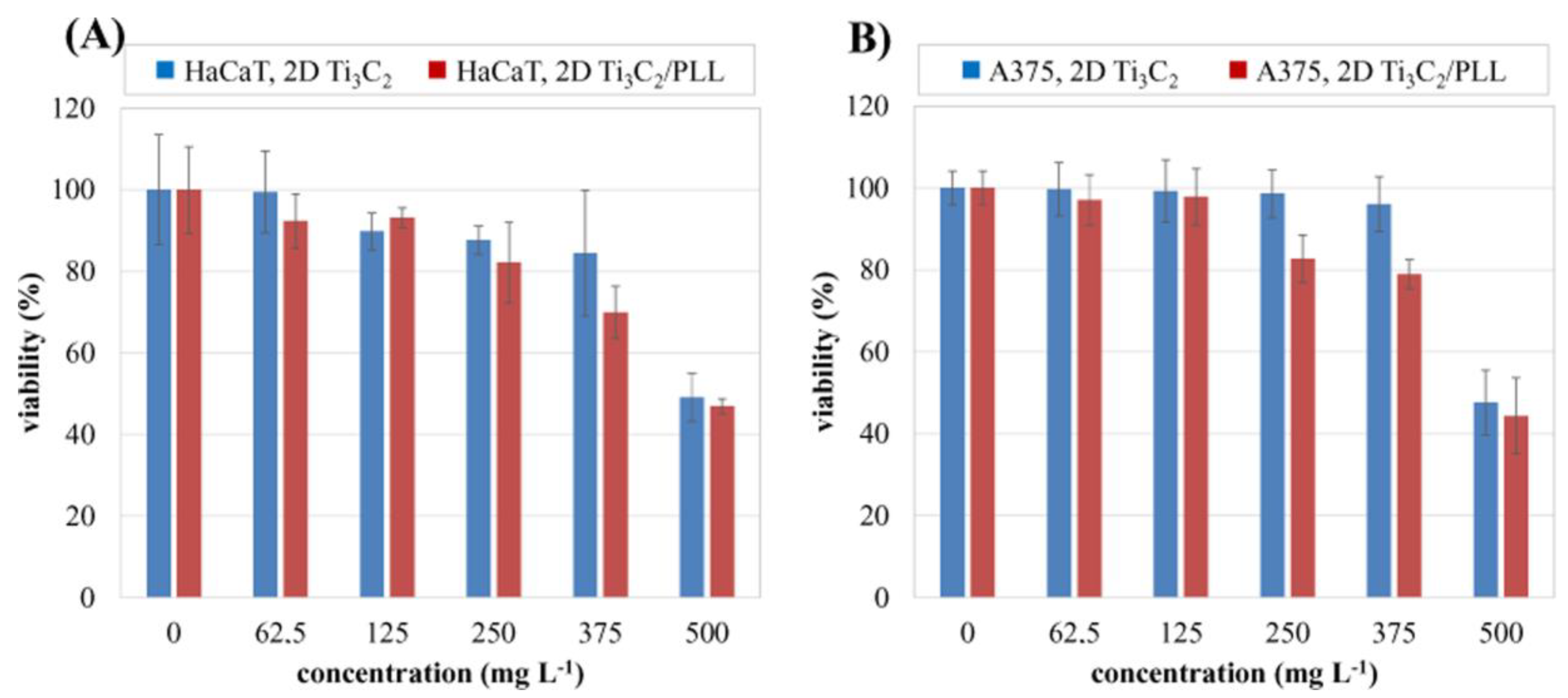
3.5. Analysis of Potential Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Szydłowska, B.M.; Harvey, A.; Yuan, S.; Vega-Mayoral, V.; Davies, B.R.; Zhao, P.; Hanlon, D.; Santos, E.J.G.; Katsnelson, M.I.; et al. Production of Highly Monolayer Enriched Dispersions of Liquid-Exfoliated Nanosheets by Liquid Cascade Centrifugation. ACS Nano 2016, 10, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Anasori, B.; Gogotsi, Y. 2D Transition Metal Carbides and Nitrides (MXenes); Springer International Publishing AG: Cham, Switzerland, 2019; pp. 3–12. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 1–17. [Google Scholar] [CrossRef]
- Mayerberger, E.A.; Urbanek, O.; McDaniel, R.M.; Street, R.M.; Barsoum, M.W.; Schauer, C.L. Preparation and characterization of polymer-Ti3C2Tx (MXene) composite nanofibers produced via electrospinning. J. Appl. Polym. Sci. 2017, 134, 45295. [Google Scholar] [CrossRef]
- Persson, I.; Halim, J.; Lind, H.; Hansen, T.W.; Wagner, J.B.; Naslund, L.A.; Darakchieva, V.; Palisaitis, J.; Rosen, J.; Persson, P.O.A. 2D Transition Metal Carbides (MXenes) for Carbon Capture. Adv. Mater. 2019, 31, 1805472. [Google Scholar] [CrossRef] [PubMed]
- Szuplewska, A.; Kulpińska, D.; Dybko, A.; Chudy, M.; Jastrzębska, A.M.; Olszyna, A.; Brzózka, Z. Future Applications of MXenes in Biotechnology, Nanomedicine and Sensors. Trends Biotechnol. 2019, 38, 264–279. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Karwowska, E.; Wojciechowski, T.; Ziemkowska, W.; Rozmysłowska, A.; Chlubny, L.; Olszyna, A. The Atomic Structure of Ti2C and Ti3C2 MXenes is Responsible for Their Antibacterial Activity Toward, E. coli Bacteria. J. Mater. Eng. Perform. 2018, 28, 1272. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Shi, J. Insights into 2D MXenes for Versatile Biomedical Applications: Current Advances and Challenges Ahead. Adv. Sci. 2018, 5, 1800518. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, A.M.; Szuplewska, A.; Wojciechowski, T.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Rozmysłowska, A.; Olszyna, A. In vitro studies on cytotoxicity of delaminated Ti3C2 MXene. J. Hazard. Mater. 2017, 339, 1–8. [Google Scholar] [CrossRef]
- Zhao, X.; Vashisth, A.; Prehn, E.; Sun, W.; Shah, S.A.; Habib, T.; Chen, Y.; Tan, Z.; Lutkenhaus, J.L.; Rodovic, M.; et al. Antioxidants Unlock Shelf-Stable Ti3C2Tx (MXene) Nanosheet Dispersions. Matter 2019, 1, 513–526. [Google Scholar] [CrossRef]
- Natu, V.; Hart, J.L.; Sokol, M.; Chiang, H.; Taheri, M.L.; Barsoum, M.W. Capping of 2D-MXene Sheets with Polyanionic Salts to Mitigate Oxidation in Aqueous Colloidal Suspensions. Angew. Chem. Int. Ed. 2019, 58, 12655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Ansori, B.; Long, E.; Park, S.-H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Wojciechowski, T.; Ziemkowska, W.; Chlubny, L.; Olszyna, A.; Jastrzębska, A.M. Surface interactions between 2D Ti3C2/Ti2C MXenes and lysozyme. Appl. Surf. Sci. 2019, 473, 409. [Google Scholar] [CrossRef]
- Szuplewska, A.; Kalinowska, D.; Dybko, A.; Jastrzębska, A.M.; Wojciechowski, T.; Rozmysłowska, A.; Chudy, M.; Grabowska-Jadach, I. 2D Ti2C (MXene) as a novel, highly efficient agent for photothermal therapy. Mater. Sci. Eng. C 2019, 98, 874. [Google Scholar] [CrossRef]
- Shima, S.; Matsuoka, H.; Iwamoto, T.; Sakai, H. Antimicrobial action of ε-poly-L-lysine. J. Antibiotics. 1984, 37, 1449–1455. [Google Scholar] [CrossRef]
- Yang, D.H.; Kim, H.J.; Park, K.; Kim, J.K.; Chun, H.J. Preparation of poly-L-lysine-based nanoparticles with pH-sensitive release of curcumin for targeted imaging and therapy of liver cancer in vitro and in vivo. Drug Deliv. 2018, 25, 950–960. [Google Scholar] [CrossRef]
- Rozmysłowska, A.; Wojciechowski, T.; Ziemkowska, W.; Chlubny, L.; Olszyna, A.; Poźniak, S.; Tomkiewicz, K.; Jastrzębska, A.M. Colloidal Properties and Stability of 2D Ti3C2 and Ti2C MXenes in Water. Int. J. Electrochem. Sci. 2018, 13, 10837–10847. [Google Scholar] [CrossRef]
- Cui, G.; Wei, X.; Olevsky, E.A.; German, R.M.; Chen, J. The manufacturing of high porosity iron with an ultra-fine microstructure via free pressureless spark plasma sintering. Materials 2016, 9, 495. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate methods for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Lao, J.; Lv, R.; Gao, J.; Wang, A.; Wu, J.; Luo, J. Aqueous Stable Ti3C2 MXene Membrane with Fast and Photo-Switchable Nanofluidic Transport. ACS Nano 2018, 12, 12464–12471. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.; Naslund, L.A.; Halim, J.; Barsoum, M.W.; Darakchieva, V.; Palisaitis, J.; Rosen, J.; Persson, P.O.A. On the organization and thermal behavior of functional groups on Ti3C2 MXene surfaces in vacuum. 2D Mater. 2018, 5, 015002. [Google Scholar] [CrossRef]
- Persson, P.O.A.; Rosen, J. Current state of the art on tailoring the MXene composition, structure, and surface chemistry. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100774. [Google Scholar] [CrossRef]
- Jastrzębska, A.; Szuplewska, A.; Rozmysłowska, A.; Chudy, M.; Olszyna, A.; Birowska, M.; Popielski, M.; Majewski, J.A.; Scheibe, B.; Barsoum, M.W.; et al. On tuning the cytotoxicity of Ti3C2 (MXene) flakes to cancerous and benign cells by post-delamination surface modifications. 2D Mater. 2020, 7, 025018. [Google Scholar] [CrossRef]
- Tam, S.K.; Dusseault, J.; Polizu, S.; Me´nard, M.; Halle, J.-P.; Yahia, L. Physicochemical model of alginate–poly-L-lysine microcapsules defined at the micrometric/nanometric scale using ATR-FTIR, XPS, and ToF-SIMS. Biomaterials 2005, 26, 6950–6961. [Google Scholar] [CrossRef]
- Rafieerad, A.; Yan, W.; Sequiera, G.L.; Sareen, N.; Abu-ElRub, E.; Moudgil, M.; Dhingra, S. Applicationof Ti3C2 MXene Quantum Dots for Immunomodulation and Regenerative Medicine. Adv. Healthc. Mater. 2019, 8, 1900569. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Szuplewska, A.; Wojciechowski, T.; Poźniak, S.; Mitrzak, J.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Olszyna, A.; Jastrzębska, A.M. A Simple, Low-Cost Green Method for Controlling the Cytotoxicity of Mxenes. Mater. Sci. Eng. C 2020, 111, 110790. [Google Scholar] [CrossRef]
- Efremova, L.; Vasilchenko, A.; Rakov, E.; Deryabin, D. Toxicity of Graphene Shells, Graphene Oxide and Graphene Oxide Paper Evaluated with Escherichia Coli Biotest. Biomed Res. Int. 2015, 2015, 869361. [Google Scholar] [CrossRef]
- Deryabin, D.G.; Efremova, L.V.; Vasilchenko, A.S.; Saidakova, E.V.; Sizova, E.A.; Troshin, P.A.; Zhilenkov, A.V.; Khakina, E.A. A zeta potential value determines the aggregate’s size of penta-substituted [60]fullerene derivatives in aqueous suspensionwhereas positive charge is required for toxicity against bacterial cells. J. Nanobiotechnol. 2015, 13, 13–50. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Vad, B.S.; Stenvang, M.; Otzen, D.E.; Meyer, R.L. The antimicrobial mechanism of action of epsilon-poly-l-lysine. Appl. Environ. Microbiol. 2014, 80, 7758–7770. [Google Scholar] [CrossRef] [PubMed]
- Mashino, T.; Usui, N.; Okuda, K.; Hirota, T.; Mochizuki, M. Respiratory chain inhibition by fullerene derivatives: Hydrogen peroxide production caused by fullerene derivatives and a respiratory chain system. Bioorg. Med. Chem. 2013, 11, 1433–1438. [Google Scholar] [CrossRef]
- Rojas-Andrade, M.D.; Chata, G.; Rouholiman, D.; Liu, J.; Saltikov, C.; Chen, S. Antibacterial mechanisms of graphene-based composite nanomaterials. Nanoscale 2017, 9, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Mashino, T.; Okuda, K.; Hirota, T.; Hirobe, M.; Nagano, T.; Mochizuki, M. Inhibition of E. coli growth by fullerene derivatives and inhibition mechanism. Bioorg. Med. Chem. Lett. 1999, 9, 2959–2962. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and Faster “Nano Darts” Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef]
- Shamsabadi, A.A.; Sharifian Gh., M.; Anasori, B.; Soroush, M. Antimicrobial Mode-of-Action of Colloidal Ti3C2Tx MXene Nanosheets. J. Am. Chem. Soc. 2018, 6, 16586–16596. [Google Scholar] [CrossRef]
- Titov, A.V.; Kral, P.; Pearson, R. Sandwiched graphene--membrane superstructures. ACS Nano 2010, 4, 229–234. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid. Interface. Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef]
- Zabadaj, M.; Szuplewska, A.; Kalinowska, D.; Chudy, M.; Ciosek-Skibińska, P. Studying pharmacodynamic effects in cell cultures by chemical fingerprinting—SIA electronic tongue versus 2D fluorescence soft sensor. Sens. Actuators B Chem. 2018, 272, 264–273. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozmysłowska-Wojciechowska, A.; Mitrzak, J.; Szuplewska, A.; Chudy, M.; Woźniak, J.; Petrus, M.; Wojciechowski, T.; Vasilchenko, A.S.; Jastrzębska, A.M. Engineering of 2D Ti3C2 MXene Surface Charge and its Influence on Biological Properties. Materials 2020, 13, 2347. https://doi.org/10.3390/ma13102347
Rozmysłowska-Wojciechowska A, Mitrzak J, Szuplewska A, Chudy M, Woźniak J, Petrus M, Wojciechowski T, Vasilchenko AS, Jastrzębska AM. Engineering of 2D Ti3C2 MXene Surface Charge and its Influence on Biological Properties. Materials. 2020; 13(10):2347. https://doi.org/10.3390/ma13102347
Chicago/Turabian StyleRozmysłowska-Wojciechowska, Anita, Joanna Mitrzak, Aleksandra Szuplewska, Michał Chudy, Jarosław Woźniak, Mateusz Petrus, Tomasz Wojciechowski, Alexey S. Vasilchenko, and Agnieszka M. Jastrzębska. 2020. "Engineering of 2D Ti3C2 MXene Surface Charge and its Influence on Biological Properties" Materials 13, no. 10: 2347. https://doi.org/10.3390/ma13102347
APA StyleRozmysłowska-Wojciechowska, A., Mitrzak, J., Szuplewska, A., Chudy, M., Woźniak, J., Petrus, M., Wojciechowski, T., Vasilchenko, A. S., & Jastrzębska, A. M. (2020). Engineering of 2D Ti3C2 MXene Surface Charge and its Influence on Biological Properties. Materials, 13(10), 2347. https://doi.org/10.3390/ma13102347