Hydrogen Permeation in X65 Steel under Cyclic Loading
Abstract
1. Introduction
2. Experimental Section
- A first series of tests was performed by applying the cyclic load before the activation of the charging cathodic current, and then it was maintained simultaneously to hydrogen permeation transient in order to evaluate the effect of cyclic loading on the apparent hydrogen permeation coefficient (Table 1).
- A second series of tests was conducted by applying the cyclic load after the achievement of the steady state of hydrogen permeation to evidence its effect on the hydrogen flux at equilibrium. These tests were also performed in the absence of a hydrogen charge to evaluate the effect of the cyclic load on the passivity current density (Table 2).
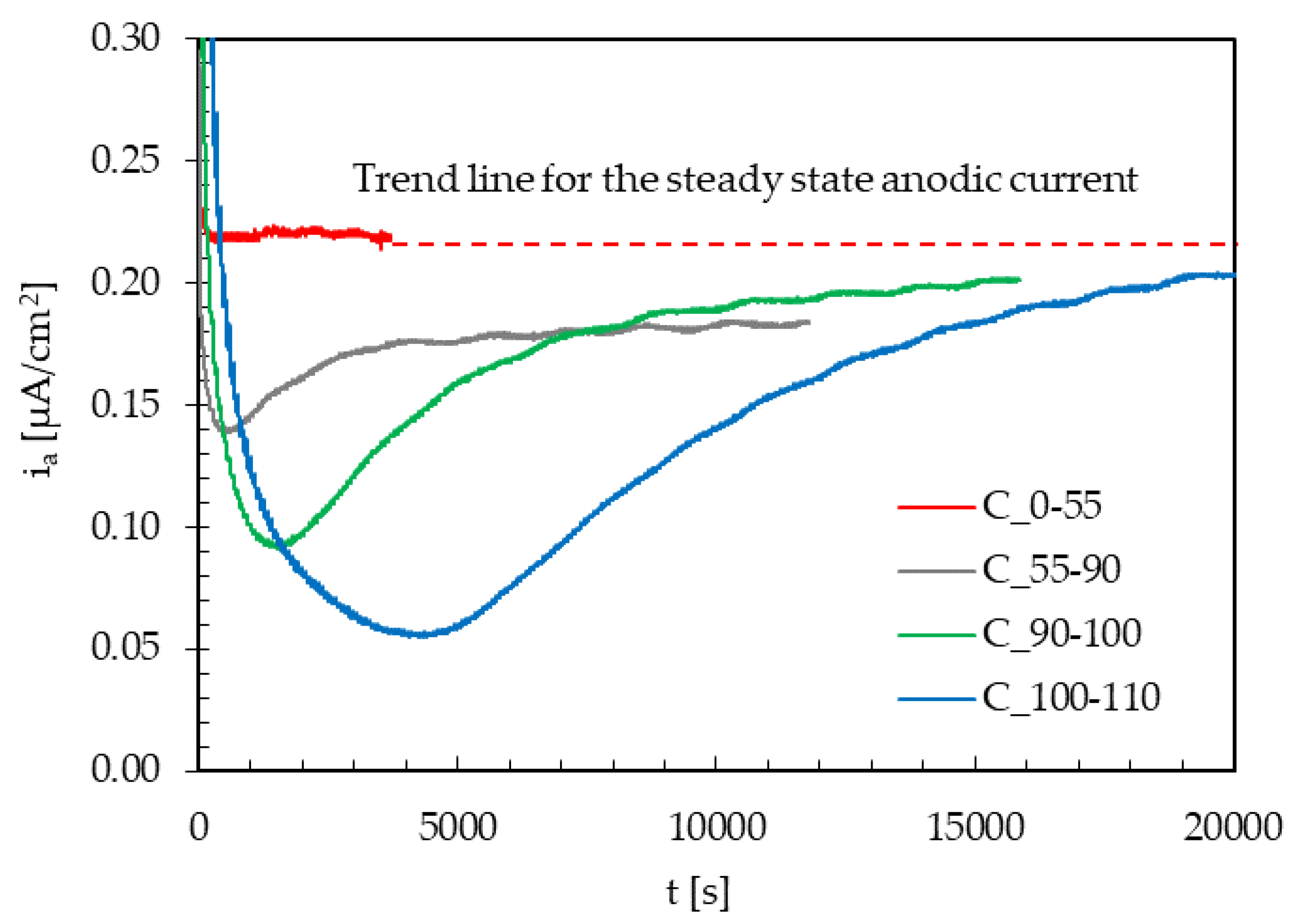
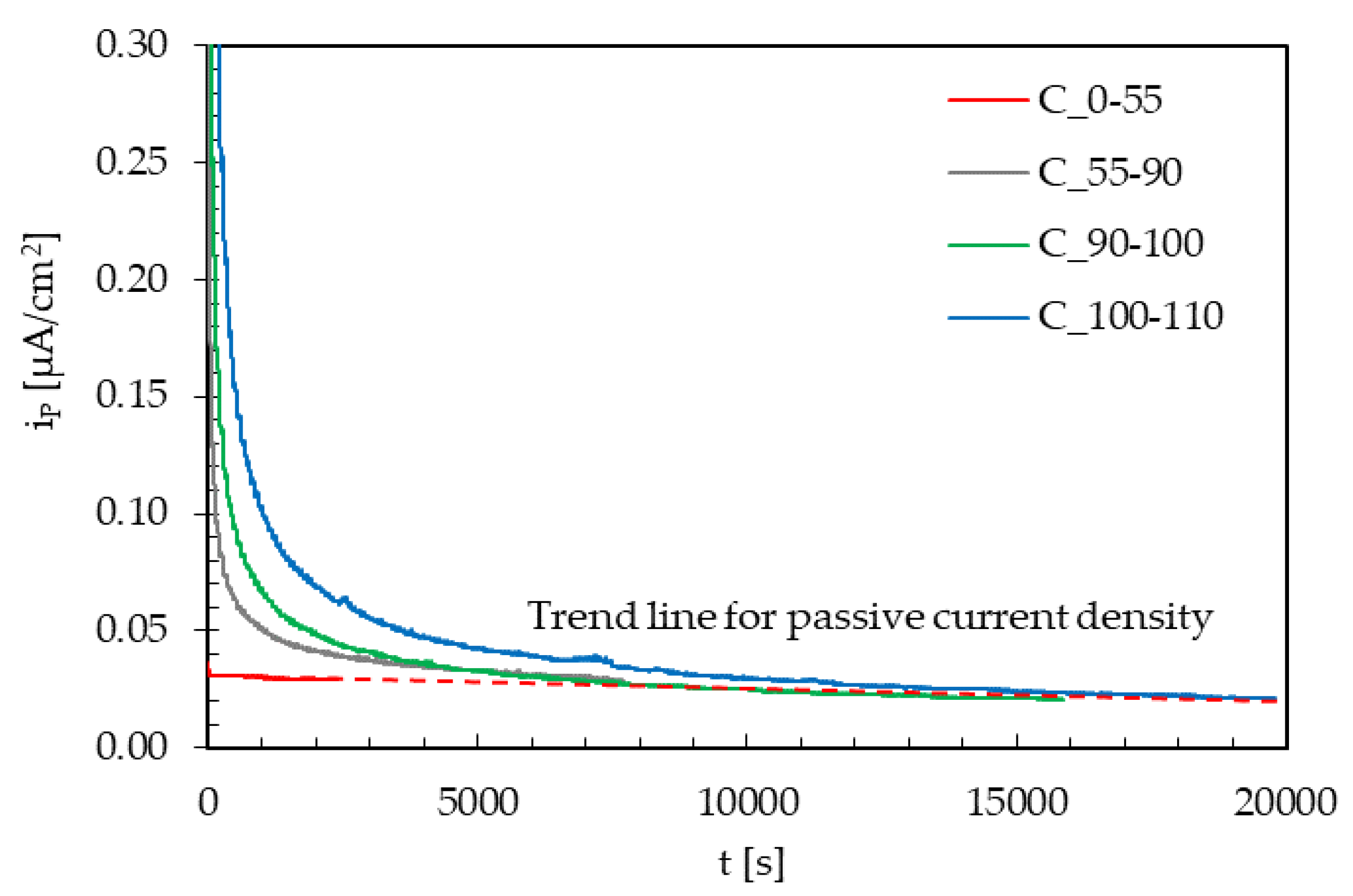
- A third series of tests was performed in order to evaluate the transient of the hydrogen steady state flux and of the passive current, increasing on the specimen the mean value of the applied load from 0% to 110% TYS, maintaining constant the amplitude and the frequency (Table 3).
- Some tests were carried out on a specimen subject to the cyclic loading for the first time from 0% to 55% TYS and from 55% to 90% TYS, then unloaded and loaded again from 0% to 90% TYS.
3. Results and Discussion
4. Conclusions
- The apparent diffusion coefficient (Dapp) ranges between 6.2 × 10−11 and 8.9 × 10−11 m2/s, with an average value of 7.5 × 10−11 m2/s. Under cyclic loading conditions, the application of a tensile stress up to yield determines a slight decrease in Dapp. When exceeding such a value—i.e., at 110% TYS—Dapp decreases to values around 1.4 × 10−11 m2/s due to the creation of a large quantity of new reversible and irreversible trapping sites.
- An applied cyclic load causes a very small decrease in the hydrogen permeation current at the steady state, mainly hindered by the increase in the passive current. This is slight as the cycle frequency decreases, and it significantly increases as the load amplitude increases.
- Increase in the maximum load causes a temporary decrease in the permeation flux, which becomes larger and larger as the applied load increases. Once the new traps generated are saturated with diffusible hydrogen, the steady state current returns to the value prior to load increase.
- No transient modifications in the permeation flux are detected after subsequent load variations on the specimens already subject to the same load variation (already strain-hardened), probably because of the local strain-hardening of the material and no creation of new trapping sites.
Author Contributions
Funding
Conflicts of Interest
References
- Eliassen, S. New concept for cathodic protection of offshore pipelines to reduce hydrogen induced stress cracking (HISC) in high strength 13%Cr stainless steels. Corros. Eng. Sci. Technol. 2004, 39, 31–37. [Google Scholar] [CrossRef]
- Orazem, M.E.; Esteban, J.M.; Kennelley, K.J.; Degerstedt, R.M. Mathematical Models for Cathodic Protection of an Underground Pipeline with Coating Holidays: Part 1—Theoretical Development. Corrosion 1997, 53, 264–272. [Google Scholar] [CrossRef]
- Kang, D.H.; Lee, J.K.; Kim, T.W. Corrosion fatigue crack propagation of high-strength steel HSB800 in a seawater environment. Procedia Eng. 2011, 10, 1170–1175. [Google Scholar] [CrossRef]
- Cheng, A.; Chen, N.Z. An extended engineering critical assessment for corrosion fatigue of subsea pipeline steels. Eng. Fail. Anal. 2018, 84, 262–275. [Google Scholar] [CrossRef]
- Knop, M.; Heath, J.; Sterjovski, Z.; Lynch, S.P. Effects of cycle frequency on corrosion-fatigue crack growth in cathodically protected high-strength steels. Procedia Eng. 2010, 2, 1243–1252. [Google Scholar] [CrossRef]
- Cabrini, M.; Pastore, T. Hydrogen diffusion and EAC of pipeline steels under cathodic protection. In Proceedings of the Fracture of Nano and Engineering Materials and Structures—Proceedings of the 16th European Conference of Fracture, Alexandroupolis, Greece, 3–7 July 2006. [Google Scholar]
- Cabrini, M.; Lorenzi, S.; Pellegrini, S.; Pastore, T. Environmentally assisted cracking and hydrogen diffusion in traditional and high-strength pipeline steels. Corros. Rev. 2015, 33, 529–545. [Google Scholar] [CrossRef]
- Lorenzi, S.; Pastore, T.; Bellezze, T.; Fratesi, R. Cathodic protection modelling of a propeller shaft. Corros. Sci. 2016, 108, 36–46. [Google Scholar] [CrossRef]
- Shipilov, S.A.; Le May, I. Structural integrity of aging buried pipelines having cathodic protection. Eng. Fail. Anal. 2006, 13, 1159–1176. [Google Scholar] [CrossRef]
- Shipilov, S. Critical Assessment of the Role of Cathodic Protection in Pipeline Integrity and Reliability. In Engineering Structural Integrity Assessment: Needs and Provision; EMAS Publishing: Sheffield, UK, 2002; pp. 155–162. ISBN 0-9543454-3-6. [Google Scholar]
- Energy Institute (Great Britain). Guidance for Corrosion Management in Oil and Gas Production and Processing; Energy Institute: London, UK, 2019; ISBN 9781787250635. [Google Scholar]
- DNV GL. Offshore Standard DNV GL as Design of Offshore Steel Structures, General-LRFD Method; DNV: Oslo, Norwey, 2015. [Google Scholar]
- ISO 19902: 2007. Petroleum and Natural Gas Industries—Fixed Steel Offshore Structures; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Hardie, D.; Charles, E.A.; Lopez, A.H. Hydrogen embrittlement of high strength pipeline steels. Corros. Sci. 2006, 48, 4378–4385. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Li, T.; Zhao, Y.; Deng, Q.; Wang, Y.; Jiang, W. Comparison of hydrogen embrittlement susceptibility of three cathodic protected subsea pipeline steels from a point of view of hydrogen permeation. Corros. Sci. 2018, 131, 104–115. [Google Scholar] [CrossRef]
- Świerczyńska, A.; Łabanowski, J.; Michalska, J.; Fydrych, D. Corrosion behavior of hydrogen charged super duplex stainless steel welded joints. Mater. Corros. 2017, 68, 1037–1045. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Sijacki Zeravcic, V.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Kyriakopoulou, H.; Karmiris-Obratański, P.; Tazedakis, A.; Daniolos, N.; Dourdounis, E.; Manolakos, D.; Pantelis, D. Investigation of Hydrogen Embrittlement Susceptibility and Fracture Toughness Drop after in situ Hydrogen Cathodic Charging for an X65 Pipeline Steel. Micromachines 2020, 11, 430. [Google Scholar] [CrossRef]
- Lynch, S. Mechanisms of hydrogen assisted cracking—A review. In Hydrogen Effects on Material Behaviour and Corrosion Deformation Interactions; TMS/AIME: Pittsburgh, PA, USA, 2003; pp. 449–466. [Google Scholar]
- Xing, X.; Zhou, J.; Zhang, S.; Zhang, H.; Li, Z.; Li, Z. Quantification of Temperature Dependence of Hydrogen Embrittlement in Pipeline Steel. Materials 2019, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Troiano, A.R. The Role of Hydrogen and Other Interstitials in the Mechanical Behavior of Metals: (1959 Edward De Mille Campbell Memorial Lecture). Metallogr. Microstruct. Anal. 2016, 5, 557–569. [Google Scholar] [CrossRef]
- Oriani, R.A. A mechanistic theory of hydrogen embrittlement of steels. Berichte Bunsengesellschaft Phys. Chem. 1972, 76, 848–857. [Google Scholar]
- Du, Y.A.; Ismer, L.; Rogal, J.; Hickel, T.; Neugebauer, J.; Drautz, R. First-principles study on the interaction of H interstitials with grain boundaries in α- and γ-Fe. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 84, 144121. [Google Scholar] [CrossRef]
- Zhou, X.; Song, J. Effects of alloying elements on vacancies and vacancy-hydrogen clusters at coherent twin boundaries in nickel alloys. Acta Mater. 2018, 148, 9–17. [Google Scholar] [CrossRef]
- Robertson, I.M.; Birnbaum, H.K.; Sofronis, P. Chapter 91 Hydrogen Effects on Plasticity. Dislocat. Solids 2009, 15, 249–293. [Google Scholar]
- Song, J.; Curtin, W.A. Mechanisms of hydrogen-enhanced localized plasticity: An atomistic study using α-Fe as a model system. Acta Mater. 2014, 68, 61–69. [Google Scholar] [CrossRef]
- Sofronis, P.; Liang, Y.; Aravas, N. Hydrogen induced shear localization of the plastic flow in metals and alloys. Eur. J. Mech. A Solids 2001, 20, 857–872. [Google Scholar] [CrossRef]
- Ayas, C.; Deshpande, V.S.; Fleck, N.A. A fracture criterion for the notch strength of high strength steels in the presence of hydrogen. J. Mech. Phys. Solids 2014, 63, 80–93. [Google Scholar] [CrossRef]
- Leyson, G.P.M.; Grabowski, B.; Neugebauer, J. Multiscale description of dislocation induced nano-hydrides. Acta Mater. 2015, 89, 50–59. [Google Scholar] [CrossRef]
- Zakroczymski, T.; Szklarska-śmiałowska, Z.; Śmiałowski, M. Effect of Promoters on the Permeation of electrolytic hydrogen through steel. Mater. Corros. Korrosion 1976, 27, 625–630. [Google Scholar] [CrossRef]
- Nagumo, M. Hydrogen related failure of steels—A new aspect. Mater. Sci. Technol. 2004, 20, 940–950. [Google Scholar] [CrossRef]
- Srinivasan, R.; Neeraj, T. Hydrogen embrittlement of ferritic steels: Deformation and failure mechanisms and challenges in the oil and gas industry. JOM 2014, 66, 1377–1382. [Google Scholar] [CrossRef]
- Carter, C.; Hyatt, M. Review of Stress Corrosion Cracking in Low Alloy Steels with Yield Strength Below 150 ksi. In Proceedings of the Stress Corrosion Cracking and Hydrogen Embrittlement of Iron Base Alloys—International Corrosion Conference Series, Unieux, Firminy, France, 12–16 June 1973; pp. 524–600. [Google Scholar]
- Punter, A.; Fikkers, A.T.; Vanstaen, G. Hydrogen-Induced Stress Corrosion Cracking on a Pipeline. Mater. Perform. 1992, 31, 24–28. [Google Scholar]
- Cabrini, M.; Pistone, V.; Sinigaglia, E.; Tarenzi, M. Unique HSC scenario leads to gas line failure. Oil Gas J. 2000, 98, 61–65. [Google Scholar]
- Zielinski, A.; Domzalicki, P. Hydrogen degradation of high-strength low-alloyed steels. J. Mater. Process. Technol. 2003, 133, 230–235. [Google Scholar] [CrossRef]
- Bosch, C.; Bayle, B.; Magnin, T.; Longaygue, X. Proposal for a Critical Test to Classify the SCC Resistance of Materials. In Hydrogen Effects on Materials Behavior and Corrosion Deformation Interactions; Moody, N.R., Thompson, A.W., Ricker, R.E., Was, G.S., Jones, R.H., Eds.; TMS: Warrendale, PA, USA, 2003; pp. 587–596. [Google Scholar]
- Bai, L.; Gao, L.; Jiang, K. Corrosion Crack Nucleation Mechanism in the Welded Structures of X65 Steel in Natural Seawater. Adv. Mater. Sci. Eng. 2018, 2018, 8973150. [Google Scholar] [CrossRef]
- Soudani, M.; Hadj Meliani, M.; El-Miloudi, K.; Azari, Z.; Sorour, A.A.; Merah, N.; Pluvinage, G. Reduction of hydrogen embrittlement of API 5l X65 steel pipe using a green inhibitor. Int. J. Hydrogen Energy 2018, 43, 11150–11159. [Google Scholar] [CrossRef]
- Latifi, V.A.; Miresmaeili, R.; Abdollah-Zadeh, A. The mutual effects of hydrogen and microstructure on hardness and impact energy of SMA welds in X65 steel. Mater. Sci. Eng. A 2017, 679, 87–94. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, W.; Guo, W.; Wang, Y. Susceptibility to Hydrogen Embrittlement of X65 Steel Under Cathodic Protection in Artificial Sea Water. J. Chin. Soc. Corros. Prot. 2014, 34, 315–320. [Google Scholar]
- Ronevich, J.A.; Somerday, B.P.; San Marchi, C.W. Effects of microstructure banding on hydrogen assisted fatigue crack growth in X65 pipeline steels. Int. J. Fatigue 2016, 82, 497–504. [Google Scholar] [CrossRef]
- Wan, H.; Du, C.; Liu, Z.; Song, D.; Li, X. The effect of hydrogen on stress corrosion behavior of X65 steel welded joint in simulated deep sea environment. Ocean Eng. 2016, 114, 216–223. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Z.; Lu, X.; Wang, L. Effect of Temperature on Corrosion and Cathodic Protection of X65 Pipeline Steel in 3.5% NaCl Solution. Int. J. Electrochem. Sci. 2019, 14, 150–160. [Google Scholar] [CrossRef]
- Fallahmohammadi, E.; Ballinger, R.; Maruno, Y.; Fumagalli, G.; Re, G.; Lazzari, L. Effect of Plastic Deformation on Hydrogen Diffusion of X65 Pipeline Steel. In Proceedings of the NACE—International Corrosion Conference Series, San Antonio, TX, USA, 9–13 March 2014. [Google Scholar]
- Singh, V.; Singh, R.; Arora, K.S.; Mahajan, D.K. Hydrogen induced blister cracking and mechanical failure in X65 pipeline steels. Int. J. Hydrogen Energy 2019, 44, 22039–22049. [Google Scholar] [CrossRef]
- Cabrini, M.; Sinigaglia, E.; Spinelli, C.; Tarenzi, M.; Testa, C.; Bolzoni, F.M. Hydrogen Embrittlement Evaluation of Micro Alloyed Steels by Means of J-Integral Curve. Materials 2019, 12, 1843. [Google Scholar] [CrossRef]
- Barth, C.F.; Troiano, A.R. Cathodic protection and hydrogen in stress corrosion cracking. Corrosion 1972, 28, 259–263. [Google Scholar] [CrossRef]
- Kawashima, A.; Hashimoto, K.; Shimodaira, S. Hydrogen electrode reaction and hydrogen embrittlement of mild steel in hydrogen sulfide solutions. Corrosion 1976, 32, 321–331. [Google Scholar] [CrossRef]
- Ohaeri, E.; Eduok, U.; Szpunar, J. Hydrogen related degradation in pipeline steel: A review. Int. J. Hydrogen Energy 2018, 43, 14584–14617. [Google Scholar] [CrossRef]
- The adsorption and diffusion of electrolytic hydrogen in palladium. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1962, 270, 90–102.
- Song, J.; Curtin, W.A. Atomic mechanism and prediction of hydrogen embrittlement in iron. Nat. Mater. 2013, 12, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Curtin, W.A. A nanoscale mechanism of hydrogen embrittlement in metals. Acta Mater. 2011, 59, 1557–1569. [Google Scholar] [CrossRef]
- Sofronis, P.; Taha, A. Micromechanical Modeling of Hydrogen Transport; A Review. In Environmentally Assisted Cracking: Predictive Methods for Risk Assessment and Evaluation of Materials, Equipment, and Structures; Kane, R.D., Ed.; ASTM International: West Conshohocken, PA, USA, 2000; pp. 70–103. ISBN 978-0-8031-5447-6. [Google Scholar]
- Oriani, R.A. The physical and metallurgical aspects of hydrogen in metals. In Proceedings of the ICCF-4 Fourth International Conference on Cold Fusion, Maui, HI, USA, 6–9 December 1993; Electric Power Research Institute: Palo Alto, CA, USA, 1993. [Google Scholar]
- Boellinghaus, T.; Hoffmeister, H.; Dangeleit, A. A scatterband for hydrogen diffusion coefficients in microalloyed and low carbon structural steels. Weld. World 1995, 35, 83–96. [Google Scholar]
- Pressouyre, G.M.; Bernstein, I.M. A quantitative analysis of hydrogen trapping. Metall. Trans. A 1978, 9, 1571–1580. [Google Scholar] [CrossRef]
- Turnbull, A.; Carroll, M.W.; Ferriss, D.H. Analysis of hydrogen diffusion and trapping in a 13% chromium martensitic stainless steel. Acta Metall. 1989, 37, 2039–2046. [Google Scholar] [CrossRef]
- Zakroczymski, T. Electrochemical determination of hydrogen in metals. J. Electroanal. Chem. 1999, 475, 82–88. [Google Scholar] [CrossRef]
- Zakroczymski, T. Adaptation of the electrochemical permeation technique for studying entry, transport and trapping of hydrogen in metals. Electrochim. Acta 2006, 51, 2261–2266. [Google Scholar] [CrossRef]
- ISO 17081:2014 Method of Measurement of Hydrogen Permeation and Determination of Hydrogen Uptake and Transport in Metals by an Electrochemical Technique; ISO: Geneva, Switzerland, 2014.
- Fallahmohammadi, E.; Bolzoni, F.M.; Fumagalli, G.; Re, G.; Lazzari, L. Diffusione e intrappolamento dell’idrogeno in acciai per condotte. La Metall. Ital. 2013, 105, 3–10. [Google Scholar]
- Fallahmohammadi, E.; Bolzoni, F.; Fumagalli, G.; Re, G.; Benassi, G.; Lazzari, L. Hydrogen diffusion into three metallurgical microstructures of a C-Mn X65 and low alloy F22 sour service steel pipelines. Int. J. Hydrogen Energy 2014, 39, 13300–13313. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pesenti Bucella, D. Hydrogen diffusion in low alloy steels under cyclic loading. Corros. Rev. 2019, 37, 459–467. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Marcassoli, P.; Pastore, T. Effetto della diffusione dell’idrogeno sui fenomeni di environmental assisted cracking di acciai per pipeline in condizioni di protezione catodica. Metall. Ital. 2008, 100, 15–22. [Google Scholar]
- Kurkela, M.; Frankel, G.S.; Latanision, R.M.; Suresh, S.; Ritchie, R.O. Influence of plastic deformation on hydrogen transport in 2 1 4 Cr-1Mo steel. Scr. Metall. 1982, 16, 455–459. [Google Scholar] [CrossRef]
- Huang, Y.; Nakajima, A.; Nishikata, A.; Tsuru, T. Effect of mechanical deformation on permeation of hydrogen in iron. ISIJ Int. 2003, 43, 548–554. [Google Scholar] [CrossRef]
- Brass, A.M.; Chêne, J. Influence of tensile straining on the permeation of hydrogen in low alloy Cr-Mo steels. Corros. Sci. 2006, 48, 481–497. [Google Scholar] [CrossRef]
- Beck, W.; Bockris, J.O.; McBreen, J.; Nanis, L. Hydrogen Permeation in Metals as a Function of Stress, Temperature and Dissolved Hydrogen Concentration. Proc. R. Soc. A Math. Phys. Eng. Sci. 1966, 290, 220–235. [Google Scholar]
- API 5L: Specification for Line Pipe; AIP: Washington, DC, USA, 2004.
- Park, G.T.; Koh, S.U.; Jung, H.G.; Kim, K.Y. Effect of microstructure on the hydrogen trapping efficiency and hydrogen induced cracking of linepipe steel. Corros. Sci. 2008, 50, 1865–1871. [Google Scholar] [CrossRef]
- Modiano, S.; Carreño, J.A.; Fugivara, C.S.; Benedetti, A.V.; Mattos, O.R. Effect of hydrogen charging on the stability of SAE 10B22 steel surface in alkaline solutions. Electrochim. Acta 2005, 51, 641–648. [Google Scholar] [CrossRef]
- Nagumo, M.; Takai, K.; Okuda, N. Nature of hydrogen trapping sites in steels induced by plastic deformation. J. Alloys Compd. 1999, 293, 310–316. [Google Scholar] [CrossRef]
- Grabke, H.J.; Riecke, E. Absorption And Diffusion Of Hydrogen In Steels. Mater. Tehnol. 2000, 34, 331–342. [Google Scholar]
- Oriani, R.A. The diffusion and trapping of hydrogen in steel. Acta Metall. 1970, 18, 147–157. [Google Scholar] [CrossRef]
- Van den Eeckhout, E.; Depover, T.; Verbeken, K. The Effect of Microstructural Characteristics on the Hydrogen Permeation Transient in Quenched and Tempered Martensitic Alloys. Metals 2018, 8, 779. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. The effect of TiC on the hydrogen induced ductility loss and trapping behavior of Fe-C-Ti alloys. Corros. Sci. 2016, 112, 308–326. [Google Scholar] [CrossRef]
- Dadfarnia, M.; Sofronis, P.; Neeraj, T. Hydrogen interaction with multiple traps: Can it be used to mitigate embrittlement? Int. J. Hydrogen Energy 2011, 36, 10141–10148. [Google Scholar] [CrossRef]
- Ha, H.; Ai, J.; Scully, J. Effects of Prior Cold Work on Hydrogen Trapping and Diffusion in API X-70 Line Pipe Steel During Electrochemical Charging. Corrosion 2014, 70, 166–184. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pesenti Bucella, D. Effetto del carico ciclico sulla diffusione di idrogeno in acciai basso-legati. La Metall. Ital. 2018, 110, 26–31. [Google Scholar]
- Bolzoni, F.; Cabrini, M.; Spinelli, C. Hydrogen diffusion and hydrogen embrittlement behaviour of two high strength pipeline steels. In Proceedings of the Eurocorr 2001, Riva del Garda, Lake Garda (BS), Italy, 30 September–4 October 2001. [Google Scholar]
- Frankel, G.S.; Latanision, R.M. Hydrogen transport during deformation in nickel: Part i. polycrystalline nickel. Metall. Trans. A Phys. Metall. Mater. Sci. 1986, 17, 861–867. [Google Scholar] [CrossRef]
- Hashimoto, M.; Latanision, R.M. Experimental study of hydrogen transport during plastic deformation in iron. Metall. Trans. A 1988, 19, 2789–2798. [Google Scholar] [CrossRef]
- Razzini, G.; Cabrini, M.; Maffi, S.; Mussati, G.; Peraldo Bicelli, L. Photoelectrochemical visualization in real-time of hydrogen distribution in plastic regions of low-carbon steel. Corros. Sci. 1999, 41, 203–208. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Marcassoli, P.; Pastore, T. Hydrogen embrittlement behavior of HSLA line pipe steel under cathodic protection. Corros. Rev. 2011, 29, 261–274. [Google Scholar] [CrossRef]
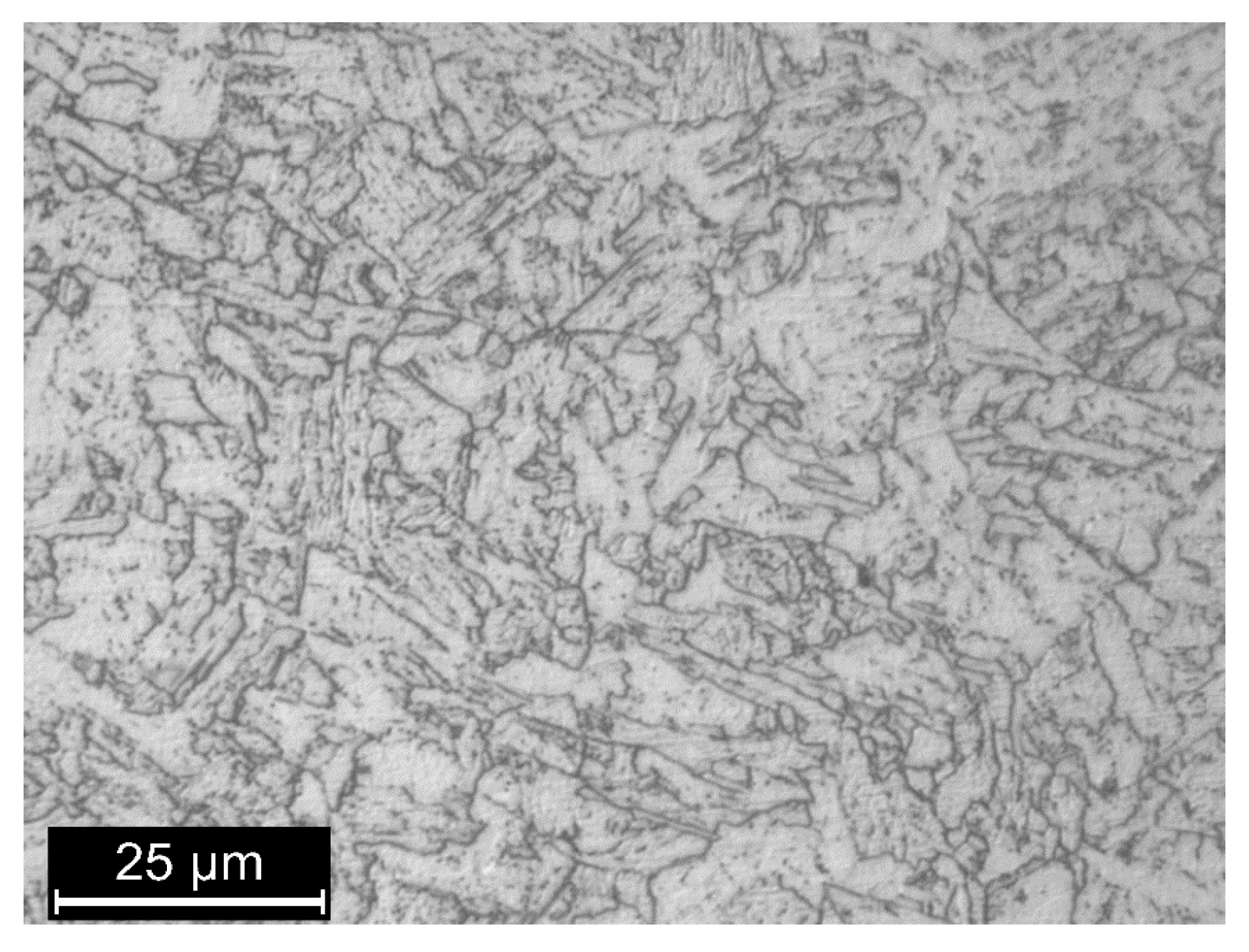

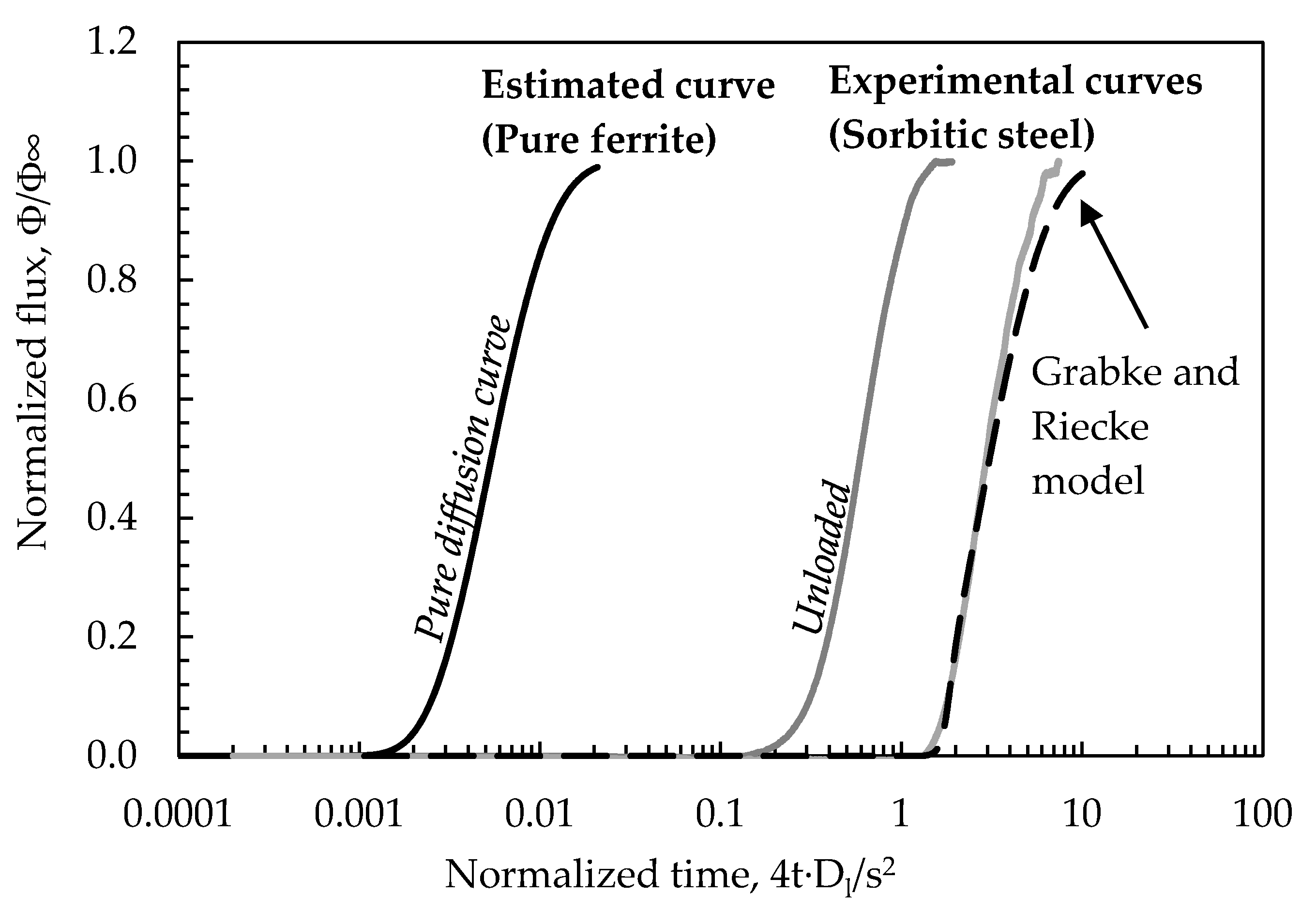
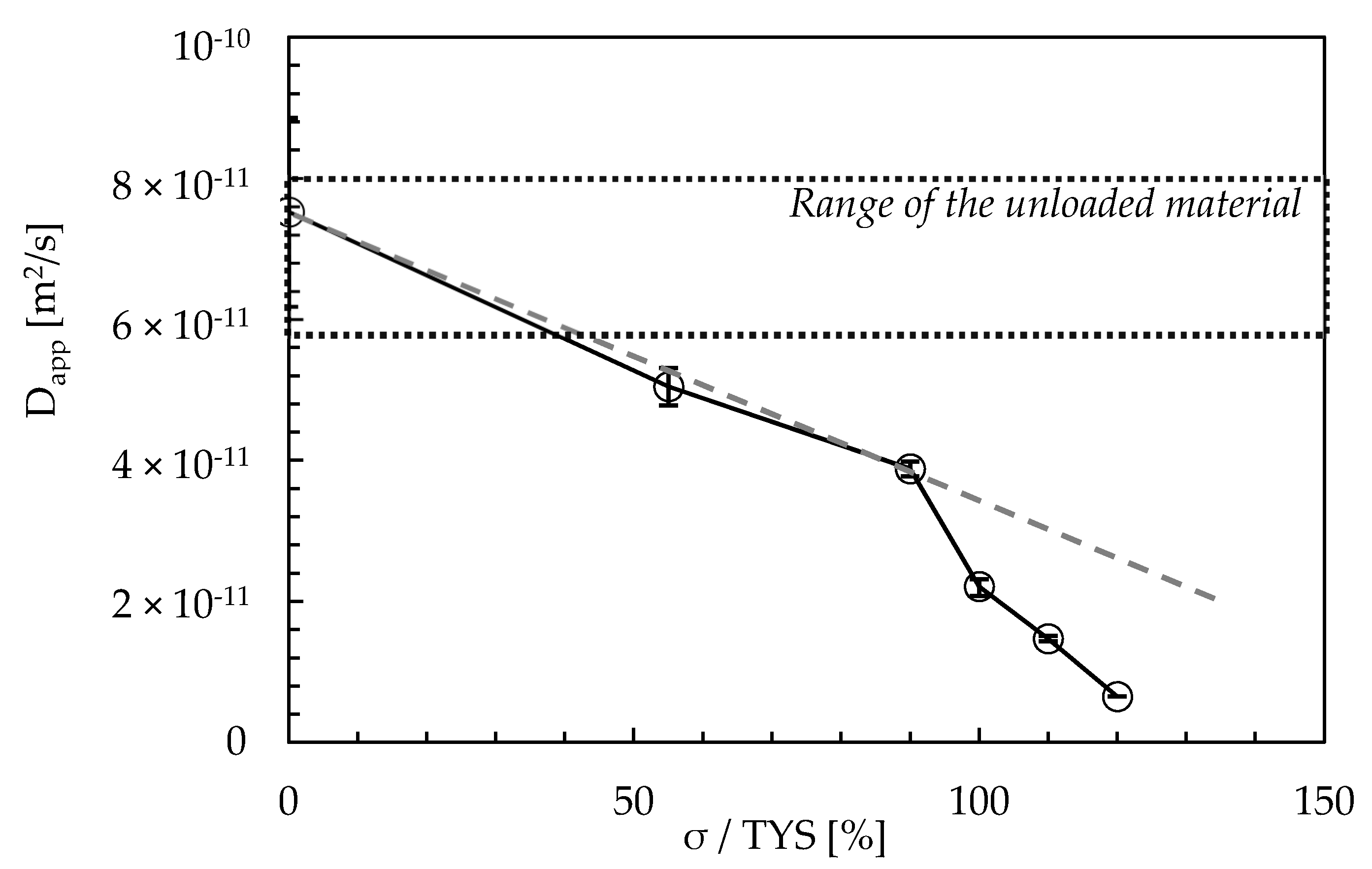
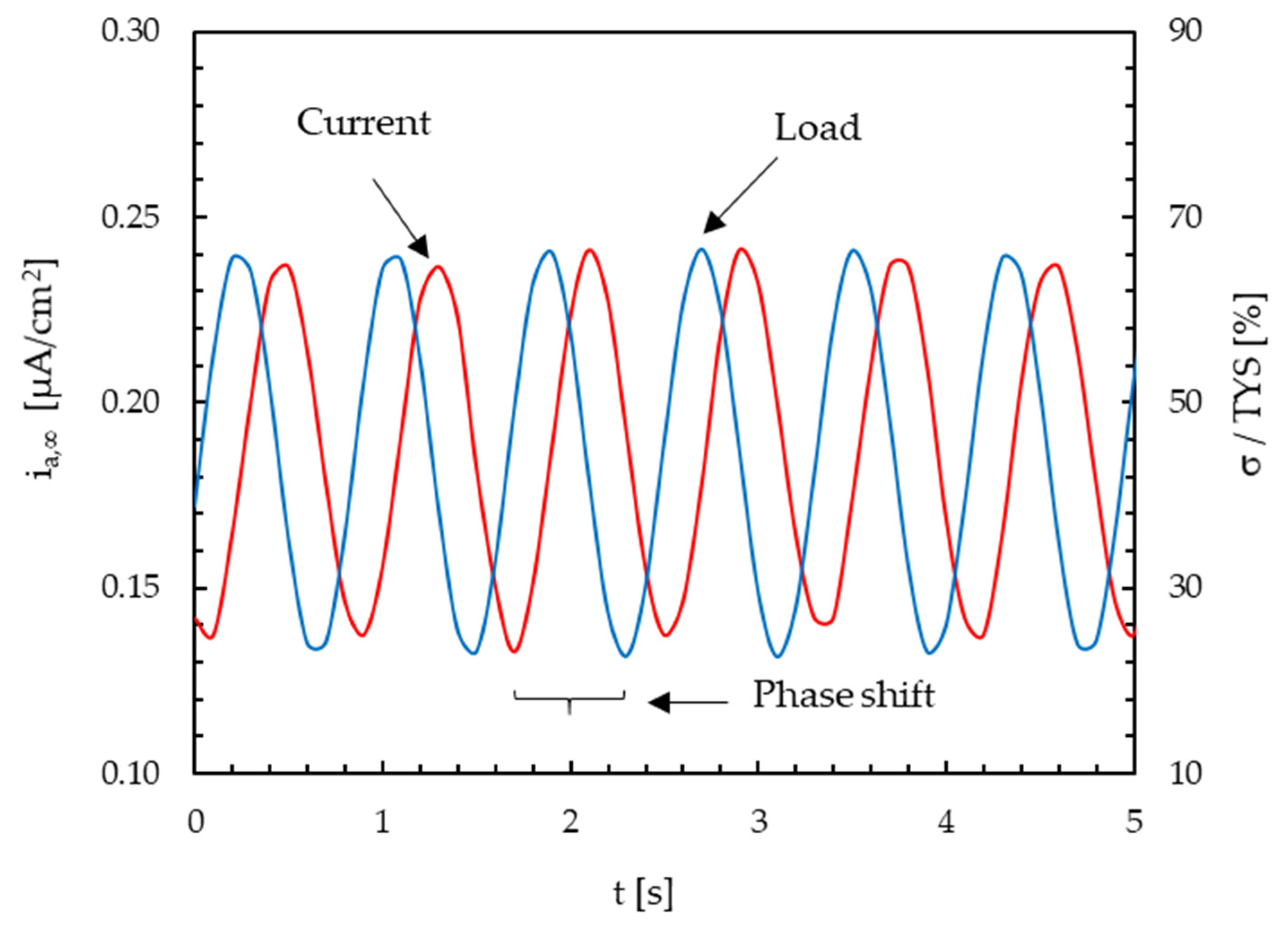



| Label | Maximum Load | Amplitude | Frequency | iC (mA/cm2) |
|---|---|---|---|---|
| C_55 | 55% TYS | ±10% TYS | 1.22 × 10−2 Hz | −0.50 |
| C_90 | 90% TYS | ±10% TYS | 1.22 × 10−2 Hz | −0.50 |
| C_100 | 100% TYS | ±10% TYS | 1.22 × 10−2 Hz | −0.50 |
| C_110 | 110% TYS | ±10% TYS | 1.22 × 10−2 Hz | −0.50 |
| Label | Maximum Load | Amplitude | Frequency | iC (mA/cm2) |
|---|---|---|---|---|
| C_45_10_f1_H | 45% TYS | ±10% TYS | 1.22 × 10−2 Hz | −0.50 |
| C_45_10_f2_H | 45% TYS | ±10% TYS | 1.22 × 10−1 Hz | −0.50 |
| C_45_10_f3_H | 45% TYS | −10% TYS | 1.22 Hz | −0.50 |
| C_45_20_f1_H | 45% TYS | ±20% TYS | 1.22 × 10−2 Hz | −0.50 |
| C_45_20_f2_H | 45% TYS | ±20% TYS | 1.22 × 10−1 Hz | −0.50 |
| C_45_20_f3_H | 45% TYS | ±20% TYS | 1.22 Hz | −0.50 |
| C_45_10_f1 | 45% TYS | ±10% TYS | 1.22 × 10−2 Hz | 0 |
| C_45_10_f2 | 45% TYS | ±10% TYS | 1.22 × 10−1 Hz | 0 |
| C_45_10_f3 | 45% TYS | ±10% TYS | 1.22 Hz | 0 |
| C_45_20_f1 | 45% TYS | ±20% TYS | 1.22 × 10−2 Hz | 0 |
| C_45_20_f2 | 45% TYS | ±20% TYS | 1.22 × 10−1 Hz | 0 |
| C_45_20_f3 | 45% TYS | ±20% TYS | 1.22 Hz | 0 |
| Label | Increasing Od Mean Load | Amplitude | Frequency | iC (mA/cm2) |
|---|---|---|---|---|
| C_0_55 | From 0% to 55% TYS | ±10% TYS | 1.22 × 10−2 Hz | −0.50 |
| C_55_90 | From 55% to 90% TYS | ±10% TYS | 1.22 × 10−2 Hz | −0.50 |
| C_90_100 | From 90% to 100% TYS | ±10% TYS | 1.22 × 10−2 Hz | −0.50 |
| C_100_110 | From 100% to 110% TYS | ±10% TYS | 1.22 × 10−2 Hz | −0.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrini, M.; Coppola, L.; Lorenzi, S.; Testa, C.; Carugo, F.; Bucella, D.P.; Pastore, T. Hydrogen Permeation in X65 Steel under Cyclic Loading. Materials 2020, 13, 2309. https://doi.org/10.3390/ma13102309
Cabrini M, Coppola L, Lorenzi S, Testa C, Carugo F, Bucella DP, Pastore T. Hydrogen Permeation in X65 Steel under Cyclic Loading. Materials. 2020; 13(10):2309. https://doi.org/10.3390/ma13102309
Chicago/Turabian StyleCabrini, Marina, Luigi Coppola, Sergio Lorenzi, Cristian Testa, Francesco Carugo, Diego Pesenti Bucella, and Tommaso Pastore. 2020. "Hydrogen Permeation in X65 Steel under Cyclic Loading" Materials 13, no. 10: 2309. https://doi.org/10.3390/ma13102309
APA StyleCabrini, M., Coppola, L., Lorenzi, S., Testa, C., Carugo, F., Bucella, D. P., & Pastore, T. (2020). Hydrogen Permeation in X65 Steel under Cyclic Loading. Materials, 13(10), 2309. https://doi.org/10.3390/ma13102309








