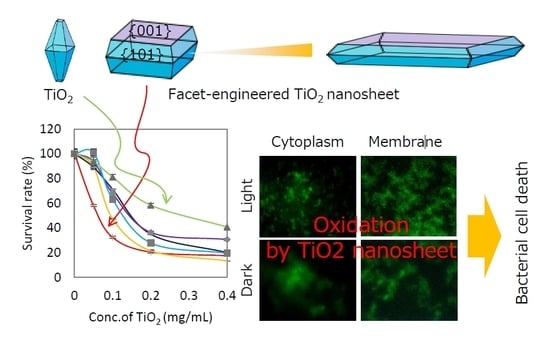
Enhanced Antibacterial Property of Facet-Engineered TiO2 Nanosheet in Presence and Absence of Ultraviolet Irradiation
Abstract
1. Introduction
2. Results
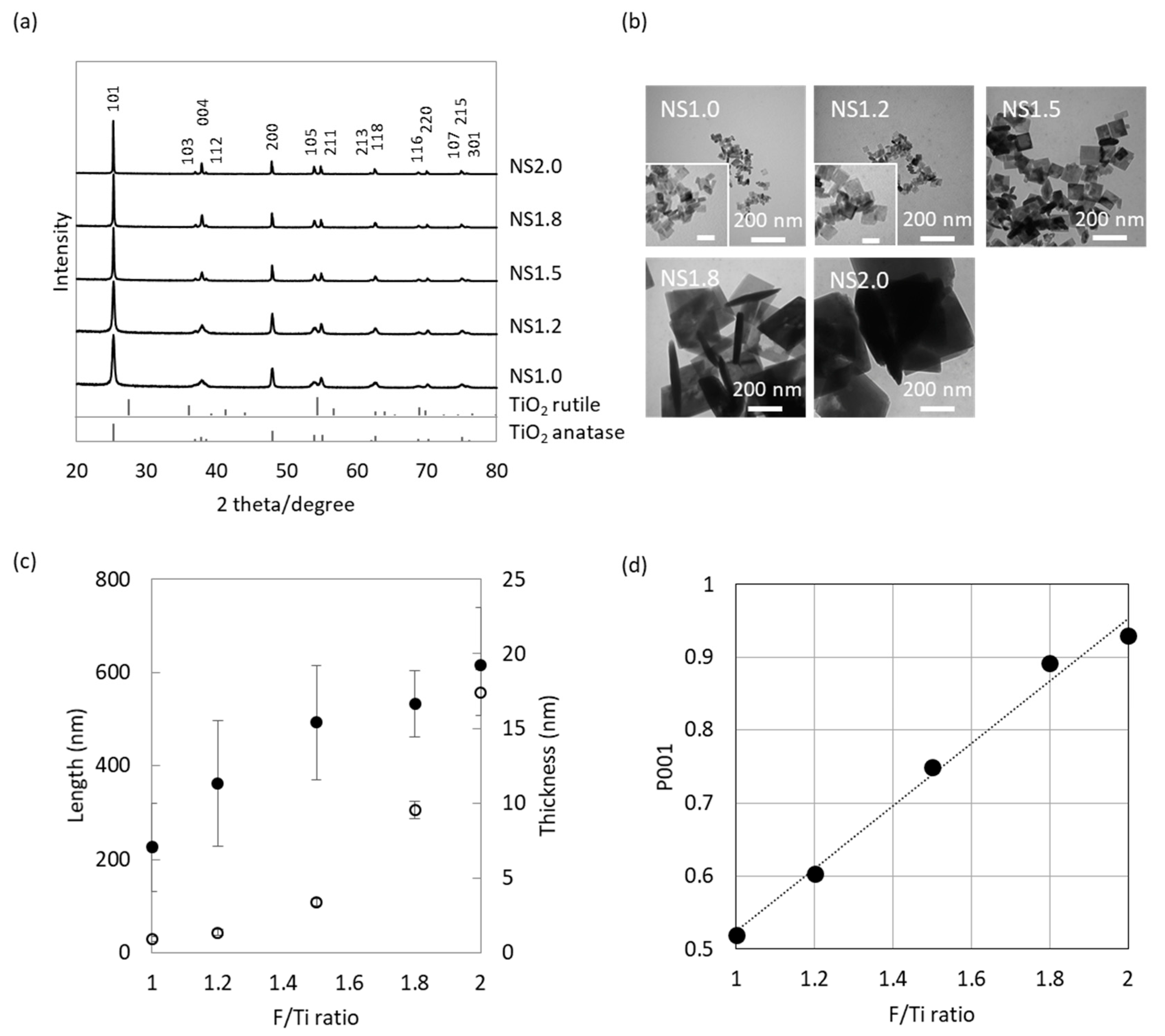
2.1. Characterization of TiO2 Nanosheet
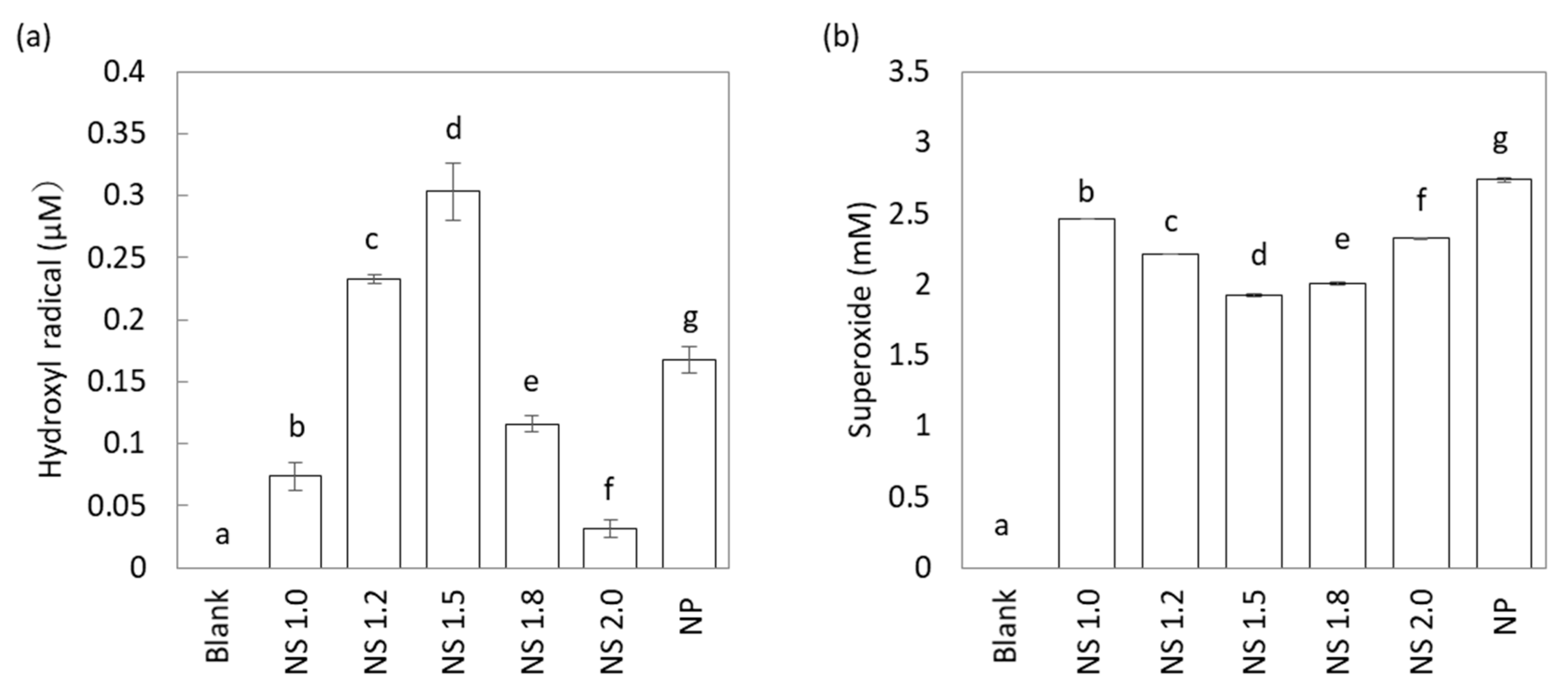
2.2. Effect of TiO2 NS on UV-Stimulated ROS Generation
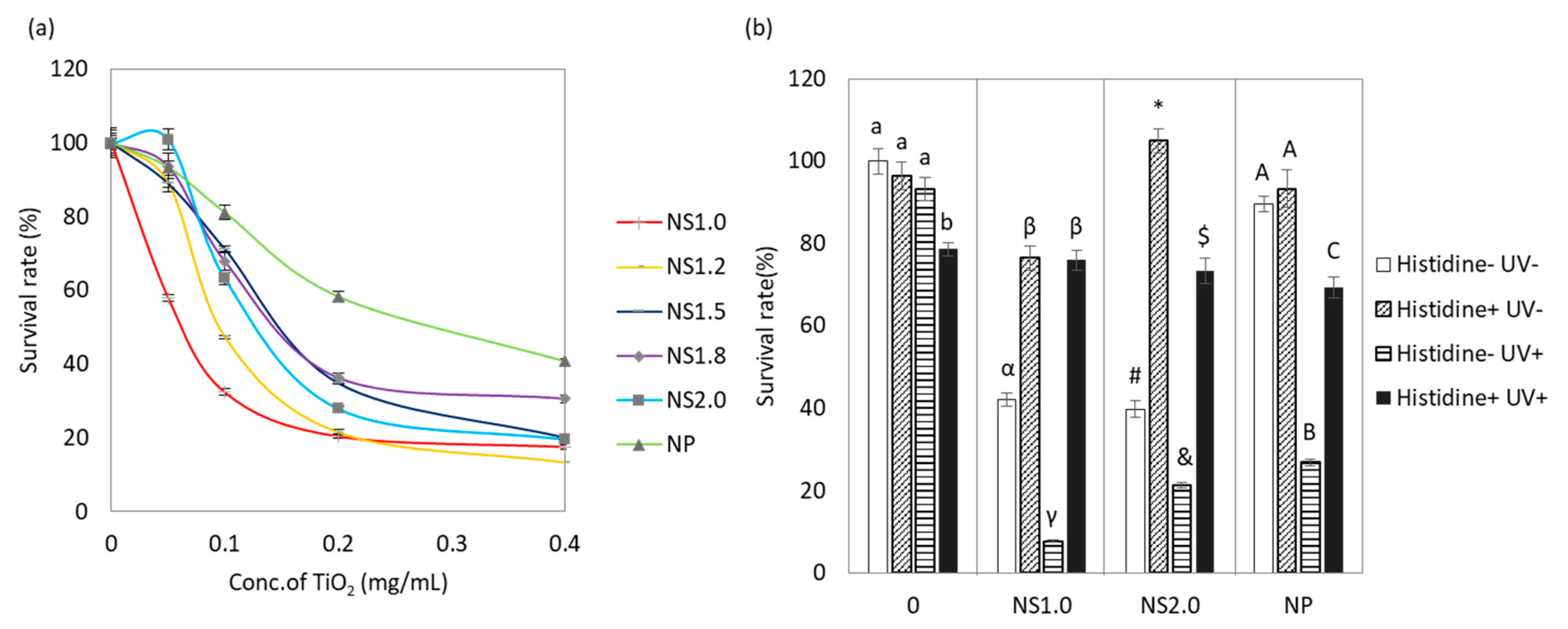
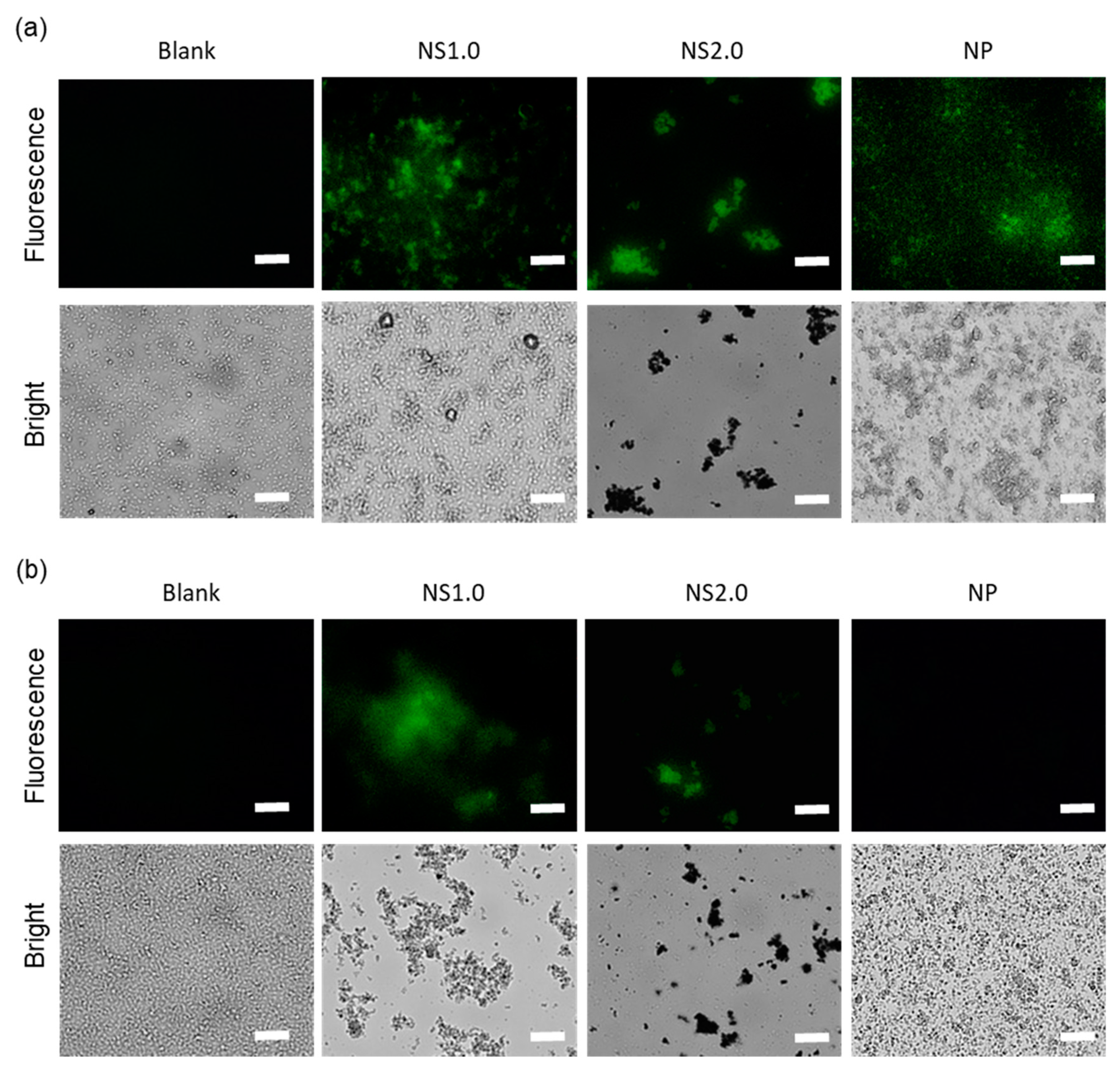
2.3. Evaluation of Antibacterial Properties
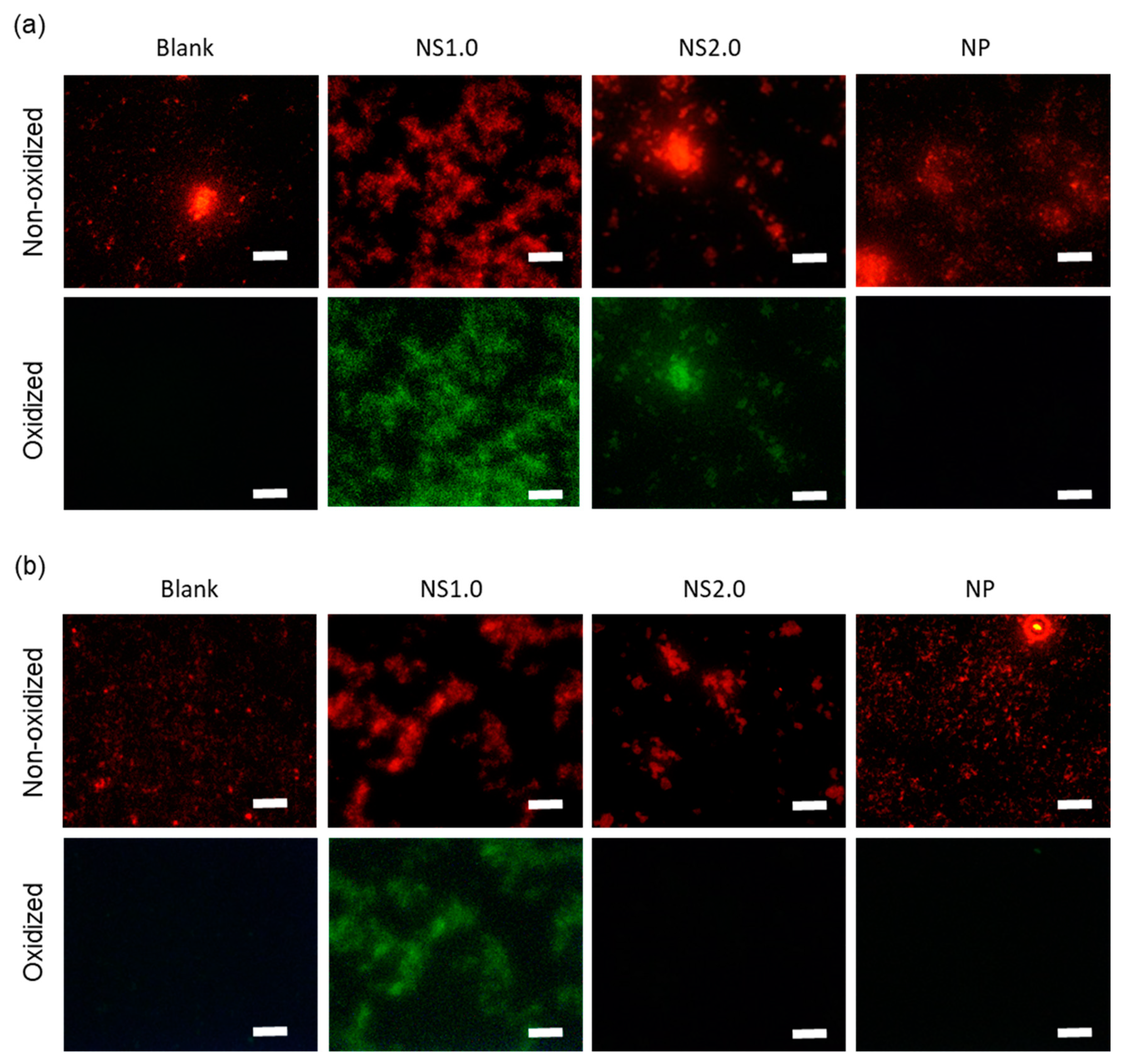
2.4. Evaluation of Oxidation State of Bacteria
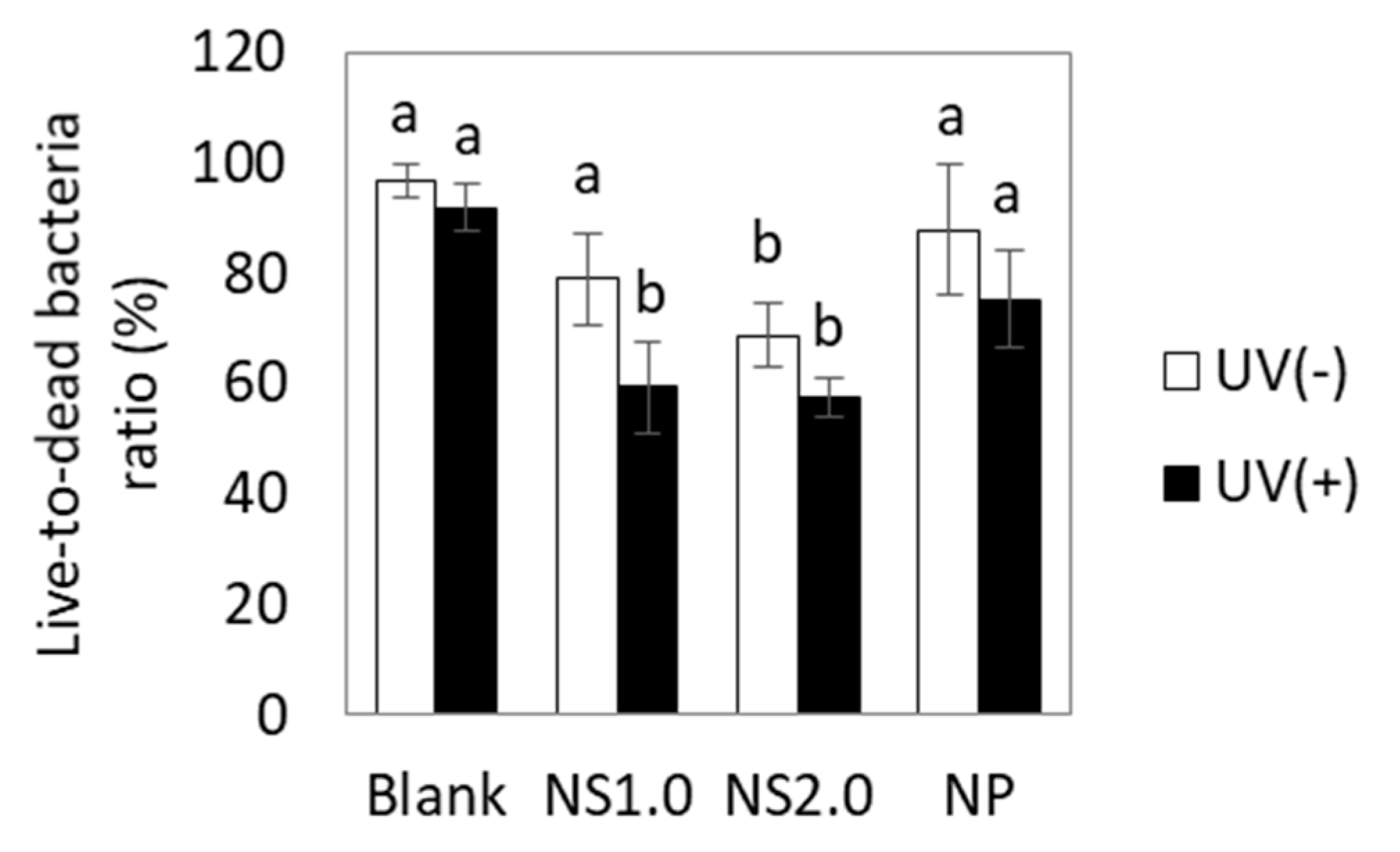
2.5. Evaluation of Cell Membrane Damage
3. Discussion
4. Materials and Methods
4.1. Synthesis of TiO2 Nanosheet
4.2. Characterization of TiO2 Nanosheet
4.3. Measurement of Reactive Oxygen Species
4.3.1. Measurement of Hydroxyl Radical
4.3.2. Measurement of Superoxide
4.4. Evaluation of Antibacterial Activity
4.4.1. Evaluation of Antibacterial Properties of TiO2 Nanosheet
4.4.2. Antioxidation Assay
4.4.3. Oxidative Stress on S. mutans by TiO2 Nanosheet
4.4.4. Cell Membrane Damage
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsai, T.-M.; Chang, H.-H.; Chang, K.-C.; Liu, Y.-L.; Tseng, C.-C. A comparative study of the bactericidal effect of photocatalytic oxidation by TiO2 on antibiotic-resistant and antibiotic-sensitive bacteria. J. Chem. Technol. Biotechnol. 2010, 85, 1642–1653. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Laxma Reddy, P.V.; Kavitha, B.; Kumar Reddy, P.A.; Kim, K.-H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef]
- McEvoy, J.G.; Zhang, Z. Antimicrobial and photocatalytic disinfection mechanisms in silver-modified photocatalysts under dark and light conditions. J. Photochem. Photobiol. C 2014, 19, 62–75. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef]
- Bai, S.; Wang, L.; Li, Z.; Xiong, Y. Facet-engineered surface and interface design of photocatalytic materials. Adv. Sci. 2017, 4, 1600216. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B 2001, 63. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef]
- Cheng, X.-L.; Hu, M.; Huang, R.; Jiang, J.-S. HF-free synthesis of anatase TiO2 nanosheets with largely exposed and clean {001} facets and their enhanced rate performance as anodes of lithium-ion battery. ACS Appl. Mater. Interfaces 2014, 6, 19176–19183. [Google Scholar] [CrossRef] [PubMed]
- Amano, F.; Prieto-Mahaney, O.-O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral single-crystalline particles of anatase titanium(IV) oxide with high photocatalytic activity. Chem. Mater. 2009, 21, 2601–2603. [Google Scholar] [CrossRef]
- Zhang, D.; Li, G.; Yang, X.; Yu, J.C. A micrometer-size TiO2 single-crystal photocatalyst with remarkable 80% level of reactive facets. Chem. Commun. 2009, 4381–4383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, S.; Bian, Z.; Xie, S.; Cai, C.; Wang, J.; Yang, H.; Li, H. Solvothermally controllable synthesis of anatase TiO2 nanocrystals with dominant {001} facets and enhanced photocatalytic activity. CrystEngComm 2010, 12, 2219–2224. [Google Scholar] [CrossRef]
- Alivov, Y.; Fan, Z.Y. A method for fabrication of pyramid-shaped TiO2 nanoparticles with a high {001} facet percentage. J. Phys. Chem. C 2009, 113, 12954–12957. [Google Scholar] [CrossRef]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous synthesis of TiO2 nanocrystals using TiF4 to engineer morphology, oxygen vacancy concentration, and photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef]
- Tan, Z.; Sato, K.; Takami, S.; Numako, C.; Umetsu, M.; Soga, K.; Nakayama, M.; Sasaki, R.; Tanaka, T.; Ogino, C.; et al. Particle size for photocatalytic activity of anatase TiO2 nanosheets with highly exposed {001} facets. RSC Adv. 2013, 3, 19268–19271. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Liu, N.; Chang, Y.; Feng, Y.; Cheng, Y.; Sun, X.; Jian, H.; Li, X.; Zhang, H. {101}– {001} surface heterojunction-enhanced antibacterial activity of titanium dioxide nanocrystals under sunlight irradiation. ACS Appl Mater. Interfaces 2017, 9, 5907–5915. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Lu, J.; Qin, X.; Zhang, X.; Dai, Y. Hierarchical TiO2 microspheres: Synergetic effect of {001} and {101} facets for enhanced photocatalytic activity. Chem. A Eur. J. 2011, 17, 15032–15038. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, X.; Xie, S.; Kuang, Q.; Ouyang, J.; Xie, Z.; Zheng, L. Carbonate ions-assisted syntheses of anatase TiO2 nanoparticles exposed with high energy (001) facets. RSC Adv. 2012, 2, 3251–3253. [Google Scholar] [CrossRef]
- Xiang, Q.; Lv, K.; Yu, J. Pivotal role of fluorine in enhanced photocatalytic activity of anatase TiO2 nanosheets with dominant (001) facets for the photocatalytic degradation of acetone in air. Appl. Catal. B Environ. 2010, 96, 557–564. [Google Scholar] [CrossRef]
- Chen, W.; Kuang, Q.; Wang, Q.; Xie, Z. Engineering a high energy surface of anatase TiO2 crystals towards enhanced performance for energy conversion and environmental applications. RSC Adv. 2015, 5, 20396–20409. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Ng, A.M.; Xu, X.Y.; Lee, P.K.; Degger, N.; Wu, R.S. Toxicity of metal oxide nanoparticles: Mechanisms, characterization, and avoiding experimental artefacts. Small 2015, 11, 26–44. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant characteristics of L-histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Dadjour, M.F.; Ogino, C.; Matsumura, S.; Shimizu, N. Kinetics of disinfection of Escherichia coli by catalytic ultrasonic irradiation with TiO2. Biochem. Eng. J. 2005, 25, 243–248. [Google Scholar] [CrossRef]
- Dalai, S.; Pakrashi, S.; Kumar, R.S.S.; Chandrasekaran, N.; Mukherjee, A. A comparative cytotoxicity study of TiO2 nanoparticles under light and dark conditions at low exposure concentrations. Toxicol. Res. 2012, 1, 116–130. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Yu, W.; Jiang, F.; Zhang, E.; Wang, H.; Kong, Z.; Xi, J.; Ji, Z. Novel dual heterojunction between MoS2 and anatase TiO2 with coexposed {101} and {001} facets. J. Am. Ceram. Soc. 2017, 100, 5274–5285. [Google Scholar] [CrossRef]
- Sahni, M.; Locke, B.R. Quantification of hydroxyl radicals produced in aqueous phase pulsed electrical discharge reactors. Ind. Eng. Chem. Res. 2006, 45, 5819–5825. [Google Scholar] [CrossRef]
- Ishibashi, K.-I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantum yields of active oxidative species formed on TiO2 photocatalyst. J. Photochem. Photobiol. A Chem. 2000, 134, 139–142. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed]
- Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef]
- Nozaki, K.; Koizumi, H.; Horiuchi, N.; Nakamura, M.; Okura, T.; Kimihiro Yamashita, K.; Nagai, A. Suppression effects of dental glass-ceramics with polarization-induced highly dense surface charges against bacterial adhesion. Dent. Mater. J. 2015, 34, 671–678. [Google Scholar] [CrossRef]
- Yamada, R.; Nozaki, K.; Horiuchi, N.; Yamashita, K.; Nemoto, R.; Miura, H.; Nagai, A. Ag nanoparticle–coated zirconia for antibacterial prosthesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1054–1060. [Google Scholar] [CrossRef]
- Nakano, K.; Nomura, R.; Nakagawa, I.; Hamada, S.; Ooshima, T. Demonstration of Streptococcus mutans with a cell wall polysaccharide specific to a new serotype, k, in the human oral cavity. J. Clin. Microbiol. 2004, 42, 198–202. [Google Scholar] [CrossRef]
- Minami, T.; Fujiwara, T.; Ooshima, T.; Nakajima, Y.; Hamada, S. Interaction of structural isomers of sucrose in the reaction between sucrose and glucosyltransferases from mutans streptococci. Oral Microbiol. Immunol. 1990, 5, 189–194. [Google Scholar] [CrossRef]
- Teles, F.R.; Teles, R.P.; Sachdeo, A.; Uzel, N.G.; Song, X.Q.; Torresyap, G.; Singh, M.; Papas, A.; Haffajee, A.D.; Socransky, S.S. Comparison of microbial changes in early redeveloping biofilms on natural teeth and dentures. J. Periodontol. 2012, 83, 1139–1148. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, K.; Nozaki, K.; Tan, Z.; Fujita, K.; Nemoto, R.; Yamashita, K.; Miura, H.; Itaka, K.; Ohara, S. Enhanced Antibacterial Property of Facet-Engineered TiO2 Nanosheet in Presence and Absence of Ultraviolet Irradiation. Materials 2020, 13, 78. https://doi.org/10.3390/ma13010078
Hayashi K, Nozaki K, Tan Z, Fujita K, Nemoto R, Yamashita K, Miura H, Itaka K, Ohara S. Enhanced Antibacterial Property of Facet-Engineered TiO2 Nanosheet in Presence and Absence of Ultraviolet Irradiation. Materials. 2020; 13(1):78. https://doi.org/10.3390/ma13010078
Chicago/Turabian StyleHayashi, Kenichiro, Kosuke Nozaki, Zhenquan Tan, Kazuhisa Fujita, Reina Nemoto, Kimihiro Yamashita, Hiroyuki Miura, Keiji Itaka, and Satoshi Ohara. 2020. "Enhanced Antibacterial Property of Facet-Engineered TiO2 Nanosheet in Presence and Absence of Ultraviolet Irradiation" Materials 13, no. 1: 78. https://doi.org/10.3390/ma13010078
APA StyleHayashi, K., Nozaki, K., Tan, Z., Fujita, K., Nemoto, R., Yamashita, K., Miura, H., Itaka, K., & Ohara, S. (2020). Enhanced Antibacterial Property of Facet-Engineered TiO2 Nanosheet in Presence and Absence of Ultraviolet Irradiation. Materials, 13(1), 78. https://doi.org/10.3390/ma13010078