Role of the Plasma Activation Degree on Densification of Organosilicon Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus and Procedures
2.2. Characterization Techniques
2.2.1. Profilometer
2.2.2. Infrared and X-ray Energy Dispersive Spectroscopies
2.2.3. Contact Angle
2.2.4. Nanoindentation and Atomic Force Microscopy
2.2.5. Electrochemical Impedance Spectroscopy
3. Results and Discussion
3.1. Thickness and Deposition Rate
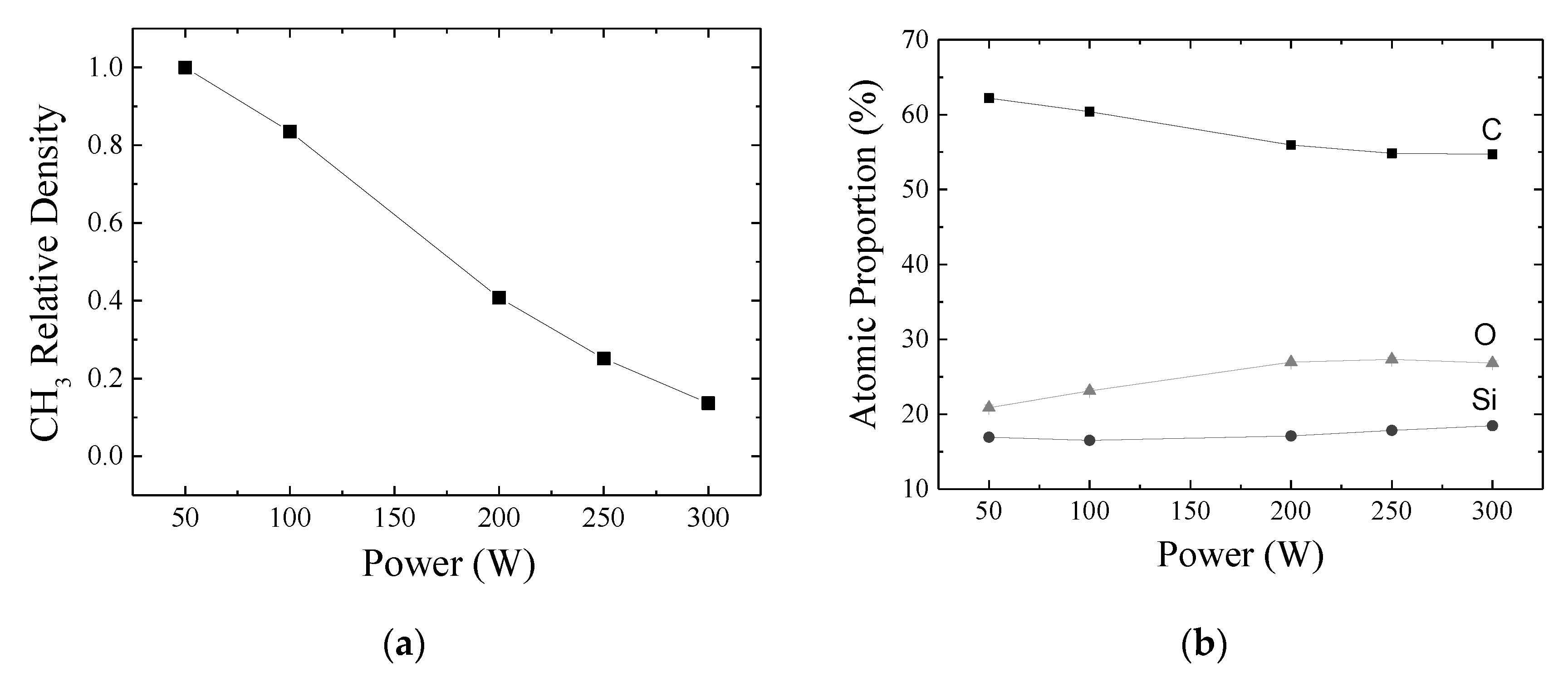
3.2. Molecular Structure and Chemical Composition
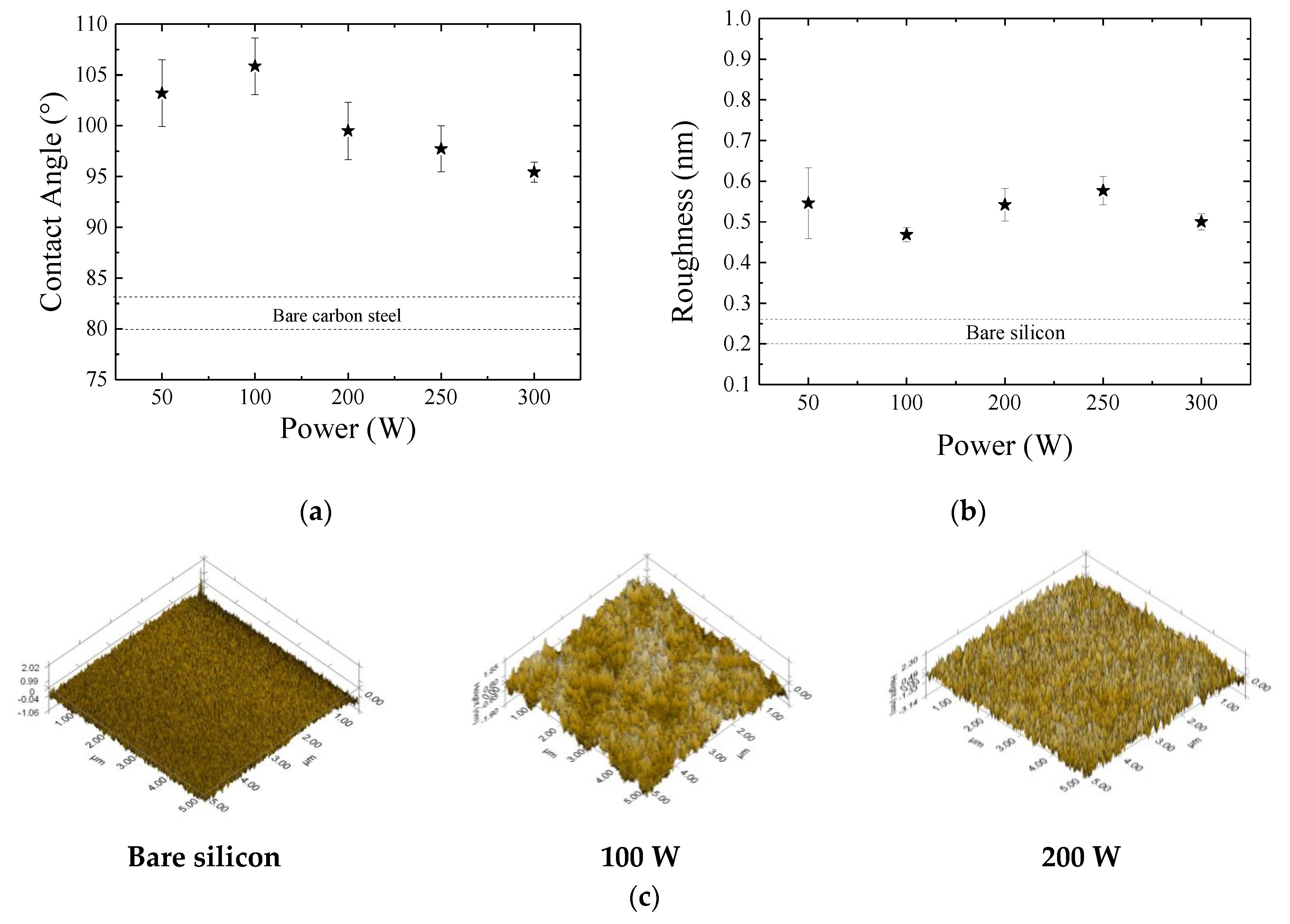
3.3. Wettability and Surface Topography
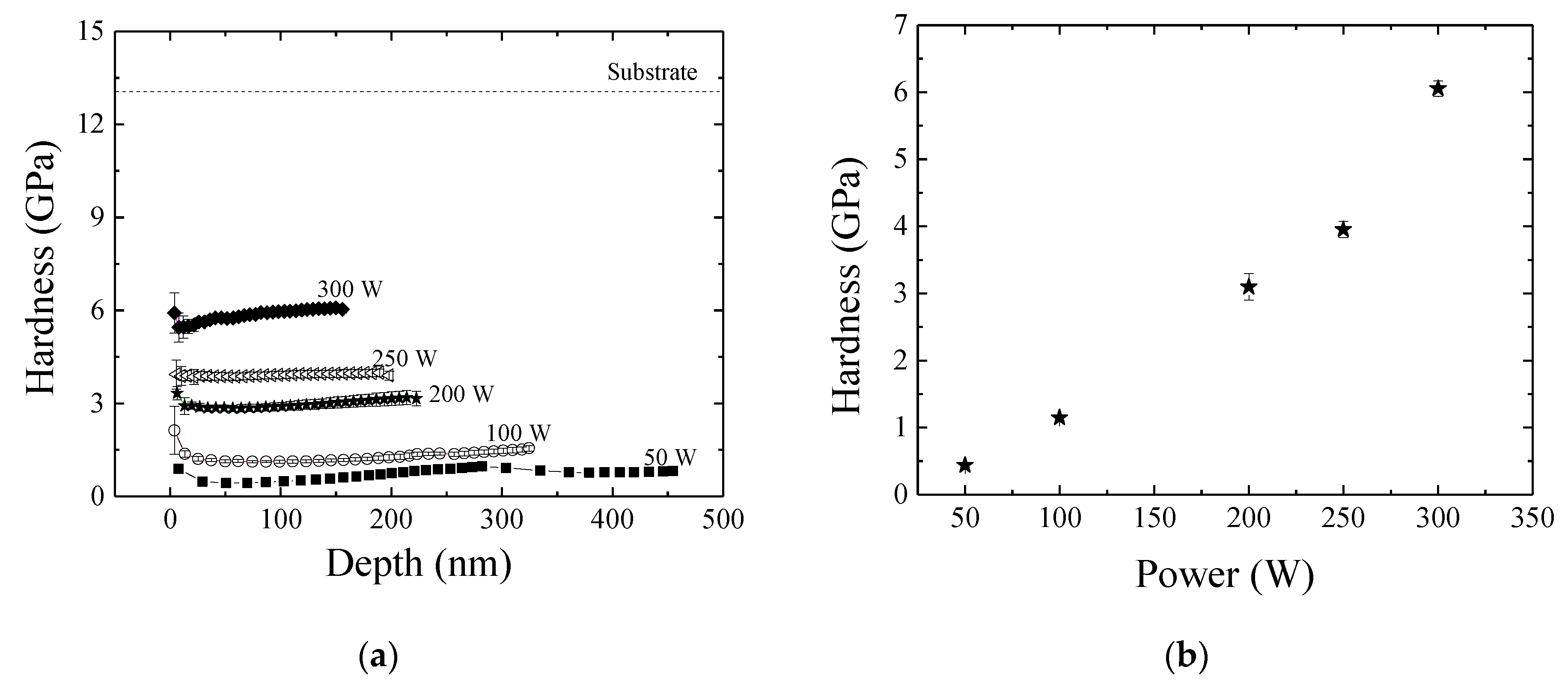
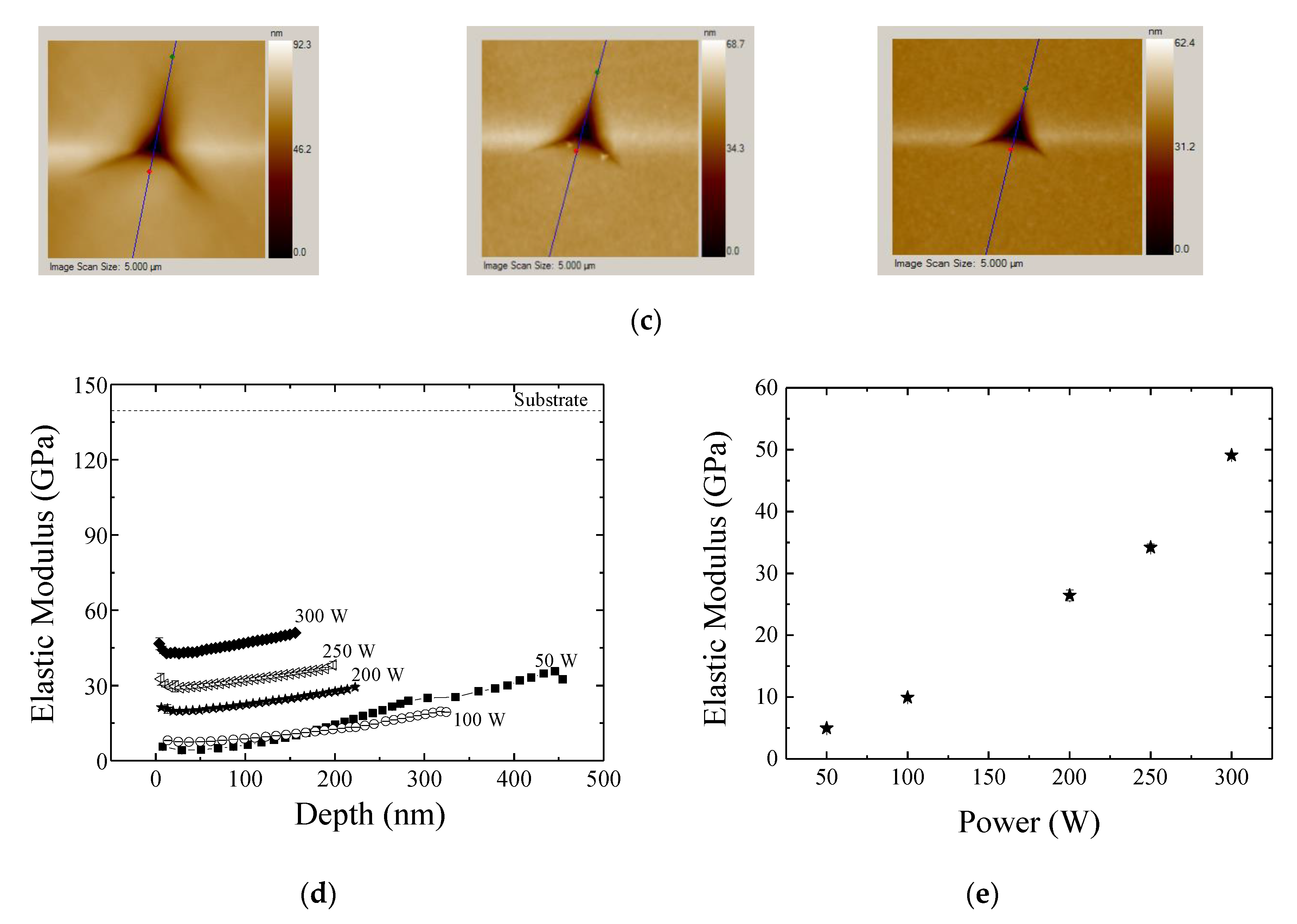
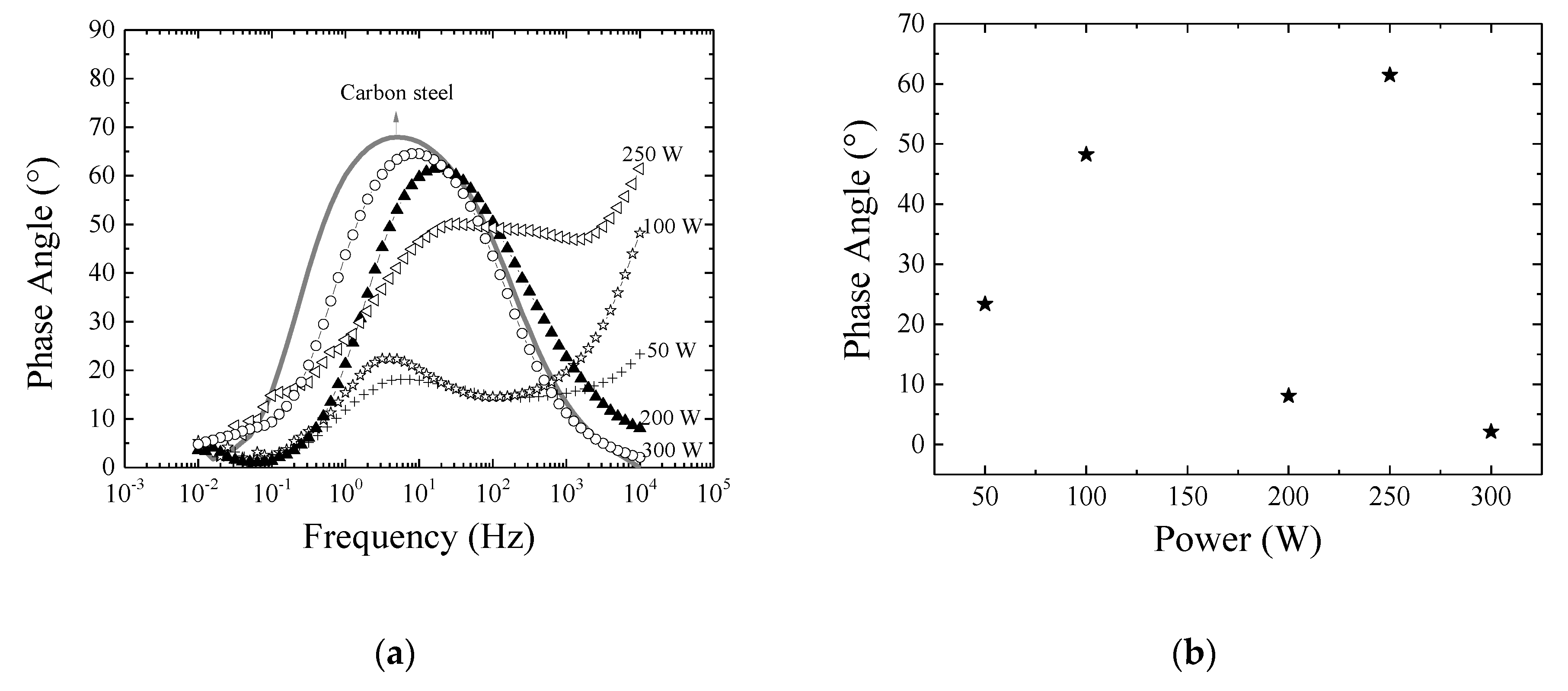
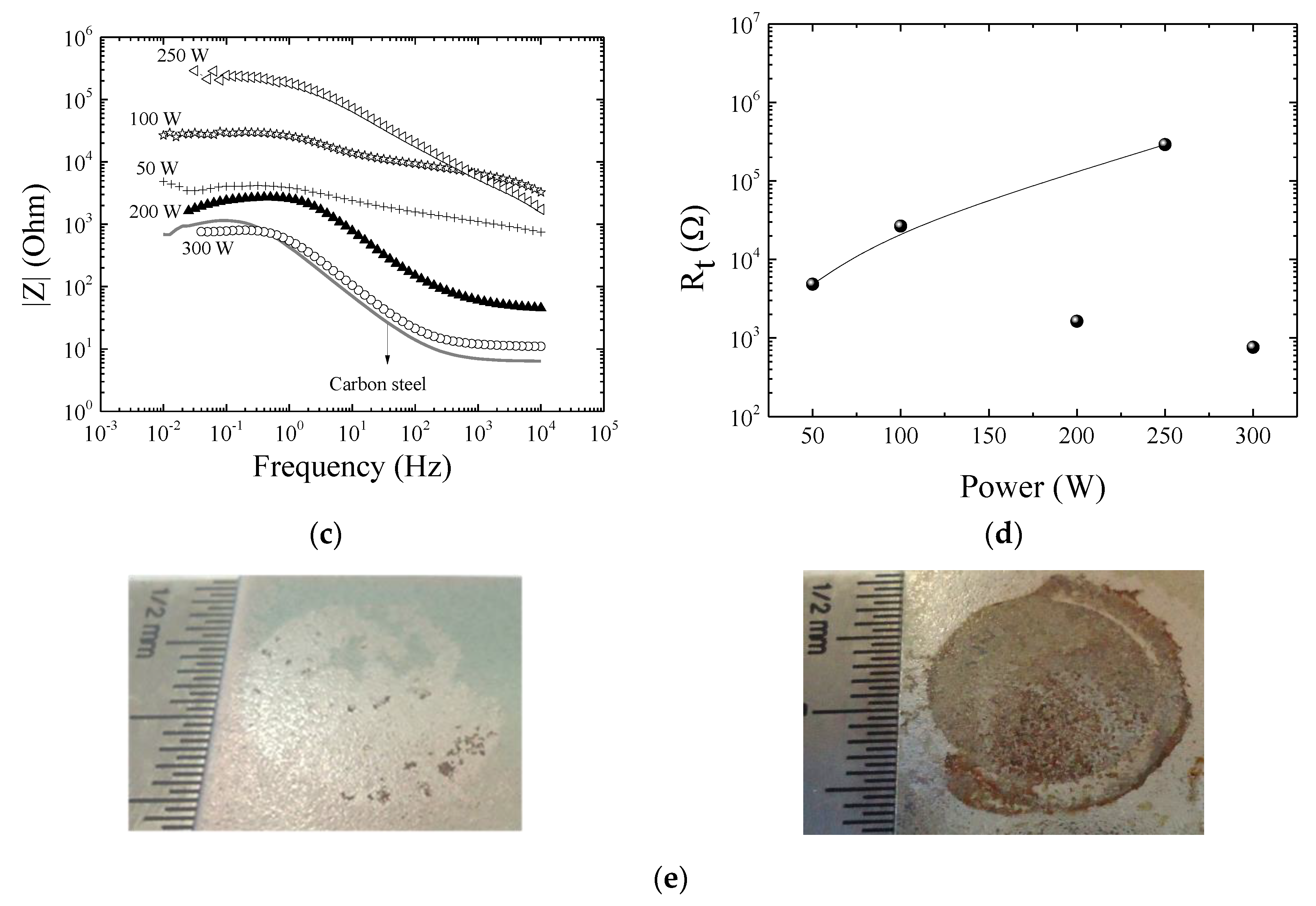
3.4. Mechanical and Barrier Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Fuerst, T.F.; Reese, M.O.; Wolden, C.A. PECVD Synthesis of Flexible Optical Coatings for Renewable Energy Applications. Plasma Proc. Polym. 2016, 13, 184–190. [Google Scholar] [CrossRef]
- Yu, Y. Package Structure of Flexible OLED Device and Display Device. U.S. Patent 20170352833, 12 July 2017. [Google Scholar]
- Hermosa, C.S.; Top, M.; Dagar, J.; Fahlteich, J.; Brown, T.M. Quantifying Performance of Permeation Barrier—Encapsulation Systems for Flexible and Glass-Based Electronics and Their Application to Perovskite Solar Cells. Adv. Electron. Mater. 2019, 1800978. [Google Scholar] [CrossRef]
- Wu, C.Y.; Liao, R.M.; Lai, L.W.; Jeng, M.S.; Liu, D.S. Organosilicon/silicon oxide gas barrier structure encapsulated flexible plastic substrate by using plasma-enhanced chemical vapor deposition. Surf. Coat. Technol. 2012, 206, 4685–4691. [Google Scholar] [CrossRef]
- Vendemiatti, C.; Hosokawa, R.S.; Rangel, R.C.C.; Bortoleto, J.R.R.; Cruz, N.C.; Rangel, E.C. Wettability and surface microstructure of polyamide 6 coated with SiOXCYHZ films. Surf. Coat. Technol. 2015, 275, 32–40. [Google Scholar] [CrossRef]
- Gosar, Ž.; Kovač, J.; Mozetič, M.; Primc, G.; Vesel, A.; Zaplotnik, R. Deposition of SiOxCyHz Protective Coatings on Polymer Substrates in an Industrial-Scale PECVD Reactor. Coatings 2019, 9, 234. [Google Scholar] [CrossRef]
- Cui, X.; Zhu, G.; Pan, Y.; Zhang, Y. Preparation and anticorrosion properties of Polydimethylsiloxane (PDMS). In Proceedings of the 4th Annual International Conference on Material Engineering and Application (ICMEA 2017), Wuhan, China, 15–17 December 2017; pp. 15–17. [Google Scholar]
- Santos, N.M.; Gonçalves, T.M.; de Amorim, J.; Freire, C.M.A.; Bortoleto, J.R.R.; Durrant, S.F.; Ribeiro, R.P.; Cruz, N.C.; Rangel, E.C. Effect of the plasma excitation power on the properties of SiOxCyHz films deposited on AISI 304 steel. Surf. Coat. Technol. 2017, 311, 127–137. [Google Scholar] [CrossRef]
- Xue, C.H.; Bai, X.; Jia, S.T. Robust, self-healing superhydrophobic fabrics prepared by one-step coating of PDMS and octadecylamine. Sci. Rep. 2016, 6, 27262. [Google Scholar] [CrossRef]
- Liu, B.; Tian, T.; Yao, J.; Huang, C.; Tang, W.; Xiang, Z.; Xu, X.; Min, J. Superhydrophobic organosilicon-based coating system by a novel ultraviolet-curable method. Nanomater. Nanotechnol. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Dos Reis, M.C.; Silva, V.R.M.; Sgura, R.; da Cruz, N.C.; Rangel, E.C.; Medeiros, I.S. Surface characteristics and optical properties of plasma deposited films on indirect aesthetic restorative dental materials. Surf. Coat. Technol. 2018, 348, 55–63. [Google Scholar] [CrossRef]
- Lou, B.S.; Wang, S.B.; Hung, S.B.; Wang, C.J.; Lee, J.W. Characterization of plasma polymerized organosilicon thin films deposited on 316L stainless steel. Thin Solid Films 2018, 660, 637–645. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Cordeiro, J.M.; Bertolini, M.; de Almeida, A.B.; Cavalcante, R.C.B.R.; Junior, F.H.N.; Feres, M.; Rangel, E.C.; Barão, V.A.R. Silicon-doped superhydrophobic coating to reduce polymicrobial biofilm on titanium surface. Biomaterials 2019. submitted. [Google Scholar]
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of Ni-Fe alloys, composites, and nano coatings–A review. J. Alloy. Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- Yang, T.C.; Chang, J.K.; Lin, C.S. The Influence of Aluminum Nitrate Pre-Treatment on High Temperature Oxidation Resistance of Dip-Coated Silica Coating on Galvanized Steel. ECS Trans. 2018, 85, 67–73. [Google Scholar] [CrossRef]
- Dalibón, E.L.; Escalada, L.; Simison, S.; Forsich, S.; Heim, D.; Brühl, S.P. Mechanical and corrosion behavior of thick and soft DLC coatings. Surf. Coat. Technol. 2017, 312, 101–109. [Google Scholar] [CrossRef]
- Choudhury, A.J.; Barve, S.A.; Chutia, J.; Pal, A.R.; Kishore, R.; Pande, M.; Patil, D.S. RF-PACVD of water repellent and protective HMDSO coatings o bell metal surfaces: Correlation between discharge parameters and film properties. Appl. Surf. Sci. 2011, 257, 8469–8477. [Google Scholar] [CrossRef]
- Hegemann, D.; Korner, E.; Blanchard, N.; Drabik, M.; Guimond, S. Densification of functional plasma polymers by momentum transfer during film growth. Appl. Phys. Lett. 2012, 101, 21160. [Google Scholar] [CrossRef]
- Hegemann, D. Plasma polymer deposition and coatings on polymers. Compr. Mater. Process. 2014, 4, 201–228. [Google Scholar]
- Blanchard, N.E.; Hanselmann, B.; Drosten, J.; Heuberger, M.; Hegemann, D. Densification and hydration of HMDSO plasma polymers. Plasma Process. Polym. 2015, 12, 32–41. [Google Scholar] [CrossRef]
- Zhou, L.; Lv, G.H.; Pang, H.; Zhang, G.P.; Yang, S.Z. Preparation of biomedical Ag incorporated hydroxyapatite/titania coatings on Ti6Al4V alloy by plasma electrolytic oxidation. Surf. Coat. Technol. 2012, 206, 2552–2557. [Google Scholar] [CrossRef]
- Nagasawa, H.; Yamamoto, Y.; Tsuda, N.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Atmospheric-pressure plasma-enhanced chemical vapor deposition of microporous silica membranes for gas separation. J. Membr. Sci. 2017, 524, 644–651. [Google Scholar] [CrossRef]
- Lee, S.; Kang, Y.J.; Jung, S.; Kim, J.; Kim, D.G. Plasma-Deposited SiOxCyHz Barrier Coatings for Organic Device Encapsulation. Jpn. J. Appl. Phys. 2013, 52, 076001. [Google Scholar] [CrossRef]
- Nagasawa, H.; Yamamoto, Y.; Kanezashi, M.; Tsuru, T. Atmospheric-pressure plasma-enhancedchemical vapor deposition of hybridsilica membranes. J. Chem. Eng. Jpn. 2018, 51, 732–739. [Google Scholar] [CrossRef]
- Guo, M.; Kanezashi, M.; Nagasawa, H.; Yu, L.; Yamamoto, K.; Gunji, T.; Ohshita, J.; Tsuru, T. Tailoring the Microstructure and permeation properties of bridged organosilica membranes via control of the bond angles. J. Membr. Sci. 2019, 584, 56–65. [Google Scholar] [CrossRef]
- Supiot, P.; Vivien, C.; Granier, A.; Bousquet, A.; Mackova, A.; Escaich, D.; Clergereaux, R.; Raynaud, P.; Stryhal, Z.; Pavli, J. Growth and Modification of Organosilicon Films in PECVD and Remote Afterglow Reactors. Plasma Process. Polym. 2006, 3, 100–109. [Google Scholar] [CrossRef]
- Van Hest, M.F.A.M.; Mitu, B.; Schram, D.C.; Van de Sanden, M.C.M. Deposition of organosilicon thin films using a remote thermal plasma. Thin Solid Films 2004, 449, 52–62. [Google Scholar] [CrossRef]
- Martinu, L.; Poitras, D. Plasma deposition of optical films and coatings: A review. J. Vac. Sci. Technol. A Vac. Surf. Films 2000, 18, 2619–2645. [Google Scholar] [CrossRef]
- Messier, R.; Giri, A.P.; Roy, R.A. Revised structure zone model for thin film physical structure. J. Vac. Sci. Technol. A Vac. Surf. Films 1984, 2, 500–503. [Google Scholar] [CrossRef]
- Barni, R.; Zanini, S.; Riccardi, C. Characterization of the chemical kinetics in an O2/HMDSO RF plasma for material processing. Adv. Phys. Chem. 2016, 6, 205380. [Google Scholar] [CrossRef]
- Oliver, W.; Pharr, G.R. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Zhang, J.; Steen, M.L.; Fisher, E.L. Investigation of Gas Phase Species and Deposition of SiO2 Films from HMDSO/O2 Plasmas. Plasma Process. Polym. 2006, 3, 276–287. [Google Scholar] [CrossRef]
- Choudhury, A.J.; Chutia, J.; Kakati, H.; Barve, S.A.; Pal, A.R.; Sarma, N.S.; Chowdhury, D.; Patil, D.S. Studies of radiofrequency plasma deposition of hexamethyldisiloxane films and their thermal stability and corrosion resistance behavior. Vacuum 2010, 84, 1327–1333. [Google Scholar] [CrossRef]
- Durrant, S.F.; Mota, R.P.; de Moraes, M.A.B. Fluorinated polymer films from rf plasmas containing benzene and sulfur hexafluorine. Thin Solid Films 1992, 220, 295–302. [Google Scholar] [CrossRef]
- Yasuda, H. Plasma Polymerization, 1st ed.; Academic Press Inc.: Orlando, FL, USA, 1985; p. 34. ISBN 0-12-768760-2. [Google Scholar]
- Rangel, R.C.C.; Pompeu, T.C.; Barros, J.L.S., Jr.; Antonio, C.A.; Santos, N.M.; Pelici, B.O.; Freire, C.M.A.; Cruz, N.C.; Rangel, E.C. Improvement of the Corrosion Resistance of Carbon Steel by Plasma Deposited Thin Films, 1st ed.; InTech: Rijeka, Croatia, 2012; p. 91. ISBN 978-953-307-920-2. [Google Scholar]
- Fracassi, F.; d’Agostino, R.; Palumbo, F.; Angelini, E.; Grassini, S.; Rosalbino, F. Application of plasma deposited organosilicon thin films for the corrosion protection of metals. Surf. Coat. Technol. 2003, 107, 174–175. [Google Scholar] [CrossRef]
- Lucovsky, G.; Mantini, M.J.; Srivastava, J.K.; Irene, E.A. Low-temperature growth of silicon dioxide films: A study of chemical bonding by ellipsometry and infrared spectroscopy. J. Vac. Sci. Technol. B 1987, 5, 530–537. [Google Scholar] [CrossRef]
- Grill, A.; Neumayer, D.A. Structure of low dielectric constant to extreme low dielectric constant SiCOH films: Fourier transform infrared spectroscopy characterization. J. Appl. Phys. 2003, 94, 6697–6707. [Google Scholar] [CrossRef]
- Chou, J.S.; Lee, S.C. Effect of porosity on infrared-absorption spectra of silicon dioxide. J. Appl. Phys. 1995, 77, 1805–1807. [Google Scholar] [CrossRef]
- Fanelli, F.; d’Agostino, R.; Fracassi, F. GC-MS Investigation of Hexamethyldisiloxane–Oxygen Fed Cold Plasmas: Low Pressure Versus Atmospheric Pressure Operation. Plasma Proc. Polym. 2011, 8, 932–941. [Google Scholar] [CrossRef]
- Alexander, M.R.; Jones, F.R.; Short, R.D. Radio-Frequency Hexamethyldisiloxane Plasma Deposition: A Comparison of Plasma- and Deposit-Chemistry. Plasmas Polym. 1997, 2, 277–300. [Google Scholar] [CrossRef]
- Rao, A.P.; Rao, A.V. Modifying the surface energy and hydrophobicity of the low-density silica aerogels through the use of combinations of surface-modification agents. J. Mater. Sci. 2010, 45, 51–63. [Google Scholar]
- Ping, Y.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar]
- Lanford, W.A.; Rand, M.J. The hydrogen content of plasma-deposited silicon nitride. J. App. Phys. 1978, 49, 2473–2477. [Google Scholar] [CrossRef]
- Montarsolo, A.; Mossottia, R.; Innocentia, R.; Vassallo, E. A study on washing resistance of pp-HMDSO films deposited on wool fabrics for anti-pilling purposes. Surf. Coat. Technol. 2013, 224, 109–113. [Google Scholar] [CrossRef]
- Li, Y.; Huang, K.; MacGregor, J.D.; Xing, Y. The Role of PTFE in Cathode Transition Layer in Aqueous Electrolyte Li-Air Battery. Electrochim. Acta 2016, 191, 996–1000. [Google Scholar] [CrossRef]
- Benítez, F.; Martínez, E.; Esteve, J. Improvement of hardness in plasma polymerized hexamethyldisiloxane coatings by silica-like surface modification. Thin Solid Films 2000, 109, 377–378. [Google Scholar] [CrossRef]
- Gengenbach, T.E.; Griesser, H. Post-deposition ageing reactions differ markedly between plasma polymers deposited from siloxane and silazane monomers. J. Polym. 1999, 40, 5091–5094. [Google Scholar] [CrossRef]
- Huan, W.; Lizhen, Y.; Qiang, C. Investigation of Microwave Surface-Wave Plasma Deposited SiOx Coatings on Polymeric Substrates. Plasma Sci. Technol. 2014, 16, 37–40. [Google Scholar]
- Kondo, M.; Toyoshima, Y.; Matsuda, A. Substrate dependence of initial growth of microcrystalline silicon in plasma-enhanced chemical vapor deposition. J. Appl. Phys. 1996, 80, 6061–6063. [Google Scholar] [CrossRef]
- Granata, S.N.; Bearda, T.; Xu, M.L.; Abdulraheem, Y.; Gordon, I.; Mertens, R.; Poortmans, J. Influence of substrate potential on a-Si: H passivation of Si foils bonded to glass. Thin Solid Films 2015, 579, 9–13. [Google Scholar] [CrossRef]
- Guo, X.; Momota, S.; Nittac, N.; Yamaguchi, T.; Sato, M.; Tokaji, H. Modification of mechanical properties of Si crystal irradiated by Kr-beam. Appl. Surf. Sci. 2015, 349, 123–128. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, X. Nanoindentation creep of plasma-enhanced chemical vapor deposited silicon oxide thin films. Scr. Mater. 2007, 56, 249–252. [Google Scholar] [CrossRef]
- Zeng, K.; Chen, Z.K.; Shen, L.; Liu, B. Study of mechanical properties of light-emitting polymer films by nano-indentation technique. Thin Solid Films 2005, 477, 111–118. [Google Scholar] [CrossRef]
- Martinatti, J.F.; Santos, L.V.; Durrant, S.F.; Cruz, N.C.; Rangel, E.C. Lubricating coating prepared by PIIID on a forming tool. J. Phys. Conf. Series. 2012, 370, 012022. [Google Scholar] [CrossRef]
- Lopes, B.B.; Rangel, R.C.C.; Antonio, C.A.; Durrant, S.F.; Cruz, N.C.; Rangel, R.C. Mechanical and Tribological Properties of Plasma Deposited a-C:H:Si:O Films, 1st ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0802-3. [Google Scholar]
- Albuquerque, M.D.F.; Santos, E., Jr.; Perdone, R.R.T.; Simao, R.A. Effect of selfbias voltage on the wettability, chemical functionality and nanomechanical properties of hexamethyldisiloxane films. Thin Solid Films 2014, 564, 73–78. [Google Scholar] [CrossRef]
- Wang, Z.B.; Tao, N.R.; Li, S.; Wang, W.; Liu, G.; Lu, J.; Lu, K. Effect of surface nanocrystallization on friction and wear properties in low carbon steel. Mater. Sci. Eng. A 2003, 352, 144–149. [Google Scholar] [CrossRef]
- Kostov, K.G.; Ueda, M.; Lepiensky, M.; Soares, P.C., Jr.; Gomes, G.F.; Silva, M.M.; Reuther, H. Surface modification of metal alloys by plasma immersion ion implantation and subsequent plasma nitriding. Surf. Coat. Technol. 2004, 186, 204–208. [Google Scholar] [CrossRef]
- Coclite, A.M.; de Luca, F.; Gleason, K.K. Mechanically robust silica-like coatings deposited by microwave plasmas for barrier applications. J. Vac. Sci. Technol. A 2012, 30, 061502. [Google Scholar] [CrossRef]
- Mello, C.B.; Ueda, M.; Oliveira, R.M.; Garcia, J.A. Corrosion effects of plasma immersion ion implantation-enhanced Cr deposition on SAE 1070 carbon steel. Surf. Coat. Technol. 2011, 205, S151–S156. [Google Scholar] [CrossRef]
- Barranco, V.; Carpentier, J.; Grundmeier, G. Correlation of morphology and barrier properties of thin microwave plasma polymer films on metal substrate. Electrochim. Acta 2004, 49, 1999–2013. [Google Scholar] [CrossRef]

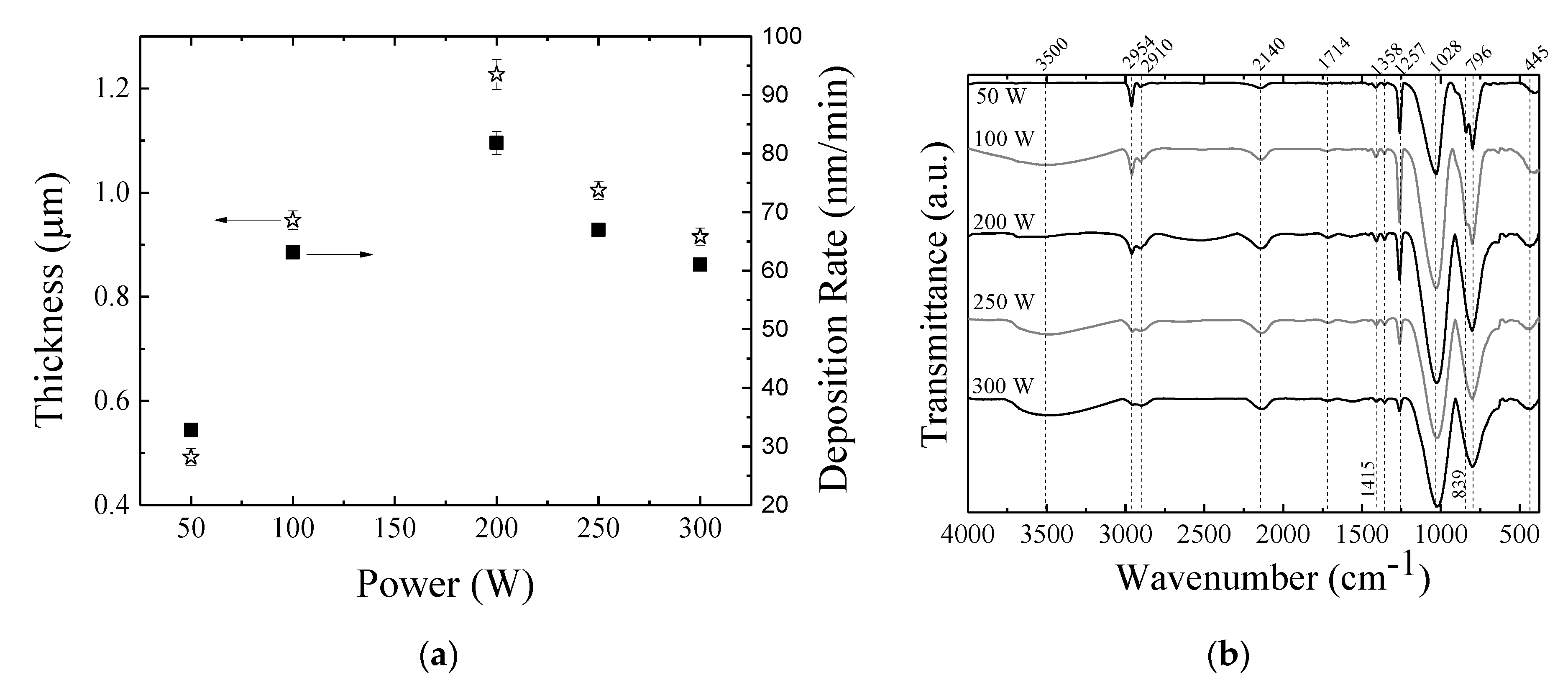

| Wavenumber (cm−1) | Mode |
|---|---|
| 3500 | OH in Si–OH |
| 2954 | ν C–H in CH3 and CH2 |
| 2910 | ν C–H in CH2 |
| 2140 | Si–H |
| 1714 | ν C=O in CH2O |
| 1415 | δ C–H in Si–(CH3)x |
| 1358 | δ C–H2 in Si–CH2–Si |
| 1257 | δ C–H3 in Si–(CH3)x |
| 1028 | ν Si–O in Si–O–Si and Si–O–C |
| 839 | ν Si–C in Si–(CH3)3 |
| 796 | ν Si–C in Si–(CH3)2 and Si–O in ν Si–O–Si |
| 445 | Si–O–Si (bending) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel, R.C.C.; Cruz, N.C.; Rangel, E.C. Role of the Plasma Activation Degree on Densification of Organosilicon Films. Materials 2020, 13, 25. https://doi.org/10.3390/ma13010025
Rangel RCC, Cruz NC, Rangel EC. Role of the Plasma Activation Degree on Densification of Organosilicon Films. Materials. 2020; 13(1):25. https://doi.org/10.3390/ma13010025
Chicago/Turabian StyleRangel, Rita C. C., Nilson C. Cruz, and Elidiane C. Rangel. 2020. "Role of the Plasma Activation Degree on Densification of Organosilicon Films" Materials 13, no. 1: 25. https://doi.org/10.3390/ma13010025
APA StyleRangel, R. C. C., Cruz, N. C., & Rangel, E. C. (2020). Role of the Plasma Activation Degree on Densification of Organosilicon Films. Materials, 13(1), 25. https://doi.org/10.3390/ma13010025