Electrochemical Evaluation of the Compact and Nanotubular Oxide Layer Destruction under Ex Vivo Ti6Al4V ELI Transpedicular Screw Implantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Oxide Film Preparation on Ti6Al4V ELI Transpedicular Screws
2.2. Implantation of Ti6Al4V ELI Transpedicular Screws
2.3. Degradation and Chemical Stability Analysis
3. Results and Discussion
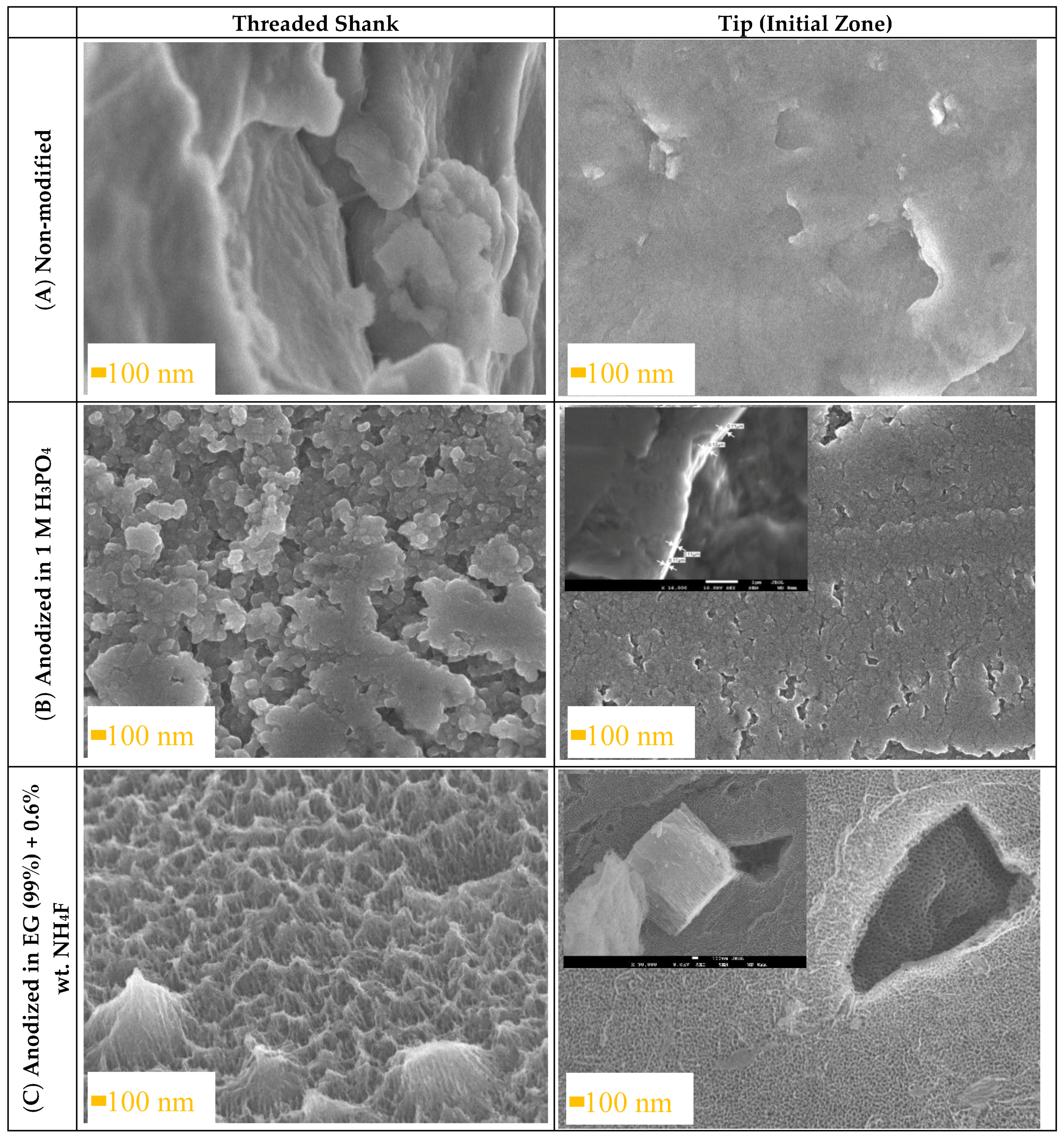
3.1. Anodizing of Ti6Al4V ELI Transpedicular Screws
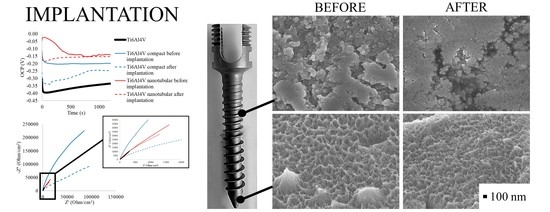
3.2. Optical Assessment of Oxide Layer Degradation after Implantation
3.3. Electrochemical Examination of Ti6Al4V ELI Transpedicular Screw after Implantation
3.3.1. Open Circuit Potential Measurements
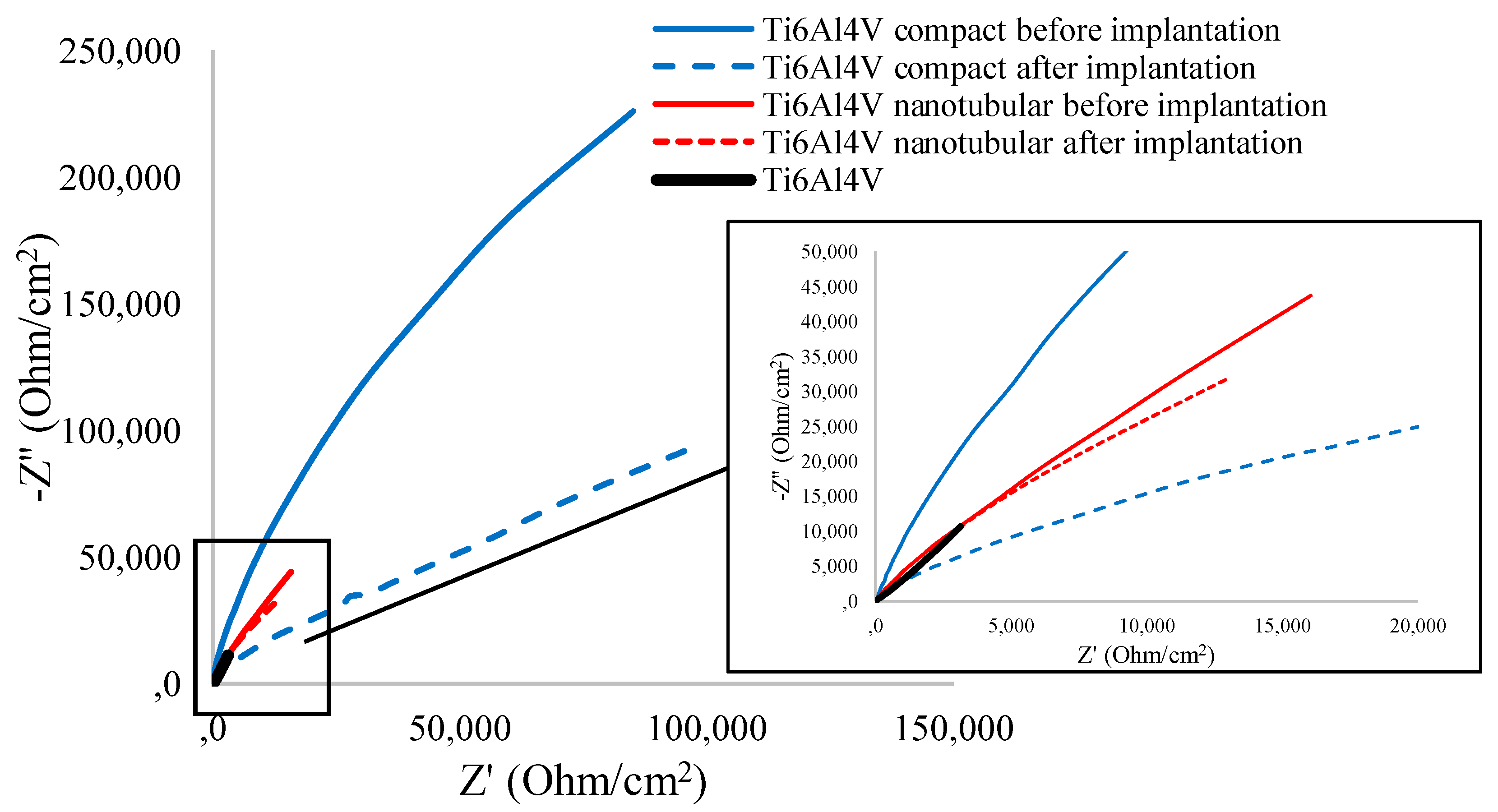
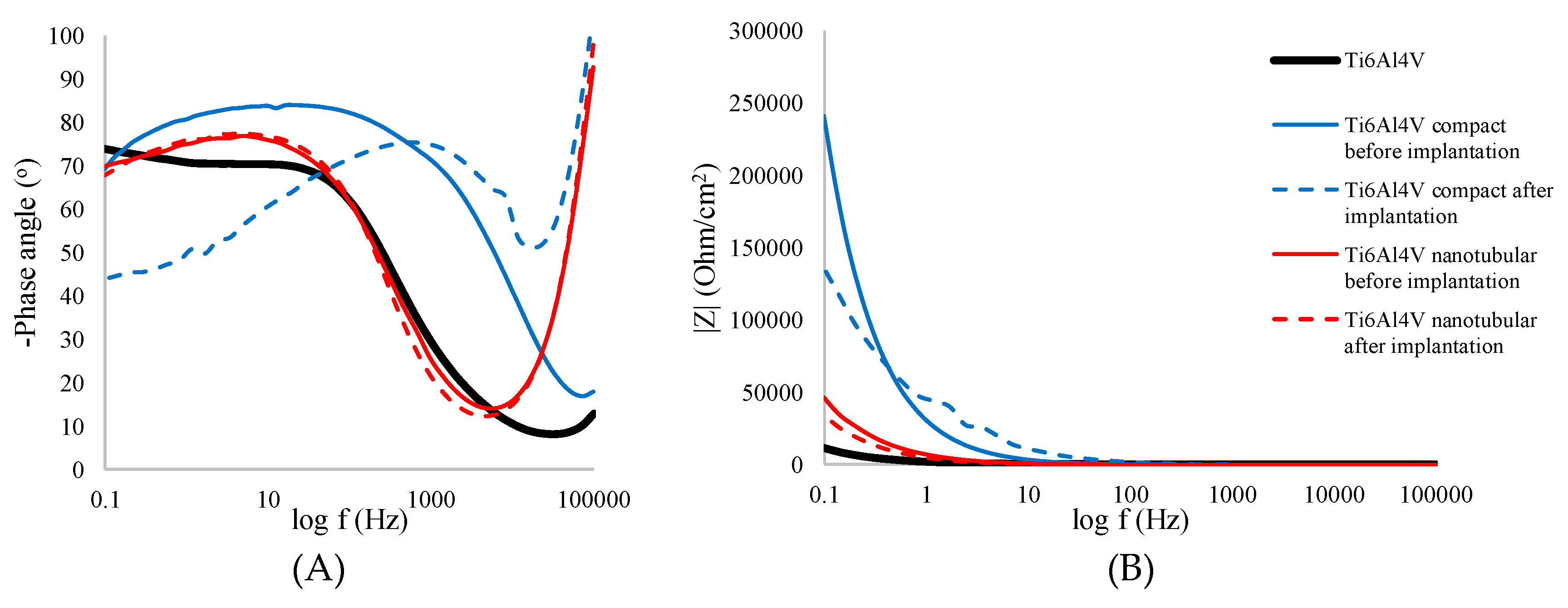
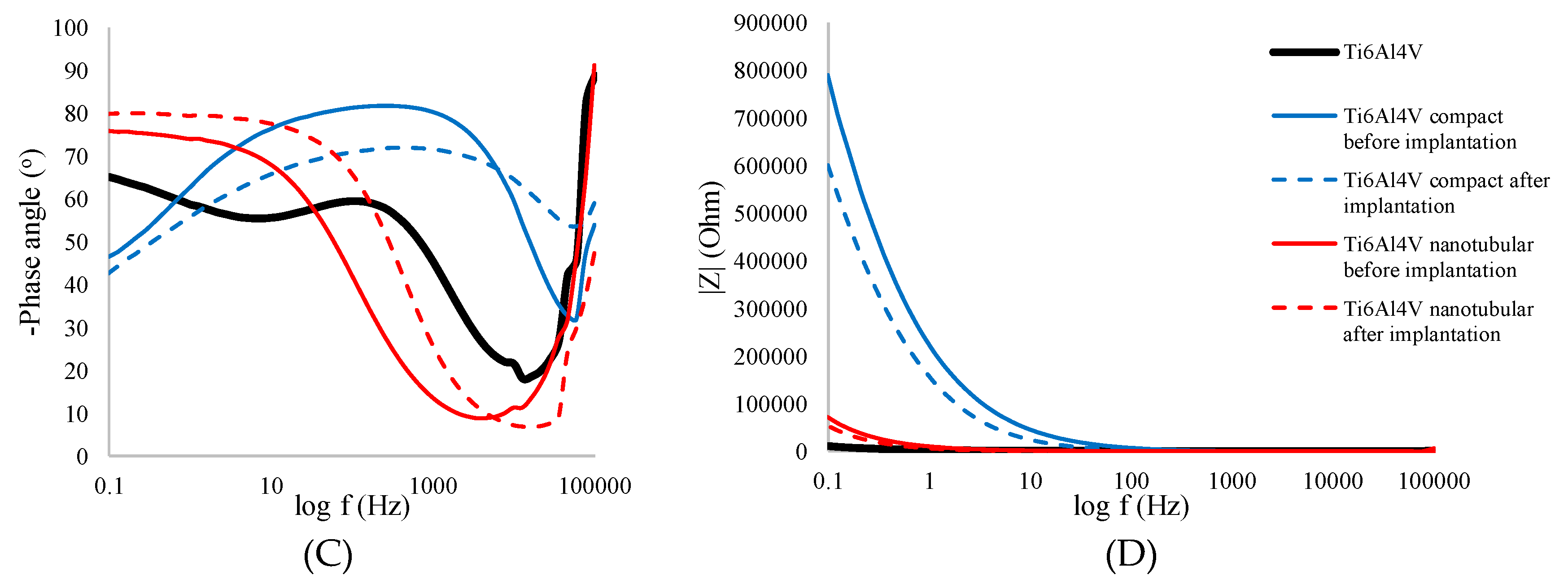
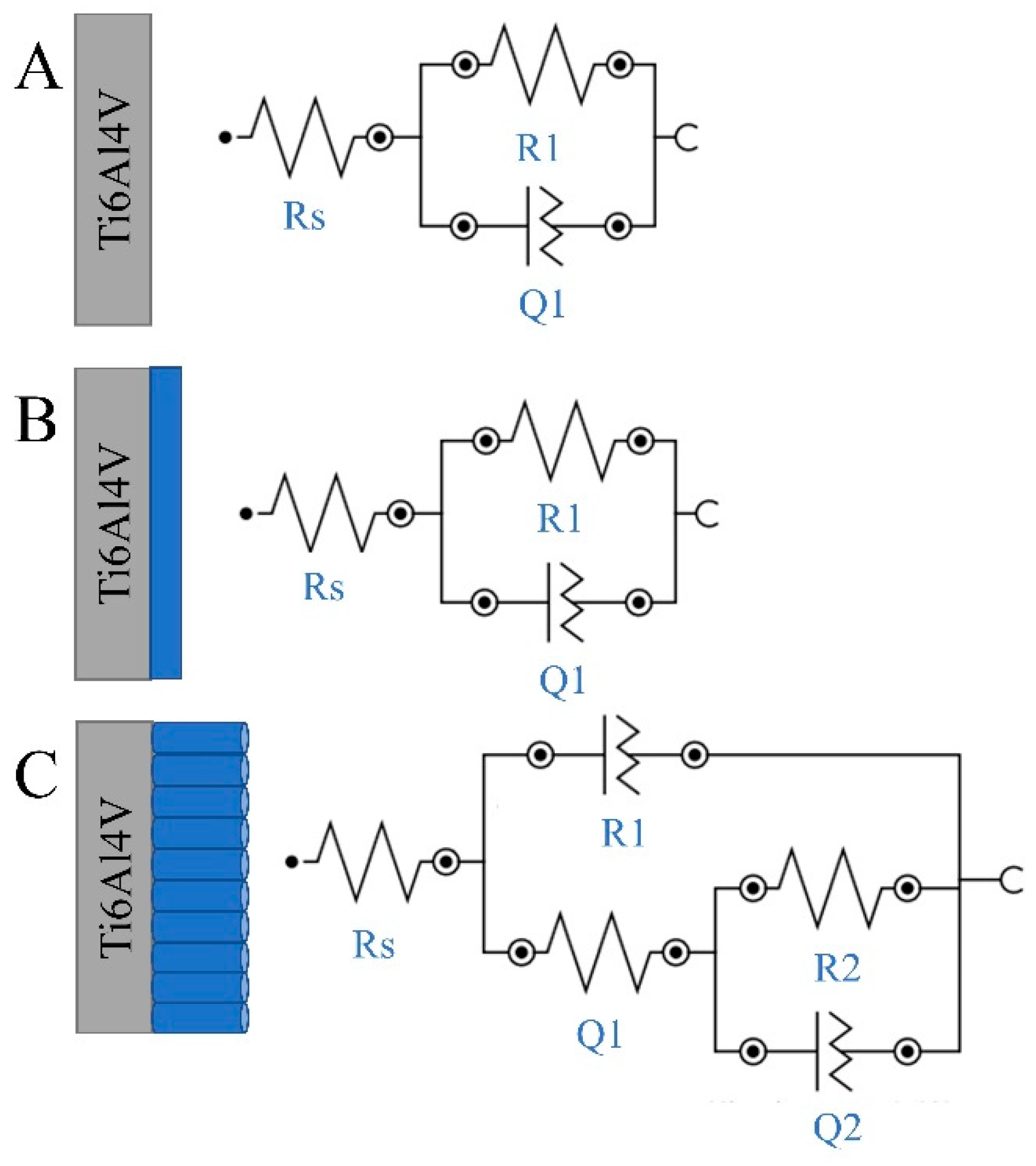
3.3.2. Electrochemical Impedance Spectroscopy Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mierzejewska, Z.A.; Hudák, R.; Sidun, J. Mechanical properties and microstructure of DMLS Ti6Al4V alloy dedicated to biomedical applications. Materials 2019, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C 2019, 96, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Lieblich, M.; Barriuso, S.; Multigner, M.; González-Doncel, G.; González-Carrasco, J.L. Thermal oxidation of medical Ti6Al4V blasted with ceramic particles: Effects on the microstructure, residual stresses and mechanical properties. J. Mech. Behav. Biomed. Mater. 2016, 54, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Bhattacharya, M.; Ratha, I.; Chakraborty, H.; Datta, S.; Ghosh, J.; Bysakh, S.; Sreemany, M.; Rane, R.; Joseph, A.; et al. Nano- and micro-tribological behaviours of plasma nitrided Ti6Al4V alloys. J. Mech. Behav. Biomed. Mater. 2018, 77, 267–294. [Google Scholar] [CrossRef]
- Kiel-Jamrozik, M.; Szewczenko, J.; Basiaga, M.; Nowińska, K. Technological capabilities of surface layers formation on implant made of Ti-6Al-4V ELI alloy. Acta Bioeng. Biomech. 2015, 17, 31–37. [Google Scholar]
- Han, B.; Zal Nezhad, E.; Musharavati, F.; Jaber, F.; Bae, S. Tribo-Mechanical Properties and Corrosion Behavior Investigation of Anodized Ti–V Alloy. Coatings 2018, 8, 459. [Google Scholar] [CrossRef]
- Atmani, D.; Saoula, N.; Abdi, A.; Azzaz, M.; Wang, Y.; Mohamedi, M. Structural, Morphological, and Electrochemical Corrosion Properties of TiO2 Formed on Ti6Al4V Alloys by Anodization. Cryst. Res. Technol. 2018, 53, 1–7. [Google Scholar] [CrossRef]
- Arkusz, K.; Paradowska, E.; Nycz, M.; Krasicka-Cydzik, E. Influence of Thermal Modification and Morphology of TiO2 Nanotubes on Their Electrochemical Properties for Biosensors Applications. J. Nanosci. Nanotechnol. 2018, 18, 3713–3721. [Google Scholar] [CrossRef]
- Kaczmarek-Pawelska, A.; Krasicka-Cydzik, E. Functionalized nanotubular oxide layer on Ti6Al4V as a regulator and biosensor of bone tissue remodeling. Arch. Mater. Sci. Eng. 2015, 73, 53–61. [Google Scholar]
- İzmir, M.; Ercan, B. Anodization of titanium alloys for orthopedic applications. Front. Chem. Sci. Eng. 2019, 13, 28–45. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Ghicov, A.; Schmuki, P. Self-organized nanotubular oxide layers on Ti-6Al-7Nb and Ti-6Al-4V formed by anodization in NH4F solutions. J. Biomed. Mater. Res.—Part A 2005, 75, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Filova, E.; Fojt, J.; Kryslova, M.; Moravec, H.; Joska, L.; Bacakova, L. The diameter of nanotubes formed on Ti-6Al-4V alloy controls the adhesion and differentiation of Saos-2 cells. Int. J. Nanomed. 2015, 10, 7145–7163. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Mazare, A.; Park, J.; Gongadze, E.; Killian, M.S.; Kralj, S.; von der Mark, K.; Iglič, A.; Schmuki, P. Protein interactions with layers of TiO2 nanotube and nanopore arrays: Morphology and surface charge influence. Acta Biomater. 2016, 45, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Park, I.S.; Oh, H.J.; Bae, T.S. Bioactivity and generation of anodized nanotubular TiO2 layer of Ti-6Al-4V alloy in glycerol solution. Thin Solid Films 2013, 548, 292–298. [Google Scholar] [CrossRef]
- Acquesta, A.; Carangelo, A.; Monetta, T. TiO2 Nanotubes on Ti Dental Implant. Part 3: Electrochemical Behavior in Hank’s Solution of Titania Nanotubes Formed in Ethylene Glycol. Metals 2018, 8, 489. [Google Scholar] [CrossRef]
- Saharudin, K.A.; Sreekantan, S.; Aziz, S.N.Q.A.A.; Hazan, R.; Lai, C.W.; Mydin, R.B.S.M.N.; Mat, I. Surface Modification and Bioactivity of Anodic Ti6Al4V Alloy. J. Nanosci. Nanotechnol. 2013, 13, 1696–1705. [Google Scholar] [CrossRef]
- Fraoucene, H.; Sugiawati, V.A.; Hatem, D.; Belkaid, M.S.; Vacandio, F.; Eyraud, M.; Pasquinelli, M.; Djenizian, T. Optical and Electrochemical Properties of Self-Organized TiO2 Nanotube Arrays From Anodized Ti−6Al−4V Alloy. Front. Chem. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, S.; Kralj-Iglic, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef]
- Arkusz, K. The influence of implantation on mechanical degradation of the nanotubular oxide layer on titanium screws. Adv. Intell. Syst. Comput. 2018, 623, 339–347. [Google Scholar]
- Descamps, S.; Awitor, K.O.; Raspal, V.; Johnson, M.B.; Bokalawela, R.S.P.; Larson, P.R.; Doiron, C.F. Mechanical Properties of Nanotextured Titanium Orthopedic Screws for Clinical Applications. J. Med. Device 2013, 7, 021005. [Google Scholar] [CrossRef]
- Monetta, T.; Acquesta, A.; Carangelo, A.; Bellucci, F. TiO2 Nanotubes on Ti Dental Implant. Part 1: Formation and Aging in Hank’s Solution. Metals 2017, 7, 167. [Google Scholar] [CrossRef]
- Oh, E.J.; Nguyen, T.D.T.; Lee, S.Y.; Jeon, Y.M.; Bae, T.S.; Kim, J.G. Enhanced compatibility and initial stability of Ti6Al4V alloy orthodontic miniscrews subjected to anodization, Cyclic precalcification, And heat treatment. Korean J. Orthod. 2014, 44, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.R.; Kolati, M.; Moser, T.; Sukotjo, C.; Shokuhfar, T. Survivability of TiO2 nanotubes on the surface of bone screws. Surf. Innov. 2013, 2, 60–68. [Google Scholar] [CrossRef]
- Cachon, T.; Pillard, P.; Odent, T.; Carozzo, C.; Viguier, E. Safe corridor for the implantation of thoracolumbar pedicle screws in growing pigs: A morphometric study. PLoS ONE 2017, 12, e0184857. [Google Scholar] [CrossRef] [PubMed]
- Stemper, B.D.; Marawar, S.V.; Yoganandan, N.; Shender, B.S.; Rao, R.D. Quantitative anatomy of subaxial cervical lateral mass: An analysis of safe screw lengths for Roy-Camille and Magerl techniques. Spine (Phila. Pa. 1976) 2008, 33, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Krishna, M. Formation of Different Titanium Oxide Morphology via Anodization and Its Effect on Hydrophillicity of the Substrate. J. Mater. Sci. Surf. Eng. 2017, 5, 581–584. [Google Scholar]
- Uttiya, S.; Contarino, D.; Prandi, S.; Carnasciali, M.; Gemme, G.; Mattera, L.; Rolandi, R.; Canepa, M.; Cavalleri, O. Anodic Oxidation of Titanium in Sulphuric Acid and Phosphoric Acid Electrolytes. J. Mater. Sci. Nanotechnol. 2014, 1, 1–8. [Google Scholar]
- Arkusz, K.; Krasicka-Cydzik, E. The effect of phosphates and fluorides, included in TiO2 nanotube layers on the performance of hydrogen peroxide detection. Arch. Metall. Mater. 2018, 63, 765–772. [Google Scholar]
- Su, E.P.; Justin, D.F.; Pratt, C.R.; Sarin, V.K.; Nguyen, V.S.; Oh, S.; Jin, S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Jt. J. 2018, 100B, 9–16. [Google Scholar] [CrossRef]
- Jäger, M.; Jennissen, H.P.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and osseointegration properties of nanostructured titanium orthopaedic implans. Materials 2017, 10, 1302. [Google Scholar] [CrossRef]
- Hao, Y.Q.; Li, S.J.; Hao, Y.L.; Zhao, Y.K.; Ai, H.J. Effect of nanotube diameters on bioactivity of a multifunctional titanium alloy. Appl. Surf. Sci. 2013, 268, 44–51. [Google Scholar] [CrossRef]
- Hansson, S.; Norton, M. The relation between surface roughness and interfacial shear strength for bone-anchored implants. A mathematical model. J. Biomech. 1999, 32, 829–836. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Krasicka-Cydzik, E.; Arkusz, K.; Kaczmarek, A. A mathematical model for detection of formation parameters of TiO2 nanotube by anodizing. Eng. Biomater. 2012, 15, 34–40. [Google Scholar]
- Cui, C.X.; Gao, X.; Qi, Y.M.; Liu, S.J.; Sun, J.B. Microstructure and antibacterial property of in situ TiO2 nanotube layers/titanium biocomposites. J. Mech. Behav. Biomed. Mater. 2012, 8, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Pina, V.G.; Dalmau, A.; Devesa, F.; Amigó, V.; Muñoz, A.I. Tribocorrosion behavior of beta titanium biomedical alloys in phosphate buffer saline solution. J. Mech. Behav. Biomed. Mater. 2015, 46, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.N.; Liu, M.N.; Wang, M.C.; Oloyede, A.; Bell, J.M.; Yan, C. Nanoindentation study of the mechanical behavior of TiO2 nanotube arrays. J. Appl. Phys. 2015, 118, 145301. [Google Scholar] [CrossRef]
- Shivaram, A.; Bose, S.; Bandyopadhyay, A. Mechanical degradation of TiO2 nanotubes with and without nanoparticulate silver coating. J. Mech. Behav. Biomed. Mater. 2016, 59, 508–518. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E.; Manabe, T.; Kobayashi, K. Comparison of metal concentrations in rat tibia tissues with various metallic implants. Biomaterials 2004, 25, 5913–5920. [Google Scholar] [CrossRef]
- Szafrańska, A.; Antolak-Dudka, A.; Baranowski, P.; Bogusz, P.; Zasada, D.; Małachowski, J.; Czujko, T. Identification of mechanical properties for titanium alloy Ti-6Al-4V produced using LENS technology. Materials 2019, 16, 886. [Google Scholar] [CrossRef]
- Flamini, D.O.; Saugo, M.; Saidman, S.B. Electrodeposition of polypyrrole on Nitinol alloy in the presence of inhibitor ions for corrosion protection. Corros. Sci. 2014, 81, 36–44. [Google Scholar] [CrossRef]
- Nycz, M.; Arkusz, K.; Pijanowska, D.G. Influence of the Silver Nanoparticles (AgNPs) Formation Conditions onto Titanium Dioxide (TiO2) Nanotubes Based Electrodes on Their Impedimetric Response. Nanomaterials 2019, 9, 1072. [Google Scholar] [CrossRef] [PubMed]
- Paradowska, E.; Arkusz, K.; Pijanowska, D.G. The Influence of the Parameters of a Gold Nanoparticle Deposition Method on Titanium Dioxide Nanotubes, Their Electrochemical Response, and Protein Adsorption. Biosensors 2019, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Mohammadi, I.; Sadrnezhaad, S.K. Hydroxyapatite based and anodic Titania nanotube biocomposite coatings: Fabrication, characterization and electrochemical behavior. Surf. Coat. Technol. 2016, 287, 67–75. [Google Scholar] [CrossRef]


| Samples | Solution | Voltage [V] | Time [min] |
|---|---|---|---|
| Ti6Al4V | - | - | - |
| Compact Ti6Al4V | 1 M H3PO4 | 20 | 20 |
| Nanotubular Ti6Al4V | EG + 0.6 wt. % NH4F + 1 wt.% H2O | 22 | 20 |
| Ti6Al4V ELI Transpedicular Screws | Ti [wt. %] | Al [wt. %] | V [wt. %] | O [wt. %] | |
|---|---|---|---|---|---|
| (A) Non-modified | 91.15 | 5.96 | 3.53 | --- | |
| (B) Anodized in 1 M H3PO4 | 72.6 | 4.56 | 3.6 | 19.19 | |
| (C) Anodized in EG (99%) + 0.6 wt. % NH4F | α-phase | 63.79 | 2.34 | --- | 33.96 |
| β-phase | 52.7 | 1.24 | 28.98 | 17.13 | |
| OCP in NaCl [V] | RSD [%] | OCP in PBS [V] | RSD [%] | |
|---|---|---|---|---|
| Ti6Al4V | −0.337 | 4.25 | −0.319 | 3.29 |
| Compact Ti6Al4V before implantation | −0.193 | 4.74 | −0.116 | 4.55 |
| Compact Ti6Al4V after implantation | −0.242 | 3.47 | −0.068 | 5.83 |
| Nanotubular Ti6Al4V before implantation | −0.129 | 7.82 | 0.021 | 6.37 |
| Nanotubular Ti6Al4V after implantation | −0.154 | 5.11 | 0.037 | 5.23 |
| |Z| [Ω/cm2] | RSD [%] | -Zphase [o] | RSD [%] | ReZ [Ω/cm2] | RSD [%] | -ImZ [Ω/cm2] | RSD [%] | |
|---|---|---|---|---|---|---|---|---|
| Ti6Al4V | 11358 | 5.45 | 73.39 | 1.65 | 3078 | 2.04 | 10594 | 4.65 |
| Before implantation | ||||||||
| Compact Ti6Al4V | 244399 | 7.68 | 69.29 | 1.80 | 85319 | 4.61 | 225727 | 7.72 |
| Nanotubular Ti6Al4V | 44261 | 6.43 | 69.81 | 2.05 | 16072 | 9.76 | 43697 | 6.39 |
| After implantation | ||||||||
| Compact Ti6Al4V | 264887 | 8.53 | 43.30 | 2.98 | 194309 | 5.18 | 183137 | 7.36 |
| Nanotubular Ti6Al4V | 37563 | 7.71 | 67.79 | 1.93 | 12994 | 9.13 | 31831 | 8.58 |
| Element | Ti6Al4V | Compact Ti6Al4V before Implantation | Compact Ti6Al4V after Implantation | Nanotubular Ti6Al4V before Implantation | Nanotubular Ti6Al4V after Implantation | |
|---|---|---|---|---|---|---|
| Rs [Ω] | 9.36 | 7.29 | 7.30 | 1.59 | 2.05 | |
| R1 [Ω] | 1.00 × 106 | 1.00 × 106 | 9.00 × 105 | 1.97 × 105 | 1.20 × 104 | |
| Q1 | C1 [F] | 1.30 × 10−4 | 6.10 × 10−6 | 8.47 × 10−6 | 2.84 × 10−5 | 3.89 × 10−5 |
| N1 | 0.79 | 0.92 | 0.55 | 0.86 | 0.87 | |
| τ1 = R1·C1 [s] | 130 | 6.1 | 7.62 | 5.59 | 0.47 | |
| R2 [Ω] | 2.64 × 105 | 0.69 × 105 | ||||
| Q2 | C2 [F] | 1.08 × 10−5 | 2.19 × 10−5 | |||
| N2 | 0.98 | 0.99 | ||||
| τ1 = R2·C2 [s] | 2.85 | 1.51 | ||||
| χ² | 0.016 | 0.0301 | 0.0320 | 0.0046 | 0.009 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkusz, K.; Nycz, M.; Paradowska, E. Electrochemical Evaluation of the Compact and Nanotubular Oxide Layer Destruction under Ex Vivo Ti6Al4V ELI Transpedicular Screw Implantation. Materials 2020, 13, 176. https://doi.org/10.3390/ma13010176
Arkusz K, Nycz M, Paradowska E. Electrochemical Evaluation of the Compact and Nanotubular Oxide Layer Destruction under Ex Vivo Ti6Al4V ELI Transpedicular Screw Implantation. Materials. 2020; 13(1):176. https://doi.org/10.3390/ma13010176
Chicago/Turabian StyleArkusz, Katarzyna, Marta Nycz, and Ewa Paradowska. 2020. "Electrochemical Evaluation of the Compact and Nanotubular Oxide Layer Destruction under Ex Vivo Ti6Al4V ELI Transpedicular Screw Implantation" Materials 13, no. 1: 176. https://doi.org/10.3390/ma13010176
APA StyleArkusz, K., Nycz, M., & Paradowska, E. (2020). Electrochemical Evaluation of the Compact and Nanotubular Oxide Layer Destruction under Ex Vivo Ti6Al4V ELI Transpedicular Screw Implantation. Materials, 13(1), 176. https://doi.org/10.3390/ma13010176